Abstract
Aims
G protein-coupled receptor kinase 2 (GRK2), which is markedly upregulated in failing human myocardium, has been implicated as a contributing factor or consequence of heart failure (HF). Importantly, cardiac-specific GRK2 knockout mice have recently proved the pathological nature of GRK2 in HF. Targeted inhibition of GRK2 is possible using a peptide inhibitor known as the βARKct, which has rescued several disparate small animal HF models. This study was designed to evaluate long-term βARKct expression in a clinically relevant large animal HF model, using stable myocardial gene delivery with adeno-associated virus serotype 6 (AAV6).
Methods and results
A porcine model of HF subsequent to left ventricular (LV) myocardial infarction (MI) was used to study the effects of retrograde injection into the anterior interventricular vein of either AAV6.βARKct or AAV6.luciferase as a control 2 weeks after MI. Echocardiography and LV hemodynamics were performed before and 6 weeks after gene transfer. Robust and long-term βARKct expression was found after AAV6-mediated delivery, leading to significant amelioration of LV haemodynamics and contractile function in HF pigs compared with AAV6.luciferase-treated control animals that showed a continued decline in cardiac function. Interestingly, the neurohormonal axis was virtually normalized in AVV6.βARKct-treated HF animals, represented by reductions in plasma norepinephrine levels, whereas AAV6.luciferase-treated pigs showed further increases in plasma catecholamine levels. As a result, LV remodelling and foetal gene expression was reversed by AVV6.βARKct gene therapy.
Conclusion
These data—showing sustained amelioration of cardiac function in a post-MI pig HF model—demonstrate the therapeutic potential of βARKct gene therapy for HF.
Keywords: Heart failure, Gene therapy, GRK2, βARKct, Adeno-associated virus
Introduction
Despite recent advances in treatment strategies, heart failure (HF) remains a leading cause of morbidity, with annual patient mortality rates ranging from 25 to 30%.1 These facts highlight an important gap between current therapeutic approaches and key underlying biological processes relating to cardiomyocytes in the setting of chronic cardiac dysfunction. A major molecular hallmark of HF is deranged cardiac β-adrenergic receptor (β-AR) signalling.2 Abnormal β-AR inotropic responsiveness leads to an increased sympathetic drive with the upregulation of tissue and plasma catecholamines in an attempt to stimulate myocardial contractile function.2 This sustains a vicious cycle, as the chronic stimulation of cardiac β-ARs perpetuates a loss of signalling due in part to a marked upregulation of G protein-coupled receptor kinase 2 (GRK2), which desensitizes β-ARs in the heart through phosphorylation.3,4 Thus, a continual state of activation and abnormal signalling is created and chronic catecholamine bombardment of the heart can contribute to further cardiac deterioration.2 The successful use of β-AR blockers in clinical HF treatment demonstrates the effectiveness of an approach aimed at interrupting this vicious cycle of sympathetic tone, by blocking chronic β-AR activation; however, these agents do not address the molecular defects in failing cardiomyocytes directly.
Indeed, myocardial GRK2 activity appears to play a profound pathological role in cardiac function. Most recently, the pathological nature of GRK2 was directly demonstrated using inducible, cardiac-specific knockout mice where GRK2 was deleted in ventricular myocytes after myocardial infarction (MI), and there was a significant improvement in overall animal survival and cardiac function.4 This gave a clear indication that lowering GRK2 was therapeutic in HF and its upregulation was not a beneficial adaptive change due to compromised β-AR signalling and cardiac function. Since no suitable small-molecule GRK2 inhibitor exists for targeted GRK2 inhibition in HF, we have engineered a peptide inhibitor that blocks the membrane translocation and activation of GRK2.5 This peptide, known as the βARKct competes with endogenous GRK2 for membrane binding to the βγ subunits of activated heterotrimeric G proteins (Gβγ) and has been shown to inhibit GRK2 activity on several receptors, including β-ARs.2,5 Further, transgenic or viral (adenovirus and adeno-associated virus, AAV) expression of the βARKct to compromised myocardium has been found to rescue several models of experimental HF.6–10 Interestingly, all data with βARKct-mediated HF rescue include normalization of β-AR signalling, including receptor upregulation (to normal levels) and GRK2 lowering. Since long-term β-AR antagonism can promote receptor upregulation and GRK2 downregulation, improved β-AR signalling is also a natural consequence of clinical β-AR blocker use.11,12 Therefore, β-AR blocker use and molecular GRK2 inhibition may act synergistically. Indeed, this has been shown in several small animal models of GRK2 inhibition and HF.4,6,8
Although data with cardiac βARKct expression, either in genetic mouse models of HF or in viral-mediated myocardial gene transfer, have been positive, studies completed to date have not been done in anything larger than a rabbit. Ultimately, to test whether βARKct and GRK2 inhibition is a potential therapeutic approach for treating HF patients, a large animal efficacy study is needed. In a clinically relevant large animal (swine) model of post-MI systolic HF, our current data demonstrate a sustained amelioration of cardiac function with adeno-associated virus serotype 6 (AAV6)-mediated βARKct delivery, with significant improvements of contractile function, reductions in plasma catecholamine levels, and subsequent reversal of cardiac remodelling and foetal gene expression. Thus, βARKct translation to human HF patients appears warranted.
Methods
For a detailed description of Methods, we refer to Supplementary material online.
Study protocol
The present investigation was carried out according to the Guide for the Care and Use of Laboratory Animals and was approved by the Animal Care and Use Committee of the state of Baden-Württemberg. Ischaemic cardiomyopathy/HF was created by left circumflex coronary artery (LCX) MI. Myocardial infarction was induced by occlusion of the LCX with a Maverick 3.0 × 12 mm PTCA balloon (Boston Scientific Natick, MA, USA) for 2 h. Two weeks later, baseline cardiac function was determined (haemodynamics, echocardiography under basal and catecholamine-stressed conditions and blood sample collection for biomarker assessment) and animals were treated with AAV6.βARKct or AAV6.luciferase as control. Forty-two days later/56 days after MI, cardiac function was determined (haemodynamics under basal and catecholamine-challenging conditions, echocardiography, as well as blood sample collection for biomarker assessment). Animals were euthanized for standardized autopsy. Tissue samples were collected for molecular analyses.
