Abstract
Objective
Selective serotonin reuptake inhibitors (SSRIs) may reduce bone mineral density (BMD). Here, we investigate whether variants of the serotonin transporter-linked polymorphic region (5-HTTLPR) of the serotonin transporter gene moderate this association in boys.
Method
Between November 2005 and August 2009, medically healthy boys, aged 7 to 17 years, were enrolled in a cross-sectional study exploring the effect of risperidone-induced hyperprolactinemia on BMD. Volumetric BMD of the ultradistal radius was measured using peripheral quantitative computed tomography, and areal BMD of the lumbar spine was estimated using dual energy x-ray absorptiometry. Multiple linear regression analysis tested whether the 5-HTTLPR genotypes interacted with SSRI treatment status to affect BMD, adjusting for relevant confounders. Participant enrollment was conducted at the University of Iowa, Iowa City.
Results
Of 108 boys (mean ± SD age = 11.7 ± 2.8 years), with DSM-IV clinical diagnoses based on chart review, 52% (n = 56) had been taking an SSRI for a median duration of 2.8 years. After adjusting for pubertal development, anthropometric measures, physical activity, calcium intake, and prolactin concentration, there was a significant 5-HTTLPR genotype × SSRI treatment interaction effect on total lumbar spine BMD z score (P < .05) in non-Hispanic whites. The interaction effect on BMD at the ultradistal radius failed to reach statistical significance. Among LS genotype carriers, those treated with SSRIs had lower lumbar BMD z score and trabecular BMD at the radius compared to those not treated (P < .02 and P < .008, respectively).
Conclusions
These findings add to the growing evidence implicating the serotonin system in bone metabolism. They suggest the potential use of 5-HTTLPR genotypes to guide the safer long-term prescribing of SSRIs in youths. However, prospective confirmation in a controlled matched population is warranted.
The use of selective serotonin reuptake inhibitors (SSRIs) in youths is widespread.1 Alone or in combination with psychotherapy, these medications effectively treat pediatric depressive and anxiety disorders and are well tolerated acutely.2,3 Their safety over longer periods of treatment is less well investigated. This gap in our knowledge is important to address, since pediatric depressive and anxiety disorders tend to recur, requiring extended treatment.4 Moreover, potentially serious adverse events might either interfere with adherence or place a child at a later risk for insidious sequelae that might increase morbidity and mortality.
More is being learned about the long-term safety of SSRIs in adults.5 One area that has gained significant attention, particularly in the elderly, is the impact of SSRIs on bone mineral density (BMD).6 This is of clinical interest, since accelerated bone loss might place an individual at an increased risk for osteoporosis and, consequently, fractures. The personal and societal costs of these sequelae are significant and include suffering, disability, reduced quality of life, and billions of dollars in health care expenditures.7
We have previously reported an inverse association between SSRI use in boys and bone mineralization at the ultradistal radius and the lumbar spine.8 SSRIs can affect BMD, because serotonin (5-HT) regulates pre-osteoblast proliferation, osteoclast differentiation and activity, bone matrix calcification, and osteoblast/osteoclast interaction.9,10 This activity is mediated through 5-HT receptors and the 5-HT transporter present on bone cells.9–11 Moreover, the administration of fluoxetine can hinder or reverse these 5-HT–mediated processes in a dose-dependent manner.9–11 Furthermore, fluoxetine-treated mice show delay in whole-body and hindlimb bone mineral accrual.12 Finally, further evidence of a potential effect of SSRIs on bone development comes from a randomized pediatric clinical trial with fluoxetine showing significant reduction in longitudinal growth over a 19-week exposure period.13,14
Like mice treated with fluoxetine, those lacking the 5-HT transporter gene exhibit reduced BMD, altered bone architecture, and inferior mechanical properties.12 Only in humans and a few primate species, the promoter region of the 5-HT transporter gene contains a polymorphic region, the serotonin transporter–linked polymorphic region (5-HTTLPR), having a short (s) and long (l) allele, the former being transcriptionally less active.15 Moreover, carriers of the s allele show a blunted serotoninergic response to neuroendocrine tests and are at a higher risk for depression in the presence of trauma, offering further support for a functional potential of these variants.15,16
Thus, since the s and l alleles of the 5-HTTLPR are commonly distributed in the population, we wished to explore whether they moderate the association we had found between SSRI treatment and low bone mineralization in youths.8 We hypothesized that the less transcriptionally-active 5-HTTLPR variant (s allele) would be associated with more accentuated failure to accrue bone mass in youths treated with SSRIs.
