Abstract
The mechanisms that drive the development of diabetic nephropathy remain undetermined. Only 30–40% of patients with diabetes mellitus develop overt nephropathy, which suggests that other contributing factors besides the diabetic state are required for the progression of diabetic nephropathy. Endothelial dysfunction is associated with human diabetic nephropathy and retinopathy, and advanced diabetic glomerulopathy often exhibits thrombotic microangiopathy, including glomerular capillary microaneurysms and mesangiolysis, which are typical manifestations of endothelial dysfunction in the glomerulus. Likewise, diabetic mice with severe endothelial dysfunction owing to deficiency of endothelial nitric oxide synthase develop progressive nephropathy and retinopathy similar to the advanced lesions observed in humans with diabetes mellitus. Additionally, inhibitors of the renin–angiotensin system fail to be renoprotective in some individuals with diabetic nephropathy (due in part to aldosterone breakthrough) and in some mouse models of the disease. In this Review, we discuss the clinical and experimental evidence that supports a role for endothelial nitric oxide deficiency and subsequent endothelial dysfunction in the progression of diabetic nephropathy and retinopathy. If endothelial dysfunction is the key factor required for diabetic nephropathy, then agents that improve endothelial function or raise intraglomerular nitric oxide level could be beneficial in the treatment of diabetic nephropathy.
Introduction
Diabetic nephropathy and retinopathy are serious complications of diabetes mellitus, and can eventually progress to end-stage renal disease and blindness. Epidemiological studies, however, show that only 30% of patients with diabetes develop overt nephropathy and that the incidence of diabetic nephropathy might be decreasing, particularly in patients with type 1 diabetes.1,2 Histological evidence also shows that only a minority of patients with diabetic nephropathy exhibit advanced glomerular injury.3,4 Therefore, it is important to note that not all patients with diabetes develop advanced stages of diabetic renal disease.
Similar observations have been noted in animal models of diabetes. Most diabetic animal models develop only early stages of diabetic nephropathy and not advanced lesions. For instance, glomerular hypertrophy, mesangial expansion, and thickening of the glomerular basement membrane—all of which are features of early diabetic nephropathy—can be induced in most animal models whereas advanced lesions (such as mesangiolysis, Kimmelstiel–Wilson nodules, and glomerular capillary microaneurysms) do not develop. Similarly, most animal models of diabetes only exhibit mild retinal vascular injury, which is characteristic of early features of diabetic retinopathy in humans. The observation that advanced diabetic lesions do not consistently develop in humans and animal models has led us to postulate that the development of these advanced lesions in diabetic nephropathy and retinopathy might require additional factors. This Review discusses the clinical and experimental evidence demonstrating that endothelial nitric oxide deficiency and subsequent endothelial dysfunction are key factors in the development of diabetic nephro-pathy and retinopathy. Therapies that could prove beneficial for the treatment of diabetic nephropathy and retinopathy are also discussed.
Diabetic nephropathy
Role of endothelial dysfunction
In 1989, Jensen et al.5,6 first described that endothelial injury, as evidenced by an increase in plasma levels of von Willebrand factor, was present in patients with incipient type 1 diabetes. Importantly, endothelial function was more severely impaired in patients with overt nephropathy than in those with incipient nephropathy.5,6 Other investigators later confirmed this association in both patients with type 1 diabetes and patients with type 2 diabetes.7–9 Fioretto et al.10 further documented that the increase in plasma levels of von Willebrand factor in patients with type 2 diabetes was positively correlated with glomerular injury whereas patients who did not exhibit endothelial dysfunction had intact renal morphology. This finding led to the hypothesis that the heterogeneous nature of glomerular injury observed in people with diabetes might be accounted for by the extent of endothelial injury in type 2 diabetes.10 In addition, endothelial injury in diabetes is accompanied by structural abnormalities in the endothelium. For instance, an impairment of endothelial glycocalyx was found to be associated with increased permeability in the glomerular basement membrane, which might partially account for the microalbuminuria observed in patients with dia-betes.11,12 loss of endothelial fenestrations is also another feature of early diabetes.13,14 Importantly, such structural abnormalities of the endothelium were also accompanied by mesangial expansion and podocyte injury in patients with diabetes.14
Tubulointerstitial injury can also be observed in patients with type 1 or type 2 diabetes, but is more common in those with type 2 diabetes. Reports suggest that approximately 30% of patients with diabetes predominantly develop tubulointerstitial injury as opposed to glomerular disease.15,16 Furthermore, the severity of the interstitial lesion is a determinant of renal disease progression in patients with type 2 diabetes.17 Interestingly, tubulointerstitial injury develops independently of glom-erular injury in type 1 diabetes.18 although the precise mechanism for diabetic tubulointerstitial injury remains unclear, some studies have suggested a role for endo-thelial dysfunction in this process.19–21 Bangstad et al.20 found an association between the ratio of asymmetric dimethylarginine: l-arginine (a marker of endothelial dysfunction) with tubulointerstitial injury in patients with type 1 diabetes. likewise, Shibata et al.19 suggested in 2009 that endothelial dysfunction could be responsible for the tubulointerstitial injury observed in rats with streptozotocin-induced diabetes. One mechanism by which endothelial dysfunction might induce tubulo-interstitial injury could be through plasma leaking from injured peritubular capillaries and eliciting an inflammatory tubulointerstitial response.22
Role of endothelial nitric oxide
Nitric oxide production is catalyzed by endothelial nitric oxide synthase (enOS) to maintain the integrity of endothelial cells. nitric oxide blocks endothelial cell activation and injury induced by cytokines (such as tumor necrosis factor), has antithrombogenic effects (for instance, by blocking the release of von Willebrand factor from Weibel–Palade bodies) and promotes vaso-dilation of the underlying vascular smooth muscle cells. Of note, one of the earliest features of endothelial dysfunction is a reduction in nitric oxide production in the endothelium.
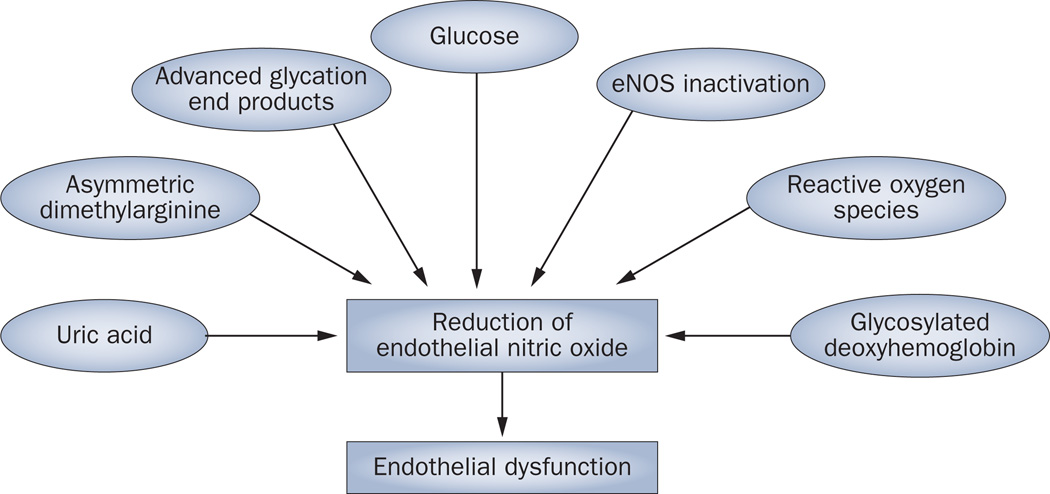
Diabetes is known to be associated with a reduction in nitric oxide bioavailability. The underlying mechanisms seem to be diverse, but include the effects of hyperglycemia,23 advanced glycation end products,24 uric acid,25 asymmetric dimethylarginine26 and oxi-dative stress27 (Figure 1). Impairment of nitric oxide generation caused by polymorphisms in enOS is also thought to cause endothelial dysfunction and nitric oxide deficiency.28 Over the past decade, several investigators have examined enOS polymorphisms in patients with diabetes. However, only some,29–32 but not all studies,33–36 have documented a positive association of specific eNOS polymorphisms with diabetic nephropathy. Hohenstein et al.37 investigated eNOS expression in patients with type 2 diabetes using immunohistochemistry of renal biopsy samples and found that eNOS expression was increased in glom-eruli that had evidence of diabetic changes. Similarly, streptozotocin-induced diabetic rats exhibit an increase in eNOS expression in endothelial cells in the afferent arterioles and glomerulus.38 Importantly, however, eNOS expression does not always correlate with eNOS activity. The generation of nitric oxide from l-arginine by eNOS requires the presence of cofactors that can be inactivated by oxidants, and when eNOS is ‘uncoupled’, superoxide as opposed to nitric oxide will be generated. Brodsky and colleagues39 found that a high level of glucose induces uncoupling of eNOS, which causes a reduction in nitric oxide bioavailability and a concurrent increase in superoxide production.39 Furthermore, Komers et al.40 found that eNOS is present primarily in the uncoupled form and localizes to the cytosolic fraction of endothelial cells from diabetic rats. Since eNOS activation also requires its translocation into the plasma membrane in its coupled form,40 it is likely that the increased level of eNOS in diabetes might be an inactivated form of the enzyme.