Gene transfer using retrograde injection into the coronary veins
Animals were anaesthetized with an intramuscular injection of ketamin 15 mg/kg body weight (ketamin 10%, Beta-pharm, Augsburg, Germany), azaperone 10 mg/kg (Stresnil, Janssen-Cilag, Neuss, Germany), 1 mg of atropinsulphate (Braun, Melsungen, Germany), and 5 mg of midazolam (Hoffmann-La Roche, Grenzach-Wyhlen, Germany), followed by intravenous injection of 10 mg of midazolam and 0.1 mg of fentanyl (Janssen-Cilag). After intubation, animals were subjected to inhalation anaesthesia using 1.5% isoflurane (Baxter, Unterschleissheim, Germany) and catheter introducer sheaths were placed in the right carotid artery and the right internal jugular vein as reported previously.13 Blood samples were collected and evaluation of cardiac function was carried out (echocardiography and haemodynamics). For selective retrograde injection, the anterior interventricular vein (AIV) was catheterized using a 6F retroinfusion catheter.13 In all animals, a 7F guiding catheter was placed in the left coronary artery and the left anterior descending coronary artery (LAD) was wired. During retrograde delivery of the AAV vectors, the LAD was occluded by a PTCA balloon distal to the first diagonal branch in all groups. Animals were randomized to treatment (n = 10 control virus AAV6.Luciferase and n = 10 AAV6.βARKct). Adeno-associated virus serotype 6 vectors [1E13 vector genomes (vg) per animal] were diluted in 50 mL of 0.9% saline solution supplemented with 0.5 mg of substance P (Sigma Aldrich, St Louis, MO, USA) and 6 mg of adenosine (Sanofi-Aventis, Frankfurt, Germany). Vector solution was injected in three portions into the AIV over each 3 min, whereas the balloon at the tip of the retroinfusion catheter was inflated to block venous outflow and the PTCA balloon in the LAD was inflated to block arterial inflow. After each injection, both balloons were deflated for 3 min to allow reflow. At the end, all catheters and introducer sheaths were removed and animals recovered.
Statistical analysis
Data are generally expressed as mean ± standard error of the mean (SEM). Data analysis was performed using SPSS 19 (SPSS, Inc., Chicago, IL, USA). An unpaired two-tailed t-test was performed when appropriate. A Kruskal–Wallis test in combination with a Wilcoxon rank-sum test was used in case of very small sample size (at most four animals/group) where a Gaussian distribution could not be assumed. One-way analysis of variance (ANOVA) or a two-way repeated measurement ANOVA was performed for between-group comparisons. The Bonferroni method was used to adjust for multiple comparisons and the computed P-values were reported.
P-values of <0.05 were considered statistically significant. The post hoc power of all ANOVAs showing significant results ranged between 0.7 and 0.98. In case of low power (1 − β < 0.8), this was indicated and absolute P-values were reported.
Results
Large animal model of post-myocardial infarction systolic heart failure
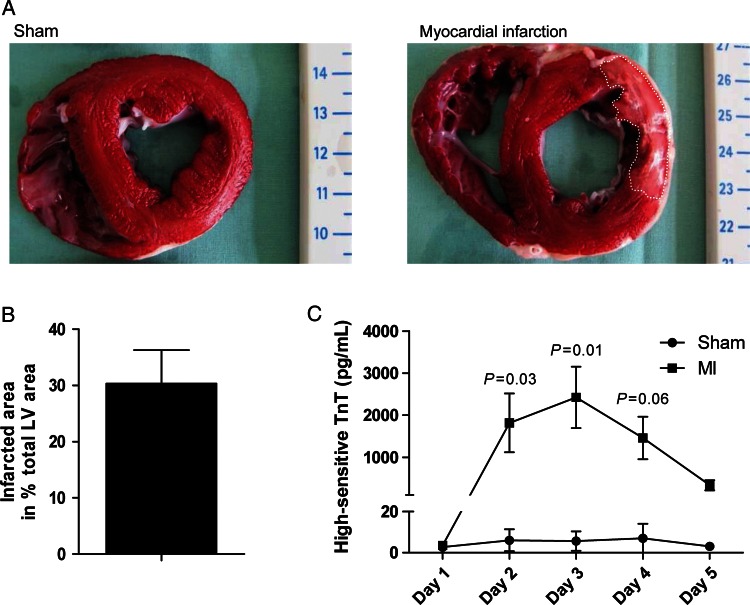
Since ischaemic cardiomyopathy is the primary cause of HF, we chose a porcine post-MI HF model to test the potential translation of our βARKct gene therapy approach. In this pre-clinical large animal model, 2h of LCX occlusion led to significant rise in serum high-sensitive troponin T (hsTnT) levels indicating significant loss of viable myocardium (Figure 1A and C). Four days after MI, an infarct size of 30.3 ± 5.9% (mean ± SEM; n = 3) of the left ventricle was observed (Figure 1B). Thus, this method of MI in the pig represents an appropriate HF model to evaluate potential therapeutics. For AAV6.βARKct gene therapy, we delivered 1 × 1013 vg to pigs 2 weeks after MI, and as a control AAV6.luciferase was delivered to a group of pigs. These groups were randomly assigned after post-MI cardiac functional assessment, and post-MI pigs in HF were followed for another 6 weeks after gene transfer.
Figure 1.
Characterization of heart failure/ischaemic cardiomyopathy model. (A) Representative triphenyltetrazolium chloride-stained slices of the heart of a sham animal and an infarcted animal 4 days after sham/myocardial infarction procedure. (B) Quantification of the infarct size 4 days post-myocardial infarction (n = 3 animals, mean ± SEM). (C) Quantification of high-sensitive troponin T serum levels for 4 consecutive days post-myocardial infarction (n = 3 animals/group). There was significant interaction between group and time (P = 0.014), indicating a different course of high-sensitive troponin T over time between myocardial infarction and sham group.
