Abstract
In this issue of Neuron, Bergquist and colleagues study a rapid form of presynaptic homeostatic regulation at the Drosophila neuromuscular junction. They show that the K+ channel genes shal and shaker are reciprocally regulated in the central nervous system and suggest a hierarchical organization of intrinsic and synaptic homeostatic regulatory processes.
Homeostasis (from Greek: ȎμOιOς, homoios, “similar”; and ȉστημι, histemi, “standing still”; defined by Claude Bernard and later by Walter Bradford Cannon in 1929 + 1932) is the property of a system, either open or closed, that regulates its internal environment and tends to maintain a stable, constant condition. Typically used to refer to a living organism, the concept came from that of milieu interieur that was created by Claude Bernard and published in 1865. Multiple dynamic equilibria adjustment and regulation mechanisms make homeostasis possible.
—Wikipedia
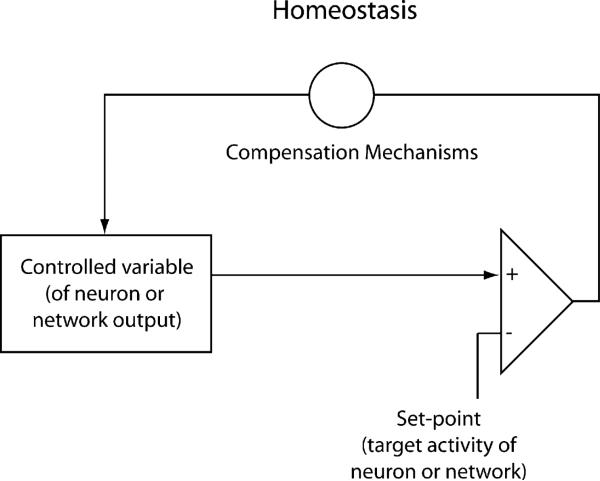
All biologists are familiar with the classical definition of homeostasis, as provided by Wikipedia (above), the source of all knowledge in today's world. As young students, we learn about the importance of the homeostatic regulation of body temperature, blood pressure, osmotic strength, etc. Homeostatic regulation is achieved most commonly with negative feedback control systems that involve some system target value (e.g., room or body temperature), some sensing mechanism (e.g., thermometer or heat-sensitive ion channel), and some mechanism to compensate for deviations between the sensor values and the target values (e.g., heat or air conditioner or perspiration or shivering) (Figure 1).
Figure 1. Cartoon Showing that Homeostasis Is Produced Using a Closed-Loop Negative Feedback.
Sensor reads the system's behavior, and this is compared to the set point with the biological analog of a differential amplifier that detects an error signal. Then either a positive or negative correction is sent back to bring the system values back to the set point.
It is therefore shocking to realize how recently neuroscientists started to concern themselves with the problems of homeostatic regulation of neuronal excitability and network function (LeMasson et al., 1993; Davis, 2006; Turrigiano, 2008; Maffei and Fontanini, 2009). We are now acutely aware of the essential conundrum posed by the fact that neurons in many animals, including humans, live for scores of years, while ion channels and receptors turn over on timescales that might range from minutes to weeks. Consequently, each long-lived neuron is faced with the task of renewing its membrane complements of ion channels and receptors many times during its lifetime. Likewise, in long-lived animals, the neuronal circuits that allow stable behavior over the lifetime of the person or animal must be constructed during development and their functional integrity must be preserved while permitting circuit modifications in the service of learning (Marder and Goaillard, 2006; Turrigiano, 2008; Maffei and Fontanini, 2009; Zhang et al., 2009).
