Abstract
In 2005, results from the Arimidex, Tamoxifen Alone or in Combination (ATAC) trial ushered in a new era of endocrine therapy for hormone-responsive malignancies. This study demonstrated that, compared to tamoxifen (a selective estrogen receptor modulator [SERM]), anastrozole (aromatase inhibitor [AI]) prolonged time to recurrence and disease-free survival for postmenopausal women with breast cancer. The advantage was even greater for those with estrogen receptor-positive (ER+) tumors, and anastrozole was better tolerated than tamoxifen. Since then, AIs have become first-line adjuvant therapy for ER+ breast cancer in postmenopausal women.
In late 2010, a trial comparing abiraterone acetate (a 17-hydroxylase/17,20-lyase [CYP17A1] inhibitor) plus prednisone versus prednisone alone in men with castration resistant-prostate cancer (CRPC) previously treated with docetaxol chemotherapy was terminated early due to the survival benefit in the abiraterone acetate arm. This result not only validated a new therapy for CRPC but, with the antecedent phase I-II abiraterone studies, shattered our understanding of the molecular mechanisms underpinning CRPC development and progression.
AIs and CYP17A1 inhibitors will be widely used by oncologists, yet fellowship programs provide little training in steroid biosynthesis, compared with training in the biology of standard chemotherapies. Consequently, these drugs might be used without an appreciation of their caveats and pitfalls. The purpose of this review is to acquaint practicing oncologists with the fundamental principles and pathways of steroid biosynthesis, to improve their understanding of how and why these drugs work, and to alert these physicians to potential problems related to the drugs’ mechanisms of action.
Introduction
The hormonal dependence of breast and prostate cancers has been exploited for decades as a therapeutic strategy. Dramatic and lifesaving tumor regression or stabilization was first achieved with surgical oophorectomy in breast cancer 1 and orchiectomy in prostate cancer 2, but recurrences were common despite elimination of all gonad-derived androgens or estrogens, respectively. Recognizing that the adrenal glands provides a source of androgen precursors, surgical adrenalectomy was added to ovariectomy for breast cancer 3, and later chemical adrenalectomy with aminoglutethimide proved equivalent 4. Efforts to provide more complete and less invasive hormone ablation strategies in prostate cancer led to the use of estrogens such as diethylstilbestrol (DES) and progestins 5, later replaced by long-acting gonadotropin-releasing hormone (GnRH) agonists such as leuprolide acetate 6. Androgen receptor (AR) antagonists such as bicalutamide and nilutimide provided the next contribution to therapy in prostate cancer 7, whereas in breast cancer, the anti-estrogen tamoxifen, which is actually a selective estrogen receptor modulator or SERM, became the reigning standard of care for many years 8 until dethroned by the aromatase inhibitors (AIs) in the past decade 9. Abiraterone acetate, a 17-hydroxylase/17,20-lyase (CYP17A1) and thus androgen biosynthesis inhibitor, has been recently approved for the treatment of castration resistant-prostate cancer (CRPC) 10. Consequently, the evolution of treatment has generally progressed from surgery to receptor antagonists to hormone synthesis inhibitors. Since these therapies should theoretically all achieve the same purpose, why are the enzyme inhibitors gaining favor over time?
To understand how these drugs work and why they might provide more complete hormone ablation than other therapies, a basic understanding of steroid biosynthesis is essential. Unfortunately, oncology training programs rarely provide more than a rudimentary introduction to steroidogenesis in their curricula. In this article, we will develop a logical and clinically relevant framework to understand steroid biosynthesis in normal and pathologic states. These basic principles will then illustrate why these drugs are more effective in some patient populations than in others, when these drugs should be combined with other agents, and how resistance develops. A comprehensive review of human steroid biosynthesis has recently appeared 11, and the reader is directed to this reference for any details not covered herein.
The big picture
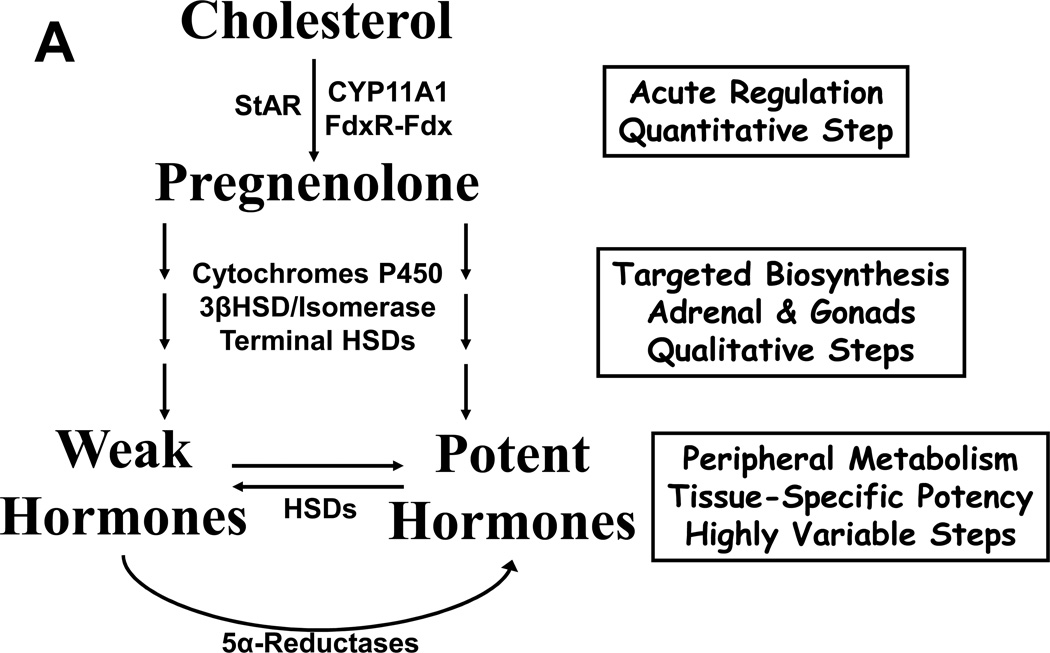
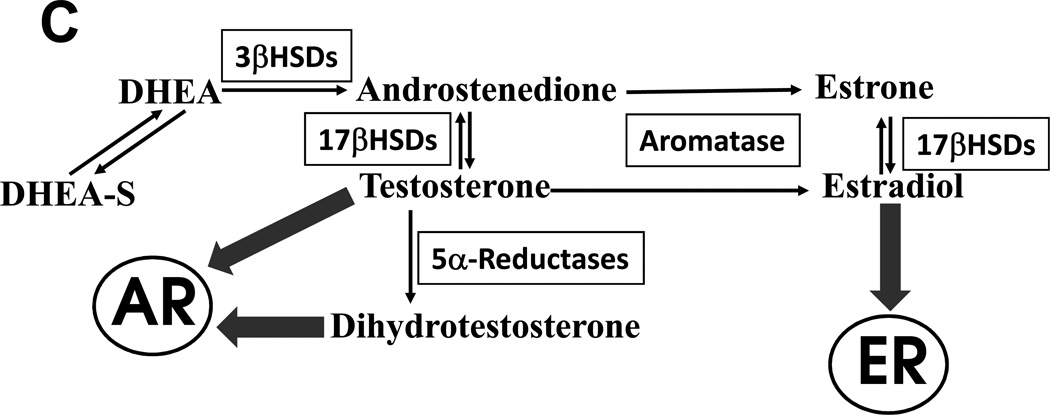
All steroid hormones derive from cholesterol, and in normal human physiology, only a few tissues convert 27-carbon cholesterol to 21-carbon pregnenolone, the first committed intermediate in steroidogenesis. The adrenal cortex, Leydig cells of the testis, theca and granulosa cells of the ovary, and trophoblasts of the placenta are the only cells in the body capable of synthesizing enough pregnenolone to contribute to circulating concentrations of steroids 12. Subsequent conversions within the steroid-producing cells yield either active hormones or hormone precursors, and the exact products depend on which downstream enzymes are expressed in those cells (Figure 1A,B). These steroids enter the circulation to act on target cells, but extensive metabolism occurs outside the steroidogenic tissues. Consequently, circulating concentrations of steroids only partially reflect their biological activity, and different cells each experience a unique hormonal milieu depending on the balance of enzymes and other factors present in each cell 13. For example, circulating testosterone (T) is actually three potential hormones. T binding to AR elicits androgenic effects in some tissues such as spermatic tubules, stimulating sperm production. In the prostate, 5α-reductase irreversibly converts T to dihydrotestosterone (DHT), which unlike T uniquely mediates formation of the prostate and the male external genitalia in fetal life, as well as development of prostatic hyperplasia 14. Conversely, aromatase activity in breast tissue metabolizes T to the potent estrogen estradiol (E2), which if excessive causes gynecomastia (Figure 1C).
