Abstract
In mice, behavioral acceptance of the bitter compound sucrose octaacetate (SOA) depends on allelic variation of a single gene, Soa. The SW.B6-Soab congenic mouse strain has the genetic background of an “SOA taster” SWR/J strain and an Soa-containing donor chromosome fragment from an “SOA nontaster” C57BL/6J strain. Using microsatellite markers polymorphic between the two parental strains, we determined that the donor fragment spans 5–10 cM of distal chromosome 6. The SWR/J mice avoided SOA in two-bottle tests with water and had strong responses to SOA in two gustatory nerves, the chorda tympani (CT) and glossopharyngeal (GL). In contrast, the SW.B6-Soab mice were indifferent to SOA in two-bottle tests and had very weak responses to SOA in both of these nerves. The SWR/J and SW.B6-Soab mice did not differ in responses of either nerve to sucrose, NaCl, HCl, or the bitter-tasting stimuli quinine, denatonium, strychnine, 6-n-propylthiouracil, phenylthiocarbamide, and MgSO4. Thus the effect of the Soa genotype on SOA avoidance is mediated by peripheral taste responsiveness to SOA, involving taste receptor cells innervated by both the CT and GL nerves.
Keywords: congenic mice, taste, bitter, chorda tympani, glossopharyngeal, electrophysiology
Gustatory sensitivity to bitter compounds varies widely among humans (24, 31) and inbred strains of mice (18, 28). Often, such differences are specific to individual bitter compounds; that is, an individual or mouse strain may be highly sensitive to one compound but insensitive to another and vice versa. One such bitter compound is the acetylated sugar, sucrose octaacetate (SOA). Mouse strains display large differences in sensitivity to SOA in two-bottle preference tests. Most dramatically, SWR/J (SWR) mice strongly avoid SOA at concentrations above 0.01 mM, whereas most other strains display no or only weak avoidance of these concentrations.
SOA acceptance is determined by allelic variation of a single gene, Soa, with three known alleles. The dominant Soaa allele determines a “taster” phenotype of strong SOA avoidance, the recessive Soab allele determines a “nontaster” phenotype of indifference to SOA, and the other recessive Soac allele determines a “demitaster” phenotype of intermediate SOA sensitivity (15). SWR mice carry the Soaa allele, and C57BL/6J (B6) mice carry the Soab allele. The SW.B6-Soab (SW.B6) congenic mouse strain was selected using a backcross-intercross system to transfer a Soa-containing donor chromosome fragment from the B6 strain onto the genetic background of the SWR strain (16). As a result, the SW.B6 mice do not avoid SOA in two-bottle tests, unlike mice from the inbred partner SWR strain. Although Soa has been mapped to distal chromosome 6 (2, 9, 20), the size of the donor chromosome fragment of the SW.B6 congenic strain is unknown.
Recent data (1, 14) suggest that the Soa locus encodes a gene for a specific bitter receptor. However, Soa effects have been characterized primarily using behavioral tests, which may depend on differences anywhere in the taste transduction pathway between taste receptors and central nervous system centers. The role of peripheral sensory mechanisms has been assessed in only one previous study (25), in which gustatory neural responses to lingual application of SOA were measured in inbred mice with different Soa genotypes. In this study, the SOA taster SWR mice had larger responses to SOA in the chorda tympani (CT) and glossopharyngeal (GL) nerves compared with demitaster LP/J and DBA/2J mice, and nontaster BDP/J mice. This study involved inbred strains, which differ in many loci throughout the genome. Therefore, the observed correlation between behavioral and neural responsiveness to SOA could be due either to the pleiotropic effect of Soa or to a fortuitous coincidence of a locus affecting behavioral sensitivity (Soa) and locus/loci affecting neural sensitivity in the inbred strains. A more direct test of whether Soa influences peripheral neural responses can be performed using congenic strains that retain only a small part of a donor genome on a genetic background of an inbred partner strain.
