Abstract
Pyramidal cells of the cerebral cortex are born in the ventricular zone and migrate radially through the intermediate zone to enter into the cortical plate. In the intermediate zone, these migrating precursors are able to move tangentially and initiate the extension of their axons by transiently adopting a characteristic multipolar morphology. We observe that expression of the forkhead transcription factor FoxG1 is dynamically regulated during this transitional period. By utilizing conditional genetic strategies, we show that the down-regulation of FoxG1 at the beginning of the multipolar cell phase induces Unc5D expression, the timing of which ultimately determines the laminar identity of pyramidal neurons. In addition, we demonstrate that the re-expression of FoxG1 is required for cells to transit out of the multipolar cell phase and to enter into the cortical plate. Thus, the dynamic expression of FoxG1 during migration within the intermediate zone is essential for the proper assembly of the cerebral cortex.
Keywords: multipolar cell phase, migration, intermediate zone, gene dosage, Unc5D, DCC, NeuroD1
Introduction
The mammalian cerebral cortex is composed of a sophisticated neuronal network that processes higher order information such as sensory perception, consciousness and memory. Construction of this network is dependent on the emergence of two major classes of cortical neurons, glutamatergic pyramidal neurons and GABAergic interneurons, both of which need to be produced and precisely assembled during the course of development (Barnes et al., 2008; Bystron et al., 2008; Kriegstein and Noctor, 2004; Marin and Rubenstein, 2003; Molyneaux et al., 2007; Nguyen et al., 2006). It is becoming increasingly clear that the coordination of tangential and radial migration is critical for the integration of both interneurons (Kriegstein and Noctor, 2004; Lodato et al., 2011; Marin and Rubenstein, 2003; Miyoshi and Fishell, 2011) and pyramidal cells into cortical circuits (Britanova et al., 2006; O'Rourke et al., 1992; Rakic, 2009; Tan and Breen, 1993; Tarabykin et al., 2001; Torii et al., 2009). Until recently, pyramidal neurons, which are generated locally within the cortical germinal zones (Gotz and Huttner, 2005), were thought to achieve their appropriate laminar positions exclusively through vertical migration along radial glial fibers. However, it is now recognized that pyramidal neuron precursors, like interneurons, tangentially disperse during their integration into the developing cortex (O'Rourke et al., 1992). During this phase, pyramidal neuron precursors within the intermediate zone transiently assume a characteristic ‘multipolar’ morphology, detach from the radial glial scaffold and initiate axonal outgrowth (Barnes et al., 2007) prior to entering the cortical plate (Noctor et al., 2004; Tabata and Nakajima, 2003). However, the importance of this multipolar migratory phase for assembling a mature cortical network and the precise genetic control of this stage are not well understood (LoTurco and Bai, 2006). Intriguingly, we have observed that the forkhead box transcription factor FoxG1, previously identified as a critical regulator of early telencephalic development (Xuan et al., 1995), is expressed in a dynamic manner as pyramidal neurons transit through these migratory phases. Here, through the use of conditional genetic strategies, we demonstrate that the dynamic regulation of FoxG1 expression that normally occurs during the pyramidal cell multipolar stage is essential for the proper assembly of cerebral cortex.
FoxG1 is known to play a central role in cortical development in that it regulates progenitor proliferation (Hanashima et al., 2002; Martynoga et al., 2005), specification and telencephalic patterning (Danesin et al., 2009; Hanashima et al., 2004; Manuel et al., 2010; Muzio and Mallamaci, 2005; Roth et al., 2010; Shen et al., 2006b). However, studying FoxG1 gene function in postmitotic cells has proven challenging, as the constitutive loss of this gene results in gross developmental abnormalities, including the complete absence of subpallial structures (Xuan et al., 1995). Hence, the function of FoxG1 during later stages of pyramidal neuron maturation has remained completely unexplored.
The central importance of FoxG1 as an essential transcriptional regulator is underscored by the observation that even subtle alterations in FoxG1 expression levels can have profound effects on brain development. Mice with heterozygous mutations in the FoxG1 gene have impaired pallial development suggesting that the cortex is highly sensitive to FoxG1 gene dosage (Eagleson et al., 2007; Shen et al., 2006a; Siegenthaler et al., 2008). Similarly, in humans, cases of Rett syndrome have been attributed to haploinsufficiency of FoxG1 (Ariani et al., 2008; Le Guen et al., 2010). Moreover, duplication of the FoxG1 locus has been found in patients with epilepsy, mental retardation and speech impairment (Brunetti-Pierri et al., 2010). These observations strongly suggest that the precise regulation of FoxG1 expression is critical for proper brain development.
We hypothesized that the dynamic expression of FoxG1 in pyramidal neuron precursors is critical for proper cortical development, and to test this, we utilized both genetic gain- and loss-of-function approaches. Remarkably, we find that the observed dynamic variation in FoxG1 expression during pyramidal cell migration is crucial for the development of the cerebral cortex. Specifically, we find that a failure to down-regulate FoxG1 at the beginning of the multipolar phase transiently stalls pyramidal neuron precursors within the lower intermediate zone, as a result of the failure to express Unc5D. Cells perturbed in this fashion were ultimately displaced to more superficial layers, and their laminar identity was re-specified accordingly. While the down-regulation of FoxG1 was essential for pyramidal cell migration, the re-initiation of FoxG1 expression following Unc5D expression was also critical for cells to leave the multipolar cell phase and to enter into the cortical plate. Taken together, our findings demonstrate that the dynamic expression of FoxG1 during the postmitotic multipolar cell phase critically regulates the assembly and integration of pyramidal neuron precursors into the cortical network.
Results
FoxG1 expression is dynamically regulated during the postmitotic multipolar cell phase
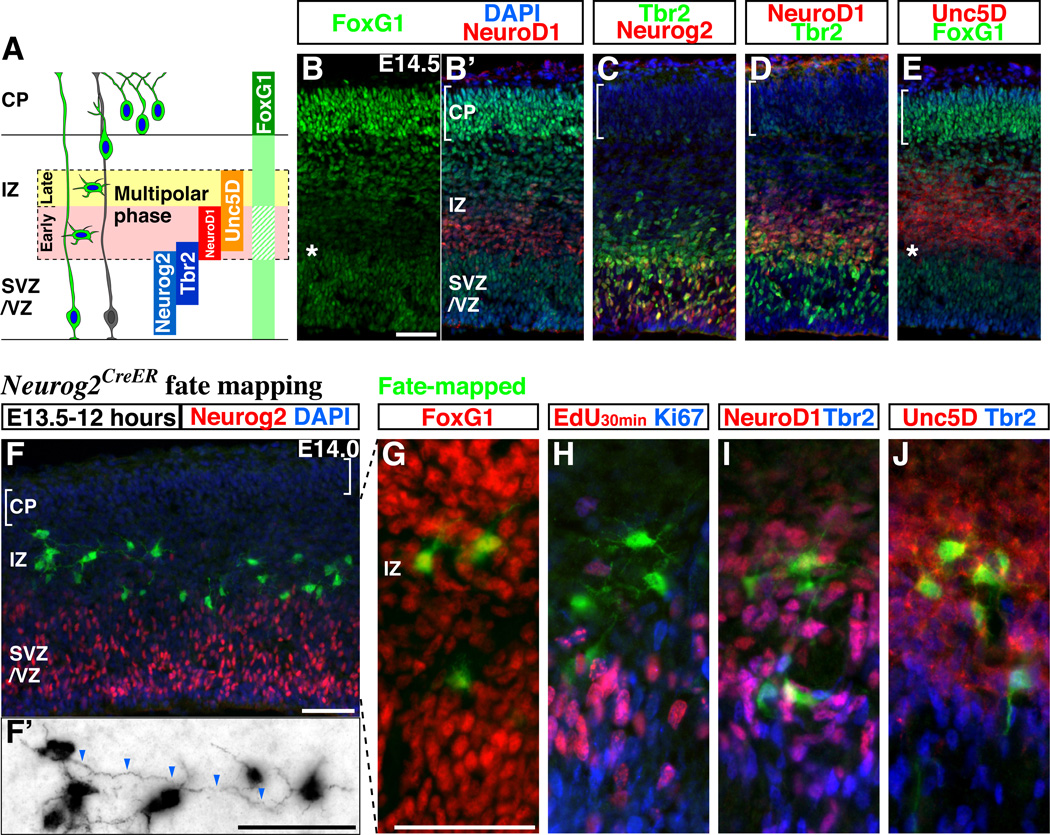
In the developing cerebral cortex, we found that FoxG1 expression is transiently down-regulated in nascent pyramidal neuron precursors located at the lower portion of the intermediate zone (E14.5 Figures 1A and 1B, see other embryonic stages for Supplemental Figures S1A to S1C). By comparing the expression of FoxG1 to other transcription factors expressed within the ventricular (VZ) and intermediate (IZ) zones (Hevner et al., 2006), such as Neurogenin2 (Neurog2) (Hand et al., 2005; Miyata et al., 2004; Nguyen et al., 2006), Tbr2 (Arnold et al., 2008; Sessa et al., 2008) (Figure 1C) and NeuroD1 (Mattar et al., 2008) (Figure 1D), we found that NeuroD1 expression, which is restricted to postmitotic cells (Mattar et al., 2008), is complementary to FoxG1 (Figure 1B and 1B’). In addition, we observed that the expression of Unc5D (Figure 1E), which has been shown to be exclusively expressed during the multipolar phase of pyramidal neuron development (Sasaki et al., 2008), is initiated at the point at which FoxG1 expression is down-regulated (Figure 1E, asterisk).
Figure 1. FoxG1 expression is dynamically regulated during the multipolar phase of pyramidal neuron development.