FIGURE 1.
A, A conceptual model for steroid biosynthesis. Classical steroid production begins with a tropic stimulus, which drives the conversion of cholesterol to pregnenolone and maintenance of the downstream enzymes in the steroidogenic cell. Peripheral tissues and target cells further metabolize these steroids. B, Major steroidogenic pathways in human beings. Enzymes are indicated above and aside arrows, and main product steroids of the adrenals, testis, and ovary are underlined. Thin line from 17-hydroxy-(17OH)-progesterone to androstenedione indicates minor pathway. C, Canonical terminal steps of T synthesis from DHEA(S) and fates of T in different tissues. T acts directly on AR or indirectly after metabolism to DHT, and aromatase changes the biological activity of T via metabolism to E2.
The first step is the hardest
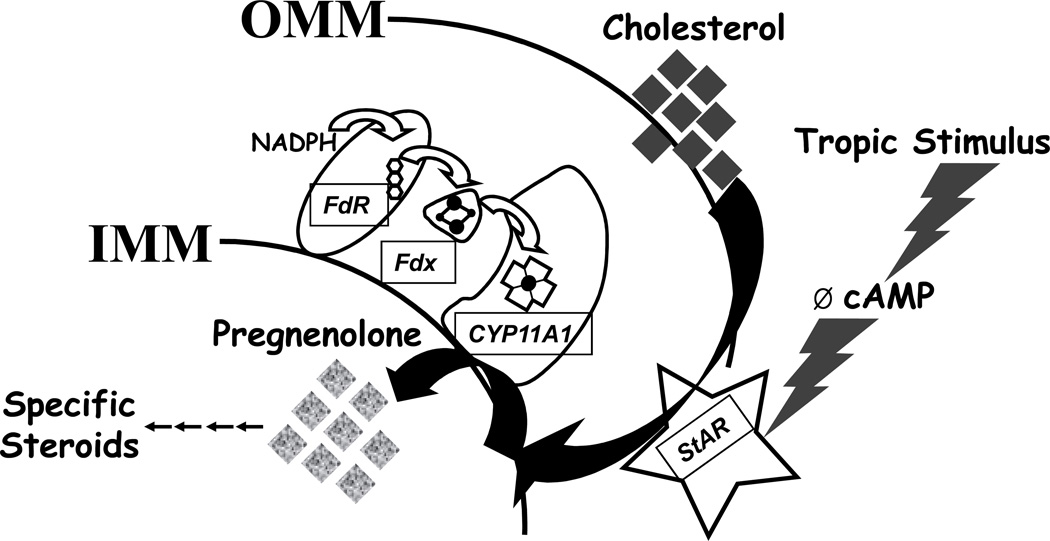
The conversion of cholesterol to pregnenolone is generally the slowest, most complex, and acutely regulated step in steroid production 15. The side chain cleavage enzyme (P450scc or CYP11A1) uniquely catalyzes this transformation, which really involves three chemical steps, at a maximum rate of roughly 7 turnovers per minute, pathetically slow for an enzyme. In addition, access of cholesterol to CYP11A1 is restricted topologically within the specialized mitochondria of steroidogenic cells. The mobile pool of cholesterol capable of entering steroid biosynthesis resides on the outer mitochondrial membrane, whereas CYP11A1 and its cofactor protein ferredoxin reductase (FdxR) are attached to the inner mitochondrial membrane (Figure 2). In the mitochondrial matrix, electrons from reduced nicotinamide adenine dinucleotide phosphate (NADPH) channel from FdxR to a soluble iron-sulfur protein ferredoxin (Fdx) and then to CYP11A1. This reaction uses two electrons and one molecule of oxygen in each of three cycles to cleave cholesterol to pregnenolone. Every pulse of luteinizing hormone (LH) yields a corresponding pulse of T via the induction of intracellular cyclic adenosine monophosphate (cAMP), which “opens the gate” for cholesterol entry to the steroidogenic pathway. The familiar cosyntropin stimulation test also elicits a sharp rise in cortisol in 30 min, due to cAMP elevation and cholesterol mobilization. How does this rapid mobilization of cholesterol occur?
FIGURE 2.
Initiation of steroid biosynthesis in the mitochondria via StAR and CYP11A1. Stimulated by increased cAMP, StAR induces the movement (large solid arrow) of cholesterol in the outer mitochondrial membrane (OMM) to the inner mitochondrial membrane (IMM), where FdR and CYP11A1 reside. Electrons from NADPH flow (open arrows) to the flavin (three hexagons) in FdR, to the iron-sulfur cluster (dots and lines) in the soluble mitochondrial matrix protein Fdx to the heme (Maltese cross and dot) of CYP11A1. These electrons plus molecular oxygen enable CYP11A1 to convert cholesterol to pregnenolone (small solid arrow) in three cycles, and pregnenolone is then liberated into pathways, which lead to specific steroids (cortisol, T, etc.).
The protein that drives cholesterol from the outer to inner mitochondrial membranes is the steroidogenic acute regulatory (StAR) protein 16 (Figure 2), whose mechanism of action is incompletely understood. The x-ray crystal structure of the StAR homolog MLN64 contains a binding pocket for one molecule of cholesterol 17, suggesting that StAR physically carries cholesterol across the intermembranous space; however, other evidence suggests that StAR acts while residing on the outer membrane 18. StAR phosphorylation is required for the activation and termination of its action 19, reflective of its transient nature. Some steroidogenic tissues such as the placenta do not express StAR yet still produce abundant steroids, possibly using MLN64 instead. In model cells, StAR co-expression increases pregnenolone production 7-fold above that observed using the CYP11A1 catalytic system alone 20.
