Abstract
AIM: To retrospectively review the results of over-the-scope clip (OTSC) use in our hospital and to examine the feasibility of using the OTSC to treat perforations after endoscopic submucosal dissection (ESD).
METHODS: We enrolled 23 patients who presented with gastrointestinal (GI) bleeding, fistulae and perforations and were treated with OTSCs (Ovesco Endoscopy GmbH, Tuebingen, Germany) between November 2011 and September 2012. Maximum lesion size was defined as lesion diameter. The number of OTSCs to be used per patient was not decided until the lesion was completely closed. We used a twin grasper (Ovesco Endoscopy GmbH, Tuebingen, Germany) as a grasping device for all the patients. A 9 mm OTSC was chosen for use in the esophagus and colon, and a 10 mm device was used for the stomach, duodenum and rectum. The overall success rate and complications were evaluated, with a particular emphasis on patients who had undergone ESD due to adenocarcinoma. In technical successful cases we included not only complete closing by using OTSCs, but also partial closing where complete closure with OTSCs is almost difficult. In overall clinical successful cases we included only complete closing by using only OTSCs perfectly. All the OTSCs were placed by 2 experienced endoscopists. The sites closed after ESD included not only the perforation site but also all defective ulcers sites.
RESULTS: A total of 23 patients [mean age 77 years (range 64-98 years)] underwent OTSC placement during the study period. The indications for OTSC placement were GI bleeding (n = 9), perforation (n = 10), fistula (n = 4) and the prevention of post-ESD duodenal artificial ulcer perforation (n = 1). One patient had a perforation caused by a glycerin enema, after which a fistula formed. Lesion closure using the OTSC alone was successful in 19 out of 23 patients, and overall success rate was 82.6%. A large lesion size (greater than 20 mm) and a delayed diagnosis (more than 1 wk) were the major contributing factors for the overall unsuccessful clinical cases. The location of the unsuccessful lesion was in the stomach. The median operation time in the successful cases was 18 min, and the average observation time was 67 d. During the observation period, none of the patients experienced any complications associated with OTSC placement. In addition, we successfully used the OTSC to close the perforation site after ESD in 6 patients. This was a single-center, retrospective study with a small sample size.
CONCLUSION: The OTSC is effective for treating GI bleeding, fistulae as well as perforations, and the OTSC technique proofed effective treatment for perforation after ESD.
Keywords: Over-the-scope clip, Gastrointestinal bleeding, Endoscopic submucosal dissection complications, Gastrointestinal fistulae, Gastrointestinal perforation
Core tip: We have reported our experiences with the over-the-scope clip (OTSC) and the outcomes of several cases in Japan. The aims of the present study were to retrospectively review the results of OTSC use in our hospital and to examine the feasibility of using the OTSC to completely close perforations after endoscopic submucosal dissection for early gastrointestinal cancers.
INTRODUCTION
Historically, the standard treatment for gastrointestinal (GI) perforations has been surgery. Recent, invasive endoscopic treatments, such as endoscopic submucosal dissection (ESD) and natural orifice transluminal endoscopic surgery (NOTES), have provided alternative approaches to surgery. Our recent attempts to develop a new device, such as a full-thickness suturing device, constitute progress in the development of NOTES[1-4]. In addition, several devices are currently available for endoscopic management[5,6]. However, there is little convenient, safety and perfect device for complication of endoscopic treatment[7,8]. Since its introduction, the over-the-scope clip (OTSC) (Ovesco Endoscopy GmbH, Tuebingen, Germany) has been used to treat GI bleeding, fistulae and perforations in the United States and several European countries. Reports describing the use and value of OTSCs have primarily consisted of animal studies and clinical cases[9-14]. Retrospective studies have demonstrated the feasibility and the preliminary safety of the OTSC for the treatment of GI bleeding and fistulae, as well as for the closure of acute GI perforations[15,16]. The OTSC was approved by the Japanese Drug Administration and was made commercially available in November 2011. We have reported on our experiences with the OTSC and the outcomes of several cases involving its use since this device became available in Japan. Here, we retrospectively report the results of using the OTSC in our hospital. We also describe the potential use of the OTSC to completely close perforations after ESD for early GI cancers.
MATERIALS AND METHODS
We retrospectively analyzed our database of all 23 patients who underwent OTSC placement (Ovesco Endoscopy GmbH, Tuebingen, Germany) in our hospital from November 2011 to September 2012, as summarized in Table 1. The indications for OTSC placement were GI bleeding, perforations, fistulae and the prevention of post-ESD duodenal artificial ulcer perforation. ESD were performed because of dissection of adenocarcinoma. Maximum lesion size was defined as lesion diameter, not lesion surface area. The number of OTSCs to be used per patient was not decided until the lesion was completely closed. We used a twin grasper (Ovesco Endoscopy GmbH, Tuebingen, Germany) as a grasping device for all the patients. A 9-mm OTSC was chosen for use in the esophagus and colon, and a 10-mm device was used for the stomach, duodenum and rectum. Clinical success was defined by the results of a computed tomography scan and a blood analysis. Cases considered to be failures were those requiring hemostasis to control GI bleeding. In technical successful cases we included not only complete closing by using OTSCs, but also partial closing where complete closure with OTSCs is almost difficult. In overall clinical successful cases we included only complete closing by using only OTSCs perfectly.
Table 1.