(A) A schematic drawing comparing the expression of genes in pyramidal neuron precursors as they proceed from the proliferative zone to the cortical plate. The area surrounded by dotted lines indicates the region in which pyramidal neuron precursors adopt a multipolar identity. CP: cortical plate, IZ: intermediate zone, SVZ/VZ: subventricular and ventricular zones (B, B’) FoxG1 protein is expressed at different levels in E14.5 cortical cells at distinct stages of their maturation (See also Supplemental Figures S1A to S1C). FoxG1 expression is down-regulated (asterisk) in cells concomitant with their initiation of NeuroD1 expression. We observe similar results from the use of two different polyclonal antibodies for FoxG1. (C) A section adjacent to (B) indicating the region where the expression of Neurog2 and Tbr2 overlap. (D) Comparison of Tbr2 and NeuroD1 expression. (E) Down-regulation of FoxG1 (indicated by asterisk) occurs in the lower IZ (early multipolar phase) slightly below (i.e., before) initiation of Unc5D expression. Notably, in the uppermost IZ (late multipolar phase) Unc5D-expressing cells re-express FoxG1 below (i.e., prior) to where Unc5D-expression is extinguished. (F–J) Multipolar cells are labeled with EGFP by an acute (12 hour) fate mapping of the Neurog2-expressing population. This is achieved by combining the Neurog2-CreER driver and the R26R-CAG-loxPstop-EGFP reporter lines (See also Supplemental Figures S1D to S1G) and initiated through tamoxifen administration at E13.5. Note that EGFP-expressing cells have already shut off Neurog2 by this time. The majority of EGFP-labeled cells are found within the intermediate zone and possess multipolar morphology. (F’) In order to better visualize the cell morphology during the multipolar phase, a picture with EGFP expression is shown with reverse contrast to highlight their morphology. Tangentially oriented process resembling axon (arrowheads) are found in the multipolar cells. (G) Dependent on the position within the intermediate zone, multipolar cells express distinct levels of FoxG1 protein. (H) Multipolar cells are neither labeled by acute pulse-chase analysis using EdU (DNA analog) nor by antibodies against the Ki67 antigen suggesting that they are postmitotic. (I, J) Many Neurog2 fate-mapped cells with multipolar morphology expressed NeuroD1 and Unc5D but only low levels of Tbr2.
Scale bars: 50µm
By taking advantage of an inducible Cre (CreER) driver under the control of proneural gene Neurog2 (Zirlinger et al., 2002), which is transiently expressed at the time progenitors become postmitotic (Bertrand et al., 2002; Miyata et al., 2004), we were able to sparsely label the multipolar cell population (Figure 1F and 1F’, see details of this method in Supplemental Figures S1D to S1G). We found two distinct levels of FoxG1 expression within these genetically labeled multipolar cells (Figure 1G), suggesting that FoxG1 expression is dynamically regulated specifically during this phase. We confirmed that the majority of multipolar cells are postmitotic as they were not labeled by an acute pulse of EdU (DNA analog) (0%, n=81) (Figure 1H) and did not express high levels of the Ki67 antigen (Miyata et al., 2004) (Figure 1H). We observed that these multipolar cells located near the ventricular zone express NeuroD1 (Figure 1I) and low levels of Tbr2, and, not surprisingly, most of them express Unc5D (Figure 1J) (Sasaki et al., 2008). We have further utilized in utero electroporation and found that FoxG1 down-regulation occurs precisely at the beginning of the multipolar cell phase, at a time coincident with when NeuroD1 expression is initiated (see detailed analysis in Supplemental Figures S1H and S1I). We refer to this NeuroD1 expressing stage as the ‘early phase’ (Figure 1A). These cells subsequently up-regulate FoxG1 levels at a period we designate as the ‘late phase’ of multipolar cell migration where NeuroD1 (but not Unc5D) has been down-regulated (Figure 1A). Based on these observations, we hypothesized that the dynamic regulation of FoxG1 activity during these multipolar cell transition phases is critical for the migration of cells through the intermediate zone and their integration into appropriate cortical layers.
Failure to down-regulate FoxG1 at the beginning of the multipolar cell phase delays migration within the intermediate zone and redirects laminar identity
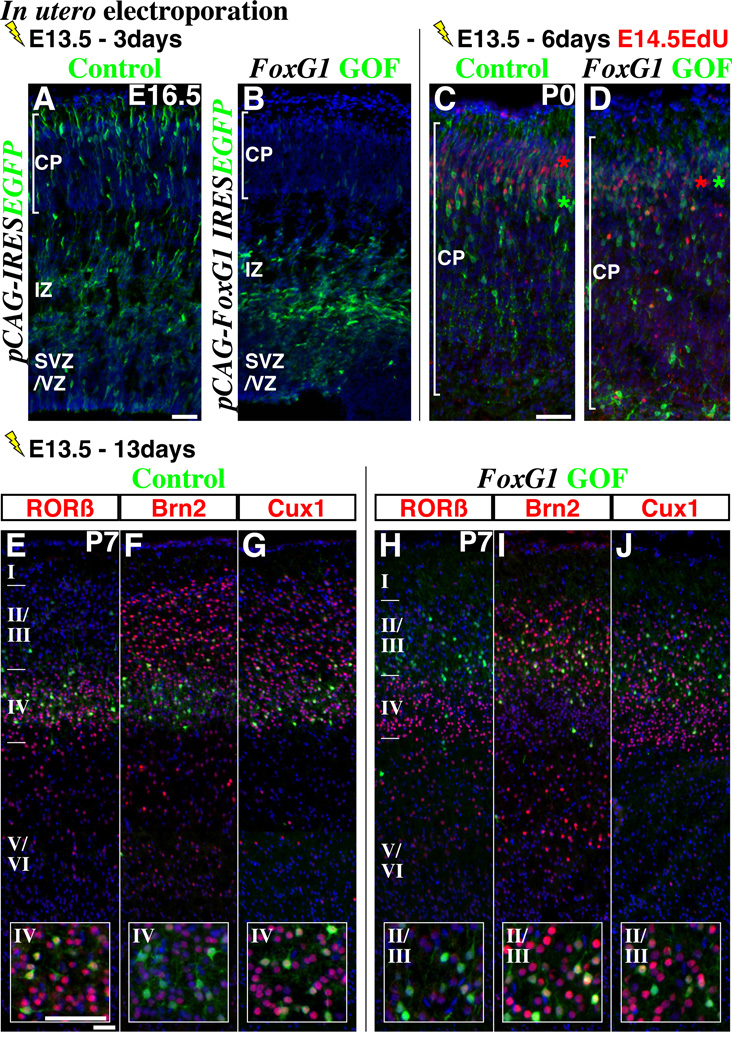
We next carried out FoxG1 gain-of-function experiments to test the importance of FoxG1 down-regulation at the beginning of the multipolar cell phase. Using in utero electroporation, we transduced the E13.5 cortical ventricular zone with a control (pCAG-IRESEGFP; Figure 2A) or a FoxG1 expression vector (pCAG-FoxG1-IRESEGFP; Figure 2B), both of which resulted in EGFP cell labeling from the ubiquitously expressed CAG promoter (Niwa et al., 1991) (see methods). Three days after this manipulation, the majority of FoxG1 gain-of-function cells remained within the lower intermediate zone and possessed multipolar morphologies (Figure 2B, compare to control in Figure 2A). However, three days later at P0, FoxG1 gain-of-function cells are found inside the cortical plate (Figures 2C and 2D, bracket indicates cortical plate), suggesting that the failure in FoxG1 down-regulation caused delay in migration but did not permanently stall cells at the multipolar phase. We compared the location of these E13.5 electroporated cells to later born cells by E14.5 EdU birthdating and found that in contrast to control cells (Figure 2C, compare the colored asterisks), FoxG1 gain-of-function cells within the cortical plate were intermingled with the population born at E14.5 (Figure 2D), suggesting that they are either still migrating or had become ectopically positioned.
Figure 2. Failure to down-regulate FoxG1 delays the migration of pyramidal neuron precursors at the multipolar phase and alters their laminar fate.
In utero electroporation of either (A) control (pCAG-IRES EGFP) or (B) FoxG1 gain-of-function (GOF) (pCAG-FoxG1-IRES EGFP) vectors under the regulation of a ubiquitous CAG promoter were carried out at E13.5 and brains were analyzed at E16.5. Three days after manipulation, while most control cells have entered the cortical plate (A), the majority of FoxG1 gain-of-function cells (B) were found in the lower part of the intermediate zone and displayed multipolar morphologies. (C, D) 6 days later, many FoxG1 gain-of-function cells delayed in migration by this manipulation now enter the cortical plate (bracket indicates cortical plate). Control cells (C) were generally found in lower positions compared to cells born one day after the electroporation (single pulse of EdU at E14.5), while FoxG1 gain-of-function cells (D) were intermingled with them. (E–G) At P7, the majority of EGFP-labeled control cells were found in layer IV and expressed molecular profiles consistent with cells within this layer (i.e., RORβ-on, Brn2-low, Cux1-on, insets). (H–J) The majority of FoxG1 gain-of-function cells were located in layers II/III and possessed molecular features consistent with this location (insets, RORβ-off, Brn2-high and Cux1-on). Note that in case of FoxG1 gain-of-function, a few cells were also found within the white matter and lacked expression of the layer specific markers we have examined. See also Supplemental Figures S2 and S3.
Scale bars: 50µm
We further fate mapped control and FoxG1 gain-of-function cells and at P3 found that while control cells were positioned below those born at E14.5, FoxG1 gain-of-function cells were located more superficially (compare Supplemental figures S2A and S2B, colored asterisks). We next analyzed the molecular expression profiles at P7, a stage at which neuronal migration is largely complete (Figures 2E – 2J). Consistent with previous findings (Takemoto et al., 2011), we found that the majority of control cells electroporated at E13.5 were located in layer IV (Figures 2E – 2G) and expressed molecular markers characteristic of that layer (RORβ-on, Brn2-low, Cux1-on Figures 2E –2G, insets) (Molyneaux et al., 2007). In contrast, the majority of FoxG1 gain-of-function cells were located in layers II/III (Figures 2H –2J) and showed molecular features consistent with their ectopic laminar location (Figures 2H –2J, insets, RORβ-off, Brn2-high and Cux1-on). We conclude that failure to down-regulate FoxG1 at the beginning of the multipolar cell phase delays cells from entering the cortical plate and results in a superficial shift in their location and marker profiles indicating a shift in their laminar identity.
We confirmed that this change in laminar identity did not result from postmitotic cells re-entering the cell cycle after FoxG1 gain-of-function (Supplemental figures S2C and S2D). We also ruled out the possibility that FoxG1 over-expression within the progenitor pool was responsible for the switch in laminar position by repeating the FoxG1 gain-of-function specifically in postmitotic multipolar cells using a NeuroD1 promoter expression vector (Supplemental Figures S1H and S1I). This manipulation resulted in a similar delay in migration after two or three days of in utero electroporation (Supplemental Figures S3A to S3D) and changes in laminar identity at postnatal stages (Supplemental Figures S3E to S3H) as was observed with the broader FoxG1 gain-of-function experiments shown in Figure 2.