Conceivably, additional tissues, including cancer cells, might convert cholesterol to pregnenolone, and evidence supporting this mechanism of T synthesis in CRPC progression has been found in some 21,22 but not all studies 23. Nevertheless, for a cell to convert cholesterol to pregnenolone, the minimum requirements include expression of CYP11A1, FdxR, and Fdx; sufficient cholesterol, oxygen, and NADPH content in a permissive mitochondrial environment; and ideally StAR and its phophorylation machinery. Of course, the quantities of pregnenolone synthesis required for local de novo T production sufficient to stimulate CRPC growth are many orders of magnitude lower than that required to raise circulating steroid concentrations, challenging the limits of detection by modern assay methods.
Steroidogenesis is directional
An important concept in steroid biosynthesis is that most steps are irreversible and that classes of endogenous steroid hormones are defined both by their biologic activities and their chemical structures. These structures, in turn, are determined by the activities of those enzymes catalyzing their biosynthesis, and the nomenclature is mercifully helpful for the novice in most cases. Most of these enzymes are cytochromes P450, and their naming is thus CYP followed by number, letter, and number (CYP17A1, CYP3A4, etc) 24. In addition, the 5α-reductase reactions are irreversible (Figure 1B).
The terminal steps of steroidogenesis and the peripheral conversions are mediated by the many hydroxysteroid dehydrogenases (HSDs) (Figure 1A,B), and these reactions are reversible, although each enzyme has a strong directional preference in intact cells 25,26. Thus, pairs of enzymes drive steroid flux in opposite directions, one from hydroxysteroid to ketosteroid and another back from ketosteroid to hydroxysteroid. An example is 17βHSD3, the critical enzyme for converting androstenedione (AD) to T in Leydig cells. Boys with 17βHSD3 deficiency are born with ambiguous genitalia from T and DHT deficiency in utero 27. Conversely, 17βHSD2 oxidizes T almost completely to AD in peripheral tissues, attenuating the androgenic activity of T in cells containing this enzyme 28. One caveat regarding the reversibility of these transformations is that the two 3βHSD-isomerase enzymes 29, by virtue of their second (Δ5→Δ4-isomerase) activity, also mediate irreversible reactions (Figure 1C). The many human HSD enzymes are each encoded by separate genes with characteristic tissue-specific expression profiles, catalytic activities, and substrate preferences 30. The function of only a few enzymes are known with certainty, particularly for those expressed in peripheral tissues, and many of these enzymes are poor catalysts for HSD reactions. Consequently, a cancer cell might contain several 17βHSD isoforms competing for steroid substrates, and the final biologic potency of an entering steroid will be determined by the net result of these activities.
Another mechanism of terminating steroid action is conjugation, similar to the “Phase II” conjugation reactions of drug metabolism, including glucuronidation and sulfation. For steroids, free hydroxyl groups are sulfated or glucuronidation by sulfotransferases (SULT) and uridine diphosphate glucuronyltransferase (UGT) enzymes, respectively, which also have a number, letter, and number nomenclature (SULT2A1, UGT2B17) 31. The UGT enzymes include UGT1A1, a major bilirubin conjugating enzyme; low UGT1A1 activity causes Gilbert’s disease and predisposes to irinotecan toxicity 32. Glucuronylation is generally an irreversible process, which tags the steroid for excretion in the urine by organic anion transporters, whereas sulfation increases protein binding and prolongs circulating half-life. At least some steroid sulfates are desulfated in specific tissues, such as estrone sulfate conversion to estrone in breast cancer cells 33.
You can go your own way
The classical pathways have been constructed based on the dominant enzyme activities and hormone concentrations observed in normal adrenal and gonadal tissues, reinforced by changes in flux observed in enzyme deficiency states. Nevertheless, minor pathways also exist, but we hide these “under the rug” since their contributions are insignificant during normal physiology. For example, 21-deoxycortisol (progesterone hydroxylated in the 17- and 11-positions) is made in the normal adrenal but is not part of a mainstream pathway and generally not measured by clinical laboratories. Elevated 21-deoxycortisol, however, is a more specific marker steroid for 21-hydroxylase deficiency than the customary 17-hydroxyprogesterone 34, and 21-deoxycortisol is used as a second-tier newborn screening test in some states.
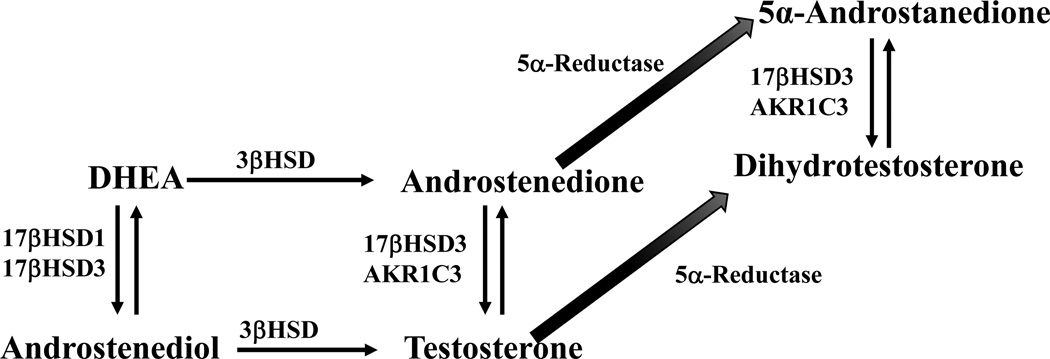
In addition, more than one pathway might converge at the same product. While pathway A might be the major route to steroid X in tissues whose primary job is to export X as a circulating hormone, other tissues might use pathway B or C. How is DHEA converted to T and DHT, as must occur to explain hirsutism in women? Well, textbooks usually say DHEA → AD → T → DHT (enzyme sequence 3βHSD, 17βHSD, SRD5A), with these reactions occurring mainly in liver and skin. There is no reason why DHEA should not be converted to androst-5-ene-3β,17β-diol (A5diol) via 17βHSD first and then by 3βHSD to T, and this sequence is probably dominant in the normal testis 35 (Figure 1B). Furthermore, we have recently shown that CRPC specimens and cell lines predominantly use the sequence DHEA → AD → 5α-androstane-3,17-dione (5αdione) → DHT (enzyme sequence 3βHSD, SRD5A1, 17βHSD), entirely bypassing T 36 (Figure 3). Even more bizarre, a complex alternate or “backdoor” pathway to DHT in the tammar wallaby pouch young testis involves 5α-reduction of 17-hydroxyprogesterone prior to the 17,20-lyase reaction of CYP17A1 37,38. In this pathway, the testis exports the 5α-reduced androgens androsterone and 5α-androstane-3α,17β-diol, that latter of which is oxidized to DHT in the target tissues to mediate male prostate and genital differentiation in that species 39. The lesson is that alternate routes might bypass blocks in classical pathways, as long as enzymes capable of catalyzing these reactions are expressed in the tissue and cells of interest.
FIGURE 3.