Efficient and specific βARKct transgene expression in the targeted myocardium
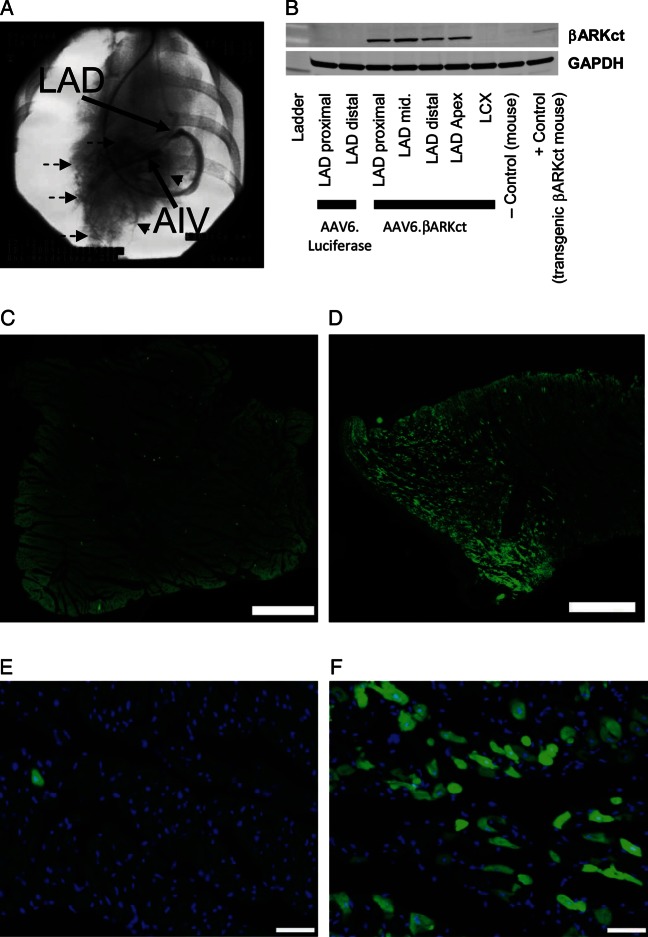
Gene delivery to post-MI pigs was done by retroinfusion into the AIV (Figure 2A; see Methods). Both transgenes (βARKct and luciferase) were under the control of a CMV-enhanced myosin light chain promoter to target expression in a cardiac-selective manner. We found robust protein expression of both transgenes specifically in the left ventricular (LV) tissue up to 10 weeks post-gene transfer either via western blotting for the βARKct (Figure 2B) or luciferase activity measurements (data not shown). Expression was restricted to the LAD target area because no expression was detected in the LCX territory (Figure 2B), and no expression was found in extracardiac tissue (data not shown). βARKct-treated pigs showed that a large area of the LV free wall (LAD territory) from basal myocardium to the apex was transduced homogenously and expressing the βARKct peptide (Figure 2B). To further visualize transgene distribution, retrograde delivery of AAV6.EGFP into the AIV was performed in an additional sham animal. Interestingly, direct immunofluorescence 6 weeks post-gene delivery showed efficient EGFP expression in the targeted (LAD) area (Figure 2D and F), whereas EGFP expression was almost absent in the non-targeted (LCX) control region (Figure 2C and E).
Figure 2.
Efficiency of βARKct gene delivery by retrograde coronary venous injection. (A) Representative delineation of the gene-transfected left anterior descending coronary artery territory (dashed arrows) by injection of contrast medium into the anterior interventricular vein (AIV); the retroinfusion catheter is blocked by a balloon at its tip, thereby preventing venous drainage. (B) βARKct transgene expression detected by western blotting in representative animals treated with AAV6.βARKct in comparison with animals treated with AAV6.luciferase control virus. Direct EGFP immunofluorescence showing transgene (EGFP-) distribution in the non-targeted (left circumflex coronary artery control, C and E) and in the targeted (LAD) area (D and F) in an additional sham animal with retrograde delivery of AAV6-EGFP into the anterior interventricular vein 6 weeks post-gene delivery; scale bar: (C and D): 1 mm; (E and F): 50 µm.
βARKct expression in the heart improves post-myocardial infarction global cardiac haemodynamic function
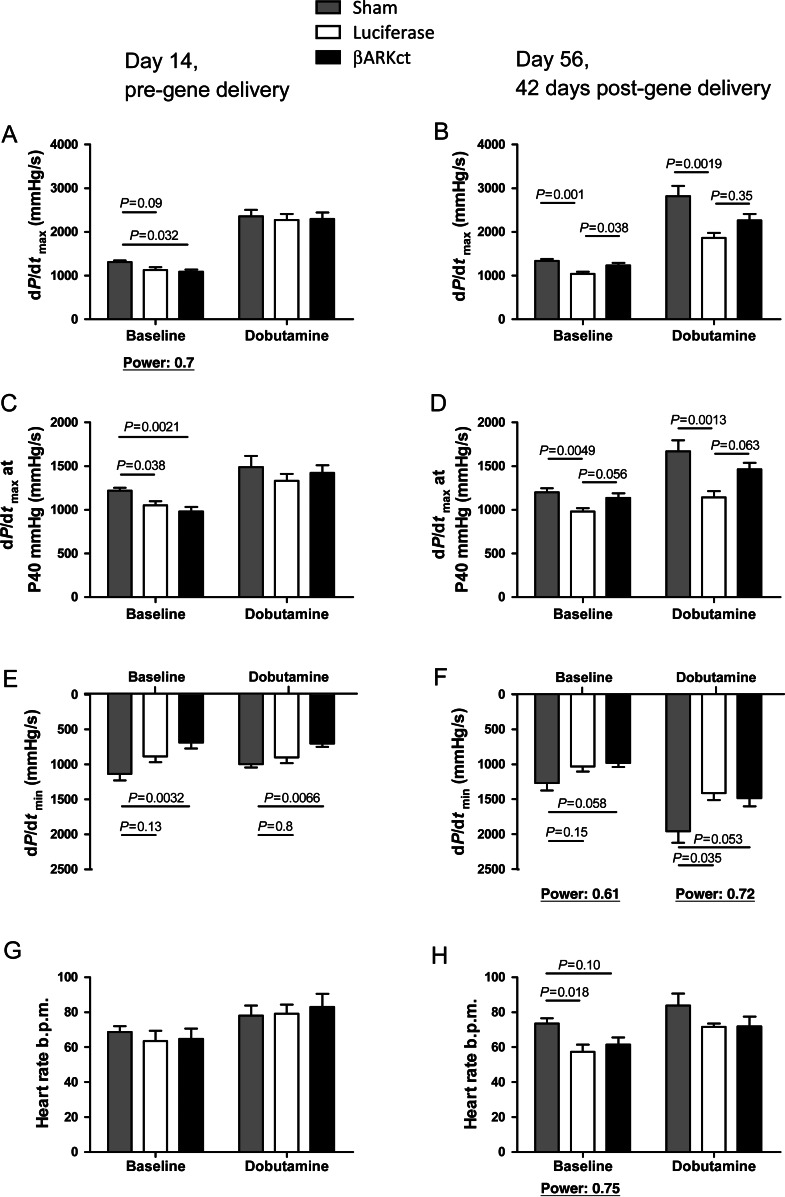
We assessed global cardiac function through catheterization of the LV and haemodynamic measurements (Figure 3, Table 1). Initially, haemodynamic cardiac function in pigs was analysed 2 weeks after MI and a significant loss of systolic and diastolic LV function was documented compared with sham-treated pigs that received no myocardial ischaemia (Figure 3A, C and E). No significant differences were observed in heart rate (Figure 3G). Post-MI pigs were then randomly assigned to receive either AAV6.βARKct or AAV6.luciferase as described above. Six weeks after retrograde gene delivery (56 days post-MI), the pigs were assessed again and these haemodynamic measurements revealed significant amelioration of global cardiac function by AAV6.βARKct treatment. Cardiac catheterization revealed preservation of β-adrenergic responsiveness in AAV6.βARKct-treated pigs represented by sustained LV contractility (LV + dP/dtmax; LV + dP/dtmax at 40 mmHg), whereas in AAV6.luciferase treated post-MI pigs β-AR contractile function worsened (Figure 3B and D). No significant differences were observed in LV relaxation (LV + dP/dtmin; Tau) (Figure 3F, Table 1) and heart rate (Figure 3H). To correct for variations in heart rate, dP/dtmax is plotted with heart rate in Supplementary material online, Figure S1.