FoxG1 down-regulation is necessary for Unc5D receptor expression and transition from the early to late multipolar phase of migration
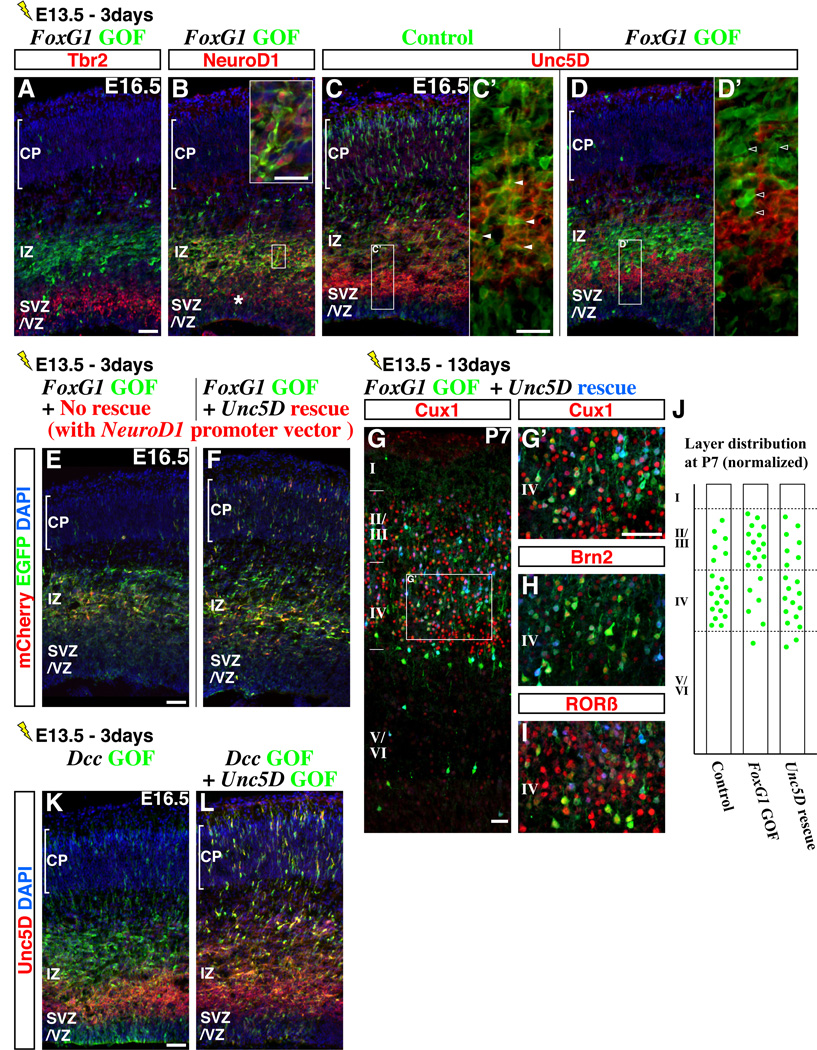
We next tried to understand why failure to down-regulate FoxG1 at the beginning of the multipolar cell phase leads to delayed migration in the intermediate zone. Consistent with their multipolar morphology, these cells had already extinguished Tbr2 (Figure 3A) but maintained NeuroD1 expression (Figure 3B, asterisk indicates the domain normally expressing NeuroD1), suggesting that they had failed to transit from the early to late multipolar cell phase (Figure 1A). Consistent with this idea, FoxG1 gain-of-function cells failed to express Unc5D (compare Figure 3C, 3C’ versus 3D, 3D’), a protein whose expression is normally initiated shortly after the onset of NeuroD1 and maintained into the late multipolar phase (Figure 1A, also see Supplemental Figures S3A to S3D for the similar absence of Unc5D expression in cells with postmitotic FoxG1 gain-of-function). Since NeuroD1 mis-expression by itself does not affect cell migration (Mattar et al., 2008), we hypothesized that the loss of Unc5D may be responsible for the delayed migration. We repeated the FoxG1 gain-of-function and rescued Unc5D expression specifically at the postmitotic multipolar phase (Figure 3F) by using a NeuroD1 promoter construct (Supplemental Figures S1H and S1I). Remarkably, restoration of Unc5D expression in NeuroD1-positive cells partially rescued the migration phenotype in that there was a dramatic increase in cells that entered the cortical plate after three days (Figure 3F) compared to the FoxG1 gain-of-function cells (Figure 3E). We further examined whether Unc5D restoration in FoxG1 gain-of-function cells could also correct their altered laminar identity (Figure 2 and Supplemental Figures S3E to S3H). When Unc5D expression was restored in FoxG1 gain-of-function cells at the multipolar phase, we observed that indeed, by P7, a substantial number of them were now appropriately located in layer IV (Figure 3G) and possessed the correct molecular profile for this layer (Figures 3G’, 3H and 3I, Cux1-on, Brn2-low, RORβ-on, See also Figure 3J).
Figure 3. FoxG1 down-regulation promotes an early to late multipolar phase transition through induction of Unc5D.
Migrating pyramidal neuron precursors that failed to down-regulate FoxG1 were delayed within the lower part of the intermediate zone (Figure 2). These cells shut off Tbr2 (A) but ectopically maintained NeuroD1 expression (B) (in a region above the normal expression domain, asterisk), suggesting that they become arrested at the early multipolar phase. (C, C’) Control cells within the intermediate zone expressed Unc5D (arrowheads in C’). (D, D’) FoxG1 gain-of-function cells, although they possess multipolar morphology, failed to express Unc5D protein (open arrowheads). (E) As we have previously shown, three days after electroporation at E13.5, FoxG1 gain-of-function cells (with pCAG-FoxG1-IRES EGFP) remained within the lower part of the intermediate zone. Note that mCherry was expressed in NeuroD1-expressing cells by co-introducing a pNeuroD1-IRES mCherry control vector. (F) When Unc5D expression was restored in NeuroD1-expressing FoxG1 gain-of-function cells (by using a pNeuroD1-Unc5D-IRES mCherry vector), a subset of these pyramidal neuron precursors migrated normally into the cortical plate after three days (green and red cells) and after 13 days at P7 (G, G’, H, I), we observed cells located in both layers II/III and layer IV (see summary in J). The partial restoration in the laminar location of this population at P7 was consistent with the degree of rescue in migration we observed after three days (F, compare with Figure 2A). In FoxG1 gain-of-function cells, both rescued (in layer IV) and non-rescued (in layers II/III) populations express molecular signatures appropriate to their laminar locations. Specifically, Unc5D-rescued cells in layer IV showed molecular expression profiles consistent with them being layer IV cells, i.e., Cux1-on (G’), Brn2-low (H) and RORβ-on (I). (J) Schematized layer distribution of Control (pCAG-IRES EGFP) (Figures 2E to 2G), FoxG1 gain-of-function (pCAG-FoxG1-IRES EGFP) (Figures 2H to 2J) and Unc5D-rescued FoxG1 gain-of-function (pCAG-FoxG1-IRES EGFP + pNeuroD1-Unc5D-IRES mCherry) experiments. Note that the numbers of EGFP-labeled cells (including the low-expressors) within the cortical plate is normalized (20 cells) and represented in this scheme. (K) Similar to FoxG1 gain-of-function, Dcc over-expression (pCAG-Dcc-IRES EGFP) delays cell migration at the intermediate zone. (L) Unc5D over-expression (pCAG-Unc5D-IRES EGFP) rescues the impaired migration phenotype observed by Dcc over-expression. Thus, a precise balance between Dcc versus Unc5D expression is important for cells to migrate through the intermediate zone (See also Supplemental Figures S4). This balance appears to be critically controlled by transient FoxG1 down-regulation as FoxG1 gain-of-function affects Unc5D (D, D’) but not Dcc expression (data not shown).
Scale bars: 50µm, except for C’ and D’: 20µm
How could Unc5D play such a critical role in regulating the early to late transitions within the multipolar cell phase? It has been shown that Unc5D is a receptor involved in Netrin-signaling in the postnatal cortex (Takemoto et al., 2011) and, in the context of axonal guidance, alters the response of Dcc (Deleted in colorectal carcinoma) to Netrins in growth cone turning assays (Hong et al., 1999). We found that both Dcc and Unc5D are expressed within the intermediate zone (Supplemental Figures S4A and S4B) and, in fact, are the only known Netrin receptor molecules expressed in this region (Unc5A, 5B, 5C, Neogenin and Dscam are not expressed within the intermediate zone, see Supplemental Figures S4C to S4H). However, unlike the down-regulation of Unc5D we have observed in FoxG1 gain-of-function cells (Figure 3D), we found that Dcc expression was not affected (data not shown). This suggests that, similar to what has been demonstrated in the context of axon guidance, disruption of the Unc5D/Dcc balance by loss of Unc5D might be responsible for the delay in migration. We directly tested this idea and found that Dcc over-expression delays cell migration at the intermediate zone (Figure 3K), in a manner similar to FoxG1 gain-of-function, and this can be rescued by simultaneously increasing the levels of Unc5D (Figure 3L). In summary, transient down-regulation of FoxG1 at the beginning of the multipolar cell phase is crucial for the maintenance of an appropriate Unc5D/Dcc balance through Unc5D induction, and this event is required for cells to transit rapidly from the early to late multipolar phase and to acquire their proper laminar position and gene expression.