Database of patients who underwent over-the-scope clip device placement
| No. | Sex | Age | Location | Primary disease | Maximum lesion size (mm) | Prior treatment history | No. of OTSCs | Operation time (min) | Time from diagnosis (wk) | Technical/overall clinical successful | Additional treatment | Complication | Stay in hospital after OTSC placement (d) | Duration of follow-up (d) |
| 1 | M | 86 | Lower esophagus | Iatrogenic perforation caused by stomach tube | 20 | None | 1 | 5 | < 1 | Yes/yes | None | None | 6 | 56 |
| 2 | M | 74 | Stomach | Delayed perforation after ESD | 40 | None | 2 | 24 | < 1 | Yes/yes | None | None | 10 | 90 |
| 3 | F | 82 | Stomach | Perforation after ESD | 25 | None | 2 | 20 | < 1 | Yes/yes | None | None | 7 | 8 |
| 4 | M | 80 | Stomach | Peptic ulcer with bleeding | 15 | Hemostatic forceps | 1 | 8 | < 1 | Yes/yes | None | None | 21 | 30 |
| 5 | F | 71 | Stomach | Peptic ulcer with perforation | 40 | None | 2 | 23 | < 1 | Yes/yes | None | None | 7 | 58 |
| 6 | F | 88 | Stomach | Gastrocutaneous fistula | 10 | None | 1 | 18 | > 4 | Yes/yes | None | None | 8 | 84 |
| 7 | M | 98 | Stomach | Bleeding due to Mallory-Weiss syndrome | 12 | None | 1 | 12 | < 1 | Yes/yes | None | None | 6 | 15 |
| 8 | M | 73 | Duodenum | Para-anastomotic ulcer bleeding | 15 | Clips | 2 | 21 | < 1 | Yes/yes | None | None | 8 | 18 |
| 9 | M | 80 | Duodenal bulb | Peptic ulcer with bleeding | 23 | Clips and hemostatic forceps | 2 | 30 | < 1 | Yes/yes | None | None | 10 | 52 |
| 10 | M | 74 | Duodenal bulb | Peptic ulcer with perforation | 5 | None | 1 | 8 | < 1 | Yes/yes | None | None | 13 | 194 |
| 11 | F | 73 | Duodenal bulb | Prevention of post-ESD perforation | 25 | None | 1 | 10 | < 1 | Yes/yes | None | None | 7 | 95 |
| 12 | M | 75 | 3rd portion of duodenum | Delayed perforation after ESD | 28 | None | 2 | 36 | < 1 | Yes/yes | None | None | 15 | 210 |
| 13 | F | 85 | Rectum | Rectovesical fistula | 15 | None | 2 | 30 | < 1 | Yes/yes | None | None | 9 | 28 |
| 14 | F | 88 | Rectum | Iatrogenic rectal perforation/fistula | 25 | None | 1 | 8 | < 1 | Yes/yes | None | None | 10 | 30 |
| 15 | M | 73 | Stomach | Peptic ulcer with bleeding | 20 | Clips and HSE | N/A | N/A | 1-4 | No/no | Hemostatic forceps | N/A | N/A | N/A |
| 16 | M | 64 | Stomach | Peptic ulcer with bleeding | 50 | Hemostatic forceps | N/A | N/A | 1-4 | No/no | Hemostatic forceps | N/A | N/A | N/A |
| 17 | F | 65 | Sigmoid colon | Perforation after ESD | 35 | None | 1 | 7 | < 1 | Yes/yes | None | None | 8 | 160 |
| 18 | F | 83 | Sigmoid colon | Perforation after ESD | 40 | None | 3 | 16 | < 1 | Yes/yes | None | None | 8 | 90 |
| 19 | F | 88 | Stomach | Perforation caused by a local injection needle | 50 | None | 3 | 51 | 1-4 | Yes/no | Surgery | N/A | N/A | N/A |
| 20 | M | 65 | Rectum | Postoperative anastomotic ulcer bleeding | 5 | Hemostatic forceps | 1 | 6 | < 1 | Yes/yes | None | None | 8 | 14 |
| 21 | M | 65 | Rectum | Postoperative anastomotic ulcer bleeding | 5 | Hemostatic forceps | 2 | 19 | < 1 | Yes/yes | None | None | 7 | 30 |
| 22 | M | 73 | Sigmoid colon | Refractory diverticular bleeding | 5 | Clips | 1 | 7 | < 1 | Yes/yes | None | None | 5 | 10 |
| 23 | M | 73 | Stomach | Gastrobronchial fistula | 28 | Bronchial embolization | 1 | 40 | > 4 | Yes/no | May be given in future | N/A | N/A | N/A |
OTSC: Over-the-scope clip; ESD: Endoscopic submucosal dissection; M: Male; F: Female; N/A: Not available; HSE: Hypertonic saline-epinephrine injection therapy.
All the OTSCs were placed by 2 experienced endoscopists. The sites closed after ESD included not only the perforation site but also all defect ulcers.
We obtained written, informed consent related to the use of OTSCs from all the patients.
Institution participating in the study
Kagawa University Hospital, Kagawa, Japan, participated in the study.
RESULTS
A total of 23 patients [mean age 77 years (range 64-98 years)] underwent OTSC placement during the study period. Of the 23 patients, 14 were male (60%) and 9 were female (40%). The indications for OTSC placement were GI bleeding (n = 9), perforation (n = 10), fistula (n = 4) and the prevention of post-ESD duodenal artificial ulcer perforation (n = 1). One patient had a perforation caused by a glycerin enema, after which a fistula formed. The perforations that were observed included iatrogenic perforations (n = 8) and hemorrhagic peptic ulcers (n = 2). The iatrogenic perforations included post-ESD artificial ulcer perforations (n = 6), a perforation by a local steroid injection into an ulcer following ESD to prevent gastric stenosis (n = 1), an esophageal perforation by a nasogastric tube (n = 1) (Figure 1) and a rectal perforation by a glycerin enema (n = 1). The fistulae included rectal fistulae (n = 2), a stomach-to-skin fistulae following percutaneous endoscopic gastronomy (PEG) tube removal (n = 1) (Figure 2) and a stomach-to-brachial tube fistula (n = 1) (Figure 3).
Figure 1.