This report consists of a reanalysis of partially published data regarding the combined, cross-sectional effect of risperidone and SSRIs on bone mineralization in boys.8 It specifically examines whether variants of the 5-HTTLPR moderate the SSRIs’ effect on BMD, found at the baseline visit.
In this study, 7- to 17-year-old individuals, treated with risperidone for at least 6 months were recruited from psychiatry outpatient clinics between November 2005 and August 2009. The study was conducted at the Department of Psychiatry, the University of Iowa, Iowa City. Genotyping was conducted at the College of Pharmacy, the University of Michigan, Ann Arbor. On enrollment, participants could be receiving other psychotropic medications but no antipsychotics other than risperidone. Specifically, current or past treatment with SSRIs was allowed. Participants with serious medical conditions, including those affecting bone metabolism, were not eligible.8,17
This study was approved by the University of Iowa Institutional Review Board. Written assent was obtained from children 13 years old and younger, and consent was obtained from adolescents and all parents or legal guardians.
The psychiatric diagnoses were clinical ones, presumably made following the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR),18 extracted from the charts. The start and stop times of each medication as well as all changes in the dosage and formulation were recorded.8 At recruitment, all participants were queried about medication adherence, smoking, and calcium and multivitamin supplementation. Daily calcium and vitamin D intake during the week prior to enrollment was estimated using the 2004 Block Kids Food Frequency Questionnaire.19 Physical activity was assessed by asking the parent to compare the child’s usual level of physical activity to their peers’ levels using a 5-point Likert scale.20
At enrollment, Tanner stage of sexual development was evaluated by physical examination as well as a self-completed form.8 Height and weight were recorded following standard procedures.8 Age- and gender-specific z scores for height, weight, and body mass index (BMI) were calculated using the Centers for Disease Control and Prevention normative data.21
A morning fasting (in 93%) blood sample was obtained to measure prolactin concentration by electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, Indiana).
Using a Stratec XCT-2000 scanner (Stratec, Inc, Pforzheim, Germany), volumetric BMD (vBMD) at the ultradistal (4%) site of the nondominant radius was measured with peripheral quantitative computed tomography (pQCT).8 All scans were reviewed to exclude movement artifacts (n = 14, 13%). Trabecular vBMD was measured as the mean density of the 90% central area of the bone’s cross-section (ie, 10% inward from the endosteum). Total vBMD, which combines cortical and trabecular bone at that site, was also measured. Total areal BMD (aBMD) in the lumbar spine (L1–L4) was estimated using dual energy x-ray absorptiometry (DXA), with a Hologic QDR DELPHI-4500A unit (Hologic, Inc, Bedford, Massachusetts). Individual measurements were converted into age- and gender-adjusted z scores using the manufacturer-supplied software and normative values. The scans were obtained by qualified technicians (reliability data available on request). Quality-control and calibration of the equipment were performed daily.
Genomic DNA was extracted from white blood cells (88%) or saliva (12%), using a Puregene (Gentra Systems, Minneapolis, Minnesota) or Oragene Kit (DNA Genotak, Kanata, Ontario, Canada). After DNA sample yield and purity were established through spectrophotometry and polymerase chain reaction (PCR) amplification, DNA samples were diluted to 20 ng/µL and stored at −20°C. PCR primers and determination of the 5-HTTLPR genotypes followed established methods.22 PCR products were visualized by electrophoresis on 1.8% agarose gels stained with ethidium bromide. For ambiguous samples, the PCR amplicons were gel-extracted and direct-sequenced.
Data Analysis
The sample was divided based on SSRI treatment status at enrollment. Those not receiving SSRIs consisted of participants who had never taken an SSRI (n = 28) and those who had previously done so but were not at enrollment (n = 24). The mean duration of SSRI treatment among formerly treated participants was 0.9 months, and the mean interval between discontinuing the SSRI and enrollment was 3.1 years. Due to the relatively short duration of exposure and long interval since the last SSRI use, these participants were combined with the never-treated ones in order to optimize statistical power. Differences in demographic and clinical variables between boys taking SSRIs or not were compared using Student t test for continuous variables and Fisher exact test for categorical ones. If the assumption of normality was violated, based on the Kolmogorov-Smirnov test, the Wilcoxon rank sum test was used.