Figure 3.
Amelioration of left ventricular haemodynamics in post-myocardial infarction heart failure by AAV6.βARKct gene therapy. (A and B) Haemodynamic assessment of left ventricular contractility under basal conditions and β-adrenergic receptor stimulation with dobutamine (dP/dtmax) pre (A) and 42 days post (B) gene delivery. (C and D) Haemodynamic assessment of left ventricular contractility under basal conditions and dobutamine stimulation at 40 mmHg (dP/dtmax at P40 mmHg) pre (C) and 42 days post (D) gene delivery. (E and F) Haemodynamic assessment of left ventricular relaxation under basal conditions and dobutamine stimulation (dP/dtmin) pre (E) and 42 days post (F) gene delivery. (G and H) Assessment of heart rate under basal conditions and dobutamine stimulation (dP/dtmin) pre (G) and 42 days post (H) gene delivery. n = 10 animals/group.
Table 1.
Haemodynamics and echocardiography
| Baseline |
Dobutamine |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | Luciferase |
βARKct |
Sham | Luciferase |
βARKct |
|||||||
| Mean ± SEM | Mean ± SEM | P-value vs. sham | Mean ± SEM | P-value vs. sham | P-value vs. luciferase | Mean ± SEM | Mean ± SEM | P-value vs. sham | Mean ± SEM | P-value vs. sham | P-value vs. luciferase | |
| Day 14 | ||||||||||||
| HR (b.p.m.) | 68 ± 3 | 64 ± 6 | 1.00 | 65 ± 6 | 1.00 | 1.00 | 81 ± 5 | 79 ± 5 | 1.00 | 83 ± 7 | 1.00 | 1.00 |
| LVP sys (mmHg) | 79 ± 2 | 72 ± 2 | 0.48 | 68 ± 3 | 0.06 | 0.91 | 91 ± 2 | 86 ± 2 | 0.99 | 83 ± 3 | 0.17 | 0.99 |
| LVP dia (mmHg) | 1 ± 1 | 0 ± 0 | 1.00 | 0 ± 1 | 1.00 | 1.00 | 0 ± 1 | 0 ± 0 | 1.00 | 0 ± 1 | 1.00 | 1.00 |
| LVEDP (mmHg) | 7 ± 0.6 | 7.6 ± 0.7 | 1.00 | 6.3 ± 0.5 | 1.00 | 1.00 | 6.8 ± 0.5 | 7.6 ± 0.7 | 1.00 | 6.8 ± 0.7 | 1.00 | 1.00 |
| dP/dtmax (mmHg/s) | 1335 ± 40 | 1135 ± 70 | 0.09 | 1092 ± 48 | 0.032 | 1.00 | 2399 ± 139 | 2280 ± 124 | 1.00 | 2300 ± 137 | 1.00 | 1.00 |
| dP/dt+ at P40mmHg (mmHg/s) | 1219 ± 30 | 1052 ± 45 | 0.038 | 981 ± 48 | 0.0021 | 0.79 | 1489 ± 120 | 1334 ± 75 | 0.84 | 1422 ± 82 | 1.00 | 1.00 |
| dP/dtmin (mmHg/s) | 1124 ± 89 | 884 ± 80 | 0.13 | 686 ± 81 | 0.0032 | 0.40 | 987 ± 44 | 896 ± 79 | 0.80 | 700 ± 46 | 0.0066 | 0.10 |
| Tau Mirsky (ms) | 44 ± 4 | 46 ± 4 | 1.00 | 58 ± 5 | 0.16 | 0.29 | 44 ± 6 | 63 ± 7 | 0.50 | 69 ± 5 | 0.17 | 1.00 |
| EDD (cm) | 4 ± 0.2 | 4.3 ± 0.2 | 0.56 | 4.1 ± 0.1 | 1.00 | 1.00 | ||||||
| ESD (cm) | 2.6 ± 0.2 | 3.2 ± 0.2 | 0.10 | 3.1 ± 0.1 | 0.18767 | 1.00 | ||||||
| FS (%) | 34.1 ± 2 | 27 ± 2 | 0.026 | 24 ± 1 | 0.00188 | 0.93 | ||||||
| Day 56 | ||||||||||||
| HR (b.p.m.) | 73 ± 3 | 57 ± 4 | 0.018 | 61 ± 4 | 0.10 | 1.00 | 81 ± 6 | 72 ± 2 | 0.33 | 72 ± 5 | 0.34 | 1.00 |
| LVP sys (mmHg) | 80 ± 2 | 76 ± 2 | 0.69 | 81 ± 3 | 1.00 | 0.30 | 97 ± 3 | 85 ± 1 | 0.0037 | 90 ± 2 | 0.15 | 0.40 |
| LVP dia (mmHg) | −1 ± 0 | 1 ± 0 | 0.028 | 1 ± 1 | 0.12 | 1.00 | −2 ± 1 | 1 ± 0 | 0.0044 | 0 ± 1 | 0.026 | 1.00 |
| LVEDP (mmHg) | 6.1 ± 0.5 | 9.6 ± 1.2 | 0.027 | 8.1 ± 0.7 | 0.36 | 0.71 | 7 ± 0.6 | 7.6 ± 0.7 | 0.018 | 6.8 ± 0.7 | 0.32 | 0.59 |
| dP/dtmax (mmHg/s) | 1383 ± 43 | 1042 ± 48 | 0.0011 | 1234 ± 53 | 0.51 | 0.038 | 2662 ± 223 | 1864 ± 109 | 0.0019 | 2262 ± 143 | 0.10 | 0.35 |
| dP/dt+ at P40mmHg (mmHg/s) | 1202 ± 44 | 979 ± 37 | 0.0049 | 1139 ± 47 | 0.99 | 0.056 | 1669 ± 118 | 1142 ± 68 | 0.0013 | 1463 ± 68 | 0.39 | 0.06 |
| dP/dtmin (mmHg/s) | 1251 ± 101 | 1031 ± 69 | 0.15 | 980 ± 57 | 0.06 | 1.00 | 1112 ± 78 | 852 ± 61 | 0.035 | 871 ± 82 | 0.053 | 1.00 |
| Tau Mirsky (ms) | 48 ± 9 | 36 ± 2 | 0.64 | 47 ± 5 | 1.00 | 0.67 | 58 ± 7 | 48 ± 6 | 0.61 | 55 ± 4 | 1.00 | 1.00 |
| EDD (cm) | 4.2 ± 0.1 | 5.2 ± 0.3 | 0.0074 | 5.5 ± 0.2 | 0.00044 | 0.87 | ||||||
| ESD (cm) | 2.6 ± 0.1 | 3.8 ± 0.3 | 0.00066 | 3.9 ± 0.2 | 0.00017 | 1.00 | ||||||
| FS (%) | 38.2 ± 1.8 | 27.5 ± 2.2 | 0.0014 | 28.5 ± 1.3 | 0.0039 | 1.00 | ||||||
| % Change of FS | 18 ± 11 | 2 ± 4.5 | 0.96 | 25 ± 14.1 | 1.00 | 0.45 | ||||||
n = 10 for each group, P values based on Bonferroni procedure for multiple comparisons. Significant P-values are shown in bold; non-significant P-values are italic.