Figure 1.
Factors that contribute to the development of endothelial dysfunction in patients with diabetes. Factors including reactive oxygen species, eNOS inactivation and uric acid contribute to a reduction in the levels of nitric oxide in the endothelium, which in turn leads to endothelial dysfunction. Abbreviation: eNOS, endothelial nitric oxide synthase.
Experimental evidence
Streptozotocin-induced diabetes in mice
One of the current favored models for the study of diabetic nephropathy associated with type 1 diabetes is the model of streptozotocin-induced nephropathy in eNOS-knockout mice. However, numerous regimens for the induction of diabetes by streptozotocin are currently being used.41–43 The animal Models of Diabetic Complications Consortium (AMDCC) recommends the injection of 50 mg/kg streptozotocin per day for 5 consecutive days to induce diabetes;44 however, regimens that use 100 mg/kg streptozotocin per day for 2–3 days are also commonly used (and will be referred to as the ‘2-day method’ in this article).45,46 Importantly, both methods are considered ‘low-dose streptozotocin’ compared with historical regimens.41–43,47–49 Comparisons of the AMDCC-recommended regimen and the 2-day method in eNOS-knockout mice showed that although both induce diabetes effectively in mice, the 2-day protocol induced diabetes within 1 week while the AMDCC-recommended regimen induced diabetes after 2 weeks. Moreover, the glomerular injury induced by the AMDCC-recommended regimen tended to be somewhat milder than that induced by the 2-day method (T. Nakagawa, unpublished work). although one might argue that the high doses of streptozotocin used by the 2-day method could cause tubulointerstitial injury owing to streptozotocin toxicity,50 the AMDCC regimen also induced tubulointerstitial injury (T. Nakagawa, unpublished work). In addition, given our finding that insulin treatment substantially blocked tubulointerstitial and glomerular lesions,49 it seems that tubulointerstitial injury occurred secondary to the diabetic state, and not from streptozotocin toxicity.
Diabetic nephropathy in eNos-knockout mice
The 2-day method has also been used to evaluate the role of eNOS in diabetic nephropathy. Consistent with previous findings, diabetic wild-type mice developed mild proteinuria but had preserved renal function. By contrast, diabetic eNOS-knockout mice developed clinical manifestations that resembled overt diabetic nephro pathy in humans. For example, hypertension, nephrotic albuminuria, and renal dysfunction were induced by administration of streptozotocin to eNOS-knockout mice.49 Diabetic eNOS-knockout mice also exhibited higher mortality from progressive renal disease than other groups, including nondiabetic wild-type mice, diabetic wild-type mice and nondiabetic eNOS-knockout mice.49 This finding is important because life expectancy is reduced in patients who have type 1 diabetes with advanced nephropathy51 and mortality corre lates with the severity of renal injury in patients with diabetes.52 These data indicate that a lack of endothelial nitric oxide could be a key factor in the development of advanced diabetic nephropathy.
Histological manifestations of diabetic nephropathy in diabetic eNOS-knockout mice also mimic those of human diabetic nephropathy. Importantly, this mouse model develops not only the early manifestations of diabetic nephropathy, such as mesangial expansion and thickening of the glomerular basement membrane, but also advanced lesions including mesangiolysis, Kimmelstiel–Wilson-like nodules, arteriolar hyalinosis, and tubulointerstitial disease.49 In addition, abnormal angiogenesis is now recognized as a manifestation of human diabetic nephropathy;53 the vessels that result from abnormal angiogenesis likely have a pathological role as antiangiogenic treatment has been shown to prevent proteinuria and renal hypertrophy in experimental models of diabetic nephropathy.54–56 abnormal extraglomerular vessels are observed in wild-type mice with diabetic renal disease,55,57 but the increase in capillary number is more marked in diabetic eNOS-knockout mice.49 The mechanism for the development of abnormal angiogenesis might involve the effects of vascular endothelial growth factor (VEGF). In particular, the effects of VEGF on endothelial cells and macrophages can be enhanced by eNOS deficiency, which could contribute to the excessive proliferation of endothelial cells and abnormal angiogenesis observed in experimental models of diabetic nephropathy.58,59