Up-regulation of FoxG1 is required for pyramidal neuron precursors to exit the multipolar phase and to enter the cortical plate
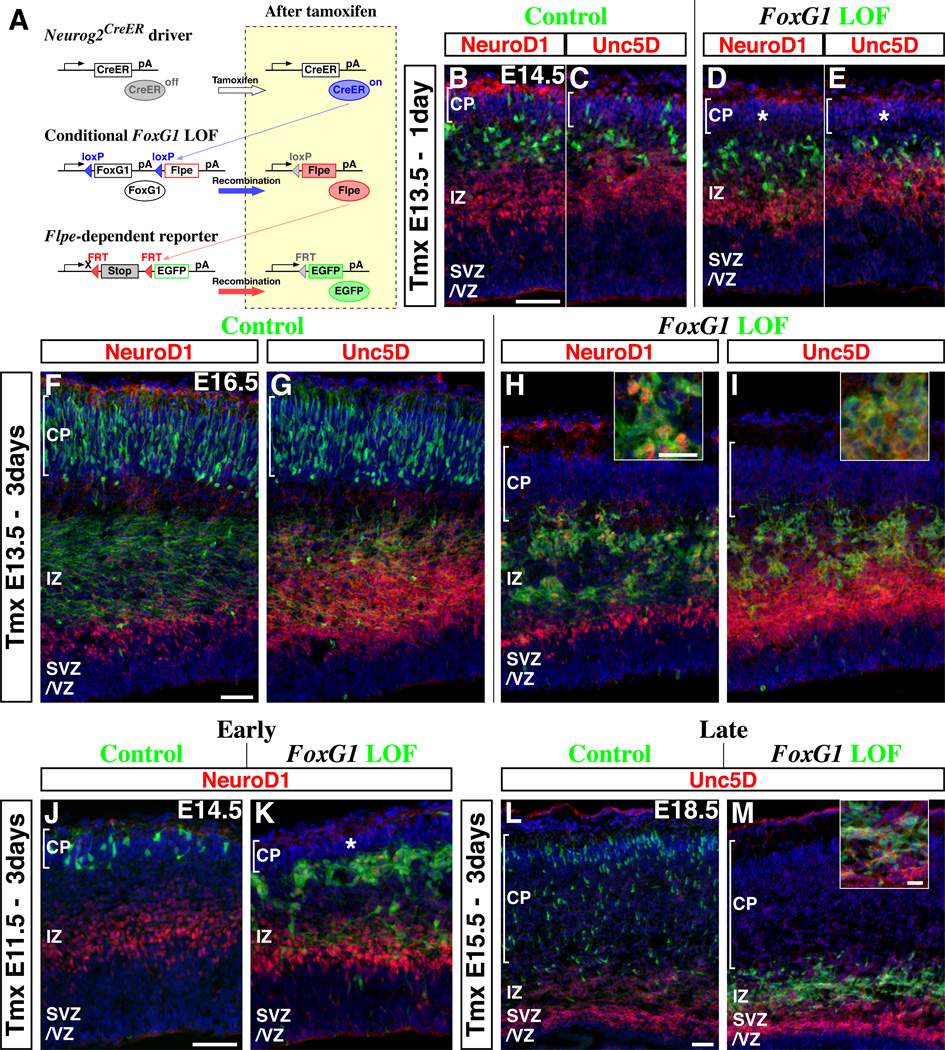
As noted above, a prominent feature of the dynamic regulation of FoxG1 is its up-regulation in cells in the late multipolar phase prior to their migration into the cortical plate (Figure 1A). To explore the significance of this up-regulation, we have generated a Cre-dependent conditional loss-of-function allele of FoxG1 (Supplemental Figure S5) in order to allow us to remove FoxG1 expression at specific stages of pyramidal cell migration. In constructing this conditional allele, the Flpe recombinase was inserted into the FoxG1 locus such that its expression is initiated upon removal of the loxP flanked FoxG1 gene (Figure 4A scheme, Supplemental Figure S5). Prior to Cre-mediated recombination, the expression of Flpe is attenuated by the FoxG1 coding and 3’UTR domains, which act as a transcriptional stop cassette (Dymecki and Kim, 2007; Joyner and Zervas, 2006; Luo et al., 2008; Miyoshi and Fishell, 2006). By combining this conditional allele with a Flpe-dependent reporter line (R26R-CAG-FRTstop-EGFP; Figure 4A bottom) (Miyoshi et al., 2010; Sousa et al., 2009), recombined cells can be selectively and permanently labeled with EGFP. To mediate the selective removal of FoxG1 (and the initiation of Flpe expression) in postmitotic multipolar cells, we used a Neurog2-CreER driver line (Figure 4A, top, also see Figures 1F to 1J).
Figure 4. Up-regulation of FoxG1 is required for pyramidal neuron precursors to transit out of the multipolar cell phase and to enter into the cortical plate.
(A) Schematic drawings of our genetic strategy to mosiacally remove FoxG1 at the multipolar cell phase and to selectively follow the fate of recombined cells. (Top) Neurog2-CreER driver was used to target multipolar cells at specific time points. (Middle) A conditional FoxG1 loss-of-function (LOF) allele that expresses Flpe after Cre-mediated recombination (Supplemental Figure S5). (Bottom) A Flpe-dependent EGFP reporter line was utilized to visualize the cells in which the FoxG1 conditional loss-of-function allele has been recombined. (Right) Subsequent to tamoxifen administration, the CreER expressed in Neurog2-positive cells becomes active (top) and recombines the FoxG1 conditional allele resulting in the removal of FoxG1 coding region and initiation of Flpe expression from the FoxG1 locus (middle), Flpe in turn removes a stop cassette in the reporter, allowing us to permanently trace the fate of manipulated cells with EGFP (Bottom). (B–I) Comparison of control (FoxG1 heterozygous) versus FoxG1 loss-of-function cells at various time points after tamoxifen administration at E13.5. (B, C) One day after tamoxifen administration, some control EGFP-labeled cells are already found inside the cortical plate (See also Supplemental Figure S1E). (D, E) By contrast, FoxG1-null cells remain excluded from the cortical plate (asterisk) and generally maintained multipolar morphologies. Note that neither control nor FoxG1-null cells located right below the cortical plate express NeuroD1 (B, D) or Unc5D (C, E). (F, G) Three days after tamoxifen administration, the majority of control cells are found within the cortical plate. Note that in these cortices, EGFP-labeled axonal fibers derived from the cortical plate cells are readily evident within the intermediate zone. (H, I) FoxG1-null cells in littermate embryos were only found below the cortical plate and maintained multipolar morphologies. Note also that the majority of FoxG1-null cells now express NeuroD1 (H, inset) and Unc5D (I, inset) suggesting that these cells have reverted back to the early multipolar phase. In addition, we observed aggregation of FoxG1-null cells at this time point (H, I). (J–M) Similar FoxG1 loss-of-function experiments were carried out at earlier (E11.5: J, K) or later (E15.5: L, M) time points. At both early and late time points, FoxG1-null cells maintained multipolar morphologies, did not enter the cortical plate (asterisk in K), re-initiated genes specifically expressed during the early multipolar phase (NeuroD1 in K and Unc5D in M, inset) and formed aggregates. Hence, FoxG1 up-regulation during the late multipolar cell phase seems universally required for pyramidal cells throughout development. See also Supplemental Figures S6A and S6B for an analysis after 7 days of manipulation.
Scale bars: 50µm, except for insets in H, I and M: 20µm
Experimentally, we compared the migration behavior of the recombined FoxG1-C:Flpe/+ cells (heterozygous controls) with FoxG1-C:Flpe/− cells (FoxG1 loss-of-function mutants). One day after tamoxifen administration at E13.5, many of the control cells were found in both the intermediate zone (Figures 4B and 4C) and the cortical plate (Figures 4B and 4C, brackets). By contrast, although the mutant cells had successfully down-regulated NeuroD1 and Unc5D (Figures 4D and 4E), they maintained a multipolar morphology and were restricted to a position below the cortical plate (Figures 4D and 4E, asterisks). Moreover, while three days after tamoxifen administration at E13.5 the majority of control cells had entered into the cortical plate (Figures 4F and 4G), all of the FoxG1 loss-of-function cells were still positioned within the intermediate zone and maintained a multipolar morphology (Figures 4H and 4I). Interestingly, at this stage many of the mutant cells expressed NeuroD1 (Figure 4H) and Unc5D (Figure 4I), strongly suggesting that they had regressed back to the early multipolar phase (Figure 1A). In addition, mutant cells had begun to form aggregates within the intermediate zone (Figures 4H and 4I). To ascertain if these results can be generalized to other stages of cortical development, we carried out similar experiments at different embryonic stages (E11.5 and E15.5) and obtained results comparable to those we observed after a E13.5 manipulation (Figures 4J to 4M). Furthermore, even 7 days after FoxG1 removal, EGFP-labeled cells were restricted to positions below the cortical plate (E11.5 to E18.5 survivals, Supplemental Figures S6A and S6B, and from E15.5 to P3 in FoxG1 conditional homozygous background, data not shown). These data suggest that when multipolar cells fail to re-express FoxG1 they permanently lose their ability to enter into the cortical plate. In addition, we observed this mutant phenotype across all neocortical areas examined. These data further support the idea that all pyramidal neurons transit through the multipolar cell phase during development and that up-regulation of FoxG1 at the end of this phase is universally required.
FoxG1 re-expression facilitates the integration of pyramidal neuron precursors into the cortical plate by regulating the late multipolar cell phase
When cells fail to up-regulate FoxG1 during the late multipolar phase, in addition to failing to enter the cortical plate, they revert/regress to the early multipolar phase by re-expressing genes associated with this phase (NeuroD1 and Unc5D) and form aggregates (Figure 4). One possibility is that cells re-enter the multipolar phase simply as a consequence of their failure to migrate properly into the cortical plate. Alternatively, this phenotype may be due to a direct requirement of FoxG1 for exiting from the multipolar phase. In order to distinguish these possibilities, we took advantage of our inducible genetic mosaic loss-of-function strategy (Figure 4A, scheme) and compared gene expression profiles in control versus FoxG1 conditional mutant cells using microarray analysis. Two days after administrating tamoxifen to E11.5 pregnant dams, we dissected out the cortices from control and mutant embryos (E13.5) and isolated EGFP-expressing cells using fluorescently-activated cell sorting. We then extracted total RNAs from these samples and carried out microarray gene expression analyses (n=3 each) using Affymetrix MOE 430A.2 arrays.
Before the analysis of the results, we first tested whether FoxG1 acts as a transcriptional activator or repressor during this transition period. We found that, both a wild type or a constitutive repressor form of FoxG1 allow mutant cells to enter the cortical plate, suggesting that either can largely rescue the postmitotic loss-of-function phenotype (Supplemental Figures S7A and S7B). By contrast, mis-expression of an activator form of FoxG1 in the wild type cortex prevented EGFP-labeled cells from entering the cortical plate, suggesting that this construct acts in a dominant negative fashion (See detailed analysis in Supplemental Figures S7D). We conclude that FoxG1 functions as a transcriptional repressor (Yao et al., 2001) in the postmitotic multipolar cells.