To test our hypotheses, we used multiple linear regression analysis predicting, separately, lumbar spine aBMD z score and radius total and trabecular vBMD while adjusting for Tanner stage, prolactin concentration, SSRI treatment status at enrollment, 5-HTTLPR genotype, and the SSRI × 5-HTTLPR genotype interaction effect as predictor variables.8 Additional covariates were considered based on their known association with bone mineralization. These included height, weight, and BMI z scores, estimated daily intake of calcium and vitamin D, and physical activity. Among these covariates, we included in each regression model predicting individual bone-related variables those factors that were correlated with the dependent variable at a P value < .2.8
Due to the different racial/ethnic composition of the 2 SSRI groups (Table 1) and the known differences in bone mass across racial/ethnic groups23 and since non-Hispanic whites represented the majority of the sample (91%), we initially restricted the analyses to this racial subgroup and then repeated them in the entire sample.
Table 1.
Demographic and Clinical Characteristics of Male Subjects Taking and Not Taking SSRIs
| Characteristic | Taking SSRIs (n = 56) | Not Taking SSRIs (n = 52) | Statistical Analysis | valuea |
|---|---|---|---|---|
| Age, mean ± SD, y | 12.3 ± 2.9 | 11.0 ± 2.6 | t106 = −2.3 | < .03 |
| At Tanner stage I/II/III/IV/V, %b | 32/18/20/20/11 | 50/15/15/17/2 | Fisher exact | > .2 |
| Non-Hispanic white/African American/Hispanic/other, % | 91/5/4/0 | 77/17/0/6 | Fisher exact | < .02 |
| Height z score, mean ± SD | 0.2 ± 0.9 | 0.2 ± 0.9 | t106 = 0.3 | > .7 |
| Weight z score, mean ± SD | 0.5 ± 1.1 | 0.5 ± 1.1 | t106 = −0.2 | > .8 |
| Body mass index z score, mean ± SD | 0.6 ± 1.1 | 0.5 ± 1.1 | t106 = −0.2 | > .8 |
| Cigarette smoking, n (%) | 2 (4) | 1 (2) | Fisher exact | > .99 |
| Daily calcium intake, mean ± SD, mg/d | 959 ± 363 | 1045 ± 363 | t106 = 1.2 | > .2 |
| Daily vitamin D intake, median (quartiles), IU/d | 259 (186–361) | 274 (184–347) | Wilcoxon = 2,883 | > .7 |
| Physical activity, median (quartiles) | 3.0 (2.0–4.0) | 4.0 (3.0–5.0) | Wilcoxon = 1,899 | < .01 |
| Prolactin concentration, median (quartiles), ng/mL | 18.5 (13.1–28.4) | 16.1 (9.7–23.1) | Wilcoxon = 2,549 | < .08 |
| 5-HTTLPR genotype, n (%) | ||||
| ll | 17 (30) | 17 (33) | Fisher exact | > .9 |
| ls | 26 (46) | 25 (48) | ||
| ss | 13 (23) | 10 (19) |
Statistically significant findings are in bold.
Percentages do not sum to 100 due to rounding.
Abbreviations: 5-HTTLPR = serotonin transporter gene–linked polymorphic region, l = long allele, s = short allele.
Data Analysis
The sample was divided based on SSRI treatment status at enrollment. Those not receiving SSRIs consisted of participants who had never taken an SSRI (n = 28) and those who had previously done so but were not at enrollment (n = 24). The mean duration of SSRI treatment among formerly treated participants was 0.9 months, and the mean interval between discontinuing the SSRI and enrollment was 3.1 years. Due to the relatively short duration of exposure and long interval since the last SSRI use, these participants were combined with the never-treated ones in order to optimize statistical power. Differences in demographic and clinical variables between boys taking SSRIs or not were compared using Student t test for continuous variables and Fisher exact test for categorical ones. If the assumption of normality was violated, based on the Kolmogorov-Smirnov test, the Wilcoxon rank sum test was used.
To test our hypotheses, we used multiple linear regression analysis predicting, separately, lumbar spine aBMD z score and radius total and trabecular vBMD while adjusting for Tanner stage, prolactin concentration, SSRI treatment status at enrollment, 5-HTTLPR genotype, and the SSRI × 5-HTTLPR genotype interaction effect as predictor variables.8 Additional covariates were considered based on their known association with bone mineralization. These included height, weight, and BMI z scores, estimated daily intake of calcium and vitamin D, and physical activity. Among these covariates, we included in each regression model predicting individual bone-related variables those factors that were correlated with the dependent variable at a P value < .2.8
Due to the different racial/ethnic composition of the 2 SSRI groups (Table 1) and the known differences in bone mass across racial/ethnic groups23 and since non-Hispanic whites represented the majority of the sample (91%), we initially restricted the analyses to this racial subgroup and then repeated them in the entire sample.