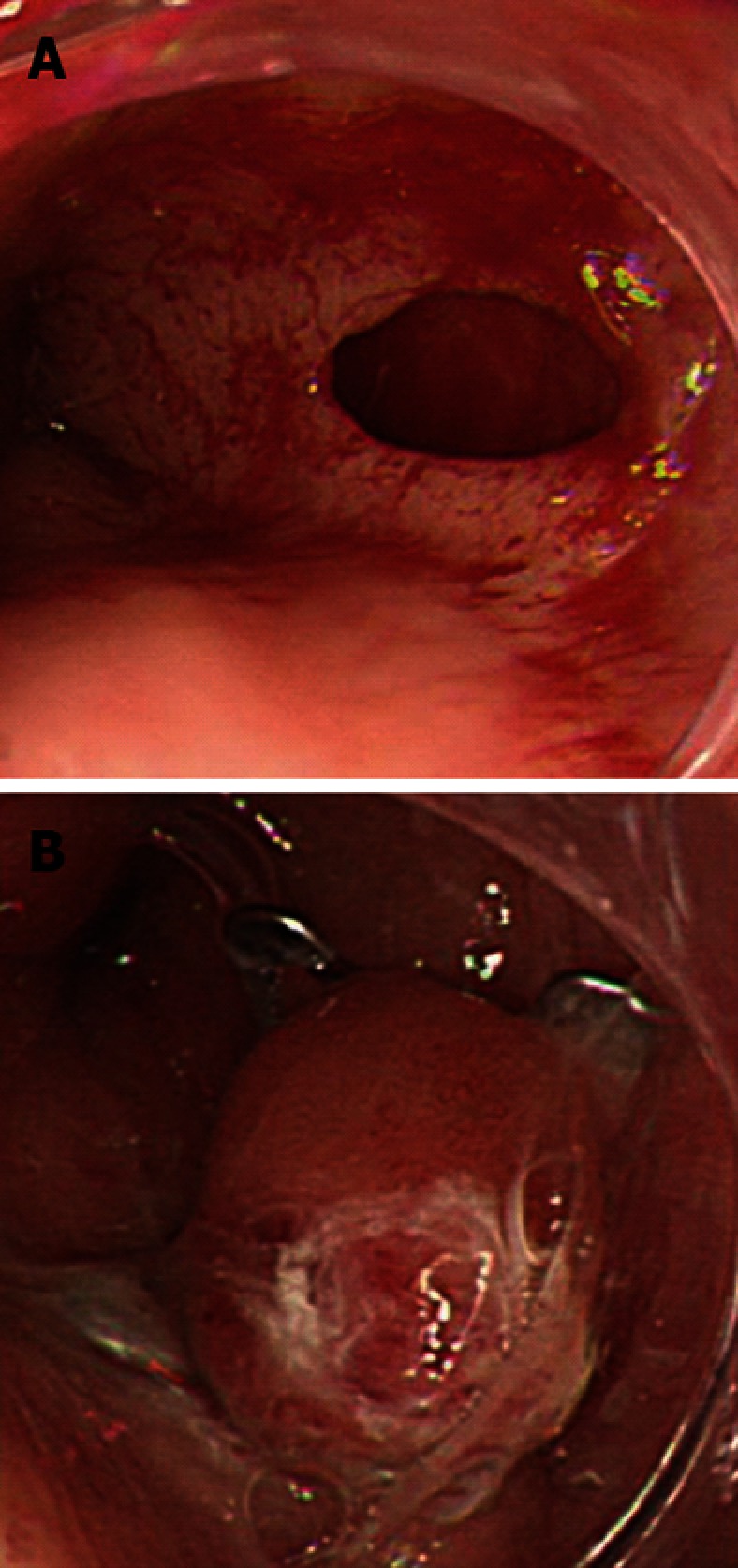
Esophageal perforation caused by a nasogastric tube. A: During the insertion of a nasogastric tube, the tip of the tube perforated the lower esophagus; B: The wound was successfully closed with a single over-the-scope clip, and there was no leakage.
Figure 2.
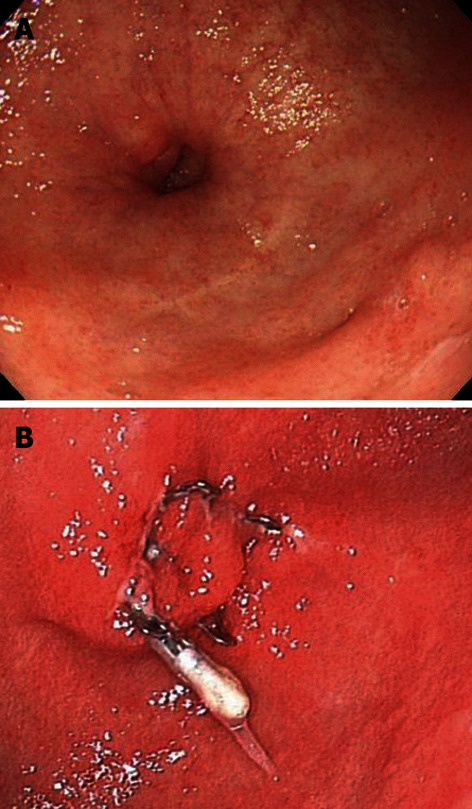
Gastrocutaneous fistula that occurred after percutaneous endoscopic gastronomy removal. A: After the removal of a gastrostomy tube, the patient was able to eat orally, but a gastrocutaneous fistula was diagnosed; B: The wound was slightly hardened, but was successfully closed with a single over-the-scope clip.
Figure 3.