Previous studies demonstrated consistency between stronger behavioral avoidance of SOA and larger neural responses to SOA (25). However, the effects of Soa on responsiveness to other bitter compounds are less obvious. For example, compared with SOA-insensitive mice, SOA-sensitive mice also had stronger avoidance of quinine and strychnine in preference tests (7, 16, 19, 28), but they did not differ in responses of the CT and GL gustatory nerves to the same compounds (8, 25).
The goal of the study reported here was to further characterize the mechanisms underlying the effects of Soa on bitter taste responsiveness using the SW.B6 congenic mice. This involved: 1) specifying the location and size of the Soa-containing donor chromosome fragment in the SW.B6 congenic mice and 2) evaluating electrophysiological responses in their CT and GL nerves to SOA and other bitter compounds.
MATERIALS AND METHODS
Animals
Mice from the SWR and B6 inbred strains were purchased from The Jackson Laboratory (Bar Harbor, ME). SW.B6-Soab (SW.B6) mice were obtained from Dr. G. Whitney (Florida State University, Tallahassee, FL) in generation NE10F23 and bred at the Monell Chemical Senses Center. The mice were kept in a temperature-controlled room at 23°C on a 12:12-h light-dark cycle and had free access to deionized water and Teklad Rodent Diet 8604 (Harlan Teklad, Madison, WI).
Genotyping
We used DNA from SWR, B6, and NE10F23 SW.B6 mice (n = 3 for each strain). For all tested markers, genotypes were identical within the strains. Genomic DNA was purified from mouse tails using the NaOH/Tris method (27). Genotyping was performed using methods described in detail elsewhere (3). Briefly, microsatellite (simple sequence length polymorphisms, or SSLP) markers were amplified using PCR with primers purchased from Research Genetics (Huntsville, AL). The denatured PCR products were electrophoresed on a polyacrylamide sequencing gel and visualized using autoradiography. Chromosomal positions of the markers relative to the centromere were obtained from the Mouse Genome Database (23).
Two-bottle tests
Male SWR, B6, and NE10F25 SW.B6 mice, 2–5 mo old (n = 8 for each strain), were caged individually. Construction of drinking tubes and other experimental details have been described previously (5). The drinking tubes were positioned to the right of the feeder with their tips 15 mm apart, and each extended 25 mm into the cage. Each tube had a stainless steel tip with a 3.175-mm diameter hole from which the mice could lick fluids.
SOA (0.1 and 1 mM) and quinine hydrochloride (QHCl, 0.01–1 mM; Sigma Chemical, St. Louis, MO) were dissolved in deionized water. The mice were presented with one tube containing a solution and the other tube containing deionized water. The solutions were tested in order of increasing concentrations. Each concentration was tested for 48 h with the positions of the tubes switched at 24 h to control for side preferences. Daily measurements were made in the middle of the light period by reading fluid volume to the nearest 0.2 ml. There were no breaks between testing different concentrations of the same compound, but between testing SOA and quinine, the mice received deionized water in both drinking tubes for 2 days.
Preference scores were calculated for each mouse as the ratio of the average daily solution intake to the average daily total fluid (solution + water) intake, in percent. The data for SOA and quinine were analyzed separately using two-way ANOVAs, with strain as a between-group factor and concentration as a within-group factor. Scheffé post-hoc tests were used to evaluate differences between individual means. The statistical tests used a two-tailed criterion for significance of P < 0.05. The significance of a taste solution preference or avoidance was determined by comparing the solution and water intakes using paired t-tests. Because we calculated 21 t-scores, we used a Bonferroni correction to account for multiple comparisons, which set threshold of significance at P < 0.05/21 = 0.00238.