Another histological manifestation of human diabetic nephropathy is tubulointerstitial injury. Diabetic mice that lack eNOS also develop tubulointerstitial injury that is more severe than that observed in diabetic wild-type mice.21 Insulin treatment can prevent this tubulo interstitial injury,21 which indicates a role for hyperglycemia in mediating this type of injury. By contrast, lowering blood pressure does not prevent tubulo interstitial injury in diabetic eNOS-knockout mice.21 These studies suggest that the diabetic state, coupled with the presence of eNOS deficiency or subsequent endo thelial dysfunction,60 might have a key role in inducing tubulointerstitial injury in individuals with diabetes and that this phenomenon is independent of blood pressure.21,49
Diabetic retinopathy in eNos-knockout mice
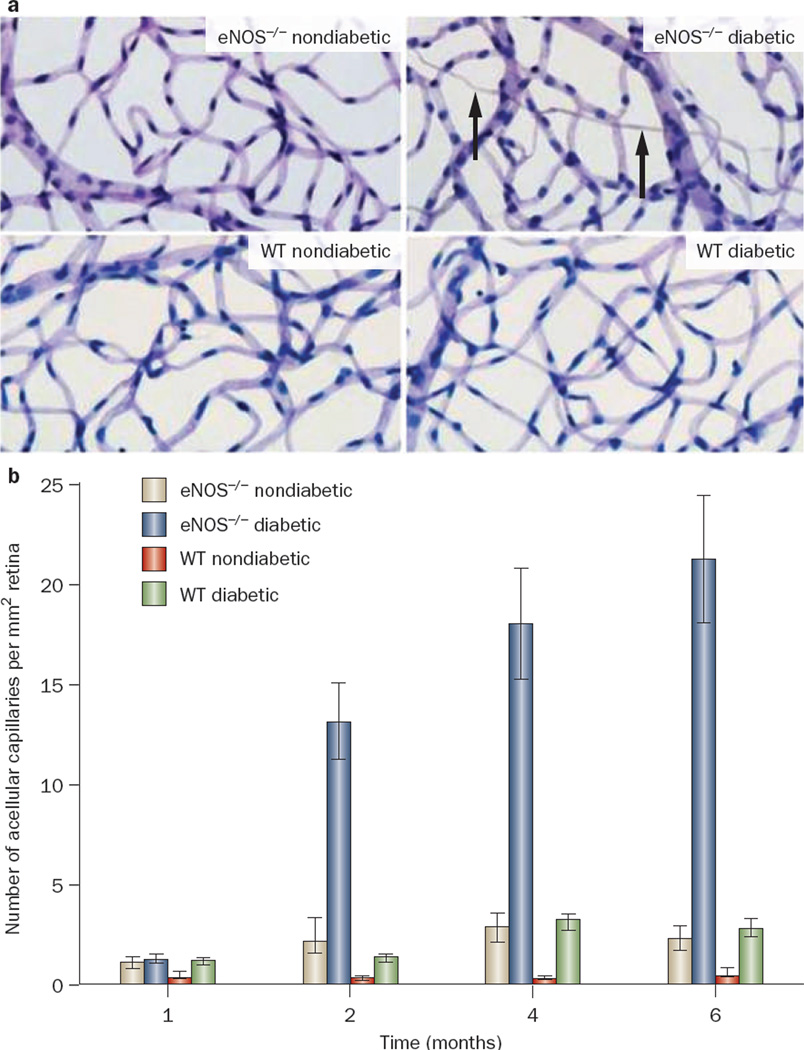
Similar to diabetic nephropathy, most animal models of diabetes do not develop several features of retinal injury that are occasionally observed in humans with diabetic retinopathy. In fact, most models of diabetic mice develop only early stages of diabetic retinopathy: increased capillary permeability, breakdown of the blood retinal barrier, thickening of the capillary basement membrane, the appearance of pericyte ghosts, and the formation of acellular capillaries.61,62 However, these manifestations are mild and do not completely replicate human diabetic retinopathy. In particular, upregulation of glial fibrillary acidic protein expression is associated with Müller cell activation in individuals with early diabetic retino pathy63–65 while expression of glial fibrillary acidic protein is not dramatically different in streptozotocin-induced diabetic mice compared with wild-type mice62 and is not increased in the Ins2Akita mouse model of diabetes.61 By contrast, diabetic eNOS-knockout mice display features of retinopathy that have not been observed in wild-type mice with streptozotocin-induced diabetes. These features resemble most of the major vascular hallmarks of human diabetic retino pathy, including increased thickness of the retinal capillary basement membrane, increased retinal vessel leakage, gliosis, and an increased number of acellular retinal capillaries (Figure 2).66 These findings support the hypothesis that deficiency of endothelial nitric oxide contributes to endothelial dysfunction, which in turn has a critical role in the pathogenesis of diabetic retinopathy.67
Figure 2.
Development of acellular capillaries in the retina of diabetic eNOS-knockout mice. a | Representative images of retinal vessels from nondiabetic wild-type (WT nondiabetic) and diabetic wild-type (WT diabetic) mice 6 months after onset of diabetes treatment, and nondiabetic eNOS-knockout (eNOS−/− nondiabetic) and diabetic eNOS-knockout (eNOS−/− diabetic) mice 4 months after onset of diabetes. The arrows indicate the presence of acellular capillaries in diabetic eNOS-knockout mice. b | Quantitative measurements of acellular capillaries in the various mice. Abbreviations: eNOS, endothelial nitric oxide synthase; W T, wild-type C57BL/6 mice. Permission obtained from Investigative Ophthalmology & visual Science © Li, Q. et al. Invest. Ophthalmol. Vis. Sci. 51, 5240–5246 (2010).
Mechanisms of accelerated nephropathy
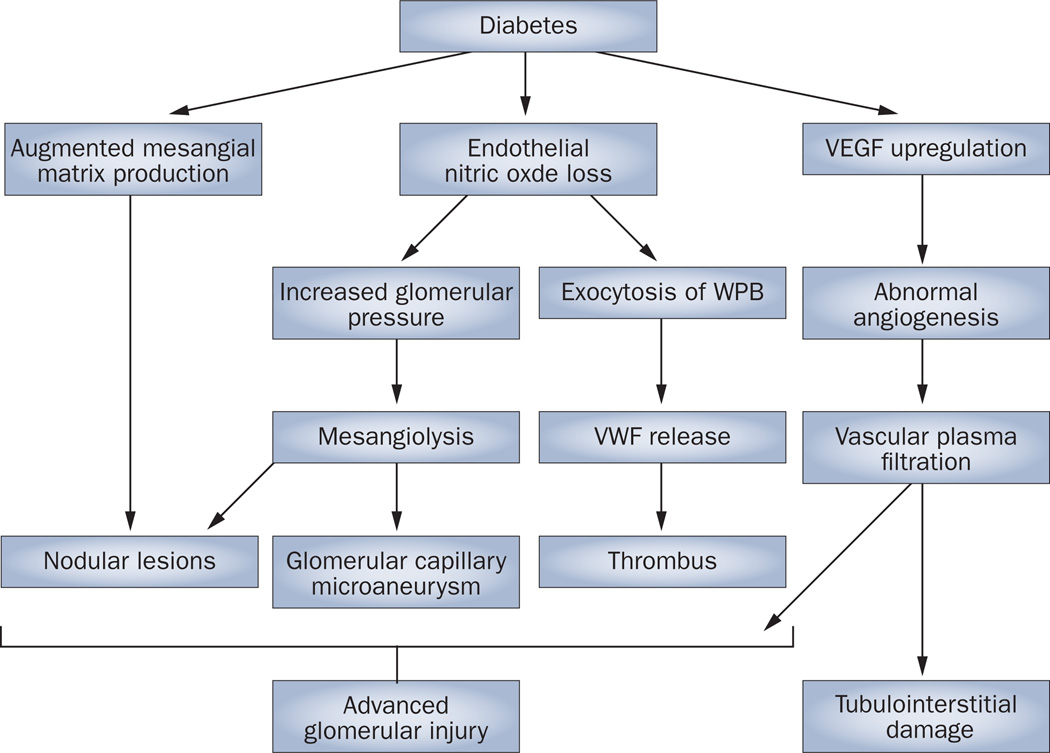
Several potential mechanisms exist through which a lack of endothelial nitric oxide could accelerate the development of diabetic nephropathy (Figure 3). First, endothe-lial dysfunction is known to alter renal autoregulation. Endothelial nitric oxide is produced by endothelial cells in afferent arterioles and the glomerulus, and to a lesser extent in efferent arterioles, and a lack of nitric oxide causes arteriolar disease.68 under physio logical conditions, endothelial nitric oxide regulates intraglomerular pressure.69,70 In settings in which production of nitric oxide is inhibited, however, an increase in systolic blood pressure coupled with altered arteriolar autoregulation results in the increased transmission of pressure to the glomerulus, which results in glomerular hypertension.71 as such, an increase in glomerular pressure owing to endothelial nitric oxide deficiency might partially account for the development of mesangiolysis that is observed in human diabetic nephropathy. Consistent with this hypothesis, we have reported that lowering blood pressure dramatically prevented the progression of glomerular injury in diabetic eNOS-knockout mice.21
Figure 3.
Potential mechanisms by which endothelial nitric oxide deficiency causes advanced renal injury in diabetes. A number of mechanisms contribute to the development of diabetic nephropathy in patients with diabetes. Changes to the production of the mesangial matrix, loss of endothelial nitric oxide and upregulation of VEGF ultimately lead to advanced glomerular injury and tubulointerstitial damage. Abbreviations: VEGF, vascular endothelial growth factor; VWF, von Willebrand factor; WPB, Weibel–Palade bodies.