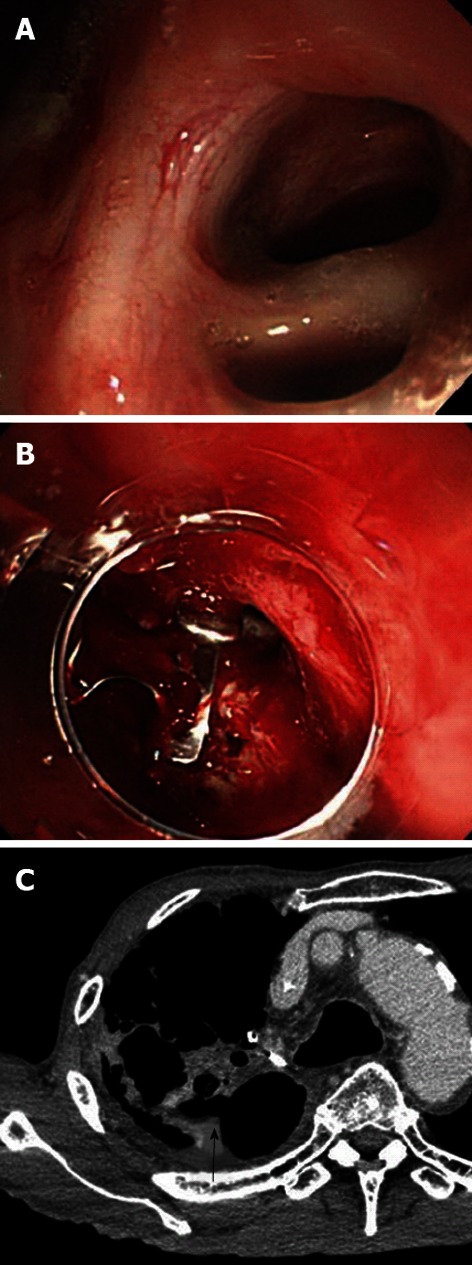
A gastric tube bronchial fistula following a subtotal esophagectomy for esophageal cancer. A: A gastric tube bronchial fistula occurred after a subtotal esophagectomy for esophageal cancer. Bronchial embolization was performed, but it failed to close the fistula; B: The authors attempted to close the fistula using over-the-scope clip (OTSC) but were unsuccessful. Although 1 OTSC was placed, mucosal hardening (resulting from the prolonged duration of the untreated ulcer) prevented the placement of the additional OTSCs required for closure; C: A chest-abdominal computed tomography scan revealed a gastrobronchial fistula (arrow).
The mean maximum size of the lesions was 23.1 mm (range 5 to 50 mm). The lesions were located in the esophagus (n = 1), the stomach (n = 10), the duodenum (n = 5), the sigmoid colon (n = 3) and the rectum (n = 4). The time required for the procedure was measured from the time that the OTSC was applied to the time that it was released in the lesion. The median operation time for the successful cases was 18 min (range 5 to 51 min). The numbers of OTSC devices used per session were one (n = 11 patients), two (n = 8 patients) and three (n = 2 patients). The post-OTSC placement mean observation period was 67 d (range 8-210 d).
The technical success rate for OTSC placement was 91.3% (21/23). The OTSC was successfully and safely released in all the cases except for 2, in which we did not release the OTSC because the ulcer was extremely stiff due to being a chronic hemorrhagic gastric ulcer. We could not include the entire thickness of the mucosa of these ulcers within the applicator cap. Ultimately, we stopped the bleeding by reapplying the hemostatic forceps (Coagrasper, FD-410LR, Olympus, Tokyo, Japan). The overall clinical success rate using the OTSC alone was 82.6% (19/23). A large lesion size (greater than 20 mm) and a delayed diagnosis (more than 1 wk) were the major contributing factors in the overall unsuccessful clinical cases. The location of the unsuccessful lesion was in the stomach (Table 2). In 2 patients, we were unable to place the OTSC on the refractory peptic ulcer. In 1 patient, a perforation occurred because of a local steroid injection into the artificial ulcer after ESD to prevent stenosis. We could not place the OTSC correctly because the wound was large and the ulcer was exceptionally stiff. In another patient, who was suffering from a gastrobrachial fistula (Figure 3), we were able to place only one OTSC, but not the additional OTSCs necessary for the complete closure of the fistula.
Table 2.
Relationship between each characters and overall clinical success rate n (%)
| Patients | Overall clinical success | |
| Location | ||
| Esophagus | 1 | 1 (100) |
| Stomach | 10 | 6 (60) |
| Duodenum | 5 | 5 (100) |
| Colon | 3 | 3 (100) |
| Rectum | 4 | 4 (100) |
| Primary disease | ||
| GI bleeding | 9 | 7 (77) |
| Chronic fistulae | 4 | 3 (75) |
| Perforation | 11 | 10 (90) |
| Maximum lesion size (mm) | ||
| < 20 | 9 | 9 (100) |
| 20-30 | 8 | 6 (75) |
| > 30 | 6 | 4 (66) |
| Time from diagnosis (wk) | ||
| < 1 | 18 | 18 (100) |
| 1-4 | 3 | 0 (0) |
| > 4 | 2 | 1 (50) |
There was no adjunct therapy used for OTSC placement in the 19 overall clinical success cases. In the two technical failure cases, we used coagulation forceps for additional hemostasis. All the patients were hospitalized for observation after the OTSC placement. The median hospital stay was 9 d (range 6-21 d).
Complications
During the observation period, none of the patients experienced any complications associated with OTSC placement.