Having ascertained this, we reasoned that one explanation for the observed phenotype is that genes associated with radial migration are up-regulated in mutant cells. To our surprise, comparison in control versus mutant populations revealed no change in the expression of genes known to regulate the migration of pyramidal neuron precursors (Table1A), including Doublecortin (Gleeson et al., 1999; Ramos et al., 2006), Filamin A (Nagano et al., 2004), Pafah1b1 (Lis1) (Tsai et al., 2007), Ndel1 (Hippenmeyer et al., 2010; Shu et al., 2004), Rnd2 (Alfano et al., 2011; Heng et al., 2008; Nakamura et al., 2006), Rnd3 (Pacary et al., 2011) and Tubb2b (Jaglin et al., 2009), suggesting that none of these genes are directly regulated by FoxG1. One exception to this overall trend was an observed 10-fold reduction in Dab1, which encodes an adaptor protein that mediates Reelin-signaling (Table 1B) (Franco et al., 2011; Morimura and Ogawa, 2009; Olson and Walsh, 2008; Sanada et al., 2004). However, studies of Dab1 indicate that it is required in early (layers V/VI) but not late (layers II/III/IV) born pyramidal neuron precursors to enter into the cortical plate (Franco et al., 2011). Since we found FoxG1 to be required for the development of all pyramidal neurons (Figure 4), Dab1 is an unlikely down-stream mediator of FoxG1 loss-of-function. Consistent with this prediction, restoration of Dab1 alone or even together with Csk, a kinase that stimulates Dab1 activity (Bock and Herz, 2003), did not allow FoxG1 mutant cells to leave the multipolar phase and enter into the cortical plate (see detailed analysis in Supplemental Figures S7E and S7F). These data suggest that neither changes in the cell’s migration apparatus nor changes in Reelin signaling could account for the failure of FoxG1 mutant cells to enter the cortical plate.
Table 1.
| A Cell migration and polarity related genes | |||
|---|---|---|---|
| Gene Symbol | Fold Change (Mut/Ctrl) | Gene Title | References |
| Cdk5 | 0.958, 0.944 | cyclin-dependent kinase 5 | (Chae et al., 1997) (Howell et al., 1997) |
| Dclk1 | 0.959,0.934,0.871, 0.800, 0.756, 0.738 | doublecortin-like kinase 1 | (Deuelet al.,2006) (Koizumi et al., 2006) |
| Dcx | 0.985, 1.191,0.673, 0.868 | doublecortin | (Gleeson et al., 1999) (Ramos et al., 2006) |
| Flna | 1.166 | filamin, alpha | (Nagano et al., 2004) |
| Mapk8 (Jnk1) | 1.180, 1.126,0.948 | mitogen-activated protein kinase 8 | (Westerlund et al., 2011) |
| Ndel1 | 1.097, 1.044 | nuclear distribution gene E-like homolog 1 | (Shu et al., 2004) (Hippenmeyer et al., 2010) |
| Pafah1b1 (Lis1) | 1.239, 1.072, 1.028, 0.853 | platelet-activating factor acetylhydrolase, isoform 1b, beta1 subunit | (Hirotsune et al., 1998) (Tsai et al., 2007) (Hippenmeyer et al., 2010) |
| Rac1 | 1.082,0.973,0.943 | RAS-related C3 botulinum substrate 1 | (Kawauchi et al., 2003) |
| Rhoa1 | 0.954 | ras homolog gene family, member A | (Govek et al., 2011) (Pacary et al., 2011) |
| Rnd2 | 1.985 | Rho family GTPase 2 | (Heng et al., 2008) |
| Rnd3 | 0.940, 0.850 | Rho family GTPase 3 | (Pacary et al., 2011) |
| Stmn2 (SCG10) | 1.110,0.969 | stathmin-like 2 | (Westerlund et al., 2011) |
| Tubb2b | 0.985 | tubulin, beta 2b | (Jaglin et al., 2009) |
| B Reelin signaling pathway related genes | |||
|---|---|---|---|
| Gene Symbol | Fold Change (Mut/Ctrl) | Gene Title | References |
| Dab1 | 0.138, 0.138, 0.109, 0.106, 0.105 | disabled homolog 1 | (Morimura and Ogawa, 2009) (Franco et al., 2011) |
| Fyn | 0.901, 0.900 | Fyn proto-oncogene | (Howell et al., 1997) |
| Lrp8 (ApoER2) | 0.925 | low density lipoprotein receptor-related protein 8, apolipoproteine receptor | (Trommsdorff et al., 1999) |
| Reln | 17.249 | reelin | (Uchida et al., 2009) (Kubo et al., 2010) |
| Src | 1.156, 1.080 | Rous sarcoma oncogene | (Howell et al., 1997) |
| Vldlr | 0.977,0.614, 0.593, 0.551 | very low density lipoprotein receptor | (Trommsdorff et al., 1999) |
No significant changes were observed in these genes. Fold change indicates intensity of the signals of Mutant / Control raw values observed in our microarray expression analysis. Note that multiple values are shown when several probes were assigned for a particular gene.
Having ruled out that FoxG1 acts by regulating radial migration, we examined the alternative hypothesis that it is required for cells to exit from the multipolar phase. In concordance with this idea, we observed a marked up-regulation of genes normally restricted to pyramidal neuron precursors within the intermediate zone (Table 2 and Supplemental Figures S8). In addition to NeuroD1, Unc5D (Figure 4) and Reelin (Table 1B) (Kubo et al., 2010; Uchida et al., 2009), we observed up-regulation of Cdh10, Nhlh1 and Slc17a6 (vGlut2). We thus conclude that the most parcimonious explanation of our findings is that FoxG1 up-regulation during the late multipolar phase is directly controlling the exit from this cellular state.
Table 2.
Intermediate zone expressed genes
| Gene Symbol | Fold Change (Mut/Ctrl) | Gene Title | Gene Paint ID |
|---|---|---|---|
| 1190002N15Rik | 47.62, 41.66 | RIKEN cDNA 1190002N15 gene /// hypothetical protein LOC100044725 | EH4432 |
| Cdh8 | 0.4937 | cadherin 8 | DA115 |
| Cdh10 | 3.219 | cadherin 10 | DA84 |
| NeuroD1 | 4.142, 4.104 | neurogenic differentiation 1 | DA125 |
| Nhlh1 | 3.909 | nescient helix loop helix 1 | EN679 |
| Pcp4 | 7.795 | Purkinje cell protein 4 | EB2090 |
| St8sia4 | 5.160, 4.640 | ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase4 | EG45 |
| Slc17a6 (vGlut2) | 10.70, 8.819 | solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter), member 6 | EB854 |
We have cross-correlated genes showing over 2-fold changes in our microarray expression analysis to the E14.5 expression database at Genepaint (www.genepaint.org). From this analysis, we have identified several genes showing specific expression within the intermediate zone (Listed in this table, also see Supplemental Figures S8) consistent with our hypothesis that FoxG1 regulates the exit from the late multipolar phase.
Postmigratory pyramidal neuron precursors do not require FoxG1 function to prevent reentry into the multipolar phase
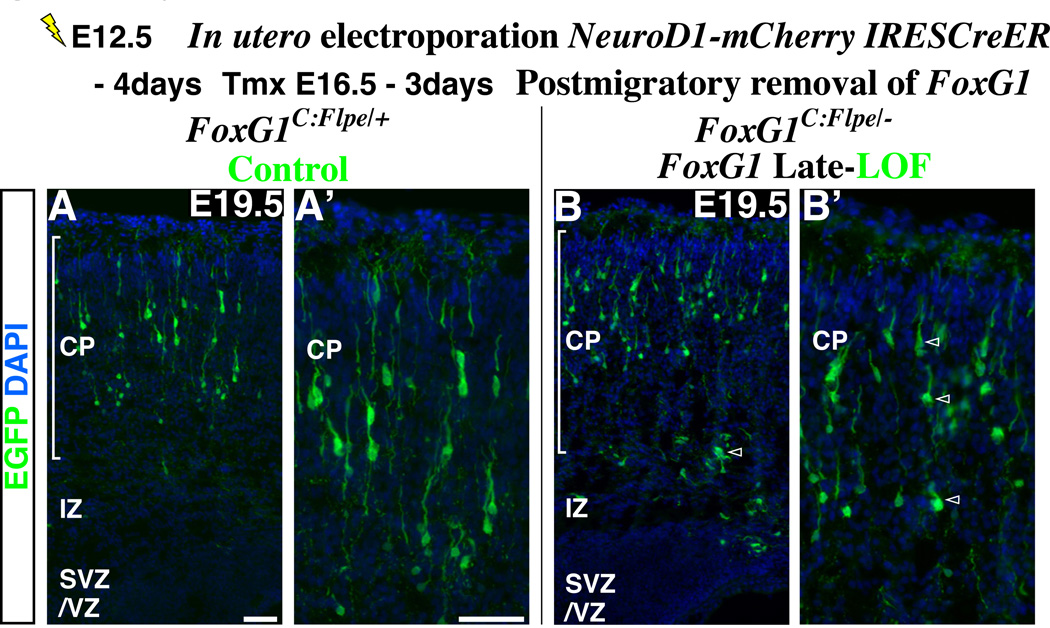
While we have shown that FoxG1 up-regulation is specifically required during the late multipolar cell phase, FoxG1 expression levels are further increased within the postmigratory cells inside the cortical plate (Figures 1A and 1B, Supplemental Figures S1A to S1C). This raised the possibility that FoxG1 up-regulation is required not only at the multipolar cell phase but also during later stages of maturation. In order to test this hypothesis, we conditionally removed FoxG1 from postmigratory pyramidal neurons located within the cortical plate (see details of this method in the legend of Supplemental Figures S6C and S6D). At E19.5, compared to the control cells (heterozygous in FoxG1-C:Flpe/+ background) (Figures 5A and 5A’), cells three days after the postmigratory removal of FoxG1 generally maintained pyramidal cell morphologies and did not resemble multipolar cells, although their dendritic branching pattern was somewhat diminished (Figures 5B and 5B’) (Also, see Supplemental Figures S6C and S6D for detailed comparison of wild type, heterozygous and null mutant cells for FoxG1 in two genetic backgrounds). Moreover, FoxG1 removal in postmigratory cells did not result in the re-expression of the multipolar cell markers NeuroD1 and Unc5D (data not shown). These data strongly suggest that FoxG1 has a specialized function during the transition from the late multipolar cell phase into the cortical plate and does not play similar roles in the postmigratory populations.