A second mechanism through which deficiency of endothelial nitric oxide could accelerate the development of diabetic nephropathy is by augmenting the response of many types of cells, including endothelial cells and macro phages, to VEGF. The diabetic state is known to cause an upregulation of VEGF expression, which induces new vessel formation in the kidney and retina,53,72 stimulates renal hypertrophy73 and causes protein uria in experimental models of diabetic nephro-pathy. 74,75 although diabetes is associated with a reduction in nitric oxide bioavailability (Figure 1), in vitro studies from our research group demonstrated that nitric oxide deficiency enhanced endothelial proliferation as well as macrophage chemotaxis in response to VEGF.59,76 Similarly, Bussolati et al.77 used a matrigel angiogenesis assay to demonstrate that nitric oxide inhibited VEGF-induced capillary growth and tube formation. We also documented that the effects of VEGF were enhanced in diabetic eNOS-knockout mice, as evidenced by increased macrophage infiltration and abnormal angiogenesis compared with diabetic wild-type mice.59,76 although VEGF is deleterious in this model, VEGF is known to exert beneficial effects in many chronic and acute kidney diseases.78,79 Such benefits could perhaps occur through nitric-oxide-dependent pathways, in which VEGF is able to induce the expression of endothelial nitric oxide to protect the kidney. By contrast, because eNOS is not available in this model, VEGF could engage nitric-oxide-independent pathways and cause excessive endothelial proliferation or macrophage infiltration that might account for some of the deleterious effects of VEGF on the endothelium.58
Nitric oxide is also known to negatively regulate the release of von Willebrand factor and P-selectin by inhibiting exocytosis of Weibel–Palade bodies in endothelial cells;80 lack of eNOS results in the accelerated release of Weibel–Palade bodies in the mouse kidney.81 This finding might account for the occasional observation of fibrin deposits in the glomeruli of patients with diabetic nephropathy.82
Therapeutic effects of RAS inhibition
Multiple vascular complications are present in 20% of patients with diabetes.83 In particular, observations that diabetic retinopathy and nephropathy are closely associated with one another84,85 have fostered the assumption that treatment of one complication could be also beneficial to the other. Yet, only a limited number of large-scale clinical trials have attempted to systematically address the therapeutic efficacy of simultaneously treating both diabetic retinopathy and nephropathy. Of note is the Diabetes Control and Complications Trial, which examined whether intensive control of blood glucose can reduce retinopathy and nephropathy in patients with type 1 diabetes.86 another example is the UK Prospective Diabetes Study, which examined the longitudinal effects of blood glucose control or lowering of blood pressure on diabetic complications in patients with type 2 diabetes. Importantly, these studies demonstrated that treatments were effective in the prevention of both nephro pathy and retinopathy in their early stages regardless of diabetes type.87
Several large clinical trials have reported that inhibition of the renin–angiotensin system (RAS) slows the progression of renal disease in diabetes; however, the effect on diabetic retinopathy was not examined in these studies.88–90 In turn, clinical studies from 2000 and 2007, respectively, examined the effects of RAS inhibitors on both diabetic nephropathy and retino pathy and have documented that the beneficial effect of RAS inhibition was observed only in diabetic nephro pathy and not in diabetic retinopathy.91,92 For instance, the MICRO-HOPE study91 documented that ramipril might have some beneficial effects on diabetic nephro pathy, but not on diabetic retinopathy. Similarly, the ADVANCE study showed that perindopril, when combined with indapamide, reduced the risk of new onset of micro-albuminuria, but had no effect on retino pathy in patients with type 2 diabetes.92 By contrast, a 2009 study performed by Mauer et al.93 documented an opposite outcome. The study included patients with type 1 diabetes and preclinical glomerulo pathy, and examined the effects of RAS-inhibitor treatment (over 5 years) on renal morphology and retino pathy.93 Interestingly, in contrast to the results of the aforementioned studies,91,92 this study documented that RAS inhibition blocked the progression of diabetic retino pathy, but not glomerulo pathy.93 likewise, the DIRECT study reported that candesartan prevented retinopathy to some degree,94,95 but did not prevent the development of microalbuminuria in normo-tensive patients with either type 1 or 2 diabetes.96 Given these contradictory findings, controversy still exists as to whether RAS inhibitors are useful for the treatment of both diabetic nephropathy and retinopathy.
RaS blockade is considered a key treatment for diabetic nephropathy based on evidence that this approach could provide additional protective effects beyond that of lowering blood pressure.90,97 However, lowering blood pressure in patients with diabetes seems to be difficult.98 Target blood-pressure levels can only be achieved in 33% of patients with diabetes and overt nephropathy even with maximum doses of angiotensin-converting-enzyme (ACE) inhibitors or angiotensin-receptor blockers.99 One potential reason for the difficulty in controlling blood pressure in patients with diabetes is that clinicians tend to manage blood pressure less aggressively in patients with diabetes than in those without,98 possibly owing to concerns about adverse effects, such as hyperkalemia or transient reductions in renal function. another potential explanation could be the development of ‘aldosterone breakthrough’. This phenomenon occurs in approximately 40% of patients with diabetes who are treated with drugs that block the RaS.100,101 Mehdi et al.102 reported in 2009 that the addition of an aldosterone-receptor blocker to the treatment regimen of patients with diabetes who failed to respond to a maximum dose of an ACE inhibitor substantially reduced albuminuria.102 However, the important question of why a sizable portion of patients with diabetic nephropathy exhibit a reduced response to RAS inhibitors and develop aldosterone breakthrough remains unanswered.
RAS inhibition and endothelial dysfunction
Brachial artery reactivity and flow-mediated vasodilatation, both of which are often used to evaluate endothelial function in clinical studies, are reduced in patients with diabetes.103–105 In particular, african americans exhibit impaired brachial artery vasodilatation106 and respond poorly to RAS blockade,107–109 which suggests that the poor response of patients with diabetes to RAS inhibitors may be associated with endothelial dysfunction. Schalkwijk and colleagues105 reported that ACE-inhibitor treatment for 5 weeks did not improve flow-mediated vasodilatation in patients with type 1 diabetes, but that this treatment effectively reduced blood pressure. Furthermore, Jawa et al.110 compared the effects of ACE-inhibitor treatment in African Americans with type 2 diabetes who had persistent microalbuminuria or in whom microalbuminuria had resolved, and found that the poor response of patients with persistent micro-albuminuria to ACE-inhibitor therapy was associated with severely impaired vascular reactivity.
The precise mechanisms for the reduced effectiveness of ACE inhibitors in patients with diabetes remain unclear; however, endothelial dysfunction presumably contributes to aldosterone breakthrough. In general, aldosterone is predominantly produced in the adrenal grand where its synthesis is regulated by nitric oxide. Indeed, a lack of endothelial nitric oxide accelerates aldosterone synthesis in the human adrenal grand.111 In addition, nitric oxide deficiency enhances the vaso-constrictive effects of aldosterone in renal afferent arterioles,112,113 which could contribute to renal disease progression. In support of this hypothesis, Ikeda et al.114 have demonstrated that chronic inhibition of nitric oxide increased plasma concentrations of aldosterone, which mediated renal inflammation and fibrosis in rats. Conversely, nitric oxide inhibits the synthesis of aldo sterone in glomerulosa cells from bovine adrenal gland.115 Thus, endothelial dysfunction could have a key role in inducing aldosterone breakthrough.
Over the past 2 years, the aldosterone–mineralocorticoid receptor system has been found to exist in the rat kidney and retina, and has been postulated to have a causal role in diabetic nephropathy and retinopathy. In the kidney, podocytes have been found to produce aldosterone, and this production is further enhanced in response to high levels of glucose to cause podocyte apoptosis in the diabetic rat kidney.116 In support of these findings, Wilkinson-Berka et al.117 demonstrated that blocking the mineralocorticoid receptor prevented retinal angiogenesis and attenuated the inflammatory response in a rat model of oxygen-induced retinopathy that has features of prematurity in humans. Hence, blocking the aldosterone system could be a novel therapeutic target in diabetic retinopathy and nephropathy.