Cardiac AAV6.βARKct gene transfer preserves myocardial β-AR inotropic responsiveness in post-MI heart failure through normalization of the catecholaminergic axis
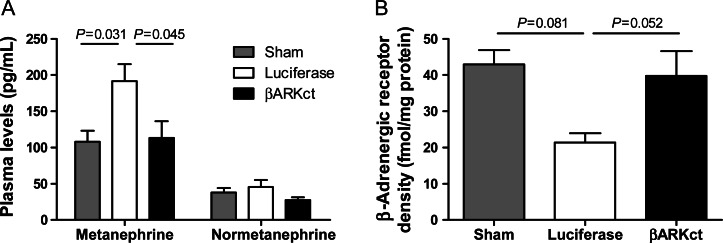
To determine whether the preservation of β-adrenergic responsiveness and improved cardiac function was due to a lowering of sympathetic overdrive due to a negative feedback after GRK2 inhibition and less receptor desensitization, we measured plasma catecholamine levels at the end of the study period. Indeed, plasma metanephrine levels after AAV6.βARKct treatment were significantly lower compared with AAV6.luciferase-treated control HF pigs and were in the same range as in sham-treated normal pigs without HF (Figure 4A). These results are consistent with a long-term reduction of sympathetic drive in HF when myocardial GRK2 is inhibited.
Figure 4.
Normalization of the catecholaminergic axis and myocardial β-adrenergic receptor responsiveness in heart failure post-myocardial infarction by AAV6.βARKct gene therapy. (A) Detected plasma levels of metanephrine and normetanephrine as direct degradation products of adrenalin and noradrenalin; blood samples were drawn 56 days post-myocardial infarction/42 days post gene therapy, n= 8 animals/group. (B) β-Adrenergic receptor-density by radioligand binding assay 56 days post-myocardial infarction/42 days post-gene therapy, n = 3 animals/sham and AAV6.luciferase and n = 4 animals/AAV6.βARKct group.
To further delineate whether the preservation of β-adrenergic responsiveness is indeed due to altered β-AR-density, β-AR-binding was determined by a radio immunosorbent assay in myocardial snap-frozen tissue samples at the study end. In control-treated (AAV6.luciferase) animals post-MI, a reduction in total β-AR-density was observed (Figure 4B). Also in AAV6.βARKct-treated animals, a small reduction in total β-AR density was found, but total β-AR density was higher compared with control-treated pigs (Figure 4B). The missing significance of the between-group comparisons is probably due to the low power of the Wilcoxon–Mann–Whitney test. Nevertheless, these data are underscoring the assumption that overall sympathetic tone is reduced in AAV6.βARKct-treated animals contributing to the preservation of cardiac function.
βARKct expression in the post-myocardial infarction failing heart reverses adverse left ventricular remodelling
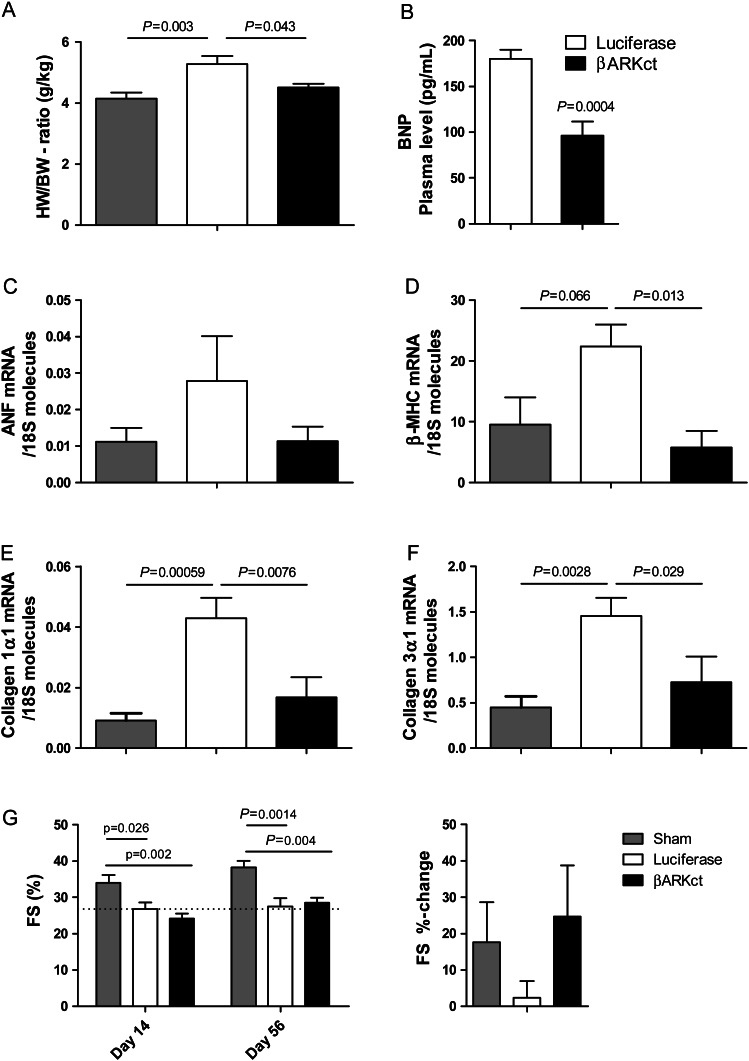
Heart failure is associated with adverse LV remodelling leading to the dilation of the LV and maladaptive hypertrophy with an increase in the heart-to-body weight ratio. The heart-to-body weight ratio was measured 56 days post-MI (42 days post-gene therapy) and control HF pigs (treated with AAV6.luciferase) showed a significant increase in cardiac mass compared with sham animals (Figure 5A). Interestingly, AAV.βARKct-treated HF animals had hearts with significantly lower cardiac mass that was indistinguishable from sham controls (Figure 5A). In addition to cardiac mass, molecular markers of maladaptive cardiac hypertrophy and adverse LV remodelling were quantified by RT-PCR in myocardial tissue samples from the targeted non-infarcted (LAD) area harvested at the study end. Interestingly, markers of foetal gene activation were found to be significantly lower in HF pigs treated with AAV6.βARKct compared with luciferase-treated HF animals. This includes plasma BNP (brain natriuretic peptide) levels (Figure 5B), which indicates a reduction of LV volume overload and wall stress in AAV6.βARKct-treated pigs. In line with this, adverse remodelling gene expression as represented by marked upregulation of the foetal gene programme [atrial natriuretic factor (ANF) and β-myosin heavy chain (β-MHC)] was observed in AAV6.luciferase-treated pigs post-MI, whereas chronic βARKct expression resulted in reductions in ventricular ANF mRNA levels (Figure 5C) and β-MHC gene expression (Figure 5D), which indicates a reversal of foetal gene expression in post-MI HF.
Figure 5.