RESULTS
Clinical Sample
This analysis includes 108 male participants with a mean age of 11.7 years (SD = 2.8 years). Tables 1 and 2 detail the demographic and clinical characteristics of those receiving SSRIs at enrollment (n = 56, 52%) and those not. Participants prescribed SSRIs were more likely to have an internalizing disorder or a pervasive developmental disorder. SSRI-treated participants were also older and less physically active, probably signaling the increased prevalence of mood disorders and physical inactivity with age.24 The trend for prolactin concentration to be higher in the SSRI-treated participants is also likely to be the result of their older age.17
Table 2.
Psychiatric Characteristics of Male Subjects Taking and Not Taking SSRIs
| Characteristic | Taking SSRIs (n = 56) |
Not Taking SSRIs (n = 52) | Statistical Analysis |
Valuea |
|---|---|---|---|---|
| Psychiatric diagnosis, n (%) | ||||
| Attention-deficit/hyperactivity disorder | 45 (80) | 48 (92) | Fisher exact | .1 |
| Disruptive behavior disorder | 32 (57) | 39 (75) | Fisher exact | < .07 |
| Pervasive developmental disorder | 13 (23) | 3 (6) | Fisher exact | < .02 |
| Depressive disorder | 20 (36) | 2 (4) | Fisher exact | < .0001 |
| Bipolar disorder | 0 (0) | 1 (2) | Fisher exact | > .4 |
| Anxiety disorder | 29 (52) | 10 (19) | Fisher exact | < .0006 |
| Psychotic disorder | 1 (2) | 1 (2) | Fisher exact | > .99 |
| Tic disorder | 12 (21) | 14 (27) | Fisher exact | > .6 |
| Pharmacotherapy | ||||
| SSRI dose, median (quartiles), unit/db | 1.0 (0.5–1.5) | NA | ||
| SSRI treatment duration, median (quartiles), y | 2.8 (1.4–4.5) | NA | ||
| Risperidone dose, median (quartiles), mg/kg/d | 0.02 (0.01–0.04) | 0.03 (0.02–0.04) | Wilcoxon = 3,008 | >.2 |
| Risperidone treatment duration, median (quartiles), y | 2.3 (0.9–3.6) | 2.2 (1.1–4.1) | Wilcoxon = 2,933 | >.5 |
| Psychostimulant treatment, n (%) | 35 (63) | 39 (75) | Fisher exact | >.2 |
| Psychostimulant dose, median (quartiles), mg/kg/d | 1.1 (0.9–1.7) | 1.3 (1.0–1.7) | Wikoxon = 1,192 | .2 |
| Psychostimulant treatment duration, median (quartiles), y | 4.8 (3.0–7.3) | 5.0 (2.8–6.7) | Wi1coxon = 1,348 | .7 |
| α2-Agonists, n (%) | 17 (30) | 15 (29) | Fisher exact | >.99 |
Statistically significant findings are in bold.
A participant could be taking any one of the SSRIs: fluoxetine (n = 29 [52%]), sertraline (n = 13 [23%]), citalopram (n = 9 [16%]), escitalopram (n = 5 [9%]). One SSRI unit was defined as being equivalent to a daily dose of 20 mg of fluoxetine or citalopram, 50 mg of sertraline, or 10 mg of escitalopram.
Abbreviations: NA = nut applicable, SSRI = selective serotonin reuptake inhibitor.
The 5-HTTLPR genotype distribution was as follows: ll = 31% (n = 34), ls= 47% (n = 51), and ss = 21% (n = 23) and in Hardy-Weinberg equilibrium (χ2 = 0.2, P > .6). This genotype composition is comparable to those reported by others25 and is not different between the 2 SSRI groups (Table 1).