RAS blockade in diabetic eNos-knockout mice
The response of diabetic eNOS-knockout mice to anti-hypertensive agents seems to be similar to that observed in patients with diabetes. We found that hydralazine markedly lowered systemic blood pressure and prevented the progression of diabetic glomerular injury as well as retinopathy in this experimental mouse model.21 By contrast, and in line with some of the findings from studies of ACE inhibition in humans with diabetes, the ACE inhibitor enalapril blocked retinopathy but not nephro pathy in diabetic eNOS-knockout mice (T. nakagawa, unpublished work). Blood pressure was only trans iently reduced by enalapril (10 mg/kg per day) in the first 2 weeks, with blood pressure levels later returning to levels similar to those in untreated diabetic eNOS-knockout mice after this time.118 likewise, enalapril had only minor effects in reducing urinary albumin excretion in diabetic eNOS-knockout mice although the same treatments markedly reduced renal disease in diabetic wild-type mice.118 Of note, enalapril had no effect in preventing mesangial expansion and reducing extracellular matrix deposition in diabetic eNOS-knockout mice. One could argue that the refractoriness of blood pressure and glomerular injury in diabetic eNOS-knockout mice to these ACE inhibitors could be due to the low dose used. However, support for this explanation is limited, since an excessive dose of enalapril (up to 50 mg/kg per day) also failed to lower blood pressure and had no effect on glomerular injury.118 An intriguing finding is that ACE-inhibitor treatment blocked the forma tion of the acellular capillaries in the retina of diabetic eNOS-knockout mice despite having no effect on blood pressure (T. nakagawa, unpublished work). Hence, the precise mechanisms by which diabetic nephropathy and retinopathy develop could differ slightly, although eNOS deficiency or endothelial dysfunction might still contribute to the development of both lesions in an indivi dual with diabetes.
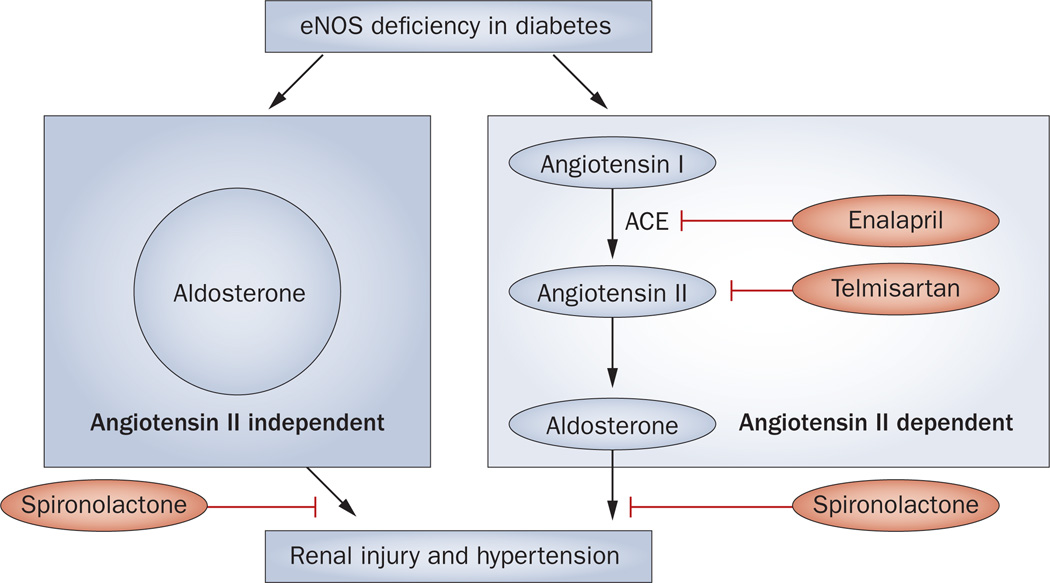
Of interest, the diabetic eNOS-knockout mice that received ACE-inhibitor therapy probably developed aldosterone breakthrough since the treatment did not suppress but, rather paradoxically, increased serum aldosterone concentration.118 In support of this hypothesis, the aldosterone antagonist spironolactone substantially lowered blood pressure and reduced renal injury in this model of diabetic nephropathy.118 The fact that diabetic wild-type mice did not show such paradoxical response to RAS inhibition supports the notion that endothelial dysfunction is involved in the pathogenesis of aldosterone breakthrough (Figure 4). A possible pathological role of the retinal aldosterone system in retinal vasculopathy has also been implicated;119 however, its involvement in the pathogenesis of retinopathy in diabetic eNOS-knockout mice has not been studied.
Figure 4.
Proposed mechanism by which aldosterone causes renal injury and hypertension in diabetes independent of angiotensin II. According to data from studies in eNOS-knockout mouse, eNOS deficiency in combination with diabetes leads to angiotensin-II-independent increases in aldosterone levels, which in turn contributes to renal injury and hypertension. The aldosterone antagonist spironolactone is able to prevent renal injury and hypertension by blocking angiotensin-II-independent and angiotensin-II-dependent aldosterone action while enalapril (an ACE inhibitor) and telmisartan (an angiotensin-receptor blocker) are able to prevent angiotensin-II-dependent renal injury and hypertension. Abbreviations: ACE, angiotensin-converting enzyme; eNOS, endothelial nitric oxide synthase.
Conclusions
Diabetic eNOS-knockout mice have several disease manifestations that resemble those of human diabetic nephropathy and retinopathy. In particular, this model displays advanced lesions that are rarely seen in other animal models of diabetes. Data from experiments using diabetic eNOS-knockout mice indicate that endothelial nitric oxide deficiency or endothelial dysfunction could contribute to advanced nephropathy and retinopathy in patients with diabetes. agents that stimulate endothelial function, such as statins and agonists of peroxi-some proliferator-activated receptors120 might benefit patients with diabetes in part because of these actions. More importantly, we suggest that new therapies specifically aimed at increasing nitric oxide levels within the endothelium are developed to determine whether such agents provide additional benefit in treating diabetic nephropathy and retinopathy.
Key points.
Not all patients with diabetes mellitus develop advanced diabetic nephropathy
Similar to some patients with diabetes, animal models of the disease usually develop only early manifestations of diabetic nephropathy (such as mesangial expansion) and nephropathy (such as pericyte ghost formation)
Severe endothelial dysfunction might be required for the development of advanced diabetic nephropathy in humans as well as animals
Not all patients with diabetic nephropathy benefit from treatment with inhibitors of the renin-angiotensin system (RAS)
The effects of RAS inhibitors on diabetic nephropathy and retinopathy might differ
Failure of RAS inhibitors to prevent the progression of diabetic nephropathy might be accounted for by endothelial dysfunction
Footnotes
Competing interests
The authors declare no competing interests.
Review criteria
Information for this Review was obtained by searching PubMed for full-text papers published in English between 1975 and 2010 using the following search terms: “type 1 diabetes”, “type 2 diabetes”, “diabetic nephropathy”, “ACE inhibitor”, “angiotensin receptor inhibitor”, “endothelial dysfunction”, “endothelial nitric oxide”, “diabetic retinopathy” and “aldosterone”.
Author contributions
T. Nakagawa, K. Tanabe, B. P. Croker, T. Kosugi and Q. Li contributed equally to researching data for this article. T. Nakagawa and M. B. Grant contributed equally to discussions of the content. T. Nakagawa wrote the article, and T. Nakagawa and R. J. Johnson contributed equally to reviewing/editing the manuscript before submission.