AAV6.βARKct gene therapy inhibits adverse left ventricular remodelling in post-myocardial infarction heart failure. (A) Heart-to-body weight ratio 56 days post-myocardial infarction/42 days post-gene therapy. (B) Detected brain natriuretic peptide plasma levels 56 days post-myocardial infarction/42 days post-gene therapy. AAV6.βARKct gene therapy inhibits foetal gene expression in post-myocardial infarction heart failure; quantitative real-time PCR analysis on cardiac tissue isolated from the targeted non-infarcted left ventricular myocardium at day 56 post-myocardial infarction/day 42 post-gene therapy. (C) Atrial natriuretic factor mRNA molecules normalized to 18S molecules. (D) β-Myosin heavy chain mRNA molecules normalized to 18S molecules. (E) Collagen 1α1 mRNA molecules normalized to 18S molecules. (F) Collagen 3α1 mRNA molecules normalized to 18S molecules. (G) Determination of fractional shortening in non-targeted left ventricular areas (for the determination of global left ventricular function and remodelling) by echocardiography 14 days post-myocardial infarction (day of gene delivery) and 56 days post-myocardial infarction/42 days post-gene delivery and %change in fractional shortening 56 days post-myocardial infarction compared with 14 days post-myocardial infarction, n = 8 animals/group (A–F), n = 10 animals/group (G).
Furthermore, cardiac fibrosis was addressed by determination of collagen 1α1 and collagen 3α1 mRNA levels from myocardial tissue samples from the targeted non-infarcted (LAD) area harvested at the study end. Interestingly, despite significant upregulation of these fibrosis markers in AAV6.luciferase-treated pigs, AAV6.βARKct treatment normalized expression of collagen 1α1 and collagen 3α1 mRNA (Figure 5E and F).
In addition to these molecular indices of reverse remodelling, echocardiography was used to determine cardiac function in the left ventricle. We specifically assessed cardiac function in the LV area that was not targeted for gene delivery (lateral short-axis M-Mode through septal and lateral walls) in order to gain a measure of regional LV remodelling distant from the MI and treatment area. Importantly, 2 weeks post-MI, echocardiography revealed a similar reduction in fractional shortening in both MI groups before gene delivery (Figure 5G). Six weeks after gene delivery (56 days post-MI), AAV6.βARKct treatment improved regional LV fractional shortening even in non-targeted areas (Figure 5G), whereas AAV6.luciferase-treated control hearts displayed impaired function in the same areas. As fractional shortening was determined in non-targeted areas, these results most likely indicate secondary inhibition of adverse LV remodelling through attenuated catecholamine overdrive. Taken together, these results demonstrate global repression of adverse LV remodelling by βARKct gene transfer in porcine post-MI HF.
Safety of AAV6.βARKct myocardial gene delivery
To delineate any potential systemic effects of our gene therapy approach, a broad clinical chemistry workup and blood count were performed. Interestingly, 42 days post-gene transfer, no difference was observed in AAV6.βARKct- or AAV6.luciferase-treated groups when compared with sham animals regarding blood count and serum clinical chemistry (see Supplementary material online, Table S1). Plasma hsTnT levels were only mildly elevated after AAV6.luciferase gene delivery; hsTnT levels after βARKct treatment were comparable with levels observed in sham animals. Thus, it appears that our method of AAV6 gene transfer does not adversely affect the animals.
Discussion
The aim of this study was to analyse the therapeutic potential of cardiac-targeted βARKct gene transfer in a clinically relevant large animal HF model. Retroinfusion of AAV6 vectors harbouring a cDNA encoding the βARKct into the LAD area 2 weeks after MI (through LCX balloon occlusion) resulted in efficient and long-term βARKct expression in the target area, which was found to cause significant amelioration of cardiac function and LV haemodynamics, normalization of the catecholaminergic axis, and repression of adverse LV remodelling. Thus, our study represents a positive final step in the translation of GRK2 inhibition for HF therapy.
Transgenic or viral (adenovirus and AAV) expression of βARKct in the myocardium has led to a protective effect on the development of HF in different small animal models of HF including mice, rat, and rabbits.6–10 However, rodent and human cardiac physiologies differ significantly, thereby preventing extrapolation of data from rodent models to humans. The pig represents a large animal model that more closely approximates human cardiac physiology and has been used successfully to study gene therapy targets.14 Therefore, a post-MI porcine HF model was selected herein to test the therapeutic potential of the GRK2 inhibitory βARKct. A model of LCX posterolateral MI was chosen in order to leave viable tissue in the anterior and anteroseptal LV wall as target area for our gene delivery methodological approach.
