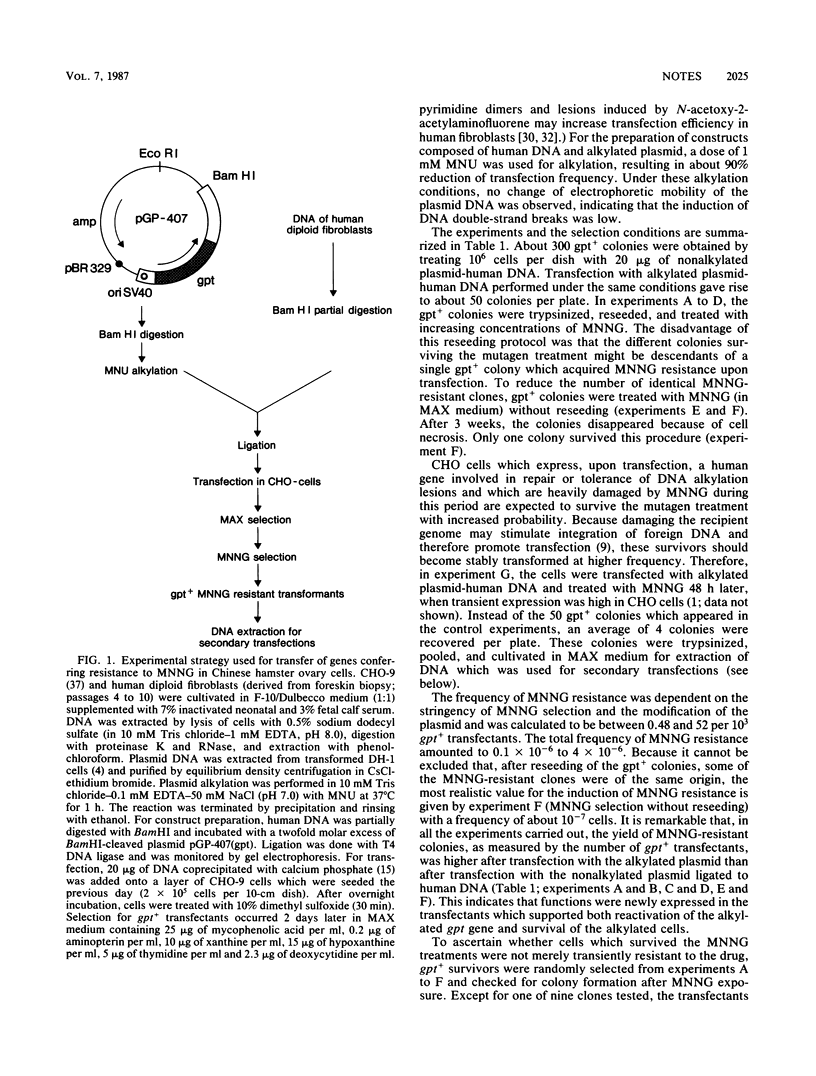
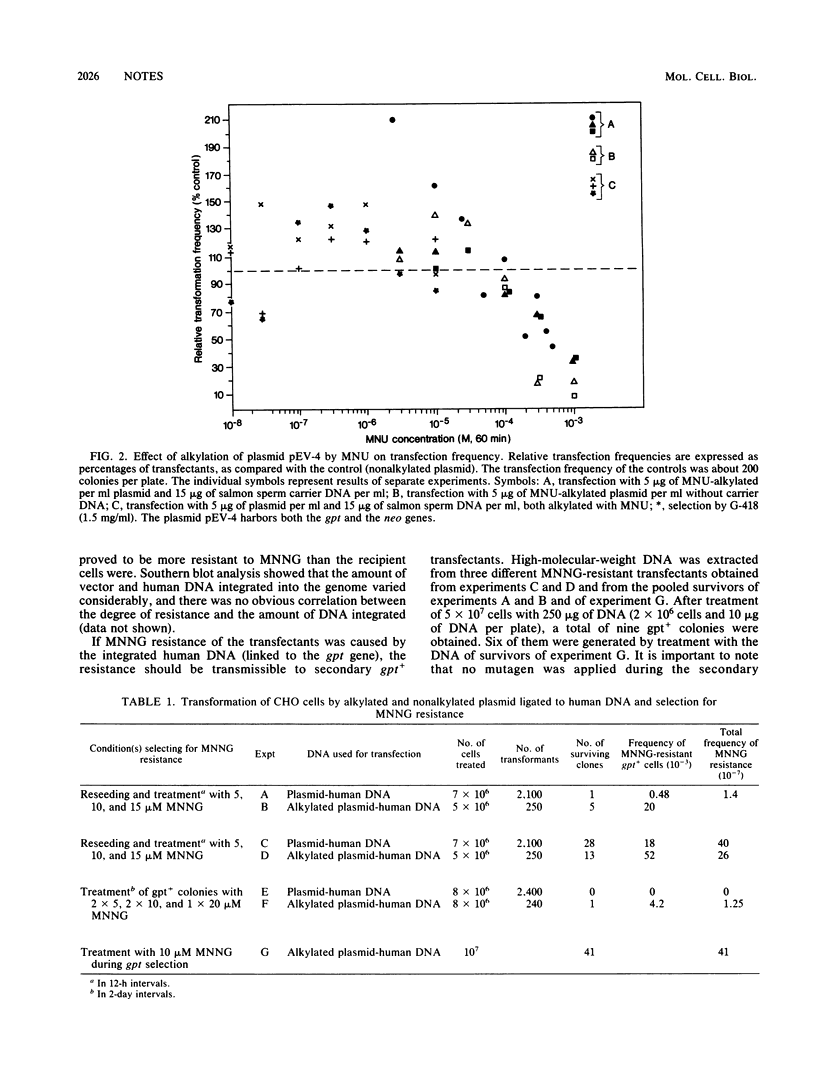
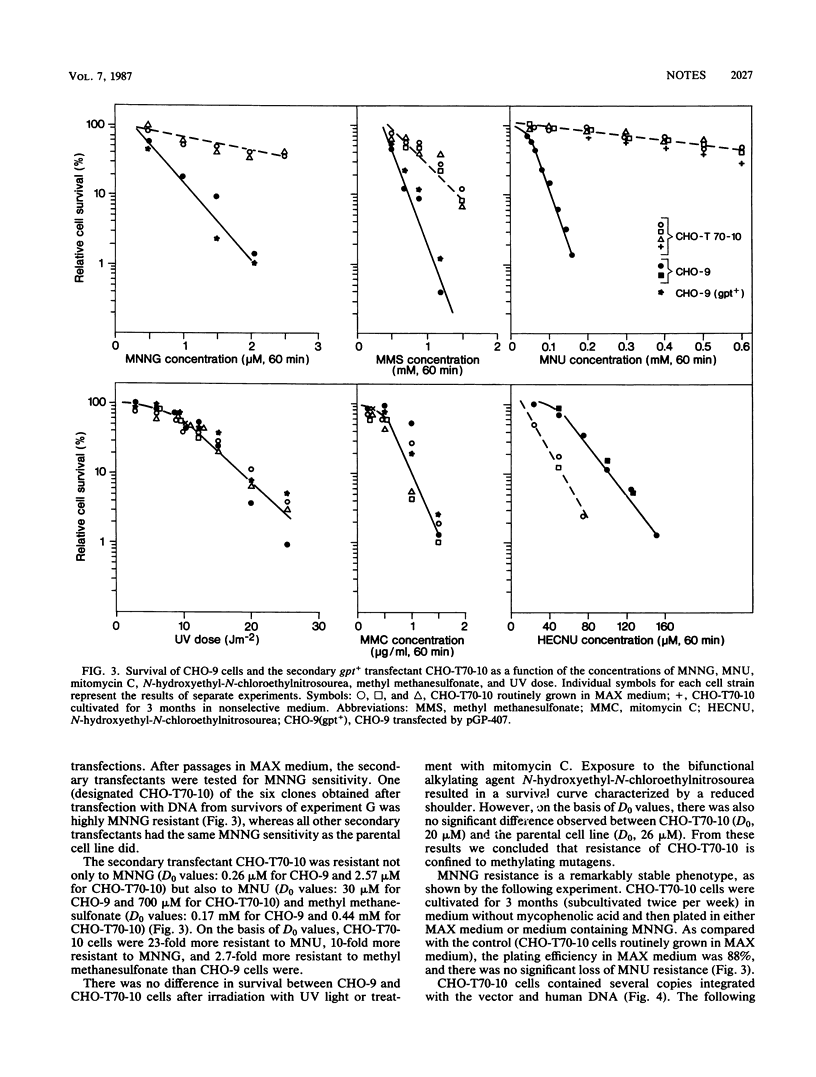
Abstract
Chinese hamster ovary cells were transfected by human DNA ligated to the bacterial gpt (xanthine-guanine-phosphoribosyltransferase) gene which was used either in its native form or after partial inactivation with methylnitrosourea. The gpt+ transfectants were screened for resistance to high doses of N-methyl-N'-nitro-N-nitrosoguanidine. Using this approach, we showed that Chinese hamster ovary cells can acquire N-methyl-N'-nitro-N-nitrosoguanidine resistance upon transfection with DNA from diploid human fibroblasts, that this resistance is transferable by secondary transfection and is specific for methylating mutagens, and that it is not caused by increased removal of O6-methylguanine, 3-methyladenine, and 7-methylguanine from DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Hidaka K., Siminovitch L. Expression of bacterial beta-galactosidase in animal cells. Mol Cell Biol. 1982 Dec;2(12):1628–1632. doi: 10.1128/mcb.2.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. M., Van Voorhis W. C., Spencer L. A. HeLa cell variants that differ in sensitivity to monofunctional alkylating agents, with independence of cytotoxic and mutagenic responses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5249–5253. doi: 10.1073/pnas.76.10.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranek D. T., Weis C. C., Swenson D. H. A comprehensive quantitative analysis of methylated and ethylated DNA using high pressure liquid chromatography. Carcinogenesis. 1980 Jul;1(7):595–606. doi: 10.1093/carcin/1.7.595. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand J., Margison G. P. Reduction of the toxicity and mutagenicity of alkylating agents in mammalian cells harboring the Escherichia coli alkyltransferase gene. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6292–6296. doi: 10.1073/pnas.83.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H. Human brain tumour cell strains with deficient host-cell reactivation of N-methyl-N'-nitro-N-nitrosoguanidine-damaged adenovirus 5. Nature. 1979 Jun 28;279(5716):797–799. doi: 10.1038/279797a0. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Debenham P. G., Webb M. B. The effect of X-rays and ultraviolet light on DNA-mediated gene transfer in mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Nov;46(5):555–568. doi: 10.1080/09553008414551761. [DOI] [PubMed] [Google Scholar]
- Ding R., Ghosh K., Eastman A., Bresnick E. DNA-mediated transfer and expression of a human DNA repair gene that demethylates O6-methylguanine. Mol Cell Biol. 1985 Nov;5(11):3293–3296. doi: 10.1128/mcb.5.11.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote R. S., Mitra S. Lack of induction of O6-methylguanine-DNA methyltransferase in mammalian cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. Carcinogenesis. 1984 Feb;5(2):277–281. doi: 10.1093/carcin/5.2.277. [DOI] [PubMed] [Google Scholar]
- Frei E., 3rd, Cucchi C. A., Rosowsky A., Tantravahi R., Bernal S., Ervin T. J., Ruprecht R. M., Haseltine W. A. Alkylating agent resistance: in vitro studies with human cell lines. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2158–2162. doi: 10.1073/pnas.82.7.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth-Goldstein R. Inability of Chinese hamster ovary cells to excise O6-alkylguanine. Cancer Res. 1980 Jul;40(7):2623–2624. [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Karran P., Lindahl T. O6-Methylguanine-DNA methyltransferase of human lymphoid cells: structural and kinetic properties and absence in repair-deficient cells. Cancer Res. 1983 Jul;43(7):3247–3252. [PubMed] [Google Scholar]