References
- 1.Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1994;330:15–18. doi: 10.1056/NEJM199401063300103. [DOI] [PubMed] [Google Scholar]
- 2.Hovind P, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 3.Saito Y, et al. Mesangiolysis in diabetic glomeruli: its role in the formation of nodular lesions. Kidney Int. 1988;34:389–396. doi: 10.1038/ki.1988.193. [DOI] [PubMed] [Google Scholar]
- 4.Stout LC, Kumar S, Whorton EB. Insudative lesions—their pathogenesis and association with glomerular obsolescence in diabetes: a dynamic hypothesis based on single views of advancing human diabetic nephropathy. Hum. Pathol. 1994;25:1213–1227. doi: 10.1016/0046-8177(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 5.Jensen T. Increased plasma concentration of von Willebrand factor in insulin dependent diabetics with incipient nephropathy. BMJ. 1989;298:27–28. doi: 10.1136/bmj.298.6665.27-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen T, Bjerre-Knudsen J, Feldt-Rasmussen B, Deckert T. Features of endothelial dysfunction in early diabetic nephropathy. Lancet. 1989;1:461–463. doi: 10.1016/s0140-6736(89)91365-2. [DOI] [PubMed] [Google Scholar]
- 7.Stehouwer CD, et al. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992;340:319–323. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- 8.Stehouwer CD, Stroes ES, Hackeng WH, Mulder PG, Den Ottolander GJ. von Willebrand factor and development of diabetic nephropathy in IDDM. Diabetes. 1991;40:971–976. doi: 10.2337/diab.40.8.971. [DOI] [PubMed] [Google Scholar]
- 9.Parving HH, et al. Macro-microangiopathy and endothelial dysfunction in NIDDM patients with and without diabetic nephropathy. Diabetologia. 1996;39:1590–1597. doi: 10.1007/s001250050619. [DOI] [PubMed] [Google Scholar]
- 10.Fioretto P, et al. Heterogeneous nature of microalbuminuria in NIDDM: studies of endothelial function and renal structure. Diabetologia. 1998;41:233–236. doi: 10.1007/s001250050895. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwdorp M, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwdorp M, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo . Diabetes. 2006;55:480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 13.Dávila-Esqueda ME, vertiz-Hernández AA, Martínez-Morales F. Comparative analysis of the renoprotective effects of pentoxifylline and vitamin E on streptozotocin-induced diabetes mellitus. Ren. Fail. 2005;27:115–122. [PubMed] [Google Scholar]
- 14.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 15.Fioretto P, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39:1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 16.Gambara v, Mecca G, Remuzzi G, Bertani T. Heterogeneous nature of renal lesions in type II diabetes. J. Am. Soc. Nephrol. 1993;3:1458–1466. doi: 10.1681/ASN.V381458. [DOI] [PubMed] [Google Scholar]
- 17.White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J. Am. Soc. Nephrol. 2000;11:1667–1673. doi: 10.1681/ASN.V1191667. [DOI] [PubMed] [Google Scholar]
- 18.Lane PH, Steffes MW, Fioretto P, Mauer SM. Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int. 1993;43:661–667. doi: 10.1038/ki.1993.95. [DOI] [PubMed] [Google Scholar]
- 19.Shibata R, et al. Involvement of asymmetric dimethylarginine (ADMA) in tubulointerstitial ischaemia in the early phase of diabetic nephropathy. Nephrol. Dial. Transplant. 2009;24:1162–1169. doi: 10.1093/ndt/gfn630. [DOI] [PubMed] [Google Scholar]
- 20.Bangstad HJ, Seljeflot I, Berg TJ, Hanssen KF. Renal tubulointerstitial expansion is associated with endothelial dysfunction and inflammation in type 1 diabetes. Scand. J. Clin. Lab. Invest. 2009;69:138–144. doi: 10.1080/00365510802444080. [DOI] [PubMed] [Google Scholar]
- 21.Kosugi T, et al. Lowering blood pressure blocks mesangiolysis and mesangial nodules but not tubulointerstitial injury, in diabetic eNOS knockout mice. Am. J. Pathol. 2009;174:1221–1229. doi: 10.2353/ajpath.2009.080605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Temm C, Dominguez JH. Microcirculation: nexus of comorbidities in diabetes. Am. J. Physiol. Renal Physiol. 2007;293:F486–F493. doi: 10.1152/ajprenal.00503.2006. [DOI] [PubMed] [Google Scholar]
- 23.Brodsky SV, Morrishow AM, Dharia N, Gross SS, Goligorsky MS. Glucose scavenging of nitric oxide. Am. J. Physiol. Renal Physiol. 2001;280:F480–F486. doi: 10.1152/ajprenal.2001.280.3.F480. [DOI] [PubMed] [Google Scholar]
- 24.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J. Clin. Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khosla UM, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 26.Xiong Y, Lei M, Fu S, Fu Y. Effect of diabetic duration on serum concentrations of endogenous inhibitor of nitric oxide synthase in patients and rats with diabetes. Life. Sci. 2005;77:149–159. doi: 10.1016/j.lfs.2004.10.062. [DOI] [PubMed] [Google Scholar]
- 27.Ceriello A, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834–838. doi: 10.1007/s001250100529. [DOI] [PubMed] [Google Scholar]
- 28.Noiri E, et al. Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension. 2002;40:535–540. doi: 10.1161/01.hyp.0000033974.57407.82. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, et al. T-786C polymorphism of the endothelial nitric oxide synthase gene is associated with albuminuria in the diabetes heart study. J. Am. Soc. Nephrol. 2005;16:1085–1090. doi: 10.1681/ASN.2004100817. [DOI] [PubMed] [Google Scholar]
- 30.Neugebauer S, Baba T, Watanabe T. Association of the nitric oxide synthase gene polymorphism with an increased risk for progression to diabetic nephropathy in type 2 diabetes. Diabetes. 2000;49:500–503. doi: 10.2337/diabetes.49.3.500. [DOI] [PubMed] [Google Scholar]
- 31.Shin Shin Y, et al. Relations between eNOS Glu298Asp polymorphism and progression of diabetic nephropathy. Diabetes Res. Clin. Pract. 2004;65:257–265. doi: 10.1016/j.diabres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Zanchi A, et al. Risk of advanced diabetic nephropathy in type 1 diabetes is associated with endothelial nitric oxide synthase gene polymorphism. Kidney Int. 2000;57:405–413. doi: 10.1046/j.1523-1755.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 33.Degen B, Schmidt S, Ritz E. A polymorphism in the gene for the endothelial nitric oxide synthase and diabetic nephropathy. Nephrol. Dial. Transplant. 2001;16:185. doi: 10.1093/ndt/16.1.185. [DOI] [PubMed] [Google Scholar]
- 34.Lin S, Qu H, Qiu M. Allele A in intron 4 of ecNOS gene will not increase the risk of diabetic nephropathy in type 2 diabetes of Chinese population. Nephron. 2002;91:768. doi: 10.1159/000065048. [DOI] [PubMed] [Google Scholar]
- 35.Rippin JD, et al. Nitric oxide synthase gene polymorphisms and diabetic nephropathy. Diabetologia. 2003;46:426–428. doi: 10.1007/s00125-003-1046-3. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu T, Onuma T, Kawamori R, Makita Y, Tomino Y. Endothelial nitric oxide synthase gene and the development of diabetic nephropathy. Diabetes Res. Clin. Pract. 2002;58:179–185. doi: 10.1016/s0168-8227(02)00156-0. [DOI] [PubMed] [Google Scholar]
- 37.Hohenstein B, et al. Analysis of NO-synthase expression and clinical risk factors in human diabetic nephropathy. Nephrol. Dial. Transplant. 2008;23:1346–1354. doi: 10.1093/ndt/gfm797. [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto H, et al. Increased expression of endothelial cell nitric oxide synthase (ecNOS) in afferent and glomerular endothelial cells is involved in glomerular hyperfiltration of diabetic nephropathy. Diabetologia. 1998;41:1426–1434. doi: 10.1007/s001250051088. [DOI] [PubMed] [Google Scholar]
- 39.Brodsky SV, Gao S, Li H, Goligorsky MS. Hyperglycemic switch from mitochondrial nitric oxide to superoxide production in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2130–H2139. doi: 10.1152/ajpheart.00196.2002. [DOI] [PubMed] [Google Scholar]