Termninal steps of androgen biosynthesis from DHEA. In this pseudo-three-dimensional diagram, reactions to the right (3βHSD) and to the back (5α-reductase) are irreversible, whereas vertical reactions are reversible and catalyzed by several 17βHSD enzymes. Flux from DHEA to DHT might follow several pathways, and an individual steroid molecule might cycle vertically many times before finally moving through the 3βHSD and/or 5α-reductase reactions.
Feedback mechanisms or lack thereof
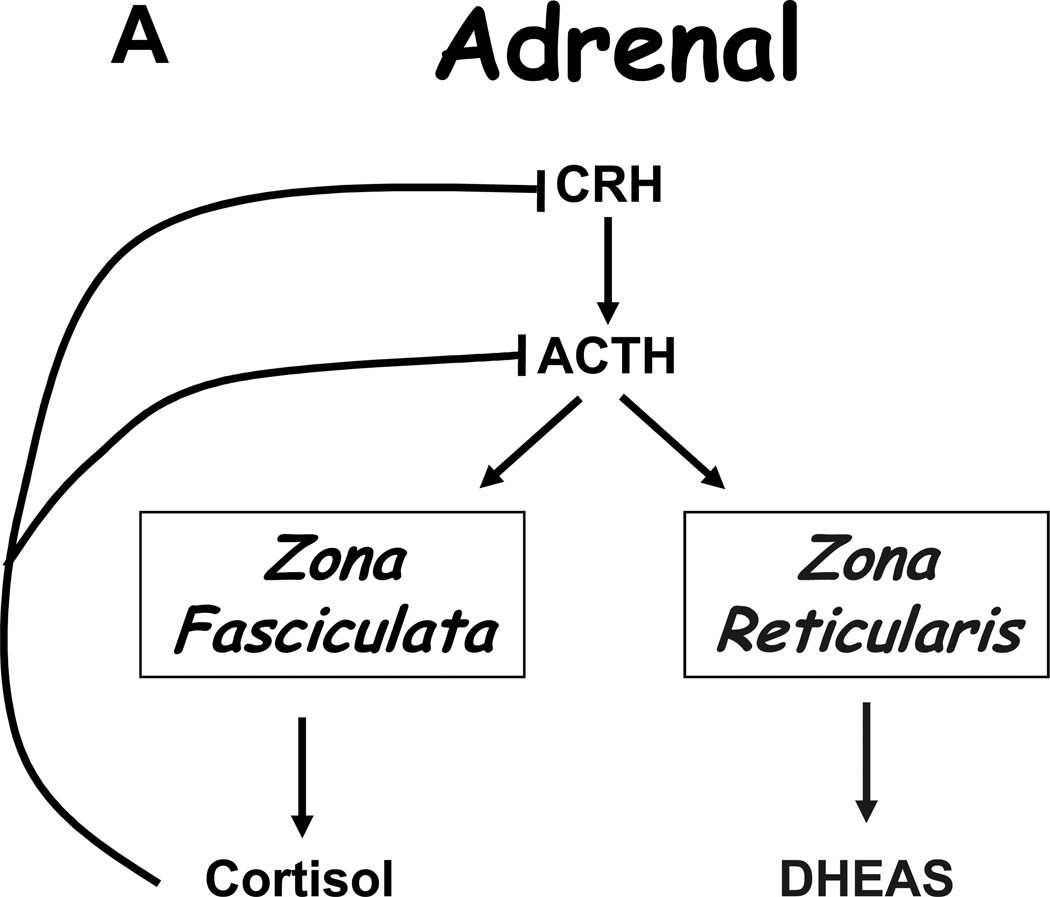
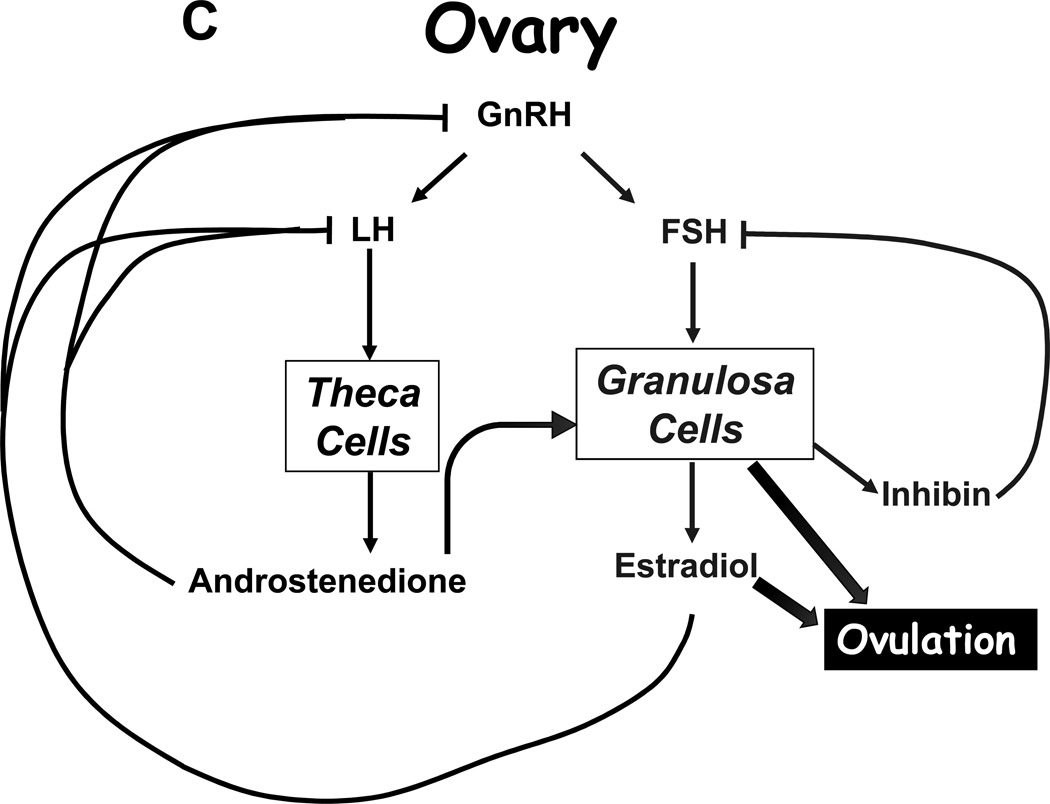
Under normal circumstances, tropic hormones regulate steroid production, and the product steroid then suppresses its own production in a classical negative feedback loop. In the adrenal, adrenocorticotropin (ACTH) stimulates production of cortisol and dehydroepiandrosterone sulfate (DHEAS), yet only cortisol provides negative feedback on ACTH (Figure 4A). Similarly, luteinizing hormone (LH) stimulates androgen production by the Leydig cells of the testis (mainly T, Figure 4B) and theca cells of the ovary (mainly AD), and these androgens suppress LH production. In the ovary, follicle-stimulating hormone (FSH) acts on the granulosa cells surrounding the developing oocyte to induce aromatase production, thus allowing conversion of AD and T to E2 (Figure 4C), and FSH also induces small amounts of E2 production in the testes. E2 and even progesterone also suppress LH production, whereas FSH negative feedback is mainly due to inhibin B, which is a protein hormone derived from the Sertoli cells of the testes and granulosa cells of the ovary.