Electrophysiological recording of taste responses in the CT and GL nerves
Male SWR (n = 12), B6 (n = 4), and NE10F24 SW.B6 (n = 12) mice, 2–4 mo old, were used in the electrophysiological experiments. The mice were anesthetized with an intraperitoneal injection (2 ml/kg, with further doses as necessary) of a mixture of ketamine (21.4 mg/ml), xylazine (4.3 mg/ml), and acepromazine (0.7 mg/ml). A cannula was inserted in the trachea, and the animal was placed supine in a nontraumatic headholder. The hypoglossal nerve was transected bilaterally to prevent inadvertent tongue movements. In six SWR, four B6, and six SW.B6 mice, the right CT nerve was exposed at its exit from the lingual nerve by removal of the internal pterygoid muscle. The CT nerve was then dissected free from surrounding tissues and cut at the point of its entry to the bulla. In six SWR and six SW.B6 mice, the right GL nerve was exposed by removal of the diagastricus muscle and posterior horn of the hyoid bone. The GL nerve was then dissected free from underlying tissues and cut near its entrance to the posterior lacerated foramen. The entire CT or GL nerve was placed on a platinum wire electrode, and a few drops of mineral oil were placed in the wound site to prevent desiccation of the nerve. An indifferent electrode was positioned in nearby muscle tissue. The whole nerve response was then amplified, integrated with a time constant of 1.0 s, and displayed on chart recorder paper.
For chemical stimulation of the fungiform taste papillae (CT recording), the anterior one-half of the animal’s tongue was enclosed in a flow chamber. For chemical stimulation of the vallate and foliate papillae in the posterior tongue (GL recording), an incision was made on each side of the animal’s face from the corner of the mouth to just above the angle of the jaw, and the papillae were exposed and their trenches opened via slight tension applied through a small suture sewn in the tip of the tongue. Solutions were delivered into the flow chamber (for the anterior part) or directly to the tongue (for the posterior part) by gravity flow at a rate of 0.5 ml/s.
Stimuli consisted of the following: 300 mM sucrose; 100 mM NaCl; 10 mM HCl; 0.01, 0.1, and 1 mM SOA; 0.1, 1, and 10 mM denatonium benzoate; 0.1, 1, and 10 mM QHCl; 0.1, 1, and 10 mM strychnine-HCl; 0.1, 1, and 3 mM 6-n-propylthiouracil (PROP); 0.1, 1, and 10 mM phenylthiocarbamide (PTC); and 10, 100, and 1,000 mM MgSO4 (Sigma). For the SWR and SW.B6 strains, responses to all stimuli were obtained from six mice from each strain for each nerve, with the exceptions of those to strychnine-HCl and MgSO4 (n = 4). For the B6 strain, only the CT responses to 300 mM sucrose, 100 mM NaCl, 10 mM HCl, 20 mM QHCl, and 0.01, 0.1, and 1 mM SOA were recorded. Stimuli were mixed in distilled water and were applied at room temperature (22°C). NH4Cl at 100 mM was presented at regular intervals to serve as a reference stimulus. For each compound, concentration series were applied in ascending order. During chemical stimulation of the tongue, the test solutions flowed for 30 s. Between taste stimuli, the tongue was rinsed with deionized water for at least 1 min.
The magnitude of the integrated response at 20 s after stimulus onset was measured and expressed as a proportion of the average of the previous and following responses to 100 mM NH4Cl (this correction was done for each nerve separately). Initial analyses were conducted to validate the use of NH4Cl as a standard stimulus for data correction using an approach described by Frank and Blizard (13). For each animal, we calculated a ratio of the magnitude of absolute response to 100 mM NH4Cl to an average of the absolute responses to four standard stimuli, 300 mM sucrose, 100 mM NaCl, 10 mM HCl, and 10 mM QHCl as follows
where R is a relative response magnitude, and A is an absolute response magnitude. The RNH4Cl was determined separately for the CT and GL nerves. The mean (±SE) relative responses to NH4Cl in the CT were 1.15 ± 0.09 for SWR strain and 1.23 ± 0.09 for the SW.B6 strain (P = 0.59, t-test). In the GL, they were 1.46 ± 0.13 for SWR strain and 1.41 ± 0.18 for the SW.B6 strain (P = 0.81). Thus we concluded that in each nerve, CT and GL, the NH4Cl responses were similar in the two strains and that it is appropriate to use 0.1 M NH4Cl as a standard stimulus in the current study.