Figure 5. The role of FoxG1 in the multipolar cells is distinct from its function in postmigratory neurons within the cortical plate.
In order to remove FoxG1 in postmigratory pyramidal neuron precursors inside the cortical plate, we have introduced a pNeuroD1-mCherry-IRES CreER vector into the ventricular zone by in utero electroporation at E12.5. This manipulation allows us to simultaneously visualize the morphologies of postmitotic cells through their expression of mCherry, as well as enabling us to recombine the conditional FoxG1 loss-of-function allele at a desired time point. As the FoxG1 loss-of-function allele expresses Flpe after gene removal (A scheme in Figure 4A), FoxG1 mutant cells can be visualized with EGFP directed from the Flpe-activated reporter line (R26R-CAG-FRTstop-EGFP) (Miyoshi et al., 2010). At E16.5, a time by which the majority of cells electroporated at E12.5 have completed migration and settled inside the cortical plate (data not shown), tamoxifen administration was carried out to remove FoxG1 in these cells. These embryos were analyzed at E19.5. (A, high magnification in A’) The control experiment was carried out in the FoxG1-C:Flpe/+ background. Pyramidal neurons labeled by EGFP are heterozygous for FoxG1. (B, B’) The loss-of-function experiment was carried out in the FoxG1-C:Flpe/− background. Note that control and FoxG1-null cells have pyramidal morphologies and occupy similar positions within the cortex. In addition, removal of FoxG1 gene function in postmigratory cells did not result in up-regulation of NeuroD1 and Unc5D (data not shown). Although the dendritic branching of mutant pyramidal neurons appears somewhat retarded compared to the control population (A, A’), they are not different from the internal control cells (Supplemental Figure S6D’, red without green cells, arrowheads). Most importantly, these cells do not morphologically or molecularly resemble the multipolar population. Note that for the purpose of presentation, cells labeled red by mCherry are not visualized in these panels. For the mCherry expression and also for the detailed comparison of wild type, heterozygous and null cells for FoxG1 in both FoxG1C:Flpe/+ and FoxG1C:Flpe/− backgrounds, see Supplemental Figures S6C and S6D.
Scale bars: 50µm
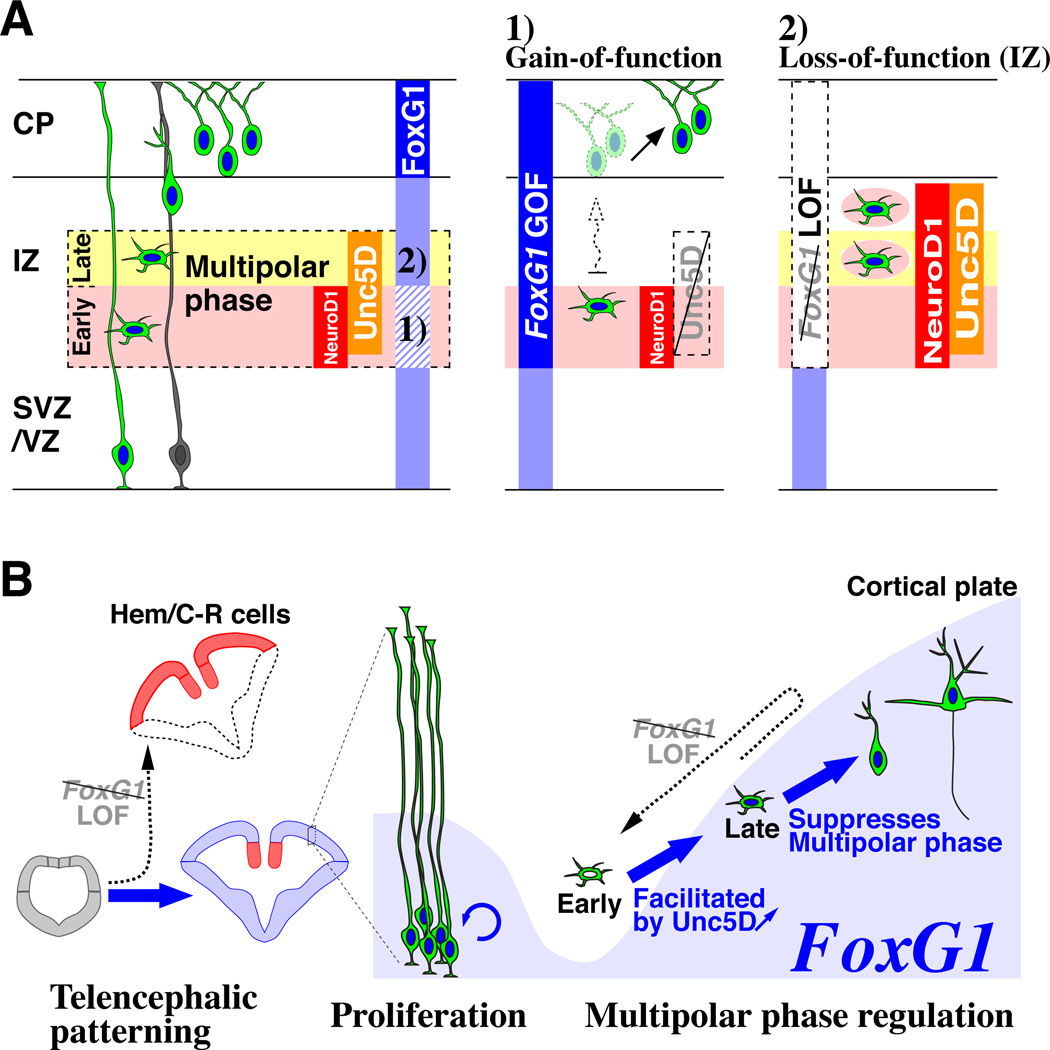
In summary, dynamic FoxG1 expression during the multipolar cell phase specifically coordinates pyramidal cell integration into the cortical plate (Figure 6A). This process appears to be mediated by two equivalently important steps: 1) a down-regulation of FoxG1, allowing pyramidal neuron precursors to promptly transit through the multipolar phase by inducing Unc5D and 2) subsequent up-regulation of FoxG1 to leave the multipolar cell phase and enter into the cortical plate.
Figure 6. Dynamic FoxG1 expression during the postmitotic multipolar cell phase coordinates pyramidal neuron development.
(A, left panel) FoxG1 expression (blue vertical bar) is dynamically regulated throughout pyramidal neuron development. Especially within the intermediate zone (IZ), FoxG1 is transiently down-regulated at the beginning of the multipolar cell phase (Pink) and subsequently re-initiated during the late multipolar phase (Yellow). (A, middle panel) FoxG1 down-regulation is necessary for multipolar cells to initiate Unc5D expression and to rapidly proceed from the early to late multipolar phase. Mis-regulation of FoxG1 at this early phase delays entry into the cortical plate and redirects the laminar fate of postmitotic multipolar cells. (A, right panel) Multipolar cells need to re-initiate FoxG1 expression during the late phase in order to enter the cortical plate; otherwise, they remain in the intermediate zone and regress to the early multipolar phase. (B) Model diagram summarizing the roles of FoxG1 during pyramidal neuron development. FoxG1 expression and function (arrows) are indicated in blue. At the early stage when the telencephalon is emerging from the anterior regions of the neural tube, FoxG1 is required for the patterning by suppressing cortical hem (Hem) / Cajal-Retzius (C-R) cell fate (Hanashima et al., 2004; Muzio and Mallamaci, 2005; Shen et al., 2006b). After this period, FoxG1 is further required for the proliferation of neocortical progenitors (Hanashima et al., 2002; Martynoga et al., 2005). In this study, we have demonstrated the requirement for FoxG1 during the postmitotic period, when pyramidal neurons transit through their multipolar phase of development. At the beginning of the multipolar phase (Early), transient down-regulation of FoxG1 allows cells to initiate Unc5D expression, which is crucial for the rapid transition from the early to late phase. Failure to undergo this transition delays entry of pyramidal neuron precursors into the cortical plate, resulting in a switch in their laminar identity (See middle panel of A). Subsequent to this, FoxG1 expression is re-initiated during the late multipolar phase (Late) and is required for pyramidal neuron precursors to enter into the cortical plate. As indicated by the dashed arrow, failure to re-express FoxG1 at this late phase results in a regression of pyramidal neuron precursors into the early multipolar phase and permanently prevents them from entering the cortical plate (See right panel of A). Hence, as illustrated here, FoxG1 has iterative roles during pyramidal neuron development in patterning, proliferation and postmitotic regulation of the multipolar cell phase.
Discussion
In the present study, we have examined the role of FoxG1 in regulating the migration and maturation of postmitotic pyramidal neuron precursors (Figures 6A). Specifically, we have observed that FoxG1 protein levels are dynamically regulated as pyramidal neurons migrate from the ventricular zone to the cortical plate. We demonstrate that the transient down-regulation of FoxG1 at the beginning of the multipolar phase enables cells to initiate Unc5D expression, which facilitates their transition from the early to late multipolar phase and is thus critical for their migration through the intermediate zone. Failure to down-regulate FoxG1 during this period delays the entrance into the cortical plate, resulting in a superficial shift in both the laminar position and marker expression of pyramidal neurons. Subsequently, the up-regulation of FoxG1 is specifically required for cells to transit out of the multipolar state and enter into the cortical plate. Taken together, we conclude that the dynamic regulation of FoxG1 is a crucial mechanism for controlling the incorporation of pyramidal neuron precursors into the cerebral cortex (Figures 6A and 6B). These findings may have relevance to the etiology of specific classes of mental disorders observed in human patients, including congenital variants of Rett syndrome (Ariani et al., 2008; Brunetti-Pierri et al., 2010; Le Guen et al., 2010).