DISCUSSION
The development of new NOTES devices has introduced advanced therapeutic techniques that can be used in minimally invasive treatments[1-3], including various full-thickness suturing devices that are applied in clinical practice. One of these devices is the OTSC system, the clinical utility of which has been reported in Europe and the Unites States. The OTSC system shows great potential for use in endoscopic treatments that require speed and simplicity[2,3,17,18]. This system received a pharmaceutical license in Japan in November 2011. Although animal experiments and clinical studies have been performed in Europe and the United States, few clinical cases have been reported in Japan[13,14]. We used the OTSC in NOTES animal experiments prior to its approval for humans. Recognizing its potential for use in Japan, we began using the OTSC clinically immediately after the pharmaceutical license was granted. We have employed this device in 23 patients within a short period in our hospital, with an overall success rate of 82%. Animal and clinical studies of the OTSC have demonstrated that gastric, duodenal or colonic perforations up to 15 mm in diameter can be completely closed with a single OTSC. For perforations up to 20 mm in diameter, closure can also be achieved using some OTSCs, which indicates that there is sufficient working space for the unobstructed use of the endoscope during NOTES[17]. There are reports that full-thickness closures of defects 18-27 mm in diameter can be performed using OTSC; however, it is difficult to completely close defects > 30 mm in diameter[18].
In the present series, OTSC closure was successful for wounds with a maximum diameter of ≤ 30 mm but unsuccessful in 1 case of refractory GI bleeding and 1 case of gastrobronchial fistula (Figure 3). Even in cases with a maximum wound diameter of > 30 mm, we placed the OTSC successfully because the tissue was well extensible and not hardened. In our clinical experience, unsuccessful OTSC closure had a chronic course and OTSC failures were due to chronic fibrotic changes and scarring at the perforation site. Specifically, we used OTSCs in 4 chronic patients and failed to close the perforation site in 3 of them (75%). It appears that wound closure with the OTSC is suitable for wounds with easy extensibility of the surrounding tissues; such lesions have little fibrosis and can be easily grasped by the twin grasper. Considering that successful closure was also achieved in cases with a rectal fistula < 20 mm in diameter or a gastrocutaneous fistula after PEG removal (Figure 2), we believe that OTSC use should be considered when surgery is the only remaining option, provided that the lesion (even if presumably hardened) is ≤ 30 mm in diameter and can be sucked into the cap to lift the mucosa. In addition, the success of the OTSC closures in 4 cases with lesions > 30 mm suggests that this device can be used in acute cases with good extensibility of surrounding mucosa.
ESD was developed for en bloc removal of large gastric cancer, which decrease the risk of local recurrence, and specimens can be accurately evaluated by histological examination[19]. However, the procedure is associated with a high incident of complication with bleeding and perforation[20-22]. Now, bleeding has been good controlled with new hemostatic forceps and clips[23]. We also used the OTSC for the complete wound closure of post-ESD perforations in the stomachs of 2 patients who had undergone ESD for early gastric cancer. In both cases, the lesions were located in the greater curvature of the stomach (Figure 4). Because it is anatomically thinner than other parts of the stomach, the greater curvature of the stomach is considered to be easy to perforate[24-29]. In addition, because the knife is applied vertically to the mucosa during ESD, it is difficult to perform the procedure while maintaining an appropriate dissection depth into the submucosal layer. The endoscope must also be retroflexed[24]. These limitations have led to several cases in which our attempts to create a partial closure of an ulcer base using conventional clips caused the further extensive separation of the muscle layer and the subsequent need for surgery. In such cases, complete closure using the OTSC is thus preferred for a post-ESD ulcer in the greater curvature of the stomach, even if perforation is suspected.
Figure 4.
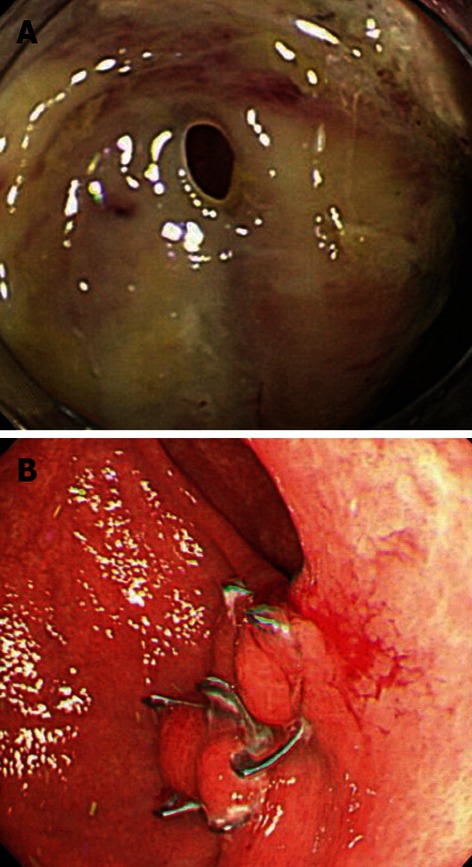
Iatrogenic perforation after endoscopic submucosal dissection for early gastric cancer in the greater curvature of the stomach. A: A post-endoscopic submucosal dissection ulcer was found in the greater curvature of the stomach. An examination by retroflex view revealed that the muscle layer was separated and perforated; B: The wound was successfully closed using an over-the-scope clip (OTSC). An upper endoscopy performed 2 mo after the closure revealed no displacement of the OTSC or complications, such as ulceration or deformation.
The use of duodenal ESD is controversial among Japanese endoscopists because the narrow lumen of the duodenum makes it difficult to perform the procedure, and the base of a post-ESD duodenal ulcer is continuously exposed to bile, causing an increased incidence of delayed perforation compared with other ESD sites[30-35]. Nevertheless, duodenal surgery is highly invasive because of the anatomical position. ESD should be preferentially performed instead of surgery if clinically indicated. The Japan Gastroenterological Endoscopy Society reported in April 2009 that the complete closure of a post-ESD duodenal ulcer using conventional clips helps prevent delayed perforation. However, conventional clips are too small and do not provide sufficient grip strength to achieve the complete closure of an ulcer base. Therefore, the OTSC, which is larger and provides greater grip strength, is recommended for the complete closure of post-ESD duodenal ulcers[36].