SSRI Treatment, BMD, and 5-HTTLPR Genotype Interaction Effect
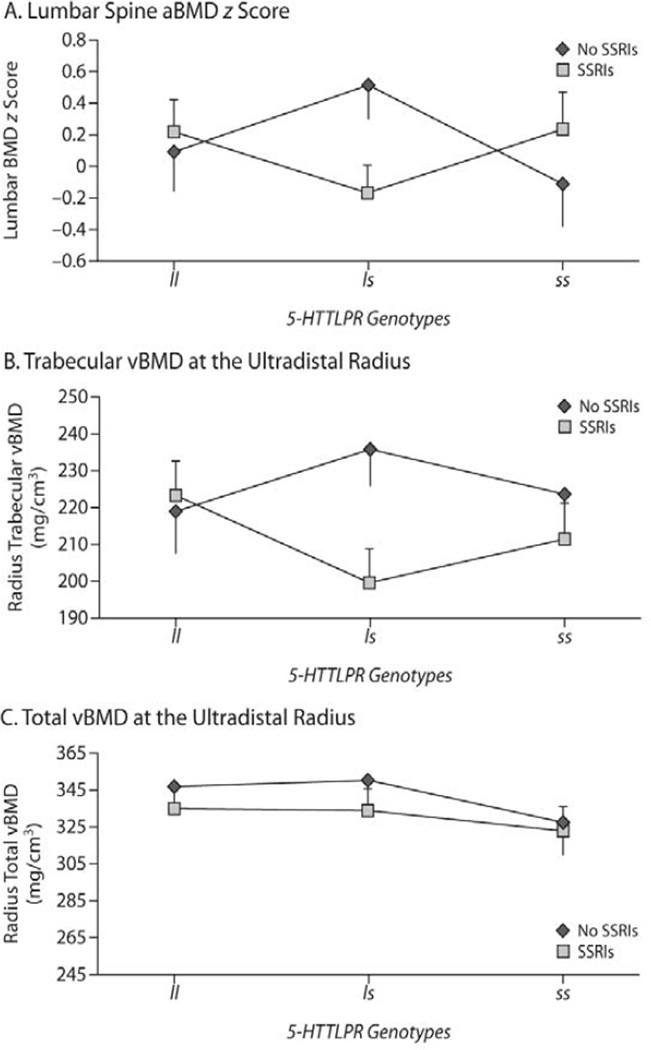
After adjusting for the stage of sexual development (β = −0.14; 95% CI, −0.29 to 0.01; P < .07), weight z score (β = 0.44; 95% CI, 0.20–0.69; P = .0006), height z score (β = −0.07; 95% CI, −0.37 to 0.24; P > .6), physical activity (β = 0.20; 95% CI, 0.02–0.38; P < .03), estimated calcium intake (β = 0.0002; 95% CI, −0.0003 to 0.0007; P > .3), and serum prolactin concentration (β = 0.005; 95% CI, −0.008 to 0.018; P > .4), there was a significant 5-HTTLPR genotype × SSRI treatment interaction effect on total lumbar spine BMD z score (P < .05). While the overall model accounted for 41% of the variance in the lumbar spine BMD z score, SSRIs, the 5-HTTLPR genotype, and their interaction effect accounted for 5% of it. Post hoc analyses to test for the effect of SSRIs within genotypes showed that the largest mean difference was in the carriers of the ls genotype, with mean z score of those taking SSRIs being 0.68 lower than those who were not (Cohen d = 0.82, P < .02). There was no significant effect of SSRIs within the ll and ss genotypes (Figure 1A). Comparable results were found when we included the entire sample in the analysis, although the interaction effect failed to reach significance (P < .07).
Figure 1.
Differences in Bone Mineral Density (BMD) in Non-Hispanic White Males Taking or Not Taking SSRIs as a Function of Genotytpea,b
aThe figure illustrates differences in BMD in non-Hispanic white males with the ll (n = 29), ls (N = 40), and ss (n = 23) 5-HTTLPR genotypes depending on SSRI treatment status (n = 51 taking SSRIs vs n = 41 not taking SSRIs).
bPost hoc analyses testing for effect of SSRIs within genotypes showed that, among carriers of the ls genotype, those who took SSRIs (n = 22) had lower areal BMD z score at the lumber spine compared to those who did not take SSRIs (n = 19) (adjusted mean areal BMD = −0.17 [SE = 0.18] vs 0.51 [SE = 0.21], P < .02). A comparable pattern was found in trabecular volumetric BMD (vBMD) at the ultradistal radius, although the difference did not reach statistical significance (n = 17 vs n = 16, adjusted mean vBMD = 199.5 [SE = 9.3] vs 235.6 [SE = 9.6], P < .008).
Abbreviations: 5-HTTLPR = serotonin transporter–linked polymorphic region, l = long allele, s = short allele, SSRI = selective serotonin reuptake inhibitor.