The importance of the multipolar cell phase in cortical circuit assembly
Only relatively recently has it been recognized that pyramidal neuron precursors transiently adopt a characteristic multipolar morphology while they are migrating within the intermediate zone (Tabata and Nakajima, 2003; Noctor et al., 2004; this study). However, the significance of this phase for the establishment of mature cortical networks remains unclear (LoTurco and Bai, 2006). Recent work suggests that the lateral dispersion of migrating pyramidal neuron precursors is mediated by EphrinA - EphA signaling, most likely during the multipolar phase, and, as a consequence, the width of radial columns is established (Torii et al., 2009). Given recent evidence that radially aligned cells arising from a common progenitor have a high probability of interconnecting (Yu et al., 2009), the tangential dispersion at the multipolar cell phase may also be critical for establishing intercolumnar cortical connectivity (Costa and Hedin-Pereira, 2010). Our work adds to these findings by demonstrating that the timing and duration of the multipolar phase is precisely regulated by FoxG1 activity.
The laminar fate of pyramidal neurons remains labile at the early multipolar cell phase
Pulse-chase studies have shown that cell birth date within the cortex predicts laminar position (Angevine and Sidman, 1961; Rakic, 1974). A now classic transplantation study found that cell fate could be altered depending on whether pyramidal neurons underwent their last neuronal division in an isochronic or heterochronic host environment (McConnell and Kaznowski, 1991). Our study adds to this finding by demonstrating that the laminar position and postnatal marker expression of pyramidal neurons remains labile at least up to the early multipolar phase. In this regard, both the laminar (Kwan et al., 2008; Lai et al., 2008) and areal (Joshi et al., 2008) identity of pyramidal neurons require the persistent expression of the transcription factors (Sox5 and BhlhB5), which are exclusively restricted to postmitotic cells. It will take further analysis to establish whether the mispositioning of pyramidal neurons upon FoxG1 gain-of-function results in changes in their hodological identity. Nonetheless, it is becoming evident that rather than being irreversibly fixed, pyramidal neurons require active maintenance in their identity, demonstrating that the line between developmental programs and adult plasticity is less absolute than previously recognized.
The role of Unc5D-mediated signaling in cell migration
In addition to its roles in axon outgrowth and growth cone turning in commissural projection neurons (Serafini et al., 1994), Netrin-signaling has been shown to mediate both attraction and repulsion during cell migration (Ackerman et al., 1997; Alcantara et al., 2000; Hu and Rutishauser, 1996; Stanco et al., 2009; Xu et al., 2010). It has also been suggested that Netrin-signaling controls axon outgrowth and cell migration through distinct downstream mechanisms (Causeret et al., 2004). Here, we show that, in the case of pyramidal neuron precursor migration, Unc5D and Dcc function in concert during the multipolar cell phase. In this context, FoxG1 appears to regulate the expression of Unc5D but not Dcc. Interestingly, in Drosophila motorneurons akin to the present context, Unc5 is positively regulated by the transcription factor Even-skipped, while Frazzled (the fly homolog of Dcc) is not (Labrador et al., 2005).
Netrins and, more recently, Flrts (Fibronectin type III domain and leucine-rich repeats transmembrane protein) have been demonstrated to interact with Unc5 receptors (Karaulanov et al., 2009; Yamagishi et al., 2011). However, we find that Unc5D-expressing multipolar cells do not undergo obvious changes in their behavior upon over-expression of either Netrin4 or Flrt2 in the ventricular zone (Data not shown due to space limitation). This suggests that Unc5D/Dcc signaling is binary rather than graded, which is consistent with it playing a role in multipolar to radial phase transition but not chemotropic guidance. An area of future interest will be to investigate whether different ligands initiate distinct down-stream signaling cascades upon Unc5D-activation.
Iterative roles of FoxG1 throughout telencephalic development
It is striking to compare the early role of FoxG1 demonstrated for suppressing the production of Cajal-Retzius cells (Hanashima et al., 2007; Hanashima et al., 2004; Shen et al., 2006b) with our present finding that FoxG1 can suppress the late multipolar cell phase of postmitotic pyramidal neuron precursors (Figure 6B). Although quite distinct lineages, Cajal-Retzius cells and pyramidal neuron precursors in the multipolar migratory phase have in common their expression of Reelin (Uchida et al., 2009; Yoshida et al., 2006) and their propensity for tangential migration. Interestingly, we observe a similar dynamic regulation of FoxG1 in telencephalic GABAergic interneuron precursors, where this gene is selectively down-regulated during the tangential phase of their migration and reinitiated when they have invaded the cortical plate (GM, unpublished observation and Supplemental Figures S1A to S1C). Furthermore, FoxG1 is also essential for the integration of interneuron precursors into the cortical plate (GM, unpublished observation). Taken together, there may be a universal requirement for FoxG1 down-regulation during the tangential phases of neuronal migration within the telencephalon. These findings lead us to conjecture that FoxG1 function has been evolutionarily adapted in mammals as a means to regulate radial versus tangential modes of neuronal migration and is therefore vital to the assembly of the laminar and columnar organization that is the hallmark of the cerebral cortex.
Supplementary Material
Acknowledgement
Research in Fishell lab is supported by NIH grants (RO1NS039007, RO1MH071679) and the Simons Foundation and New York State through its NYSTEM initiative. G.M. is supported by a grant from the National Alliance for Research on Schizophrenia and Depression. We thank the following Drs. for kindly sharing their reagents with us; David Anderson (Neurog2-CreER driver), Yoshiki Sasai (FoxG1 antibodies), Sally Temple (FoxG1 antibodies), Jean Hebert (Targeting arms for the FoxG1 locus), Toshifumi Morimura (mDab1 DNA construct), Eseng Lai (FoxG1-LacZ knock-in mutant), Pierre Mattar and Carol Schuurmans (NeuroD1 promoter pGL3 construct), Kyonsoo Hong (Rat Dcc DNA construct), Takahiko Matsuda and Connie Cepko (CAGEN vector), Rudiger Klein (Flrt1-3 DNA constructs) and Nobuhiko Yamamoto (Netrin4 DNA construct). We also wish to thank Dr. Alex Joyner for helpful inputs constructing the conditional FoxG1 loss-of-function allele and Dr. Frada Berenshteyn for her generous help in gene targeting and ES cell selection. Finally, we are extremely appreciative to all of the Fishell lab members for the support and suggestions throughout this project. We thank Lihong Yin for her technical help. We greatly appreciate Dr. Vitor Sousa for his collaborative effort in generating the RCE EGFP reporter lines. We especially wish to thank Dr. Rob Machold for his intellectual inputs in the interpretation of our data and for the generous time he devoted in discussions and assembly of this manuscript. We are also greatly appreciative of the efforts Drs. Theofanis Karayannis, Xavier Jaglin and Allison Roberta made in critically reading this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Extended experimental procedures are included in the Supplemental Information
References
- Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. doi: 10.1038/386838a0. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, De Castro F, Soriano E, Sotelo C. Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development. 2000;127:1359–1372. doi: 10.1242/dev.127.7.1359. [DOI] [PubMed] [Google Scholar]
- Alfano C, Viola L, Heng JI, Pirozzi M, Clarkson M, Flore G, De Maio A, Schedl A, Guillemot F, Studer M. COUP-TFI promotes radial migration and proper morphology of callosal projection neurons by repressing Rnd2 expression. Development. 2011;138:4685–4697. doi: 10.1242/dev.068031. [DOI] [PubMed] [Google Scholar]
- Angevine JBJ, Sidman RL. Autoradiographic Study of Cell Migration during Histogenesis of Cerebral Cortex in the Mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Ariani F, Hayek G, Rondinella D, Artuso R, Mencarelli MA, Spanhol-Rosseto A, Pollazzon M, Buoni S, Spiga O, Ricciardi S, et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am J Hum Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ, Huang GJ, Cheung AF, Era T, Nishikawa S, Bikoff EK, Molnar Z, Robertson EJ, Groszer M. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 2008;22:2479–2484. doi: 10.1101/gad.475408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Solecki D, Polleux F. New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr Opin Neurobiol. 2008;18:44–52. doi: 10.1016/j.conb.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Britanova O, Alifragis P, Junek S, Jones K, Gruss P, Tarabykin V. A novel mode of tangential migration of cortical projection neurons. Dev Biol. 2006;298:299–311. doi: 10.1016/j.ydbio.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Paciorkowski AR, Ciccone R, Mina ED, Bonaglia MC, Borgatti R, Schaaf CP, Sutton VR, Xia Z, Jelluma N, et al. Duplications of FOXG1 in 14q12 are associated with developmental epilepsy, mental retardation, and severe speech impairment. Eur J Hum Genet. 2010 doi: 10.1038/ejhg.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Causeret F, Hidalgo-Sanchez M, Fort P, Backer S, Popoff MR, Gauthier-Rouviere C, Bloch-Gallego E. Distinct roles of Rac1/Cdc42 and Rho/Rock for axon outgrowth and nucleokinesis of precerebellar neurons toward netrin 1. Development. 2004;131:2841–2852. doi: 10.1242/dev.01162. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Costa MR, Hedin-Pereira C. Does cell lineage in the developing cerebral cortex contribute to its columnar organization? Front Neuroanat. 2010;4:26. doi: 10.3389/fnana.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesin C, Peres JN, Johansson M, Snowden V, Cording A, Papalopulu N, Houart C. Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev Cell. 2009;16:576–587. doi: 10.1016/j.devcel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Deuel TA, Liu JS, Corbo JC, Yoo SY, Rorke-Adams LB, Walsh CA. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron. 2006;49:41–53. doi: 10.1016/j.neuron.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Dymecki SM, Kim JC. Molecular neuroanatomy's "Three Gs": a primer. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Schlueter McFadyen-Ketchum LJ, Ahrens ET, Mills PH, Does MD, Nickols J, Levitt P. Disruption of Foxg1 expression by knock-in of cre recombinase: effects on the development of the mouse telencephalon. Neuroscience. 2007;148:385–399. doi: 10.1016/j.neuroscience.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Govek EE, Hatten ME, Van Aelst L. The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol. 2011 doi: 10.1002/dneu.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashima C, Fernandes M, Hebert JM, Fishell G. The role of Foxg1 and dorsal midline signaling in the generation of Cajal-Retzius subtypes. J Neurosci. 2007;27:11103–11111. doi: 10.1523/JNEUROSCI.1066-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22:6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, et al. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Heng JI, Nguyen L, Castro DS, Zimmer C, Wildner H, Armant O, Skowronska-Krawczyk D, Bedogni F, Matter JM, Hevner R, Guillemot F. Neurogenin 2 controls cortical neuron migration through regulation of Rnd2. Nature. 2008;455:114–118. doi: 10.1038/nature07198. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Youn YH, Moon HM, Miyamichi K, Zong H, Wynshaw-Boris A, Luo L. Genetic mosaic dissection of Lis1 and Ndel1 in neuronal migration. Neuron. 2010;68:695–709. doi: 10.1016/j.neuron.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat Genet. 1998;19:333–339. doi: 10.1038/1221. [DOI] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Howell BW, Gertler FB, Cooper JA. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. Embo J. 1997;16:121–132. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Rutishauser U. A septum-derived chemorepulsive factor for migrating olfactory interneuron precursors. Neuron. 1996;16:933–940. doi: 10.1016/s0896-6273(00)80116-6. [DOI] [PubMed] [Google Scholar]