- Karran P., Hjelmgren T., Lindahl T. Induction of a DNA glycosylase for N-methylated purines is part of the adaptive response to alkylating agents. Nature. 1982 Apr 22;296(5859):770–773. doi: 10.1038/296770a0. [DOI] [PubMed] [Google Scholar]
- Karran P., Lindahl T., Griffin B. Adaptive response to alkylating agents involves alteration in situ of O6-methylguanine residues in DNA. Nature. 1979 Jul 5;280(5717):76–77. doi: 10.1038/280076a0. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Warren W., Medcalf A. S., Amos J. Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature. 1980 Feb 7;283(5747):596–599. doi: 10.1038/283596a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Wiest L., Foote R. S., Mitra S., Perry W. Purification and properties of O6-methylguanine-DNA transmethylase from rat liver. J Biol Chem. 1983 Feb 25;258(4):2327–2333. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rubin J. S., Joyner A. L., Bernstein A., Whitmore G. F. Molecular identification of a human DNA repair gene following DNA-mediated gene transfer. Nature. 1983 Nov 10;306(5939):206–208. doi: 10.1038/306206a0. [DOI] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Samson L., Derfler B., Waldstein E. A. Suppression of human DNA alkylation-repair defects by Escherichia coli DNA-repair genes. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5607–5610. doi: 10.1073/pnas.83.15.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero D. A., Meyer S. A., Clatterbuck B. E., Mattern M. R., Ziolkowski C. H., Day R. S., 3rd Relationship of DNA repair phenotypes of human fibroblast and tumor strains to killing by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1984 Mar;44(3):961–966. [PubMed] [Google Scholar]