For each stimulus series, a two-way ANOVA was performed with strain and concentration as factors. Nonbitter stimuli including NaCl, sucrose, and HCl were examined using two-tailed t-tests. When the interaction term of the ANOVA was significant, post-hoc t-tests were performed. The level of statistical significance used for all tests was P < 0.05.
RESULTS
Genotyping
Using markers polymorphic between the parental B6 and SWR strains, we determined the length and location of the B6 donor chromosome fragment. The SW.B6 mice were homozygous for SWR alleles of D6Mit9, D6Mit177, D6Mit36, D6Mit55, D6Mit366, D6Mit150, D6Mit12, D6Mit109, D6Mit57, D6Mit14, and D6Mit201 markers, and they were homozygous for the B6 alleles of D6Mit337, D6Mit13, and D6Mit199 markers. Therefore, the donor fragment is flanked by D6Mit109 [61.4 cM from centromere, Mouse Genome Database (23)] proximally and D6Mit57 (71.1 cM) distally and thus spans less than 9.7 cM of distal chromosome 6 (Fig. 1). The two markers inside the donor fragment and closest to its ends are D6Mit337 (62.5 cM) and D6Mit199 (68.0 cM). The distance of 5.5 cM between these two markers represents the minimum donor fragment size.
Fig. 1.

Haplotype of chromosome 6 in the SW.B6 congenic strain. Chromosomal positions of the markers were obtained from the Mouse Genome Database (23). SW.B6, SW.B6-Soab congenic mouse strain.
Two-bottle tests
The SWR mice strongly avoided 0.1 and 1 mM SOA (i.e., they drank significantly less SOA than water; paired t-tests, P < 0.001), whereas the B6 and SW.B6 mice were indifferent to SOA (Fig. 2A). Correspondingly, preference scores were lower in the SWR mice compared with the B6 and SW.B6 mice; the latter two strains did not differ from each other [effect of strain F(2,21) = 197.9, P < 0.0001].
Fig. 2.

Preference scores of SWR/J inbred (SWR), C57BL/6J inbred (B6), and SW.B6 congenic mice for sucrose octaacetate (SOA, A) and quinine hydrochloride (QHCl, B) in two-bottle tests with water. Values are means ± SE. *Significant difference between SWR and B6, P < 0.05, post-hoc test. +Significant difference between SWR and SW.B6; P < 0.05, post-hoc test.
The SWR mice strongly avoided all concentrations of quinine tested (P < 0.001; Fig. 2B). The B6 and SW.B6 mice were indifferent to 0.01 and 0.03 mM QHCl and avoided 0.1–1 mM QHCl. The SWR mice had lower preference scores than did the B6 and SW.B6 mice for 0.01 and 0.03 mM QHCl [effects of strain, F(2,21) = 23.2, P < 0.0001; concentration, F(4,84) = 53.5, P < 0.0001; and strain × concentration interaction, F(8,84) = 13.2, P < 0.0001], but the latter two strains did not differ significantly at any QHCl concentration.
Electrophysiology
Figure 3 shows sample records of integrated responses of the CT and GL nerves to 1 mM SOA, 10 mM QHCl, and 100 mM NH4Cl. In the SWR mice, both nerves gave robust responses to all three stimuli. In the SW.B6 mice, both nerves showed similarly robust responses to QHCl and NH4Cl but almost no response to 1 mM SOA. We also recorded the CT responses to SOA in four B6 mice and found that there were no detectable responses to 0.01, 0.1, or 1 mM SOA, whereas the nerve responded robustly to sucrose, NaCl, HCl, QHCl, and NH4Cl (data not shown).
Fig. 3.

Sample recordings of integrated responses of the chorda tympani (A) and glossopharyngeal (B) nerves of SWR and SW.B6 mice to 1 mM SOA, 10 mM QHCl, and 100 mM NH4Cl.