- Jaglin XH, Poirier K, Saillour Y, Buhler E, Tian G, Bahi-Buisson N, Fallet-Bianco C, Phan-Dinh-Tuy F, Kong XP, Bomont P, et al. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat Genet. 2009;41:746–752. doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PS, Molyneaux BJ, Feng L, Xie X, Macklis JD, Gan L. Bhlhb5 regulates the postmitotic acquisition of area identities in layers II-V of the developing neocortex. Neuron. 2008;60:258–272. doi: 10.1016/j.neuron.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn. 2006;235:2376–2385. doi: 10.1002/dvdy.20884. [DOI] [PubMed] [Google Scholar]
- Karaulanov E, Bottcher RT, Stannek P, Wu W, Rau M, Ogata S, Cho KW, Niehrs C. Unc5B interacts with FLRT3 and Rnd1 to modulate cell adhesion in Xenopus embryos. PLoS One. 2009;4:e5742. doi: 10.1371/journal.pone.0005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration. Embo J. 2003;22:4190–4201. doi: 10.1093/emboj/cdg413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49:55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kubo K, Honda T, Tomita K, Sekine K, Ishii K, Uto A, Kobayashi K, Tabata H, Nakajima K. Ectopic Reelin induces neuronal aggregation with a normal birthdate-dependent "inside-out" alignment in the developing neocortex. J Neurosci. 2010;30:10953–10966. doi: 10.1523/JNEUROSCI.0486-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Lam MM, Krsnik Z, Kawasawa YI, Lefebvre V, Sestan N. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci U S A. 2008;105:16021–16026. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrador JP, O'Keefe D, Yoshikawa S, McKinnon RD, Thomas JB, Bashaw GJ. The homeobox transcription factor even-skipped regulates netrin-receptor expression to control dorsal motor-axon projections in Drosophila. Curr Biol. 2005;15:1413–1419. doi: 10.1016/j.cub.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Le Guen T, Bahi-Buisson N, Nectoux J, Boddaert N, Fichou Y, Diebold B, Desguerre I, Raqbi F, Daire VC, Chelly J, Bienvenu T. A FOXG1 mutation in a boy with congenital variant of Rett syndrome. Neurogenetics. 2010 doi: 10.1007/s10048-010-0255-4. [DOI] [PubMed] [Google Scholar]
- Lodato S, Rouaux C, Quast KB, Jantrachotechatchawan C, Studer M, Hensch TK, Arlotta P. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69:763–779. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Bai J. The multipolar stage and disruptions in neuronal migration. Trends Neurosci. 2006;29:407–413. doi: 10.1016/j.tins.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Martynoga B, Yu T, West JD, Mason JO, Price DJ. The transcription factor Foxg1 regulates the competence of telencephalic cells to adopt subpallial fates in mice. Development. 2010;137:487–497. doi: 10.1242/dev.039800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev Biol. 2005;283:113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Mattar P, Langevin LM, Markham K, Klenin N, Shivji S, Zinyk D, Schuurmans C. Basic helix-loop-helix transcription factors cooperate to specify a cortical projection neuron identity. Mol Cell Biol. 2008;28:1456–1469. doi: 10.1128/MCB.01510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. Directing neuron-specific transgene expression in the mouse CNS. Curr Opin Neurobiol. 2006;16:577–584. doi: 10.1016/j.conb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Morimura T, Ogawa M. Relative importance of the tyrosine phosphorylation sites of Disabled-1 to the transmission of Reelin signaling. Brain Res. 2009;1304:26–37. doi: 10.1016/j.brainres.2009.09.087. [DOI] [PubMed] [Google Scholar]
- Muzio L, Mallamaci A. Foxg1 confines Cajal-Retzius neuronogenesis and hippocampal morphogenesis to the dorsomedial pallium. J Neurosci. 2005;25:4435–4441. doi: 10.1523/JNEUROSCI.4804-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Morikubo S, Sato M. Filamin A and FILIP (Filamin A-Interacting Protein) regulate cell polarity and motility in neocortical subventricular and intermediate zones during radial migration. J Neurosci. 2004;24:9648–9657. doi: 10.1523/JNEUROSCI.2363-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Yamashita Y, Tamamaki N, Katoh H, Kaneko T, Negishi M. In vivo function of Rnd2 in the development of neocortical pyramidal neurons. Neurosci Res. 2006;54:149–153. doi: 10.1016/j.neures.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Besson A, Roberts JM, Guillemot F. Coupling cell cycle exit, neuronal differentiation and migration in cortical neurogenesis. Cell Cycle. 2006;5:2314–2318. doi: 10.4161/cc.5.20.3381. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- O'Rourke NA, Dailey ME, Smith SJ, McConnell SK. Diverse migratory pathways in the developing cerebral cortex. Science. 1992;258:299–302. doi: 10.1126/science.1411527. [DOI] [PubMed] [Google Scholar]
- Olson EC, Walsh CA. Reelin/Dab1 Signaling in the Developing Cerebral Cortex. REELIN GLYCOPROTEIN Chapter. 2008;7:89–105. [Google Scholar]
- Pacary E, Heng J, Azzarelli R, Riou P, Castro D, Lebel-Potter M, Parras C, Bell DM, Ridley AJ, Parsons M, Guillemot F. Proneural Transcription Factors Regulate Different Steps of Cortical Neuron Migration through Rnd-Mediated Inhibition of RhoA Signaling. Neuron. 2011;69:1069–1084. doi: 10.1016/j.neuron.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos RL, Bai J, LoTurco JJ. Heterotopia formation in rat but not mouse neocortex after RNA interference knockdown of DCX. Cereb Cortex. 2006;16:1323–1331. doi: 10.1093/cercor/bhj074. [DOI] [PubMed] [Google Scholar]
- Roth M, Bonev B, Lindsay J, Lea R, Panagiotaki N, Houart C, Papalopulu N. FoxG1 and TLE2 act cooperatively to regulate ventral telencephalon formation. Development. 2010;137:1553–1562. doi: 10.1242/dev.044909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Gupta A, Tsai LH. Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron. 2004;42:197–211. doi: 10.1016/s0896-6273(04)00222-3. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Tabata H, Tachikawa K, Nakajima K. The cortical subventricular zone-specific molecule Svet1 is part of the nuclear RNA coded by the putative netrin receptor gene Unc5d and is expressed in multipolar migrating cells. Mol Cell Neurosci. 2008;38:474–483. doi: 10.1016/j.mcn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to Celegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Sessa A, Mao CA, Hadjantonakis AK, Klein WH, Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Nam HS, Song P, Moore H, Anderson SA. FoxG1 haploinsufficiency results in impaired neurogenesis in the postnatal hippocampus and contextual memory deficits. Hippocampus. 2006a;16:875–890. doi: 10.1002/hipo.20218. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006b;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Tremper-Wells BA, Miller MW. Foxg1 haploinsufficiency reduces the population of cortical intermediate progenitor cells: effect of increased p21 expression. Cereb Cortex. 2008;18:1865–1875. doi: 10.1093/cercor/bhm209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–i10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci U S A. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 2003;23:9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M, Hattori Y, Zhao H, Sato H, Tamada A, Sasaki S, Nakajima K, Yamamoto N. Laminar and Areal Expression of Unc5d and Its Role in Cortical Cell Survival. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq265. [DOI] [PubMed] [Google Scholar]
- Tan SS, Breen S. Radial mosaicism and tangential cell dispersion both contribute to mouse neocortical development. Nature. 1993;362:638–640. doi: 10.1038/362638a0. [DOI] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Torii M, Hashimoto-Torii K, Levitt P, Rakic P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461:524–528. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- Uchida T, Baba A, Perez-Martinez FJ, Hibi T, Miyata T, Luque JM, Nakajima K, Hattori M. Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages. J Neurosci. 2009;29:10653–10662. doi: 10.1523/JNEUROSCI.0345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund N, Zdrojewska J, Padzik A, Komulainen E, Bjorkblom B, Rannikko E, Tararuk T, Garcia-Frigola C, Sandholm J, Nguyen L, et al. Phosphorylation of SCG10/stathmin-2 determines multipolar stage exit and neuronal migration rate. Nat Neurosci. 2011;14:305–313. doi: 10.1038/nn.2755. [DOI] [PubMed] [Google Scholar]
- Xu B, Goldman JS, Rymar VV, Forget C, Lo PS, Bull SJ, Vereker E, Barker PA, Trudeau LE, Sadikot AF, Kennedy TE. Critical roles for the netrin receptor deleted in colorectal cancer in dopaminergic neuronal precursor migration, axon guidance, and axon arborization. Neuroscience. 2010;169:932–949. doi: 10.1016/j.neuroscience.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Hampel F, Hata K, Del Toro D, Schwark M, Kvachnina E, Bastmeyer M, Yamashita T, Tarabykin V, Klein R, Egea J. FLRT2 and FLRT3 act as repulsive guidance cues for Unc5-positive neurons. Embo J. 2011 doi: 10.1038/emboj.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Lai E, Stifani S. The winged-helix protein brain factor 1 interacts with groucho and hes proteins to repress transcription. Mol Cell Biol. 2001;21:1962–1972. doi: 10.1128/MCB.21.6.1962-1972.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458:501–504. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirlinger M, Lo L, McMahon J, McMahon AP, Anderson DJ. Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc Natl Acad Sci U S A. 2002;99:8084–8089. doi: 10.1073/pnas.122231199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.