At our hospital, patient 11 (Table 1) experienced a small perforation of a post-ESD ulcer that formed in the duodenal bulb, and the OTSC was used for its closure. In patient 12 (Table 1), the lesion occurred in the descending portion of the duodenum and was exposed to bile, indicating an increased risk of delayed perforation. Thus, the ulcer was closed using the OTSC, which helped prevent perforation and bleeding.
We experienced 2 cases of OTSC closure for post-ESD ulcers in the colon. In both cases, the lesions were located in the sigmoid colon, with post-ESD perforations requiring complete wound closure. The wound was > 30 mm in diameter in both cases and was successfully closed using the OTSC.
We experienced 9 cases of GI bleeding that were treated with OTSCs. Widely used hemostatic procedures, such as hemostatic clips and local injections, are economically advantageous but sometimes fail to achieve complete primary hemostasis. The use of coagulation hemostasis with hemostatic forceps is also increasing because the reliable coagulation of exposed vessels under direct visualization can minimize the risk of rebleeding. However, the application of coagulation hemostasis to a deep ulcer or a thin wall of the duodenum is associated with a risk of perforation. For patients who do not tolerate surgery well and are in shock due to rebleeding or who do not respond to conventional treatment, the use of the OTSC should be considered. Based on these criteria, we applied OTSCs to the 9 patients with GI bleeding and achieved complete hemostasis in 7 of them. The remaining 2 patients with failed hemostasis using the OTSC system had a personal status ≥ 3 and could not tolerate open abdominal surgery. One of the patients had a large ulcer (50 mm in diameter) to which hemostasis with hemostatic forceps was applied. However, the patient experienced 2 episodes of shock due to bleeding from other sites of neovascularization. During the third episode of bleeding, ulcer closure with the OTSC was attempted but was unsuccessful due to a hardened ulcer base. Hemostasis with hemostatic forceps was again performed, after which no rebleeding was observed.
Hemostatic treatment of an anastomotic ulcer using the OTSC for was successful in 1 patient with a duodenal lesion and for 2 patients with lesions in the sigmoid colon (Figure 5). The aggressive treatment of bleeding with hemostatic forceps may cause perforations because anastomotic sites are usually thin and fragile. We consider the OTSC to be an effective tool for the treatment of lesions at anastomotic sites.
Figure 5.
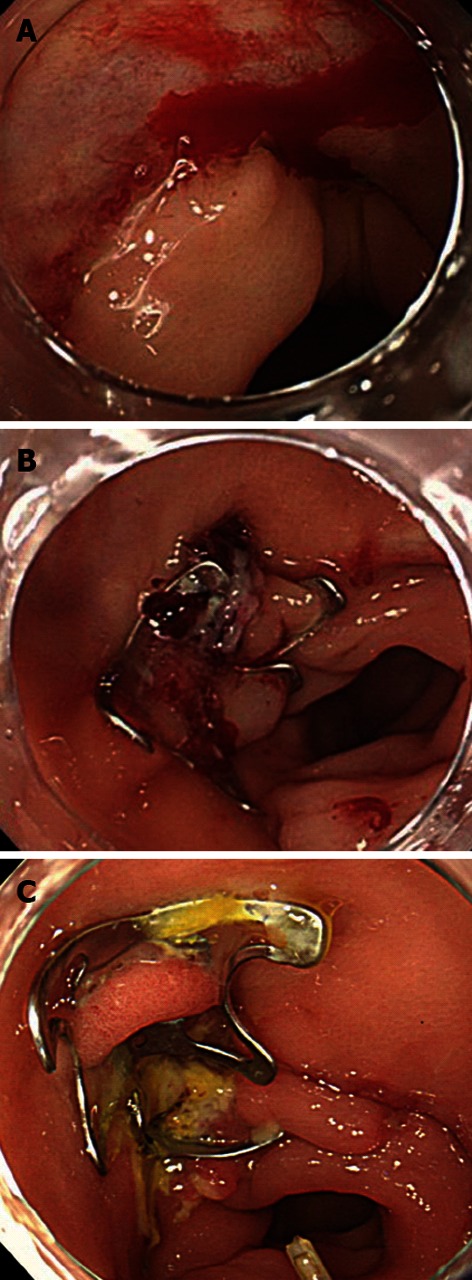
Bleeding from an anastomotic ulcer caused by a sigmoidectomy for sigmoid cancer. A: Bleeding was observed from the anastomotic site after surgery for sigmoid colon cancer; B: The wound was successfully closed using an over-the-scope clip; C: The postoperative course has been uneventful, with no rebleeding.
Regarding safety and complications, none of our patients treated with the OTSC reported any complications. Assessments performed 7 d after closure using the OTSC also revealed no displacements of the OTSC or tissue necrosis at the wound sites. Endoscopic examinations revealed OTSC losses in 2 cases: 1 occurred 1 mo after OTSC placement for duodenal lesions, and the other occurred 2 mo after OTSC placement for colonic lesions. No associated complications were observed in either case. Other possible complications include mucosal damage caused by the teeth of the OTSC protruding out of the hood top during insertion. Therefore, special care should be taken when the OTSC is inserted into physiologically narrow sites, such as the esophageal entrance, pyloric ring or anal ring. There have been no reports of OTSCs causing mucosal deformation or stenosis of the gastrointestine[9]. However, we must consider the possibility that a failure to extract the fibrotic mucosa may result in the tissue being crushed by the twin grasper during the extraction of hardened tissue.
Based on our 23 cases and those reported in the literature, we consider OTSC to be a highly useful device that can be safely utilized in the treatment of GI perforations, fistulae and refractory bleeding. However, OTSC is not suitable for the closure of chronic lesions with hard, severely fibrotic wounds because it is difficult to draw such a lesion into the top of the device. However, we have used the OTSC to close post-ESD perforations with a 100% success rate. Although our sample size was small, we believe that the OTSC is a viable treatment option for post-ESD perforation.