After we adjusted for pubertal stage (P < .1), height z score (β = −6.73; 95% CI, −18.29 to 4.83; P > .2), BMI z score (β = 7.98; 95% CI, −1.27 to 17.24; P < .09), and serum prolactin concentration (β = −0.05; 95% CI, −0.66 to 0.56; P > .8), the 5-HTTLPR genotype × SSRI treatment interaction effect on trabecular vBMD at the ultradistal radius in non-Hispanic white boys failed to reach statistical significance (P = .14), although SSRIs, the 5-HTTLPR genotype, and their interaction effect explained 9% of the variance. Post hoc analyses to test for the effect of SSRIs within genotypes showed that the largest difference in mean trabecular vBMD was again within carriers of the ls genotype, with vBMD being 36 mg/cm3 lower in those taking SSRIs compared to those who were not (Cohen d = 1.04, P < .008; Figure 1B). Similar results were obtained when we included the whole sample in the analysis.
Finally, after adjusting for pubertal stage (P = .0004), height z score (β = −30.13; 95% CI, −50.37 to −9.89; P < .004), weight z score (β = 11.30; 95% CI, −4.54 to 27.15; P > .1), physical activity (β = 10.86; 95% CI, −0.18 to 21.90; P < .06), and prolactin concentration (β = −0.43; 95% CI, −1.24 to 0.38; P > .2), there was no significant 5-HTTLPR genotype × SSRI treatment interaction effect on total vBMD at the ultradistal radius in non-Hispanic white boys (P > .9). SSRIs, the 5-HTTLPR genotype, and their interaction effect accounted for less than 3% of the variance in total vBMD. Moreover, post hoc analyses failed to show any significant differences between those treated with SSRIs and those not across the different 5-HTTLPR genotypes (Figure 1C and Table 1). The results remained unchanged when the analysis included all the participants.
DISCUSSION
Findings from in vitro, animal, and clinical research, including work from our laboratory, have linked SSRIs to lower BMD.6,8,11,12 However, to our knowledge, ours is the first study, albeit cross-sectional, to examine whether a common, functional, variant of the serotonin transporter gene moderates this association in a clinical sample. We found partial support for our hypothesis that the short, and less transcriptionally active, allele of the 5-HTTLPR variants places those treated with SSRIs at a higher risk for reduced BMD. Although the finding was most prominent at the lumbar spine, a comparable effect was observed in the trabecular bone at the ultradistal radius.
Recent evidence has implicated the serotonin system in bone metabolism.11,26 In fact, various serotonin receptors, along with the serotonin transporter, have been shown to play an active role in different aspects of bone cell functions.9,11,26 Moreover, this effect is inhibited when bone cells are treated with fluoxetine, a potent blocker of the serotonin transporter.26 In addition, studies in mice have confirmed the inhibitory effect of fluoxetine on bone mineralization by reducing bone formation and altering its architecture.12
In the elderly, a number of epidemiologic studies have found an association between SSRI use, lower BMD, and increased bone fracture, even after accounting for important confounders.6 In youths, however, little is known about the potential effect of SSRIs on bone development. In a case series of 4 adolescents (ages 11.6 to 13.7 years), longitudinal growth was found to be significantly hindered by treatment with SSRIs, recovering after the discontinuation of the medication.27 In the one case in which the SSRI (fluoxetine) was restarted, nearly 18 months after it had been discontinued, longitudinal growth was suppressed again.27 In addition, in a multiphase, double-blind relapse prevention study in pediatric depression, youths receiving fluoxetine failed to grow in height at the same rate as those assigned to placebo. While this between-group difference was significant (P = .001) at the end of the initial 19-week phase of the study,14 it failed to reach statistical significance by the end of the medication discontinuation phase (week 51), likely due to lack of statistical power (following a 65% attrition; see author reply in Calarge and Kuperman13). Interestingly, this decline in longitudinal growth was accompanied by a significant reduction in alkaline phosphatase, a marker of osteoid formation, adding credence to the finding.14