Amelioration of left ventricular function by adeno-associated virus serotype 6-mediated βARKct gene delivery
Our study shows for the first time a robust therapeutic efficacy of βARKct expression in an experimental pre-clinical large animal HF model mimicking clinical conditions. Haemodynamic analysis revealed significant improvements of contractile/systolic cardiac performance 42 days after gene transfer, suggesting a long-term effect. Despite increased adrenergic drive under resting conditions, luciferase-treated animals revealed significantly decreased cardiac performance. Therefore, the beneficial effect in the βARKct group is most likely underestimated taking into account the bias of higher resting plasma catecholamine levels in MI luciferase-treated pigs. Future studies will potentially eliminate this limitation by studying single cardiac myocyte contractility from βARKct and control-treated pigs, thereby allowing comparable ambient conditions including adrenergic stimulation.
Overall, the degree of amelioration after βARKct treatment was significant, as systolic cardiac performance improved after βARKct delivery, whereas control animals revealed further deterioration over the 6 weeks of treatment period. Further, the beneficial effects of βARKct gene therapy were also present under β-AR challenge indicating sustained preservation of β-AR signalling. These results are similar to previous studies in smaller animal models after transgenic expression of the βARKct or after viral-mediated gene delivery.6–10 Thus, our findings are significant since they are now shown to occur in a clinically relevant HF model.
Reversal of heart failure-induced sympathetic overdrive by βARKct gene therapy
Neurohormones such as catecholamines usually elevate during chronic HF, drive cardiac remodelling, and contribute to enhanced morbidity and mortality.15 Sustained systemic sympathetic tone in chronic HF creates a vicious cycle perpetuating the release of catecholamines and further GRK2-driven dampening of cardiac β-AR signalling; these circumstances contribute further to cardiac deterioration.2,16 One question we specifically wanted to address in our study was whether improved cardiac performance in βARKct-treated pigs eliminates sympathetic overdrive? Indeed, another significant finding of our study is that long-term βARKct expression after AAV6-mediated gene transfer appeared to normalize the catecholaminergic overdrive in post-MI HF. In the present study, we have focused on measuring degradation products (normetanephrine and metanephrine) of plasma catecholamines (norepinephrine and epinephrine) as they offer higher stability and reflect long-term neurohormonal activity. Interestingly, circulating plasma levels of normetanephrine and metanephrine were virtually normalized by βARKct gene therapy, indicating interruption of this pathological neurohumoral cycle. Beyond systemic catecholamine levels, we addressed local sympathetic tone in myocardial tissue by determination of β-AR density; interestingly, βARKct could preserve β-AR density at least to some extent, underscoring the motion that overall sympathetic tone is reduced contributing to the preservation of cardiac function. These findings are of utmost importance as they delineate βARKct gene therapy as the first molecular approach successfully normalizing the disturbed neurohormonal signals in a clinically relevant large animal HF model. Moreover, these results suggest that clinical β-AR blocker use and molecular GRK2 inhibition with the βARKct may act synergistically as previously shown in a mouse model of genetically caused cardiomyopathy and in a rat cryo-MI HF model.6,8
Prevention of adverse left ventricular remodelling by βARKct gene therapy
Long-term βARKct expression in the failing heart also had significant effects on cardiac post-MI adverse remodelling as indicated by a reduced heart-to-body weight ratio and repression of the activation of foetal genes, which underscores previous findings following conditional cardiac GRK2 knockdown in a mouse HF model4 and also after chronic AAV6.βARKct gene therapy in a rat model of HF.8 In addition, echocardiographic assessment of cardiac performance of the non-targeted LV areas revealed improvements in non-βARKct-treated myocardial tissue, representing indirect reverse remodelling effects. These observations could indirectly result from enhanced contractile function in the gene-targeted tissue of the heart, thereby reducing biomechanical overload and repressing the systemic sympathetic drive, which would allow reverse remodelling. Alternatively, βARKct might directly affect signal transduction pathways related to LV adverse remodelling effects. However, regardless of the mechanism, cardiac βARKct gene delivery to the post-MI heart beneficially affects LV remodelling. Future studies will address potential effects of cardiac βARKct gene therapy on additional neurohormonal signalling pathways like the renin–angiotensin–aldosterone system, which in a rat model showed significant decreases.8
Adeno-associated virus serotype 6 vector, mode of gene delivery, and biosafety
Previous studies in porcine models have identified AAV6 as candidate for a highly efficient cardiac gene transfer.13,17 Thus, there was a clear rationale for using AAV6 vectors, which enabled detection of a significant long-time βARKct protein expression in the target region compared with control myocardium. Regarding vector dose, we have chosen the highest dose currently used in humans in the CUPID trial, the first clinical cardiac gene therapy trial18 studying the safety of sarcoplasmic Ca2+ ATPase (SERCA2a) myocardial gene transfer (AAV1.SERCA2a).
A crucial point for the development of a gene therapeutic approach is the application mode of the vector. Cardiac vector delivery by selective retroinfusion through the coronary sinus13,17 is a promising and highly effective application method since it enables prolonged exposure of the vector to the endothelium and an increased intravascular pressure resulting in a homogenous gene delivery to the targeted area with all advantages of a percutaneous procedure like its minimally invasive nature. With this local delivery method in combination with the cardiac-specific promoter employed in our study, systemic transgene exposure was almost eliminated. The vector, dose, and mode of delivery chosen in our study resulted in efficient transgene expression limited to the LV target area.
In terms of safety, βARKct gene delivery and transgene expression showed no systemic side effects, as intensive blood workup and clinical chemistry disclosed no differences between sham and MI gene therapy groups. Especially, hsTnT was only mildly elevated at the study end in AAV6.luciferase-treated animals. No increase was found following AAV6.βARKct treatment. As hsTnT is a systemic marker for cardiac myocyte death, these results clearly demonstrate no harming effects on cardiac myocyte integrity by our gene therapy approach.
Clinical potential of βARKct gene therapy
AAV6.βARKct treatment resulted in similar effects on LV systolic function as previously observed in pigs receiving β-blocker therapy after MI.19 The latter study showed that β-blockade was associated with preserved myofilament phosphorylation and function in remote myocardium compared with the decrease in LV as well as myofilament function in the control MI group.