Relative responses of the CT nerve to various bitter compounds are shown in Fig. 4. The Soa genotype influenced the CT responses to SOA [strain × concentration interaction: F(2,20) = 11.48, P = 0.0005] but not to any of the other bitter compounds tested. Relative to SWR mice, SW.B6 mice had a significantly reduced CT response to 0.1 mM and 1 mM SOA. There were no differences between SWR and SW.B6 mice in relative CT responses to 300 mM sucrose, 100 mM NaCl, or 10 mM HCl or to the bitter compounds denatonium benzoate, QHCl, strychnine-HCl, PROP, PTC, and MgSO4, although responses to strychnine-HCl tended to be higher in the SW.B6 mice compared with the SWR mice (P = 0.08). Responses to even the highest concentrations of PROP and PTC were small in both strains (less than 17% of the NH4Cl response).
Fig. 4.

Chorda tympani responses (relative to 100 mM NH4Cl) in SWR and SW.B6 mice. PROP, 6-n-propylthiouracil; PTC, phenylthiocarbamide. Values are means ± SE. *P < 0.05, t-tests.
The profile of responses observed in the GL recordings (Fig. 5) was similar to that of the CT, although the relative responses of the GL nerve to bitter compounds generally tended to be higher compared with the CT nerve. The largest difference between the two nerves was observed for responses to SOA in the SWR mice. The Soa genotype influenced the responses of the GL nerve to SOA [strain × concentration interaction: F(2,20) = 11.06, P = 0.0006] but not to any of the other bitter or nonbitter solutions. The SWR mice showed robust responses to SOA solutions, and the responses to all concentrations tested were significantly higher than those of the SW.B6 mice.
Fig. 5.
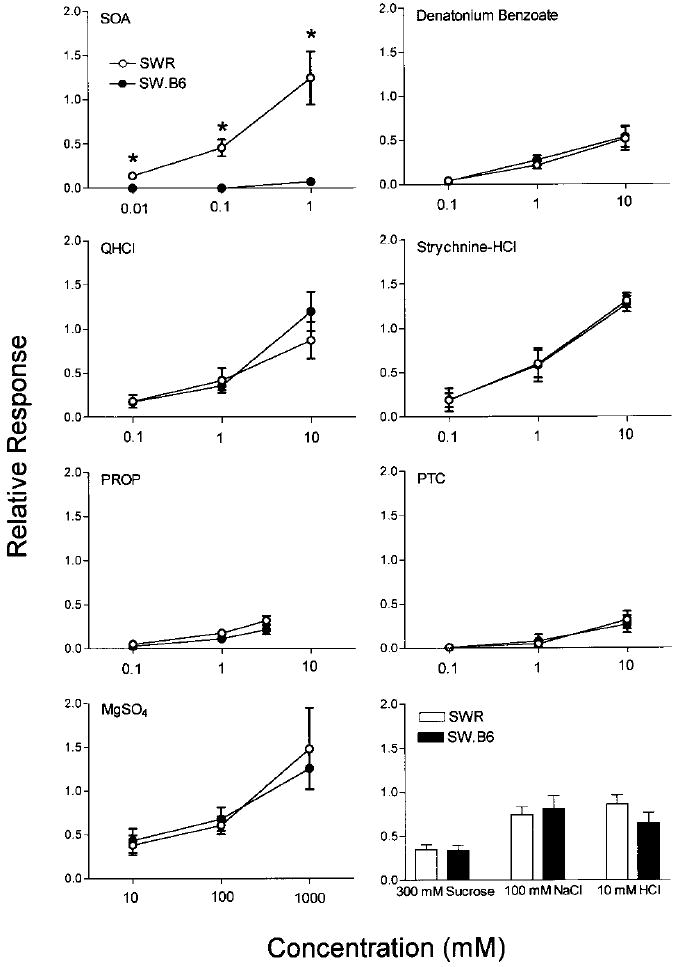
Glossopharyngeal nerve responses (relative to 100 mM NH4Cl) in SWR and SW.B6 mice. Values are means ± SE. *P < 0.05, t-tests.