Over the years a number of laboratories have studied the homeostatic regulation of both intrinsic neuronal excitability and synaptic strength (Turrigiano, 2008), and clearly both kinds of mechanisms, separately and together, contribute in times and places to achieving network stability (Pratt and Aizenman, 2007; Maffei and Fontanini, 2009). Just as all network dynamics depend on the interaction of neuronal intrinsic properties and synaptic strengths, homeostatic regulation of intrinsic neuronal properties and synaptic strength must be well-matched for appropriate circuit function and normal behavior. If, as we suspect, there are multiple homeostatic mechanisms called into play in maintaining behavioral integrity, it is an important challenge for the future to understand how these are coordinated. The paper by Bergquist et al. (2010) in this issue argues for a hierar chical interaction between an intrinsic homeostatic neuronal mechanism and a local synaptic mechanism at the larval Drosophila neuromuscular junction.
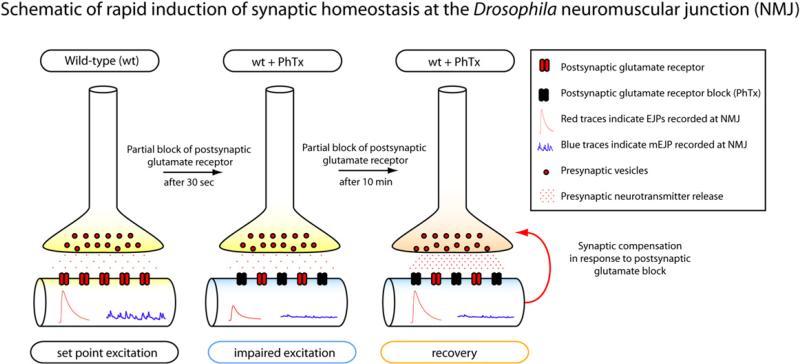
While many studies of homeostatic regulation have focused on relatively slow processes that would tend to maintain stable function over the lifetime of the animal, the Davis laboratory defined a rapid form of synaptic homeostatic regulation in which short-term block of the postsynaptic receptors produces a set of presynaptic changes that rapidly compensate for the pharmacological block (Frank et al., 2006; Dickman and Davis, 2009; Frank et al., 2009) (Figure 2). In this issue, Bergquist et al. (2010) exploit this preparation and paradigm to study the relationship between the presynaptic regulation of several K+ channel genes and the amplitude of the postsynaptic responses. In so doing, they reveal that several different homeostatic mechanisms are implicated in the control of the function of these neuromuscular junctions.
Figure 2. Schematic of Rapid Induction of Synaptic Homeostasis and Compensation at the Neuromuscular Junction of Drosophila.
(Left) Under normal conditions, the NMJ displays large evoked excitatory junctional potentials (EJP; red traces) and miniature excitatory junctional potentials (mEJP; blue traces). (Middle) Application of postsynaptic glutamate antagonist (philanthotoxin, PhTx) initially causes reduction in both EJP and mEJP amplitude at the NMJ. (Right) After 10 min in the presence of PhTx, EJP amplitude increase to baseline values while mEJP amplitude remains suppressed. Enhancement of presynaptic neurotransmitter release is responsible for this compensatory increase in EJP amplitude.
This story started with a genetic screen to look for mutations that would disrupt the recovery of synaptic transmission after postsynaptic blockade (Dickman and Davis, 2009). Surprisingly, mutations in three different K+ channel genes block the homeostasis triggered in response to postsynaptic blockade (Dickman and Davis, 2009), and this finding becomes the starting point for Bergquist et al. (2010).
Antibody staining (Baro et al., 2000) for the Shal protein showed it to be present on the initial portion of the motor neuron axons but not at the neuromuscular junction. The shal mutants showed a mild deficit in baseline transmission (a modest decrease in evoked EPSP). This is counterintuitive because one might expect that decreased Shal abundance would broaden the action potential and thus increase the evoked EPSP. Surprisingly, the shal mutant appeared to block both rapid and sustained expression of synaptic homeostasis. This is perplexing because rapid induction of synaptic homeostasis was shown to occur independently of motor neuron activity (Frank et al., 2006). The natural question that arises is why shal mutants should block a synapse-specific process that is not dependent on the activity of the motor neuron itself when the protein is expressed only in the proximal segments of the motor neurons.