COMMENTS
Background
Recently, over-the-scope clip (OTSC) devices have been used for gastrointestinal (GI) bleeding, fistulae and perforations in the United States and several European countries. OTSC devices became pharmaceutically licensed in Japan in August 2011. The authors have reported their experiences with the OTSC and the outcomes of several cases in Japan. The aims of the present study were to retrospectively review the results of OTSC use in the authors’ hospital and to examine the feasibility of using the OTSC to completely close perforations after endoscopic submucosal dissection (ESD) for early GI cancers.
Research frontiers
Historically, the standard treatment for GI perforations has been surgery. Recently, invasive endoscopic treatments, such as ESD and natural orifice transluminal endoscopic surgery (NOTES), have provided alternative approaches to surgery. However, the devices used to treat complications following endoscopic treatments are less convenient and not as safe. OTSCs have been used to treat GI bleeding, fistulae and perforations in several countries. In this study, the authors retrospectively report the results of using the OTSC in their hospital. They also discuss the potential use of the OTSC to completely close perforations after ESD for early GI cancers.
Innovations and breakthroughs
This is the first retrospective study of the OTSC in Japan, and it includes more cases of post-ESD perforation closure compared with other published reports on OTSC. All the post-ESD perforation closure cases in the present study were successes. Thus, the authors consider OTSC to be a possible tool for the treatment of perforations after ESD.
Applications
Based on the present 23 cases and those reported in the literature, the authors assert that the OTSC is useful and safe for the treatment of GI perforations, fistulae and refractory bleeding.
Terminology
ESD is the only nonsurgical, endoscopic method of treating early GI cancers; NOTES, a fusion of flexible endoscopy and operative techniques, is a less invasive form of treatment than surgery.
Peer review
The OTSC is an interesting and novel device that enhances the armamentarium of therapeutic gastroenterologists. This report illustrates the use of this novel device, which facilitates interventions that were previously impossible to perform endoscopically.
Footnotes
P- Reviewers Van Rensburg C, Kumar A S- Editor Wen LL L- Editor A E- Editor Li JY
References
- 1.Mori H, Kobara H, Kobayashi M, Muramatsu A, Nomura T, Hagiike M, Izuishi K, Suzuki Y, Masaki T. Establishment of pure NOTES procedure using a conventional flexible endoscope: review of six cases of gastric gastrointestinal stromal tumors. Endoscopy. 2011;43:631–634. doi: 10.1055/s-0030-1256227. [DOI] [PubMed] [Google Scholar]
- 2.von Renteln D, Vassiliou MC, Rothstein RI. Randomized controlled trial comparing endoscopic clips and over-the-scope clips for closure of natural orifice transluminal endoscopic surgery gastrotomies. Endoscopy. 2009;41:1056–1061. doi: 10.1055/s-0029-1215241. [DOI] [PubMed] [Google Scholar]
- 3.Voermans RP, van Berge Henegouwen MI, Bemelman WA, Fockens P. Novel over-the-scope-clip system for gastrotomy closure in natural orifice transluminal endoscopic surgery (NOTES): an ex vivo comparison study. Endoscopy. 2009;41:1052–1055. doi: 10.1055/s-0029-1215231. [DOI] [PubMed] [Google Scholar]
- 4.Kobara H, Mori H, Masaki T. Successful en bloc resection of an esophageal hemangioma by endoscopic submucosal dissection. Endoscopy. 2012;44 Suppl 2 UCTN:E134–E135. doi: 10.1055/s-0030-1256703. [DOI] [PubMed] [Google Scholar]
- 5.Raju GS. Endoscopic closure of gastrointestinal leaks. Am J Gastroenterol. 2009;104:1315–1320. doi: 10.1038/ajg.2009.34. [DOI] [PubMed] [Google Scholar]
- 6.Mori H, Kobara H, Inoue H, Kobayashi M, Muramatsu A, Nomura T, Gong J, Hagiike M, Izuishi K, Suzuki Y, et al. New technique for safer endoscopic submucosal dissection using the duodenal balloon occlusion method. J Gastroenterol Hepatol. 2012;27:81–85. doi: 10.1111/j.1440-1746.2011.06833.x. [DOI] [PubMed] [Google Scholar]
- 7.Mori H, Rafiq K, Kobara H, Fujihara S, Nishiyama N, Kobayashi M, Himoto T, Haba R, Hagiike M, Izuishi K, et al. Local steroid injection into the artificial ulcer created by endoscopic submucosal dissection for gastric cancer: prevention of gastric deformity. Endoscopy. 2012;44:641–648. doi: 10.1055/s-0032-1309815. [DOI] [PubMed] [Google Scholar]
- 8.Kobara H, Mori H, Fujiwara S, Nishiyama N, Kobayashi M, Masaki T. Bloc biopsy by tunneling method using endoscopic submucosal dissection for an upper gastrointestinal submucosal tumor. Endoscopy. 2012;44 Suppl 2 UCTN:E197–E198. doi: 10.1055/s-0031-1291821. [DOI] [PubMed] [Google Scholar]
- 9.Traina M, Curcio G, Tarantino I, Soresi S, Barresi L, Vitulo P, Gridelli B. New endoscopic over-the-scope clip system for closure of a chronic tracheoesophageal fistula. Endoscopy. 2010;42 Suppl 2:E54–E55. doi: 10.1055/s-0029-1243824. [DOI] [PubMed] [Google Scholar]
- 10.von Renteln D, Rudolph HU, Schmidt A, Vassiliou MC, Caca K. Endoscopic closure of duodenal perforations by using an over-the-scope clip: a randomized, controlled porcine study. Gastrointest Endosc. 2010;71:131–138. doi: 10.1016/j.gie.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Seebach L, Bauerfeind P, Gubler C. “Sparing the surgeon”: clinical experience with over-the-scope clips for gastrointestinal perforation. Endoscopy. 2010;42:1108–1111. doi: 10.1055/s-0030-1255924. [DOI] [PubMed] [Google Scholar]