DISCUSSION
In this study, we determined the size and location of the Soa-containing donor chromosomal fragment of the SW.B6 Soa-congenic strain and provided direct evidence that the Soa genotype selectively affects gustatory neural responding to SOA. We have found that the SW.B6 congenic strain has a 5- to 10-cM long donor fragment of distal chromosome 6 from the B6 strain, which is close to expected size (12). This donor fragment includes D6Mit13, which is a part of the Prp (salivary proline-rich protein) gene closely linked to Soa (2, 9, 20). Although the Prp gene(s) had been considered as a candidate for Soa, a recent transgenic phenotype rescue experiment did not confirm this hypothesis (14). The donor fragment probably also includes some of the recently discovered bitter taste receptor genes, mT2Rs and TRBs, mapped near the Prp locus (1, 22). These receptors are possible candidates for Soa, but this has not yet been proven. Therefore, further studies are needed to identify the Soa gene. One possible strategy is identification of Soa by positional cloning, which is based on the high-resolution genetic mapping (11). Congenic strains have proven to be an efficient tool for fine genetic mapping (21), and thus genomic characterization of the SW.B6 congenic strain is an important step toward positional cloning of Soa.
Results of the two-bottle tests with SOA are consistent with previously published data (16). Prior studies involving Soa-congenic mouse strains demonstrated that allelic variation of Soa affects avoidance of acetylated sugars (including SOA), brucine, denatonium, strychnine, PROP, and isohumulone, but not caffeine, cycloheximide, thiamine, l-phenylalanine, and humulone (7, 16, 28, 29). Sensitivity to quinine is affected by both Soa and at least one other genetically unlinked locus (4, 6, 17). Moreover, in previous studies, the effect of Soa on quinine sensitivity depended on the magnitude of differences between donor and inbred partner strains. C3HeB/FeJ mice had much lower quinine sensitivity than SWR mice, and an effect of Soa on quinine sensitivity was prominent in C3.SW-Soaa congenic mice (7). However, in previous tests, the differences in quinine sensitivity among the B6, SWR, SW.B6-Soab, and B6.SW-Soaa strains were less obvious (16, 29). Our study confirmed that the B6 and SW.B6 mice have higher quinine avoidance thresholds than the SWR mice.
Our electrophysiological results provide the first examination of neural responses to bitter compounds in the SW.B6 mice. We have shown that the SW.B6 mice have lower responses to SOA in the CT and GL nerves compared with the SWR mice. The small responses to SOA of SW.B6 mice must be due to the genetic material introgressed from the donor B6 strain onto the genetic background of the SWR strain, which is consistent with similarly low CT responses to SOA in B6 mice. In a previous study (25), the effect of Soa on neural responses to SOA was investigated by comparing different inbred mouse strains, which vary in many loci other than Soa. The present study using congenic mice provides stronger evidence that the neural responses to SOA are determined by the Soa locus. Thus our data suggest that the effect of Soa on SOA avoidance is mediated by peripheral taste responsiveness involving both the CT and GL nerves. This is consistent with results of a prior study, where SOA stimulated inositol 1,4,5-trisphosphate production in the lingual taste tissue of SOA taster SWR and B6.SW-Soaa mice but not in that of SOA nontaster B6 mice (26), suggesting that Soa affects initial steps of transduction in the taste receptor cells. Together, these electrophysiological and biochemical data indicate that Soa affects taste receptor cells innervated by both the CT and GL nerves.
Further evidence that taste receptor cells, and possibly taste receptors, are the primary site affected by Soa derives from the specificity of the Soa effects. The SW.B6 and SWR mice differed only in neural responses to SOA but not to other bitter (denatonium benzoate, QHCl, strychnine-HCl, PROP, PTC, and MgSO4) and nonbitter (sucrose, NaCl, and HCl) stimuli, which is consistent with previous reports (8, 25). Recent evidence suggests that a single taste receptor cell can express multiple different bitter receptors, each of them being selectively activated by particular bitter compounds (1, 10). Therefore, the selective effect of Soa on peripheral neural responsiveness to SOA is consistent with Soa encoding a SOA-binding bitter receptor. Expression of the bitter receptors in circumvallate, foliate, and fungiform papillae (1) is consistent with our observation that Soa affects responses of both the CT and GL nerves. Afferent responses in the gustatory nerves may depend not only on taste receptors but also on downstream intracellular or synaptic transduction components. However, changes in these downstream elements would probably result in less specific changes in taste responsiveness, such as those observed in gustducin-deficient mice (30).