One possibility is that the loss (or over-expression) of individual ion channels can initiate compensatory changes in the expression of other ion channels (MacLean et al., 2003; Swensen and Bean, 2005; Marder and Goaillard, 2006). To determine if this form of “cell-intrinsic” homeostatic compensation occurs in Drosophila motor neurons, Bergquist et al. (2010) measured shal and shaker mRNA expression (both encode IA) using qPCR in extracts of the larval central nervous systems.
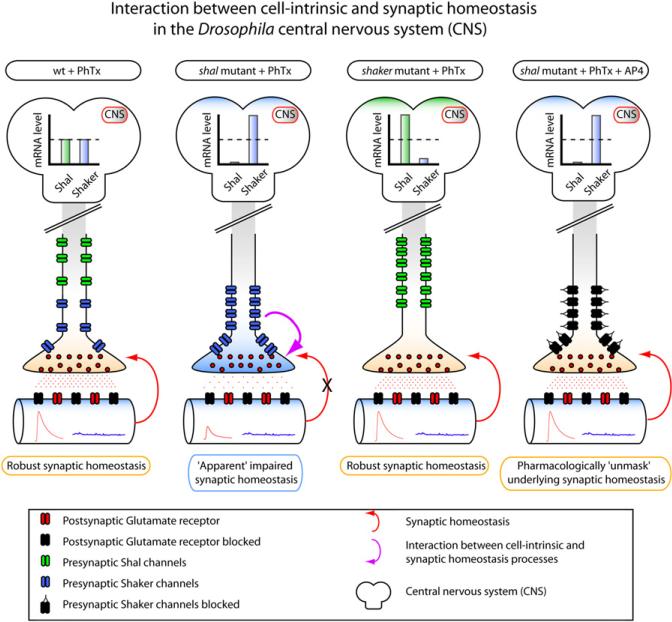
Intriguingly, the authors found an increase in shaker expression in the shal mutant. Conversely, shal expression was dramatically upregulated in the shaker mutant, demonstrating reciprocal regulation of two of the genes that contribute to the IA current (Figure 3). If we assume that this reciprocal regulation seen at the level of the entire central nervous system is also seen at the level of the motor neurons, then this would have important consequences for transmitter release.
Figure 3. Interaction between Cell-Intrinsic and Synaptic Homeostatic Processes and Homeostatic Coupling of IA Channel Expression in Drosophila.
(First panel) Wild-type response to PhTx shows robust synaptic compensation after 10 min of PhTx. (Second panel) The Shal mutant apparently impairs synaptic homeostasis. The loss of the Shal channel is accompanied by compensatory upregulation of shaker mRNA in the CNS. (Third panel) Homeostatic reciprocal regulation of two potassium channels encoding IA. Shaker mutants do not display deficits in synaptic homeostasis but do show substantial compensatory upregulation of shal mRNA in the CNS. (Fourth panel) Pharmacological unmasking of underlying synaptic homeostasis. Application of AP-4 (a specific antagonist of the Shaker channel) restores synaptic homeostasis, demonstrating that upregulation of shaker does not block the induction synaptic compensation. Rather, the Shaker channel blocks the apparent expression of synaptic homeostasis by presumably acting as a shunt that blocks increased presynaptic neurotransmitter release.
The Shaker protein is normally found in the motor axon and presynaptic nerve terminals. In shal mutants, this would result in upregulation of presynaptic Shaker, which one would predict would decrease synaptic release. This could explain the apparent failure in synaptic homeostasis and also account for the reduced baseline transmission in the shal mutant. To further solidify this hypothesis, the authors show in a variety of ways (shaker mutations, overexpression, RNAi, and pharmacology) that it is the compensatory upregulation in presynaptic Shaker that is responsible for the impairment in synaptic homeostasis. Importantly, the acute pharmacological inhibition of Shaker with 4-AP restores synaptic homeostasis (Figure 3). From this, the authors argue that the upregulation of Shaker in the motor neuron “masks” the expression of synaptic homeostasis but does not block its induction mechanisms (Figure 3).