FIGURE 4.
Hypothalamic-pituitary-target gland axes for the A, adrenal; B, testis; and C, ovary. Block arrows indicate actions of hormones, and flat end to lines indicate negative feedback. Curved block arrow in panel C indicates that androstenedione from the theca cell diffuses to the granulosa cell, where aromatization occurs.
Based on the paradigms described, damage to the pituitary (tumor, trauma, radiation) will lower LH, FSH, and ACTH production and therefore reduce production of T, E2, cortisol, DHEAS, and all the precursor steroids and metabolites as well. Surgical removal of the adrenal glands raises ACTH, which can be lowered with exogenous cortisol replacement. Gonadectomy will cause LH and FSH to rise, and androgen or estrogen replacement lowers LH but not FSH, since inhibin B remains absent. Similarly, natural menopause occurs when all follicles and thus granulosa cells are depleted, and estradiol production falls while LH and more specifically FSH rise. Long-acting GnRH agonists act by desensitizing the pituitary gonadotropes and suppressing LH (and FSH) and therefore T and E2 synthesis. Supraphysiologic doses of exogenous androgens and estrogens or progestins also lower LH, T, and E2 production—but not FSH—but directly exert hormone actions on the body. For example, high-dose DES, a potent estrogen, is used to treat prostate cancer by increasing negative feedback on the gonadal axis, which suppresses LH and thus T production. The estrogenic action of DES, however, causes gynecomastia in a high proportion of patients and predisposes to thrombotic events, limiting its utility. Similarly, dexamethasone or prednisone suppress ACTH and thus both cortisol and DHEAS synthesis. Steroid receptor antagonists relieve the negative feedback of these hormones and cause a rise in the tropic hormones from the pituitary and subsequently the endogenous steroids themselves.
T and DHT in prostate cancer
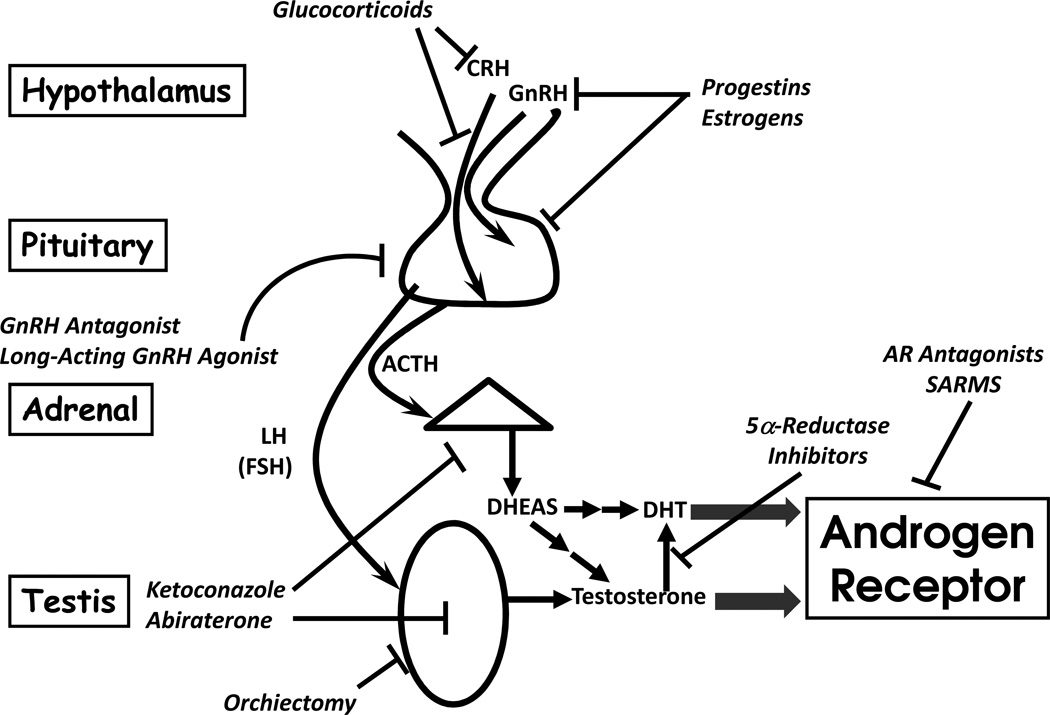
Based on the preceding discussion, several strategies to block T and DHT synthesis and/or action can be envisioned, and most of these have already found application in clinical practice (Figure 5). Surgical orchiectomy, DES, leuprolide acetate, and bicalutamide are all successful examples of different strategies to block testicular T production or action, and dutasteride inhibits conversion of T to DHT by 5α-reductase enzymes. The problem with AR antagonists is that most are partial agonists or incomplete antagonists, and up-regulation of LH and thus T might overcome the blockade, prompting the development of more potent compounds such as MDV3100 40. Gonadal suppression strategies fail to address the abundant DHEAS and DHEA derived from human adrenal gland, which are only 3 or 2 steps upstream of T (Figure 3), with redundant pathways mediating these transformations 13. Adrenal androgen elimination in CRPC probably explains the additional therapeutic benefit of ketoconazole 41, a CYP17A1 inhibitor, and dexamethasone or prednisone, which suppress ACTH and thus adrenal DHEA(S) production 42.
FIGURE 5.
Endocrine therapy strategies for prostate cancer, which all inhibit androgen production or antagonize the androgen receptor. Flat end to lines indicate site of action on process or tissues, including hypothalamus and pituitary (top), adrenal (triangle in middle), and testis (oval at bottom). Block arrows indicate receptor activation.
Similarly, abiraterone is a potent and specific CYP17A1 inhibitor, explaining its recent success in treating CRPC 10,43,44. Despite its ideal molecular target and potency, abiraterone is not used as monotherapy. Like AR antagonists, inhibition of T synthesis will remove negative feedback and increase LH production in an effort to restore homeostasis. The mechanism of abiraterone action involves irreversible binding to the heme iron in the active site of CYP17A1, so inhibition continues even after the drug has been completely cleared from the circulation, until new enzyme is synthesized. Consequently, abiraterone is used with GnRH agonist, to maintain LH suppression and prevent the synthesis of new CYP17A1 protein in the testis. When studied in noncastrate men with prostate cancer, abiraterone alone lowers T only transiently to castrate levels 45, and therefore abiraterone is unlikely to be effective as monotherapy in prostate cancer.