- 40.Komers R, et al. Altered endothelial nitric oxide synthase targeting and conformation and caveolin-1 expression in the diabetic kidney. Diabetes. 2006;55:1651–1659. doi: 10.2337/db05-1595. [DOI] [PubMed] [Google Scholar]
- 41.Mogyorosi A, Ziyadeh FN. Increased decorin mRNA in diabetic mouse kidney and in mesangial tubular cells cultured in high glucose. Am. J. Physiol. 1998;275:F827–F832. doi: 10.1152/ajprenal.1998.275.5.F827. [DOI] [PubMed] [Google Scholar]
- 42.Flyvbjerg A, Bennett WF, Rasch R, Kopchick JJ, Scarlett JA. Inhibitory effect of a growth hormone receptor antagonist (G120K-PEG) on renal enlargement, glomerular hypertrophy, and urinary albumin excretion in experimental diabetes in mice. Diabetes. 1999;48:377–382. doi: 10.2337/diabetes.48.2.377. [DOI] [PubMed] [Google Scholar]
- 43.Inada A, et al. A model for diabetic nephropathy: advantages of the inducible cAMP early repressor transgenic mouse over the streptozotocin-induced diabetic mouse. J. Cell. Physiol. 2008;215:383–391. doi: 10.1002/jcp.21316. [DOI] [PubMed] [Google Scholar]
- 44.Breyer MD, et al. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 45.Fujimoto M, et al. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem. Biophys. Res. Commun. 2003;305:1002–1007. doi: 10.1016/s0006-291x(03)00885-4. [DOI] [PubMed] [Google Scholar]
- 46.Wang A, et al. Interference with TGF-beta signaling by Smad3-knockout in mice limits diabetic glomerulosclerosis without affecting albuminuria. Am. J. Physiol. Renal Physiol. 2007;293:F1657–F1665. doi: 10.1152/ajprenal.00274.2007. [DOI] [PubMed] [Google Scholar]
- 47.Tay YC, et al. Can murine diabetic nephropathy be separated from superimposed acute renal failure? Kidney Int. 2005;68:391–398. doi: 10.1111/j.1523-1755.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 48.Kanetsuna Y, et al. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am. J. Pathol. 2007;170:1473–1484. doi: 10.2353/ajpath.2007.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa T, et al. Diabetic eNOS knockout mice develop advanced diabetic nephropathy. J. Am. Soc. Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 50.Brosius FC, et al. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. (3rd) 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borch-Johnsen K, Kreiner S, Deckert T. Mortality of type 1 (insulin-dependent) diabetes mellitus in Denmark: a study of relative mortality in 2,930 Danish type 1 diabetic patients diagnosed from 1933 to 1972. Diabetologia. 1986;29:767–772. doi: 10.1007/BF00873214. [DOI] [PubMed] [Google Scholar]
- 52.Groop PH, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651–1658. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58:1471–1478. doi: 10.2337/db09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ichinose K, et al. 2-(8-Hydroxy-6-methoxy-1-oxo-1h-2-benzopyran-3-yl) propionic acid, an inhibitor of angiogenesis, ameliorates renal alterations in obese type 2 diabetic mice. Diabetes. 2006;55:1232–1242. [PubMed] [Google Scholar]
- 55.Ichinose K, et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes. 2005;54:2891–2903. doi: 10.2337/diabetes.54.10.2891. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto Y, et al. Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes. 2004;53:1831–1840. doi: 10.2337/diabetes.53.7.1831. [DOI] [PubMed] [Google Scholar]
- 57.Guo M, et al. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J. Anat. 2005;207:813–821. doi: 10.1111/j.1469-7580.2005.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakagawa T. Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am. J. Physiol. Renal Physiol. 2007;292:F1665–F1672. doi: 10.1152/ajprenal.00495.2006. [DOI] [PubMed] [Google Scholar]
- 59.Nakagawa T, et al. Uncoupling of vascular endothelial growth factor with nitric oxide as a mechanism for diabetic vasculopathy. J. Am. Soc. Nephrol. 2006;17:736–745. doi: 10.1681/ASN.2005070759. [DOI] [PubMed] [Google Scholar]
- 60.Huang PL, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 61.Barber AJ, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest. Ophthalmol. Vis. Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 62.Feit-Leichman RA, et al. vascular damage in a mouse model of retinopathy: relation to neuronal glial changes. Invest. Ophthalmol. Vis. Sci. 2005;46:4281–4287. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
- 63.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Invest. Ophthalmol. Vis. Sci. 2000;41:3561–3568. [PubMed] [Google Scholar]
- 64.Rungger-Brändle E, Dosso AA, Leuenberger PM. Glial reactivity an early feature of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2000;41:1971–1980. [PubMed] [Google Scholar]
- 65.Mizutani M, Gerhardinger C, Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 66.Li Q, et al. Diabetic eNOS knockout mice develop accelerated retinopathy. Invest. Ophthalmol. Vis. Sci. 2010;51:5240–5246. doi: 10.1167/iovs.09-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng D, et al. von Willebrand factor and retinal circulation in early-stage retinopathy of type 1 diabetes. Diabetes Care. 2000;23:1694–1698. doi: 10.2337/diacare.23.11.1694. [DOI] [PubMed] [Google Scholar]
- 68.Quiroz Y, et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am. J. Physiol. Renal Physiol. 2001;281:F38–F47. doi: 10.1152/ajprenal.2001.281.1.F38. [DOI] [PubMed] [Google Scholar]
- 69.Edwards RM, Trizna W. Modulation of glomerular arteriolar tone by nitric oxide synthase inhibitors. J. Am. Soc. Nephrol. 1993;4:1127–1132. doi: 10.1681/ASN.V451127. [DOI] [PubMed] [Google Scholar]
- 70.Patzak A, et al. Nitric oxide counteracts angiotensin II induced contraction in efferent arterioles in mice. Acta. Physiol. Scand. 2004;181:439–444. doi: 10.1111/j.1365-201X.2004.01316.x. [DOI] [PubMed] [Google Scholar]
- 71.Sánchez-Lozada LG, et al. Mild hyperuricemia induces glomerular hypertension in normal rats. Am. J. Physiol. Renal Physiol. 2002;283:F1105–F1110. doi: 10.1152/ajprenal.00170.2002. [DOI] [PubMed] [Google Scholar]
- 72.Ferrara N. vascular endothelial growth factor: basic science clinical progress. Endocr. Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 73.Flyvbjerg A, Schrijvers BF, De vriese AS, Tilton RG, Rasch R. Compensatory glomerular growth after unilateral nephrectomy is vEGF dependent. Am. J. Physiol. Endocrinol. Metab. 2002;283:E362–E366. doi: 10.1152/ajpendo.00007.2002. [DOI] [PubMed] [Google Scholar]
- 74.de vriese AS, et al. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J. Am. Soc. Nephrol. 2001;12:993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- 75.Flyvbjerg A, et al. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090–3094. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- 76.Sato W, et al. The pivotal role of VEGF on glomerular macrophage infiltration in advanced diabetic nephropathy. Lab. Invest. 2008;88:949–961. doi: 10.1038/labinvest.2008.60. [DOI] [PubMed] [Google Scholar]
- 77.Bussolati B, et al. vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am. J. Pathol. 2001;159:993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. vascular endothelial growth factor administration reduces renal fibrosis stabilizes renal function. J. Am. Soc. Nephrol. 2001;12:1448–1457. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- 79.Shimizu A, et al. vascular endothelial growth factor165 resolves glomerular inflammation and accelerates glomerular capillary repair in rat anti-glomerular basement membrane glomerulonephritis. J. Am. Soc. Nephrol. 2004;15:2655–2665. doi: 10.1097/01.ASN.0000141038.28733.F2. [DOI] [PubMed] [Google Scholar]