These results are fascinating in many ways. First, these data demonstrate clearly that there are at least two independent homeostatic signaling systems acting at two different loci within the nervous system, and likely within the same neuron. Although this presumably must always be the case, as mechanisms of synaptic scaling (Turrigiano, 2008) must always be kept in register with mechanisms of intrinsic neuronal plasticity (O'Leary et al., 2010), the interaction of multiple mechanisms is elegantly revealed in these experiments. Second, these data indicate that the functional outcome of the synaptic homeostasis is contingent on whether or not the shaker/shal intrinsic homeostatic mechanisms have been engaged. Davis and his colleagues argue that this represents a hierarchy of homeostatic signaling systems (Bergquist et al., 2010) and that this may have ramifications for how we view neuronal homeostatic systems and their possible implications in certain neurological pathologies and their treatments.
At face value, the “hierarchy” of mechanism suggested by these results leaves us with an apparently disconcerting out come; intrinsic homeostatic mechanisms can sometimes leave the final output at the muscle uncompensated. But, we are missing a critical piece of information, namely, what the firing patterns of the motor neurons would be in a more intact physiological preparation in each of the conditions used in the study. Without this, it is difficult to evaluate what the actual target output for the homeostatic regulation is likely to be. This is not a criticism of the present paper, but a more general statement of how difficult it can be to know whether the target for homeo-static regulation is at the single neuron or circuit level, and how then to evaluate it. As the authors point out, the mechanisms described in Bergquist et al. (2010) could also be called into play somewhat differently under longer, developmental time-scales. Of course, the issue of which timescales are relevant to neuronal homeostasis may differ in short-lived animals such as worms and flies when compared to animals that live for many years, such as lobsters and humans. And certainly, there must be many additional mechanisms that contribute to long-term stability in adult nervous systems, some of which might be quite different from those revealed in developing neuromuscular system, or for that matter, in immature cortex. Nonetheless, as alluded to above, one of the big open questions in this ever-growing field is whether the target of homeostasis is the activity of single neurons or an entire circuit.
REFERENCES
- Baro DJ, Ayali A, French L, Scholz NL, Labenia J, Lanning CC, Graubard K, Harris-Warrick RM. J. Neurosci. 2000;20:6619–6630. doi: 10.1523/JNEUROSCI.20-17-06619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist S, Dickman DK, Davis GW. Neuron. 2010;66(this issue):220–234. doi: 10.1016/j.neuron.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW. Annu. Rev. Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Davis GW. Science. 2009;326:1127–1130. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Pielage J, Davis GW. Neuron. 2009;61:556–569. doi: 10.1016/j.neuron.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasson G, Marder E, Abbott LF. Science. 1993;259:1915–1917. doi: 10.1126/science.8456317. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Neuron. 2003;37:109–120. doi: 10.1016/s0896-6273(02)01104-2. [DOI] [PubMed] [Google Scholar]
- Maffei A, Fontanini A. Curr. Opin. Neurobiol. 2009;19:168–173. doi: 10.1016/j.conb.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Nat. Rev. Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- O'Leary T, van Rossum MC, Wyllie DJ. J. Physiol. 2010;588:157–170. doi: 10.1113/jphysiol.2009.181024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. J. Neurosci. 2007;27:8268–8277. doi: 10.1523/JNEUROSCI.1738-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. J. Neurosci. 2005;25:3509–3520. doi: 10.1523/JNEUROSCI.3929-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Khorkova O, Rodriguez R, Golowasch J. J. Neurophysiol. 2009;101:372–386. doi: 10.1152/jn.01290.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]