Furthermore, the βARKct construct showed comparable effects to the SERCA2a construct in rodent models.20–22 Kawase et al.14 recently tested SERCA2a gene therapy for HF in a preclinical pig model; in this model, SERCA2a revealed a positive inotropic effect along with beneficial effects on LV remodelling and BNP levels. Our present study could successfully establish βARKct as a novel gene therapeutic approach to treat the failing heart in a clinically relevant large animal HF model; beneficial effects of βARKct on cardiac function, LV remodelling, and neurohormonal signalling are significant and comparable with the SERCA2a construct. With the first clinical gene therapy study employing SERCA2a (CUPID trial),18 our present study builds the basis for further clinical investigation of βARKct gene therapy for HF treatment.
Study limitations
AAV6.βARKct treatment could ameliorate cardiac function in our preclinical ischaemic cardiomyopathy model although it could not completely restore cardiac function. Surprisingly, we found no effect of AAV6.βARKct treatment on LV relaxation. It is currently not clear whether this might be attributed to low test power (Figure 3F) or a limitation of our model itself, such as a potential influence of isoflurane anaesthesia on diastoclic function or a limited effect of βARKct expression on LV relaxation. The latter might be explained by the mechanistical allocation of βARKct effects to an increased L-type Ca-channel current,23 suggesting a predominant effect on systolic function.
Furthermore, our study shows two major limitations of gene therapy approaches of HF that need to be addressed in future studies. (i) Unlike monocausal genetic diseases, HF is a complex disease characterized by multiple intracellular signalling changes affecting the β-AR-adenylyl cyclase-proteinkinase A interactome, Ca2+ cycling, apoptosis, autophagy, etc. βARKct only affects parts of the disturbed intracellular signalling, which obviously is not enough to promote a complete rescue. Future studies combining βARKct gene therapy with other constructs affecting different intracellular signalling pathways (for example, S100A1 or SERC2a normalizing intracellular Ca2+ handling) might show potential therapeutic synergisms and might therefore obtain a more complete rescue.18,24 (ii) Gene transfer with retrograde delivery of AAV6 into the coronary veins leads to a significant, but by far not complete, expression in the targeted area. Improvements in vector design, like capsid modifications, will further enhance transduction efficiency. Furthermore, improvements in gene delivery techniques and the use of respective additives (like nitric oxide) promoting temporary capillary leakage might accelerate the clinical use of gene therapy to treat HF. Future studies will address these potential issues.
Nevertheless, our study shows for the first time that AAV6-mediated gene transfer in combination with selective retroinfusion into the coronary vein allows an efficient and safe βARKct gene therapy in a clinically relevant model. Thus, our approach may be a step towards translation of this promising therapeutic strategy into improved patient care.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (RA 1668/1-1 and RA 1668/3-1 to P.W.J.R., PM 562/1-1 to P.M., and MU 1654/3-2 to O.J.M.) and the Bundesministerium für Bildung und Forschung (01GU0527 to O.J.M., P.M., and H.A.K.). This study was further supported by grants of the National Institute of Health (RO1 HL92130 and RO1 HL92130-02 S1 to P.M.). W.J.K. is the W.W. Smith Professor of Medicine and this research was supported by his NIH grants R01 HL61690, R01 HL56205, and P01 HL075443 (Project 2).
Conflict of interest: No conflicts of interests are related to this manuscript.
Supplementary Material
Acknowledgements
We thank Barbara Leuchs and the German Cancer Research Center (DKFZ) vector core production unit for their support in generating high-titre AAV vector stocks.
References
- 1.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 2.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 3.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 4.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW, II, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 6.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac beta ARK1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci USA. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, Lefkowitz RJ, Koch WJ. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci USA. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci USA. 2000;97:5428–5433. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah AS, White DC, Emani S, Kypson AP, Lilly RE, Wilson K, Glower DD, Lefkowitz RJ, Koch WJ. In vivo ventricular gene delivery of a beta-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation. 2001;103:1311–1316. doi: 10.1161/01.cir.103.9.1311. [DOI] [PubMed] [Google Scholar]
- 11.Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert EM, Abraham WT, Olsen S, Hattler B, White M, Mealy P, Larrabee P, Bristow MR. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996;94:2817–2825. doi: 10.1161/01.cir.94.11.2817. [DOI] [PubMed] [Google Scholar]
- 13.Raake PW, Hinkel R, Müller S, Delker S, Kreuzpointner R, Kupatt C, Katus HA, Kleinschmidt JA, Boekstegers P, Müller OJ. Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther. 2008;15:12–17. doi: 10.1038/sj.gt.3303035. [DOI] [PubMed] [Google Scholar]
- 14.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF, Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Sigurdsson A, Amtorp O, Gundersen T, Nilsson B, Remes J, Swedberg K. Neurohormonal activation in patients with mild or moderately severe congestive heart failure and effects of ramipril. The Ramipril Trial Study Group. Br Heart J. 1994;72:422–427. doi: 10.1136/hrt.72.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102:1458–1470. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasano T, Kikuchi K, McDonald AD, Lai S, Donahue JK. Targeted high-efficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007;42:954–961. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncker DJ, Boontje NM, Merkus D, Versteilen A, Krysiak J, Mearini G, El-Armouche A, de Beer VJ, Lamers JM, Carrier L, Walker LA, Linke WA, Stienen GJ, van der Velden J. Prevention of myofilament dysfunction by beta-blocker therapy in postinfarct remodeling. Circ Heart Fail. 2009;2:233–242. doi: 10.1161/CIRCHEARTFAILURE.108.806125. [DOI] [PubMed] [Google Scholar]
- 20.Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Tsuji T, Takewa Y, del Monte F, Peluso R, Zsebo K, Jeong D, Park WJ, Kawase Y, Hajjar RJ. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007;42:852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Monte F, Dalal R, Tabchy A, Couget J, Bloch KD, Peterson R, Hajjar RJ. Transcriptional changes following restoration of SERCA2a levels in failing rat hearts. FASEB J. 2004;18:1474–1476. doi: 10.1096/fj.04-1714fje. [DOI] [PubMed] [Google Scholar]
- 22.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Völkers M, Weidenhammer C, Herzog N, Qiu G, Spaich K, von Wegner F, Peppel K, Müller OJ, Schinkel S, Rabinowitz JE, Hippe HJ, Brinks H, Katus HA, Koch WJ, Eckhart AD, Friedrich O, Most P. The inotropic peptide βARKct improves βAR responsiveness in normal and failing cardiomyocytes through G(βγ)-mediated L-type calcium current disinhibition. Circ Res. 2011;108:27–39. doi: 10.1161/CIRCRESAHA.110.225201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Müller OJ, Most P. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3:92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.