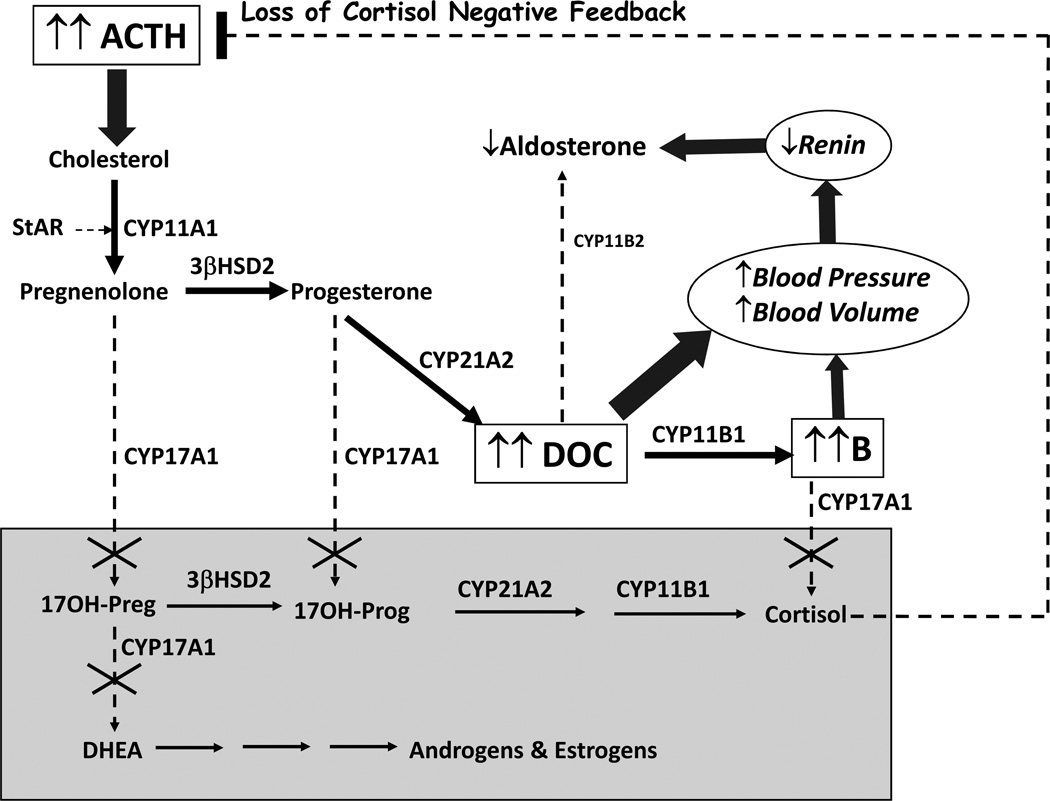
In the pivotal abiraterone trial, all participants received prednisone 5 mg twice daily as well, which was generally started during docetaxol treatment. Since abiraterone also ablates adrenal CYP17A1 activity, prednisone might seem unnecessary for efficacy, but CYP17A1 inhibition also blocks cortisol synthesis and induces a syndrome of 17-hydroxylase deficiency 46,47 (17OHD, Figure 5). Biglieri described the clinical and biochemical consequences of 17OHD, a rare form of congenital adrenal hyperplasia, nearly a half-century ago 48. Loss of negative feedback from cortisol deficiency causes ACTH to rise, activating StAR, driving cholesterol into steroidogenesis, and flooding the available downstream enzymes with pregnenolone (Figure 6). The block at CYP17A1 leaves only pathways involving 3βHSD2, 21-hydroxylase (CYP21A2), and 11β-hydroxylase (CYP11B1) and limits steroidogenesis to corticosterone and its precursor 11-deoxycorticosterone (DOC). Steroid flux in the adrenal of a patient with 17OHD resembles that of the rodent adrenal, which also lacks CYP17A1 and makes corticosterone as the principal glucocorticoid. In 17OHD, circulating corticosterone concentrations rise over 100 times normal to >10,000 ng/dL (~1 µM) in a new steady-state 47, which prevents glucocorticoid insufficiency in most patients 46. In order to make this much corticosterone, however, DOC production also rises markedly, and at these concentrations (>10 nM), DOC is a potent mineralocorticoid receptor (MR) agonist, inducing volume expansion with kaluresis and suppressing plasma renin activity (Figure 6). Consequently, the clinical presentation of complete 17OHD is sexual infantilism regardless of chromosomal sex, hypertension, and hypokalemia 48.
FIGURE 6.
Physiology of genetic 17-hydroxylase deficiency, equivalent to abiraterone acetate monotherapy. Loss of CYP17A1 activities (dashed arrows with X) eliminates production of all steroids in grey box, including androgens, estrogens, and cortisol. Loss of cortisol negative feedback increases ACTH and flux of cholesterol to pregnenolone (block arrow). The only available pathways (thick arrows) go to DOC and corticosterone (B), which expand blood volume, increase blood pressure, and suppress both plasma renin activity and aldosterone production (block arrows).
In the phase I-II trials of abiraterone, prednisone was omitted 43,44, and hypertension and hypokalemia were commonly observed. DOC and corticosterone rose markedly, as is seen in genetic 17OHD 49. These consequences were treated effectively with addition of either MR antagonist to block the action of DOC on MR (eplerenone in these studies) or corticosteroid to prevent the ACTH rise and thus lower DOC production. Thus, prednisone co-therapy with abiraterone acetate serves two purposes: to limit synthesis of new adrenal CYP17A1, which might overcome of the blockade in DHEAS synthesis in between abiraterone acetate doses, and to prevent accumulation of DOC with its propensity to cause hypertension and hypokalemia. This triple-regimen is the evolution of the “total androgen blockade” concept proposed by Labrie 50 upon considering all potential androgen sources and peripheral steroid metabolic pathways. It is possible that smaller prednisone doses and other glucocorticoids might accomplish the same purposes, but other regimens have not been studied.
In considering other targets, only three enzymes besides CYP17A1 are required for T synthesis (Figure 3). CYP11A1 and 3βHSD inhibitors would block synthesis of all steroid hormones and cause adrenal insufficiency as well. A 17βHSD inhibitor, acting on the final step, might seem ideal, but many enzymes catalyze this reaction besides testicular 17βHSD3, so the exact target(s) are unknown. Although AKR1C3 (17βHSD5) is highly expressed in many CRPC specimens 51, inhibition of this enzyme might select for clones that express alternative 17βHSD isoenzymes. In contrast, CYP17A1 is the ONLY enzyme that converts pregnenolone to DHEA, so this target leaves no alternative pathway to T open. The relative contributions of the de novo steroidogenesis from cholesterol all the way to T and DHT versus terminal metabolism of adrenal-derived DHEA(S) within the CRPC tumor to androgens is debated and likely to vary amongst specific tumors 52. Regardless of origin, complete blockade is of all CYP17A1 activity will ablate all sources of T and DHT production, but the complete endocrinologic consequences of these manipulations must be considered to optimize patient outcomes.
Estrogens in breast cancer
Similarly, steroidogenesis in the ovary of reproductive-age women derives from feedback-regulated axes as in the male, but steroid and germ cell production in the female is cyclical, adding to complexities beyond the scope of this article. Since E2 is the major hormone providing negative feedback, inhibition of aromatase will increase LH and FSH production, which increases aromatase expression in an effort to overcome the blockade (Figure 4C). When AIs are administered to premenopausal women, E2 decreases only transiently, and subsequently a new steady-state is reached. In contrast, the postmenopausal ovary makes little or no estrogen, and E2 deriveds from androgen precursors (DHEAS, AD) of ovarian and adrenal origin 53. The aromatase enzyme in postmenopausal women is expressed mainly in adipose tissues, including those of the breast 54, but also in breast cancers themselves 55. Since LH and FSH, which are already elevated in postmenopausal women, do not regulate aromatase expression in these tissues, the potent, irreversible AIs used clinically are very effective in suppressing E2 synthesis in postmenopausal women and treating ER+ breast cancers in these women 56.