In a smaller but overlapping sample with the present one, we have reported elsewhere that SSRI treatment was associated with lower bone mass at the lumbar spine and ultradistal radius.8 On the basis of evidence showing reduced BMD in mice lacking the serotonin transporter gene,12 we hypothesized that the s allele of the 5-HTTLPR variants would place carriers at a higher risk of having low bone mass when exposed to SSRIs, since this allele is less functionally active.15 As anticipated, we did find an interaction effect with ls genotype carriers being at the highest risk for low bone mass when treated with SSRIs. It is not clear why there was no significant effect of the ss genotype. It is worth noting, however, that others have found the ls heterozygote status to be associated with the poorest response to antidepressants, compared to the other genotypes.15,28
Since the extant clinical and epidemiologic studies, including ours, are observational, it is not possible to isolate the potential effect of SSRIs on BMD from that of the underlying psychiatric condition for which SSRIs were prescribed.29 In fact, depression itself has been linked to low bone mass.30 However, the fact that SSRI treatment interacts with the 5-HTTLPR variants to affect bone mass suggests that this “confounding by indication” phenomenon might not fully account for the association between SSRIs and BMD. This would be consistent with the in vitro and animal research as well as the randomized trial of fluoxetine for relapse prevention in depressed youths cited earlier.6,12,14
While generating novel and intriguing findings, our study suffers from additional limitations. First, it involves youths treated with risperidone only, which itself can affect the serotonin system.31 However, since, by design, everyone received risperidone, this cannot fully explain our results. Nevertheless, it might be that SSRIs interacted with risperidone and with the 5-HTTLPR variants to affect BMD. Second, while our hypothesis was based on the 5-HT transporter blocking activity of SSRIs, the different SSRIs bind to a variety of 5-HT and non–5-HT receptors. To what extent other pathways mediate the effect of SSRIs on bone mineralization requires further research. Third, we measured BMD at only 2 sites. Thus, it is not known whether the treatment by genotype interaction effect impacts other skeletal sites. This is relevant since the temporal trajectory of bone mass accrual varies across different bone sites.32 Fourth, our analyses were restricted to males, who were mostly non-Hispanic white. Fifth, we did not adjust the statistical significance level for the number of tests conducted. We felt this was justifiable due to the exploratory nature of this analysis and the moderate correlation (Pearson r > .34, P < .001) between BMD in the lumbar spine and ultradistal radius. Finally, though this is one of the largest studies that have investigated the effect of psychotropic medications on bone mineralization in a clinical population, the sample size remains modest for a pharmacogenetic study. Thus, future studies should be larger, include females, have a larger representation of diverse ethnic/racial groups, and involve participants treated only with SSRIs and followed prospectively. They should also explore the potential interaction effect of the dose of SSRIs and 5-HTTLPR genotypes. Due to our limited statistical power, we did not conduct such analyses, although ls genotype carriers received a lower daily dose than those with the ss genotype (0.9 versus 1.7 SSRI units, P < .003).
Since many pediatric psychiatric conditions are chronic, it is now recognized that extended treatment is necessary to maintain remission and optimal functioning. Therefore, establishing the long-term safety of psychotropic medications in growing youths is imperative. If our finding that the 5-HTTLPR variants moderate the association between SSRIs and low bone mass is replicated in a prospective study, a substantial subgroup (those with the ll and ss genotypes) of children treated with this class of drugs might be able to take these medications with less concern about their skeletal development and future fracture risk. On the other hand, further research into best practices to monitor and optimize bone mass in ls genotype carriers initiating an SSRI would be indicated.