- 12.Pohl J, Borgulya M, Lorenz D, Ell C. Endoscopic closure of postoperative esophageal leaks with a novel over-the-scope clip system. Endoscopy. 2010;42:757–759. doi: 10.1055/s-0030-1255634. [DOI] [PubMed] [Google Scholar]
- 13.Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos) Gastrointest Endosc. 2012;76:202–208. doi: 10.1016/j.gie.2012.03.250. [DOI] [PubMed] [Google Scholar]
- 14.Albert JG, Friedrich-Rust M, Woeste G, Strey C, Bechstein WO, Zeuzem S, Sarrazin C. Benefit of a clipping device in use in intestinal bleeding and intestinal leakage. Gastrointest Endosc. 2011;74:389–397. doi: 10.1016/j.gie.2011.03.1128. [DOI] [PubMed] [Google Scholar]
- 15.Repici A, Arezzo A, De Caro G, Morino M, Pagano N, Rando G, Romeo F, Del Conte G, Danese S, Malesci A. Clinical experience with a new endoscopic over-the-scope clip system for use in the GI tract. Dig Liver Dis. 2009;41:406–410. doi: 10.1016/j.dld.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 16.von Renteln D, Denzer UW, Schachschal G, Anders M, Groth S, Rösch T. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos) Gastrointest Endosc. 2010;72:1289–1296. doi: 10.1016/j.gie.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 17.Parodi A, Repici A, Pedroni A, Blanchi S, Conio M. Endoscopic management of GI perforations with a new over-the-scope clip device (with videos) Gastrointest Endosc. 2010;72:881–886. doi: 10.1016/j.gie.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 18.von Renteln D, Schmidt A, Vassiliou MC, Rudolph HU, Caca K. Endoscopic full-thickness resection and defect closure in the colon. Gastrointest Endosc. 2010;71:1267–1273. doi: 10.1016/j.gie.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 19.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 21.Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987–990. doi: 10.1055/s-2006-944716. [DOI] [PubMed] [Google Scholar]
- 22.Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980–986. doi: 10.1055/s-2006-944809. [DOI] [PubMed] [Google Scholar]
- 23.Muraki Y, Enomoto S, Iguchi M, Fujishiro M, Yahagi N, Ichinose M. Management of bleeding and artificial gastric ulcers associated with endoscopic submucosal dissection. World J Gastrointest Endosc. 2012;4:1–8. doi: 10.4253/wjge.v4.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Yoo JH, Shin SJ, Lee KM, Choi JM, Wi JO, Kim DH, Lim SG, Hwang JC, Cheong JY, Yoo BM, et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012;26:2456–2464. doi: 10.1007/s00464-012-2211-x. [DOI] [PubMed] [Google Scholar]
- 26.Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907–912. doi: 10.1111/j.1440-1746.2011.07039.x. [DOI] [PubMed] [Google Scholar]
- 27.Ohta T, Ishihara R, Uedo N, Takeuchi Y, Nagai K, Matsui F, Kawada N, Yamashina T, Kanzaki H, Hanafusa M, et al. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest Endosc. 2012;75:1159–1165. doi: 10.1016/j.gie.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Iwabuchi K. A study of the aging of the stomach: Especially from the view point of mural alteration. Tokyo Jikeikai Med J. 2008;123:197–210. [Google Scholar]
- 29.Jeon SW, Jung MK, Kim SK, Cho KB, Park KS, Park CK, Kwon JG, Jung JT, Kim EY, Kim TN, et al. Clinical outcomes for perforations during endoscopic submucosal dissection in patients with gastric lesions. Surg Endosc. 2010;24:911–916. doi: 10.1007/s00464-009-0693-y. [DOI] [PubMed] [Google Scholar]
- 30.Spira IA, Ghazi A, Wolff WI. Primary adenocarcinoma of the duodenum. Cancer. 1977;39:1721–1726. doi: 10.1002/1097-0142(197704)39:4<1721::aid-cncr2820390450>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Lim CH, Park JM, Park CH, Cho YK, Lee IS, Kim SW, Choi MG, Chung IS. Endoscopic submucosal dissection of gastric neoplasia involving the pyloric channel by retroflexion in the duodenum. Dig Dis Sci. 2012;57:148–154. doi: 10.1007/s10620-011-1863-z. [DOI] [PubMed] [Google Scholar]
- 32.Ono H, Nonaka S, Uedo N, Kaise M, Oyama T, Doyama H, Kokawa A, Kaneko K, Kodashima S, Tanabe S, et al. Clinical Issues of Duodenal EMR/ESD. Stomach and Intestine (Tokyo) 2011;46:1669–1677. [Google Scholar]
- 33.Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K, Hanatsuka K, Sugano K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270–274. doi: 10.1111/j.1443-1661.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 34.Shinoda M, Makino A, Wada M, Kabeshima Y, Takahashi T, Kawakubo H, Shito M, Sugiura H, Omori T. Successful endoscopic submucosal dissection for mucosal cancer of the duodenum. Dig Endosc. 2010;22:49–52. doi: 10.1111/j.1443-1661.2009.00917.x. [DOI] [PubMed] [Google Scholar]
- 35.Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901–2905. doi: 10.1007/s00464-011-1640-2. [DOI] [PubMed] [Google Scholar]
- 36.Mori H, Shintaro F, Kobara H, Nishiyama N, Rafiq K, Kobayashi M, Nakatsu T, Miichi N, Suzuki Y, Masaki T. Successful closing of duodenal ulcer after endoscopic submucosal dissection with over-the-scope clip to prevent delayed perforation. Dig Endosc. 2012:Aug 7; Epub ahead of print. doi: 10.1111/j.1443-1661.2012.01363.x. [DOI] [PubMed] [Google Scholar]