- Shiloh Y., Becker Y. Kinetics of O6-methylguanine repair in human normal and ataxia telangiectasia cell lines and correlation of repair capacity with cellular sensitivity to methylating agents. Cancer Res. 1981 Dec;41(12 Pt 1):5114–5120. [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spivak G., Ganesan A. K., Hanawalt P. C. Enhanced transformation of human cells by UV-irradiated pSV2 plasmids. Mol Cell Biol. 1984 Jun;4(6):1169–1171. doi: 10.1128/mcb.4.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Minkler J. L., Fuscoe J. C., Henning K. A., Carrano A. V. DNA-mediated transfer of a human DNA repair gene that controls sister chromatid exchange. Mol Cell Biol. 1985 Apr;5(4):881–884. doi: 10.1128/mcb.5.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein E. A., Cao E. H., Setlow R. B. Direct assay for O6-methylguanine-acceptor protein in cell extracts. Anal Biochem. 1982 Nov 1;126(2):268–272. doi: 10.1016/0003-2697(82)90514-0. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Burki H. J. Repair capability and the cellular age response for killing and mutation induction after UV. Mutat Res. 1982 Aug;95(2-3):505–514. doi: 10.1016/0027-5107(82)90281-0. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Simons J. W. Analysis of repair processes by the determination of the induction of cell killing and mutations in two repair-deficient Chinese hamster ovary cell lines. Mutat Res. 1986 Jul;166(1):59–69. doi: 10.1016/0167-8817(86)90041-6. [DOI] [PubMed] [Google Scholar]
- van Duin M., Westerveld A., Hoeijmakers J. H. UV stimulation of DNA-mediated transformation of human cells. Mol Cell Biol. 1985 Apr;5(4):734–741. doi: 10.1128/mcb.5.4.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeeland A. A., Mohn G. R., Neuhäuser-Klaus A., Ehling U. H. Quantitative comparison of genetic effects of ethylating agents on the basis of DNA adduct formation. Use of O6-ethylguanine as molecular dosimeter for extrapolation from cells in culture to the mouse. Environ Health Perspect. 1985 Oct;62:163–169. doi: 10.1289/ehp.8562163. [DOI] [PMC free article] [PubMed] [Google Scholar]