References
- 1.Delate T, Gelenberg AJ, Simmons VA, et al. Trends in the use of antidepressants in a national sample of commercially insured pediatric patients, 1998 to 2002. Psychiatr Serv. 2004;55(4):387–391. doi: 10.1176/appi.ps.55.4.387. [DOI] [PubMed] [Google Scholar]
- 2.Birmaher B, Brent D, Bernet W, et al. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SD, Bernstein GA Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267–283. doi: 10.1097/01.chi.0000246070.23695.06. [DOI] [PubMed] [Google Scholar]
- 4.Emslie GJ, Kennard BD, Mayes TL, et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165(4):459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersohn F, Schade R, Suissa S, et al. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166(5):591–598. doi: 10.1176/appi.ajp.2008.08071065. [DOI] [PubMed] [Google Scholar]
- 6.Haney EM, Warden SJ. Skeletal effects of serotonin (5-hydroxytryptamine) transporter inhibition: evidence from clinical studies. J Musculoskelet Neuronal Interact. 2008;8(2):133–145. [PubMed] [Google Scholar]
- 7.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 8.Calarge CA, Zimmerman B, Xie D, et al. A cross-sectional evaluation of the effect of risperidone and selective serotonin reuptake inhibitors on bone mineral density in boys. J Clin Psychiatry. 2010;71(3):338–347. doi: 10.4088/JCP.08m04595gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaglino R, Fu J, Späte U, et al. Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res. 2004;19(9):1420–1431. doi: 10.1359/JBMR.040606. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson BI, Thommesen L, Stunes AK, et al. Serotonin and fluoxetine modulate bone cell function in vitro. J Cell Biochem. 2006;98(1):139–151. doi: 10.1002/jcb.20734. [DOI] [PubMed] [Google Scholar]
- 11.Bliziotes MM, Eshleman AJ, Zhang XW, et al. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001;29(5):477–486. doi: 10.1016/s8756-3282(01)00593-2. [DOI] [PubMed] [Google Scholar]
- 12.Warden SJ, Robling AG, Sanders MS, et al. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146(2):685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 13.Calarge C, Kuperman S. Fluoxetine for depression relapse prevention. J Am Acad Child Adolesc Psychiatry. 2005;44(10):966–967. doi: 10.1097/01.chi.0000172535.24534.44. author reply 967–968. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson M, Joliat MJ, Miner CM, et al. Safety of subchronic treatment with fluoxetine for major depressive disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2004;14(3):412–417. doi: 10.1089/cap.2004.14.412. [DOI] [PubMed] [Google Scholar]
- 15.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13(2):131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 16.Smith GS, Lotrich FE, Malhotra AK, et al. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology. 2004;29(12):2226–2234. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- 17.Calarge CA, Ellingrod VL, Acion L, et al. Variants of the dopamine D2 receptor gene and risperidone-induced hyperprolactinemia in children and adolescents. Pharmacogenet Genomics. 2009;19(5):373–382. doi: 10.1097/FPC.0b013e328329a60f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 19.Block G, Murphy M, Roullet JB, et al. Pilot validation of a FFQ for children 8–10 years. Abstract presented at: Fourth International Conference On Dietary Assessment Methods; September 17–20, 2000; Tucson, Arizona. [Google Scholar]
- 20.Slemenda CW, Peacock M, Hui S, et al. Reduced rates of skeletal remodeling are associated with increased bone mineral density during the development of peak skeletal mass. J Bone Miner Res. 1997;12(4):676–682. doi: 10.1359/jbmr.1997.12.4.676. [DOI] [PubMed] [Google Scholar]
- 21.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 22.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 23.Bell NH, Shary J, Stevens J, et al. Demonstration that bone mass is greater in black than in white children. J Bone Miner Res. 1991;6(7):719–723. doi: 10.1002/jbmr.5650060709. [DOI] [PubMed] [Google Scholar]
- 24.Janz KF, Lutuchy EM, Wenthe P, et al. Measuring activity in children and adolescents using self-report: PAQ-C and PAQ-A. Med Sci Sports Exerc. 2008;40(4):767–772. doi: 10.1249/MSS.0b013e3181620ed1. [DOI] [PubMed] [Google Scholar]
- 25.Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bliziotes M, Eshleman A, Burt-Pichat B, et al. Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone. 2006;39(6):1313–1321. doi: 10.1016/j.bone.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weintrob N, Cohen D, Klipper-Aurbach Y, et al. Decreased growth during therapy with selective serotonin reuptake inhibitors. Arch Pediatr Adolesc Med. 2002;156(7):696–701. doi: 10.1001/archpedi.156.7.696. [DOI] [PubMed] [Google Scholar]
- 28.Aitchison K, Huezo-Diaz P, Williamson R, et al. Genetic association data from GENDEP, a multicentre European study. Am J Med Genetics. 2006;141B(7):690. [Google Scholar]
- 29.Saag K. Mend the mind, but mind the bones!: balancing benefits and potential skeletal risks of serotonin reuptake inhibitors. Arch Intern Med. 2007;167(12):1231–1232. doi: 10.1001/archinte.167.12.1231. [DOI] [PubMed] [Google Scholar]
- 30.Yirmiya R, Bab I. Major depression is a risk factor for low bone mineral density: a meta-analysis. Biol Psychiatry. 2009;66(5):423–432. doi: 10.1016/j.biopsych.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68(1):29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- 32.Bradney M, Karlsson MK, Duan Y, et al. Heterogeneity in the growth of the axial and appendicular skeleton in boys: implications for the pathogenesis of bone fragility in men. J Bone Miner Res. 2000;15(10):1871–1878. doi: 10.1359/jbmr.2000.15.10.1871. [DOI] [PubMed] [Google Scholar]