In contrast, the use of AIs in premenopausal women with ER+ breast cancer would be ineffective, unless combined with therapy to prevent overcoming the blockade. Such treatments might include surgical or chemical oophorectomy and suppression of LH and FSH with high-dose progestins or long-acting GnRH agonists. Preliminary reports have demonstrated the feasibility of “total estrogen blockade” in premenopausal women with ER+ breast cancers using leuprorelin and anastrazole 57. Whether such combination therapies will prove effective in this population remains unknown.
Because AI therapy in women who have ceased menses for over 12 months—and thus are clinically menopausal—effectively suppresses E2 and eliminates most negative feedback on the gonadotropes, LH and FSH might rise even higher. In some women, this gonadotropin surge, which mimics the mid-cycle surge, induces ovulation and/or menses in some women 58. Often only one cycle is experienced, but this phenomenon, which is rather distressing to patient and doctor alike, also brings into question whether the menopausal status has been correctly assigned. Will such patients still benefit from AI therapy? In general, patients with a single episode of menses on an AI can be considered postmenopausal, although serum estradiol measurements before and during AI therapy are required in this setting 59. As long as serum estradiol remains low, AI therapy remains an option.
Similar to the tumor progression typically observed in CRPC, tumor progression eventually occurs in most cases of metastatic breast cancer treated with AIs. The mechanisms responsible for the escape from control are being studied vigorously in several groups. Serum concentrations of E2 remain markedly reduced at progression in nearly all cases 60,61, although intratumoral concentrations of E2 might be sufficient to activate ER yet elude detection in the most sensitive assays. Furthermore, the steroids accumulating above the AI blockade are androgens, and AR is expressed in many breast cancers 62. Could these tumors be converting from estrogen-dependent to androgen-dependent growth mechanisms? In addition, androgen metabolites such as androstanediols also accumulate, and some of these compounds activate ER, thwarting the intention of the treatment 63. In either case, strategies similar to those employed in CRPC might be effective salvage therapies, by blocking all androgen and estrogen production, and some trials testing this hypothesis are underway.
Summary and conclusions
Endocrine therapy has been a mainstay for the treatment of breast and prostate cancer for decades, and enzyme inhibitors have taken center stage as first-line therapies in recent years. Treatment strategies to eliminate production of key steroids must consider the optimal targets, alternate pathways that might bypass the blockade, and the endocrine consequences of deranged steroid flux. In many instances, combination therapies with other agents are necessary to prevent side-effects of upstream steroids and/or physiologic adaptations, which might overcome the blockades. Every new drug brings its unique challenges, which derive from the basic physiology of how the biochemical machinery in different tissues orchestrates the synthesis of steroid hormones. Armed with an understanding of these fundamental concepts and principles of steroid biosynthesis, the practicing oncologist will be prepared to avoid these pitfalls and optimally manage the prostate and breast cancer patients on these treatments.
Acknowledgements
RJA is supported by a Clinical Scientist Award in Translational Research from the Burroughs-Wellcome Fund (#1005954). We thank Drs. Nima Sharifi and Michael McPhaul for many productive discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyd S. On oophorectomy in cancer of the breast. BMJ. 1900;2:1161–1167. [Google Scholar]
- 2.Huggins C. Prostatic cancer treated by orchiectomy; the five year results. JAMA. 1946;15:576–581. doi: 10.1001/jama.1946.02870240008003. [DOI] [PubMed] [Google Scholar]
- 3.Huggins C, Dao TL. Adrenalectomy and oophorectomy in treatment of advanced carcinoma of the breast. JAMA. 1953;18:1388–1394. [PubMed] [Google Scholar]
- 4.Santen RJ, Worgul TJ, Samojlik E, et al. A randomized trial comparing surgical adrenalectomy with aminoglutethimide plus hydrocortisone in women with advanced breast cancer. N Engl J Med. 1981;305:545–551. doi: 10.1056/NEJM198109033051003. [DOI] [PubMed] [Google Scholar]
- 5.Susan LP, Roth RB, Adkins WC. Regression of prostate cancer metastasis by high doses of diethylsilbestrol phosphate. Urology. 1976;7:598–601. doi: 10.1016/0090-4295(76)90084-4. [DOI] [PubMed] [Google Scholar]
- 6.The Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. The Leuprolide Study Group. N Engl J Med. 1984;311:1281–1286. doi: 10.1056/NEJM198411153112004. [DOI] [PubMed] [Google Scholar]
- 7.Schellhammer PF, Sharifi R, Block NL, et al. A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate carcinoma. Analysis of time to progression. CASODEX Combination Study Group. Cancer. 1996;78:2164–2169. doi: 10.1002/(sici)1097-0142(19961115)78:10<2164::aid-cncr18>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 9.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 10.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller WL. Lessons from congenital lipoid adrenal hyperplasia. Curr Opin Endo Diab. 1998;5:155–161. [Google Scholar]
- 13.Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:113–118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- 14.Griffin JE. Androgen resistance--the clinical and molecular spectrum. N Engl J Med. 1992;326:611–618. doi: 10.1056/NEJM199202273260906. [DOI] [PubMed] [Google Scholar]
- 15.Miller WL. Early steps in androgen biosynthesis: from cholesterol to DHEA. Bailliere's Clin Endocrinol Metab. 1998;12:67–81. doi: 10.1016/s0950-351x(98)80461-8. [DOI] [PubMed] [Google Scholar]
- 16.Clark BJ, Wells J, King SR, et al. The purification, cloning and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- 17.Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol. 2000;7:408–414. doi: 10.1038/75192. [DOI] [PubMed] [Google Scholar]
- 18.Bose H, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- 19.Arakane F, King SR, Du Y, et al. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem. 1997;272:32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- 20.Lin D, Sugawara T, Strauss JF, III, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-0532. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofland J, van Weerden WM, Dits NF, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70:1256–1264. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- 24.Nelson DR, Kamataki T, Waxman DJ, et al. The P450 superfamily: Update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993;12:1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- 25.Khan N, Sharma KK, Andersson S, et al. Human 17β-hydroxysteroid dehydrogenases types 1, 2, and 3 catalyze bi-directional equilibrium reactions, rather than unidirectional metabolism, in HEK-293 cells. Arch Biochem Biophys. 2004;429:50–59. doi: 10.1016/j.abb.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Mizrachi D, Auchus RJ. Androgens, estrogens, and hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2009;301:37–42. doi: 10.1016/j.mce.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Geissler WM, Davis DL, Wu L, et al. Male pseudohermaphroiditism caused by mutations of testicular 17β-hydroxysteroid dehydrogenase 3. Nature Genet. 1994;7:34–39. doi: 10.1038/ng0594-34. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Einstein M, Geissler WM, et al. Expression cloning and characterization of human 17β-hydrosteroid dehydrogenase Type 2, a microsomal enzyme possessing 20α-hydroxysteroid dehydrogenase activity. J Biol Chem. 1993;268:12964–12969. [PubMed] [Google Scholar]