- 80.Matsushita K, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakayama T, et al. Endothelial von Willebrand factor release due to eNOS deficiency predisposes to thrombotic microangiopathy in mouse aging kidney. Am. J. Pathol. 2010;176:2198–2208. doi: 10.2353/ajpath.2010.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farquhar A, MacDonald MK, Ireland JT. The role of fibrin deposition in diabetic glomerulosclerosis: a light electron immunofluorescence microscopy study. J. Clin. Pathol. 1972;25:657–667. doi: 10.1136/jcp.25.8.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgan CL, et al. The prevalence of multiple diabetes-related complications. Diabet. Med. 2000;17:146–151. doi: 10.1046/j.1464-5491.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 84.El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, Moharram OA, Kangave D. Retinopathy as a predictor of other diabetic complications. Int. Ophthalmol. 2001;24:1–11. doi: 10.1023/a:1014409829614. [DOI] [PubMed] [Google Scholar]
- 85.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care. 2002;25:859–864. doi: 10.2337/diacare.25.5.859. [DOI] [PubMed] [Google Scholar]
- 86.The Diabetes Control Complications Trial/ Epidemiology of Diabetes nterventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N. Engl. J. Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 88.Brenner BM, et al. Effects of losartan on renal cardiovascular outcomes in patients with type 2 diabetes nephropathy. N. Engl. J. Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 89.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 90.Lewis EJ, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 91.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 92.Patel A, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 93.Mauer M, et al. Renal and retinal effects of enalapril losartan in type 1 diabetes. N. Engl. J. Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaturvedi N, et al. Effect of candesartan on prevention (DIRECT-Prevent 1), progression (DIRECT-Protect1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 95.Sjølie AK, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect2): a randomised placebo-controlled trial. Lancet. 2008;372:1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 96.Bilous R, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann. Intern. Med. 2009;151:11–20. doi: 10.7326/0003-4819-151-1-200907070-00120. [DOI] [PubMed] [Google Scholar]
- 97.Mathiesen ER, Hommel E, Giese J, Parving HH. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. BMJ. 1991;303:81–87. doi: 10.1136/bmj.303.6794.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berlowitz DR, et al. Hypertension management in patients with diabetes: the need for more aggressive therapy. Diabetes Care. 2003;26:355–359. doi: 10.2337/diacare.26.2.355. [DOI] [PubMed] [Google Scholar]
- 99.Tomlinson JW, Owen KR, Close CF. Treating hypertension in diabetic nephropathy. Diabetes Care. 2003;26:1802–1805. doi: 10.2337/diacare.26.6.1802. [DOI] [PubMed] [Google Scholar]
- 100.Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41:64–68. doi: 10.1161/01.hyp.0000044937.95080.e9. [DOI] [PubMed] [Google Scholar]
- 101.Schjoedt KJ, Andersen S, Rossing P, Tarnow L, Parving HH. Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47:1936–1939. doi: 10.1007/s00125-004-1542-0. [DOI] [PubMed] [Google Scholar]
- 102.Mehdi UF, Adams-Huet B, Raskin P, vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 2009;20:2641–2650. doi: 10.1681/ASN.2009070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papaioannou GI, et al. Brachial artery reactivity in symptomatic patients with type 2 diabetes mellitus and microalbuminuria (from the Detection of Ischemia in Asymptomatic Diabetics-brachial artery reactivity study) Am. J. Cardiol. 2004;94:294–299. doi: 10.1016/j.amjcard.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 104.Stehouwer CD, et al. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int. Suppl. 2004:S42–S44. doi: 10.1111/j.1523-1755.2004.09211.x. [DOI] [PubMed] [Google Scholar]
- 105.Schalkwijk CG, Smulders RA, Lambert J, Donker AJ, Stehouwer CD. ACE-inhibition modulates some endothelial functions in healthy subjects and in normotensive type 1 diabetic patients. Eur. J. Clin. Invest. 2000;30:853–860. doi: 10.1046/j.1365-2362.2000.00721.x. [DOI] [PubMed] [Google Scholar]
- 106.Perregaux D, et al. Brachial vascular reactivity in blacks. Hypertension. 2000;36:866–871. doi: 10.1161/01.hyp.36.5.866. [DOI] [PubMed] [Google Scholar]
- 107.Dries DL, et al. Racial differences in the outcome of left ventricular dysfunction. N. Engl. J. Med. 1999;340:609–616. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 108.Dries DL, Strong MH, Cooper RS, Drazner MH. Efficacy of angiotensin-converting enzyme inhibition in reducing progression from asymptomatic left ventricular dysfunction to symptomatic heart failure in black and white patients. J. Am. Coll. Cardiol. 2002;40:311–317. doi: 10.1016/s0735-1097(02)01943-5. [DOI] [PubMed] [Google Scholar]
- 109.Julius S, et al. Cardiovascular risk reduction in hypertensive black patients with left ventricular hypertrophy: the LIFEstudy. J. Am. Coll. Cardiol. 2004;43:1047–1055. doi: 10.1016/j.jacc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 110.Jawa A, Nachimuthu S, Pendergrass M, Asnani S, Fonseca v. Impaired vascular reactivity in African-American patients with type 2 diabetes mellitus and microalbuminuria or proteinuria despite angiotensin-converting enzyme inhibitor therapy. J. Clin. Endocrinol. Metab. 2006;91:31–35. doi: 10.1210/jc.2005-1632. [DOI] [PubMed] [Google Scholar]
- 111.Muldowney JA, 3rd, Davis SN, vaughan DE, Brown NJ. NO synthase inhibition increases aldosterone in humans. Hypertension. 2004;44:739–745. doi: 10.1161/01.HYP.0000143852.48258.f1. [DOI] [PubMed] [Google Scholar]
- 112.Hanke CJ, Campbell WB. Endothelial cell nitric oxide inhibits aldosterone synthesis in zona glomerulosa cells: modulation by oxygen. Am. J. Physiol. Endocrinol. Metab. 2000;279:E846–E854. doi: 10.1152/ajpendo.2000.279.4.E846. [DOI] [PubMed] [Google Scholar]
- 113.Arima S, et al. Endothelium-derived nitric oxide modulates vascular action of aldosterone in renal arteriole. Hypertension. 2004;43:352–357. doi: 10.1161/01.HYP.0000111138.78714.1a. [DOI] [PubMed] [Google Scholar]
- 114.Ikeda H, et al. Spironolactone suppresses inflammation and prevents L-NAME-induced renal injury in rats. Kidney Int. 2009;75:147–155. doi: 10.1038/ki.2008.507. [DOI] [PubMed] [Google Scholar]
- 115.Sainz JM, et al. Effects of nitric oxide on aldosterone synthesis and nitric oxide synthase activity in glomerulosa cells from bovine adrenal gland. Endocrine. 2004;24:61–71. doi: 10.1385/ENDO:24:1:061. [DOI] [PubMed] [Google Scholar]
- 116.Lee SH, et al. Activation of local aldosterone system within podocytes is involved in apoptosis under diabetic conditions. Am. J. Physiol. Renal Physiol. 2009;297:F1381–F1390. doi: 10.1152/ajprenal.00101.2009. [DOI] [PubMed] [Google Scholar]
- 117.Wilkinson-Berka JL, Tan G, Jaworski K, Miller AG. Identification of a retinal aldosterone system and the protective effects of mineralocorticoid receptor antagonism on retinal vascular pathology. Circ. Res. 2009;104:124–133. doi: 10.1161/CIRCRESAHA.108.176008. [DOI] [PubMed] [Google Scholar]
- 118.Kosugi T, Heinig M, Nakayama T, Matsuo S, Nakagawa T. eNOS knockout mice with advanced diabetic nephropathy have less benefit from renin-angiotensin blockade than from aldosterone receptor antagonists. Am. J. Pathol. 2010;49:51–54. doi: 10.2353/ajpath.2010.090578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wilkinson-Berka JL, Tan G, Jaworski K, Miller AG. Identification of a retinal aldosterone system and the protective effects of mineralocorticoid receptor antagonism on retinal vascular pathology. Circ. Res. 2009;104:124–133. doi: 10.1161/CIRCRESAHA.108.176008. [DOI] [PubMed] [Google Scholar]
- 120.Tousoulis D, et al. Novel therapies targeting vascular endothelium. Endothelium. 2006;13:411–421. doi: 10.1080/10623320601061714. [DOI] [PubMed] [Google Scholar]