Although there was a close correspondence between the behavioral and neural data for SOA, this was not the case for other bitter compounds. The Soa genotype is known to affect behavioral avoidance of quinine, PROP, denatonium, and strychnine (7, 16, 28, 29) but had no effect on neural responses to lingual application of these stimuli in our experiment. There are several possible explanations for this discrepancy. First, SOA may bind selectively to a receptor coded by the Soa gene, whereas the other bitter compounds may also activate additional receptors. Thus a variation in the SOA receptor may cause subtle changes in the transduction induced by these other compounds, which would not be detected in multifiber nerve recordings, but would decrease behavioral sensitivity. Also, differences in neural responding to the compounds other than SOA might be present only in a subset of fibers in the CT or GL or in other gustatory nerves. Second, the Soa-containing donor fragment (or a small percentage of the remaining donor genome unlinked to Soa) might contain other genes that influence behavioral responses to these other bitter stimuli through mechanisms that are independent of peripheral gustatory responding, such as central processing or postingestive effects.
In conclusion, in this study we determined that the SW.B6 congenic strain has a 5- to 10-cM long donor fragment of distal chromosome 6 from the B6 strain, which includes the Prp (salivary proline-rich proteins) locus. We found that the Soa genotype influences behavioral avoidance of SOA and quinine and responses of the CT and GL nerves to lingual application of SOA, but not other stimuli.
Acknowledgments
We thank Glayde Whitney and David B. Harder for providing SW.B6-Soab congenic mice and for advising us on their maintenance, Maria Theodorides for breeding and behavioral testing of the mice, and Edwin A. Azen for comments on the manuscript.
This work was supported by National Institutes of Health Grants R03-DC-03853 (to A. A. Bachmanov) and R01-DC-00882 (to G. K. Beauchamp).
Footnotes
Portions of this work were presented at the XXII Annual Meeting of the Association for Chemoreception Sciences (Sarasota, FL, April 2000).
References
- 1.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 2.Azen EA. Linkage studies of genes for salivary proline-rich proteins and bitter taste in mouse and human. In: Wysocki CJ, Kare MR, editors. Genetics of Perception and Communication. New York: Dekker; 1991. pp. 279–290. [Google Scholar]
- 3.Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, Beauchamp GK. Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mamm Genome. 1997;8:545–548. doi: 10.1007/s003359900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boughter JD, Harder DB, Capeless CG, Whitney G. Polygenic determination of quinine aversion among mice. Chem Senses. 1992;17:427–434. [Google Scholar]
- 7.Boughter JD, Whitney G. Behavioral specificity of the bitter taste gene Soa. Physiol Behav. 1998;63:101–108. doi: 10.1016/s0031-9384(97)00398-3. [DOI] [PubMed] [Google Scholar]
- 8.Boughter JD, Whitney G, Contreras RJ. Chorda tympani responses to bitters in inbred and congenic mice (Abstract) Chem Senses. 1996;21:580. [Google Scholar]
- 9.Capeless CG, Whitney G, Azen EA. Chromosome mapping of Soa, a gene influencing gustatory sensitivity to sucrose octaacetate in mice. Behav Genet. 1992;22:655–663. doi: 10.1007/BF01066636. [DOI] [PubMed] [Google Scholar]
- 10.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba N. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 11.Collins FS. Positional cloning: let’s not call it reverse anymore. Nat Genet. 1992;1:3–6. doi: 10.1038/ng0492-3. [DOI] [PubMed] [Google Scholar]