- 29.Simard J, Sanchez R, Durocher F, et al. Structure-function relationships and molecular genetics of the 3β-hydroxysteroid dehydrogenase gene family. J Steroid Biochem Mol Biol. 1995;55:489–505. doi: 10.1016/0960-0760(95)00198-0. [DOI] [PubMed] [Google Scholar]
- 30.Labrie F, Luu-The V, Lin SX, et al. Role of 17β-hydroxysteroid dehydrogenases in sex steroid formation in peripheral intracrine tissues. Trends Endocrinol Metab. 2000;11:421–427. doi: 10.1016/s1043-2760(00)00342-8. [DOI] [PubMed] [Google Scholar]
- 31.Turgeon D, Carrier JS, Levesque E, et al. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology. 2001;142:778–787. doi: 10.1210/endo.142.2.7958. [DOI] [PubMed] [Google Scholar]
- 32.Iyer L, King CD, Whitington PF, et al. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–854. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasqualini JR, Chetrite G, Blacker C, et al. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J Clin Endocrinol Metab. 1996;81:1460–1464. doi: 10.1210/jcem.81.4.8636351. [DOI] [PubMed] [Google Scholar]
- 34.Tonetto-Fernandes V, Lemos-Marini SH, Kuperman H, et al. Serum 21-deoxycortisol, 17-hydroxyprogesterone, and 11-deoxycortisol in classic congenital adrenal hyperplasia: clinical and hormonal correlations and identification of patients with 11β-hydroxylase deficiency among a large group with alleged 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91:2179–2184. doi: 10.1210/jc.2005-1890. [DOI] [PubMed] [Google Scholar]
- 35.Flück CE, Miller WL, Auchus RJ. The 17, 20-lyase activity of cytochrome P450c17 from human fetal testis favors the Δ5 steroidogenic pathway. J Clin Endocrinol Metab. 2003;88:3762–3766. doi: 10.1210/jc.2003-030143. [DOI] [PubMed] [Google Scholar]
- 36.Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108:13728–13733. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Wilson JD, Auchus RJ, Leihy MW, et al. 5α-androstane-3α,17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology. 2003;144:575–580. doi: 10.1210/en.2002-220721. [DOI] [PubMed] [Google Scholar]
- 39.Wilson JD, Shaw G, Leihy ML, et al. The marsupial model for male phenotypic development. Trends Endocrinol Metab. 2002;13:78–83. doi: 10.1016/s1043-2760(01)00525-2. [DOI] [PubMed] [Google Scholar]
- 40.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan CJ, Halabi S, Ou SS, et al. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin Cancer Res. 2007;13:2030–2037. doi: 10.1158/1078-0432.CCR-06-2344. [DOI] [PubMed] [Google Scholar]
- 42.Patel SR, Kvols LK, Hahn RG, et al. A phase II randomized trial of megestrol acetate or dexamethasone in the treatment of hormonally refractory advanced carcinoma of the prostate. Cancer. 1990;66:655–658. doi: 10.1002/1097-0142(19900815)66:4<655::aid-cncr2820660409>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 43.Attard G, Reid AH, Yap TA, et al. Phase I Clinical Trial of a Selective Inhibitor of CYP17, Abiraterone Acetate, Confirms That Castration-Resistant Prostate Cancer Commonly Remains Hormone Driven. J Clin Oncol. 2008;26:463–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 44.Attard G, Reid AH, A'Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17α-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–2325. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am. 2001;30:101–119. doi: 10.1016/s0889-8529(08)70021-5. [DOI] [PubMed] [Google Scholar]
- 47.Costa-Santos M, Kater CE, Auchus RJ. Two Prevalent CYP17 Mutations and Genotype-Phenotype Correlations in 24 Brazilian Patients with 17-Hydroxylase Deficiency. J Clin Endocrinol Metab. 2004;89:49–60. doi: 10.1210/jc.2003-031021. [DOI] [PubMed] [Google Scholar]
- 48.Biglieri EG, Herron MA, Brust N. 17α-hydroxylation deficiency in man. J Clin Invest. 1966;15:1945–1954. doi: 10.1172/JCI105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attard G, Reid AHM, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. submitted. 2011 doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 50.Labrie F. Endocrine therapy of prostate cancer: optimal form and timing. J Clin Endocrinol Metab. 1995;80:1066–1071. doi: 10.1210/jcem.80.4.7714068. [DOI] [PubMed] [Google Scholar]
- 51.Penning TM, Steckelbroeck S, Bauman DR, et al. Aldo-keto reductase (AKR) 1C3: role in prostate disease and the development of specific inhibitors. Mol Cell Endocrinol. 2006;248:182–191. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Sharifi N, McPhaul MJ, Auchus RJ. "Getting from here to there"--mechanisms and limitations to the activation of the androgen receptor in castration-resistant prostate cancer. J Investig Med. 2010;58:938–944. doi: 10.231/JIM.0b013e3181ff6bb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Judd HL, Shamonki IM, Frumar AM, et al. Origin of serum estradiol in postmenopausal women. Obstet Gynecol. 1982;59:680–686. [PubMed] [Google Scholar]
- 54.Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 55.Santner SJ, Pauley RJ, Tait L, et al. Aromatase activity and expression in breast cancer and benign breast tissue stromal cells. J Clin Endocrinol Metab. 1997;82:200–208. doi: 10.1210/jcem.82.1.3672. [DOI] [PubMed] [Google Scholar]
- 56.Rugo HS. The breast cancer continuum in hormone-receptor-positive breast cancer in postmenopausal women: evolving management options focusing on aromatase inhibitors. Ann Oncol. 2008;19:16–27. doi: 10.1093/annonc/mdm282. [DOI] [PubMed] [Google Scholar]
- 57.Recchia F, Candeloro G, Necozione S, et al. Premenopausal hormone-responsive breast cancer with extensive axillary nodes involvement: total estrogen blockade and chemotherapy. Anticancer Res. 2011;31:671–676. [PubMed] [Google Scholar]
- 58.Smith IE, Dowsett M, Yap YS, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol. 2006;24:2444–2447. doi: 10.1200/JCO.2005.05.3694. [DOI] [PubMed] [Google Scholar]
- 59.Ortmann O, Pagani O, Jones A, et al. Which factors should be taken into account in perimenopausal women with early breast cancer who may become eligible for an aromatase inhibitor? Recommendations of an expert panel. Cancer Treat Rev. 2011;37:97–104. doi: 10.1016/j.ctrv.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Dixon JM, Renshaw L, Young O, et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol. 2008;26:1671–1676. doi: 10.1200/JCO.2007.13.9279. [DOI] [PubMed] [Google Scholar]
- 61.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 62.Moinfar F, Okcu M, Tsybrovskyy O, et al. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003;98:703–711. doi: 10.1002/cncr.11532. [DOI] [PubMed] [Google Scholar]
- 63.Sikora MJ, Cordero KE, Larios JM, et al. The androgen metabolite 5α-androstane-3β,17β-diol (3βAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat. 2009;115:289–296. doi: 10.1007/s10549-008-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]