- 12.Flaherty L. Congenic strains. In: Foster HL, Small JD, Fox JG, editors. The Mouse in Biomedical Research. New York: Academic; 1981. pp. 215–222. [Google Scholar]
- 13.Frank ME, Blizard DA. Chorda tympani responses in two inbred strains of mice with different taste preferences. Physiol Behav. 1999;67:287–297. doi: 10.1016/s0031-9384(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 14.Harder DB, Azen EA, Whitney G. Sucrose octaacetate avoidance in nontaster mice is not enhanced by two type-A Prp transgenes from taster mice. Chem Senses. 2000;25:39–45. doi: 10.1093/chemse/25.1.39. [DOI] [PubMed] [Google Scholar]
- 15.Harder DB, Capeless CG, Maggio JC, Boughter JD, Gannon KS, Whitney G, Azen EA. Intermediate sucrose octa-acetate sensitivity suggests a third allele at mouse bitter taste locus Soa and Soa-Rua identity. Chem Senses. 1992;17:391–401. [Google Scholar]
- 16.Harder DB, Gannon KS, Whitney G. SW.B6-Soab non-taster congenic strains completed and a sucrose octaacetate congenic quartet tested with other bitters. Chem Senses. 1996;21:507–517. doi: 10.1093/chemse/21.5.507. [DOI] [PubMed] [Google Scholar]
- 17.Harder DB, Whitney G. A common polygenic basis for quinine and PROP avoidance in mice. Chem Senses. 1998;23:327–332. doi: 10.1093/chemse/23.3.327. [DOI] [PubMed] [Google Scholar]
- 18.Lush IE. The genetics of bitterness, sweetness, and saltiness in strains of mice. In: Wysocki CJ, Kare MR, editors. Genetics of Perception and Communication. New York: Marcel Dekker; 1991. pp. 227–241. [Google Scholar]
- 19.Lush IE. The genetics of tasting in mice. II. Strychnine. Chem Senses. 1982;7:93–98. [Google Scholar]
- 20.Lush IE, Hornigold N, King P, Stoye JP. The genetics of tasting in mice. VII. Glycine revisited, and the chromosomal location of Sac and Soa. Genet Res. 1995;66:167–174. doi: 10.1017/s0016672300034510. [DOI] [PubMed] [Google Scholar]
- 21.Markel P, Shu P, Ebeling C, Carlson GA, Nagle DL, Smutko JS, Moore KJ. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet. 1997;17:280–284. doi: 10.1038/ng1197-280. [DOI] [PubMed] [Google Scholar]
- 22.Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 23.The Jackson Laboratory. Mouse Genome Informatics. Aug 25; [Online]. http://www.informatics.jax.org.
- 24.Reed DR, Bartoshuk LM, Duffy V, Marino S, Price RA. PROP tasting: determination of underlying thresholds distributions using maximum likelihood. Chem Senses. 1995;20:529–533. doi: 10.1093/chemse/20.5.529. [DOI] [PubMed] [Google Scholar]
- 25.Shingai T, Beidler LM. Interstrain differences in bitter taste responses in mice. Chem Senses. 1985;10:51–55. [Google Scholar]
- 26.Spielman AI, Nagai H, Sunavala G, Dasso M, Breer H, Boekhoff I, Huque T, Whitney G, Brand JG. Rapid kinetics of second messenger production in bitter taste. Am J Physiol Cell Physiol. 1996;270:C926–C931. doi: 10.1152/ajpcell.1996.270.3.C926. [DOI] [PubMed] [Google Scholar]
- 27.Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 28.Whitney G, Harder DB. Genetics of bitter perception in mice. Physiol Behav. 1994;56:1141–1147. doi: 10.1016/0031-9384(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 29.Whitney G, Maggio JC, Harder DB. Manifestations of the major gene influencing sucrose octaacetate (SOA) tasting among mice: classic taste qualities. Chem Senses. 1990;15:243–252. [Google Scholar]
- 30.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 31.Yokomukai Y, Cowart BJ, Beauchamp GK. Individual differences in sensitivity to bitter-tasting substances. Chem Senses. 1993;18:669–681. [Google Scholar]


