SUMMARY
Complement activation modulates DC-mediated T-cell activation, but whether complement affects DC-mediated priming of NK cells is unknown. Here we demonstrate that conventional DCs (cDCs) from C3–/– and C5aR–/– mice are hyper-responsive to polyI:C, a TLR3 ligand, leading to enhanced NK-cell activation. We found that cDCs lack C5a receptor (C5aR) and do not respond to C5a directly. Depletion of Gr-1+ myeloid cells augments polyI:C-induced cDC activation in WT but not in C3–/– or C5aR–/– mice, indicating that the effect of complement activation on cDCs is indirectly mediated through C5aR-expressing Gr-1+ myeloid cells. We further demonstrated that the mechanism by which Gr-1+ myeloid cells regulate the activity of cDCs involves C5a-dependent TGF-β1 production in Gr-1+ myeloid cells. C5a enhances and blocking C5aR decreases TGF-β1 production in cultured bone marrow Gr-1+CD11b+ cells. C5aR deficiency is associated with reduced circulating TGF-β1 levels, while depleting Gr-1+ myeloid cells abrogates this difference between WT and C5aR–/– mice. Lastly, we showed that enhanced cDC-NK cell activity in C3–/– mice led to delayed melanoma tumor growth. Thus, complement activation indirectly regulates cDC-NK cell activation in response to inflammatory stimuli such as TLR3 by promoting TGF-β1 production in Gr-1+ myeloid cells at steady state.
Keywords: Conventional DCs, NK cells, complement, myeloid cells
INTRODUCTION
NK cells are essential effector lymphocytes in anti-viral and tumor immunity. Naïve NK cells require priming from accessory cells, particularly DCs, to initiate IFN-γ secretion and degranulation [1, 2]. DCs that are activated through sensor-like pattern recognition receptors (PRRs) produce pro-inflammatory cytokines such as TNF-α, IL-12, IL-15, IL-18 and type I IFNs, and they prime or activate NK cells in both cell contact-dependent and independent manner [1]. CD11chighMHC class II+ cDCs play a pivotal role in priming and activating NK cells in vivo [2-4]. Homeostatic peripheral cDCs mainly reside in the spleen and develop from a unique committed precursor known as pre-cDCs, driven by FMS-like tyrosine kinase 3 (Flt-3) ligand [5-7]. Splenic cDCs preferentially express TLR3 and stimulation with polyI:C, an agonist for TLR3, activates NK cells mostly through cDCs [3, 8, 9].
Complement system plays an important role in modulating the DC-mediated T-cell immune response [10], yet it is unknown whether complement affects cDC-mediated NK-cell activation (cDC-NK cell activation). Activation of the complement cascade leads to degradation of C3 and C5 and subsequent generation of the anaphylatoxins C3a and C5a that mediate chemotaxis and modulation of immune cell function [10]. The most potent split product, C5a, signals through a G-protein coupled receptor C5aR and has multiple pro-inflammatory properties. The impact of C5a on DC-T-cell activation appears to be contradictory in several animal models. C5a promotes bone marrow derived DCs (BMDC)-mediated T-cell activation by providing co-stimulatory signals for maturation of BMDCs [11-13]. In contrast, C5a was inhibitory in pulmonary DC-mediated T-cell activation in a mouse model of allergen-induced asthma [14], and splenic DCs from C5aR–/– mice are more potent inducers of Th17 and regulatory T (Treg) cell differentiation than WT splenic DCs [15]. These apparently paradoxical roles of C5a on DC-mediated T-cell immune responses may be related to differences in DC subsets. GM-CSF induced BMDCs, which have been widely used for studying DCs in vitro, resemble monocyte-derived CD11cint DCs in vivo, also known as TNF/iNOS-producing DCs (TipDCs) that usually arise during inflammation [16]. In contrast, cDCs represent steady-state DCs and do not develop from monocytes [5, 17]. Although it is clear that BMDCs express C5aR [13], whether C5aR is expressed by cDCs has been a subject of controversy [18, 19]. As for NK cells, they express complement receptor CR3 and CR4 that facilitate cytolysis of complement opsonized cells, and C3a and C3b inhibit human NK cell cytotoxic activity in vitro [20, 21]. However, it is unclear whether NK cells are affected by C5a and express its receptor.
Given the importance of cDCs in NK-cell activation and the precedent role for complement in modulating DC-T cell interaction, we asked whether the complement system affects cDC-mediated NK-cell activation. To address this question, we analyzed NK-cell activation mediated by splenic cDCs triggered with polyI:C in C3–/– and C5aR–/– mice, in which either activation of complement cascade is blocked due to C3 deficiency or host cells cannot respond to the complement activation product C5a. Our data indicate that both cDCs and NK cells lack C5aR, and are not influenced by C5a directly. Surprisingly, cDCs from complement deficient mice are hyper-responsive to polyI:C stimulation and lead to enhanced NK-cell activation, mediated indirectly through Gr-1+ myeloid cells that highly express C5aR. We found that Gr-1+ myeloid cells produce TGF-β1 in a C5aR-dependent manner, which conditions cDCs for their response to polyI:C. Finally, we showed that enhanced activation of cDCs and NK cells in the absence of complement C3 contributed to harnessing tumor growth in a mouse model of melanoma.
RESULTS
C3–/– mice have enhanced NK activity in response to polyI:C stimulation
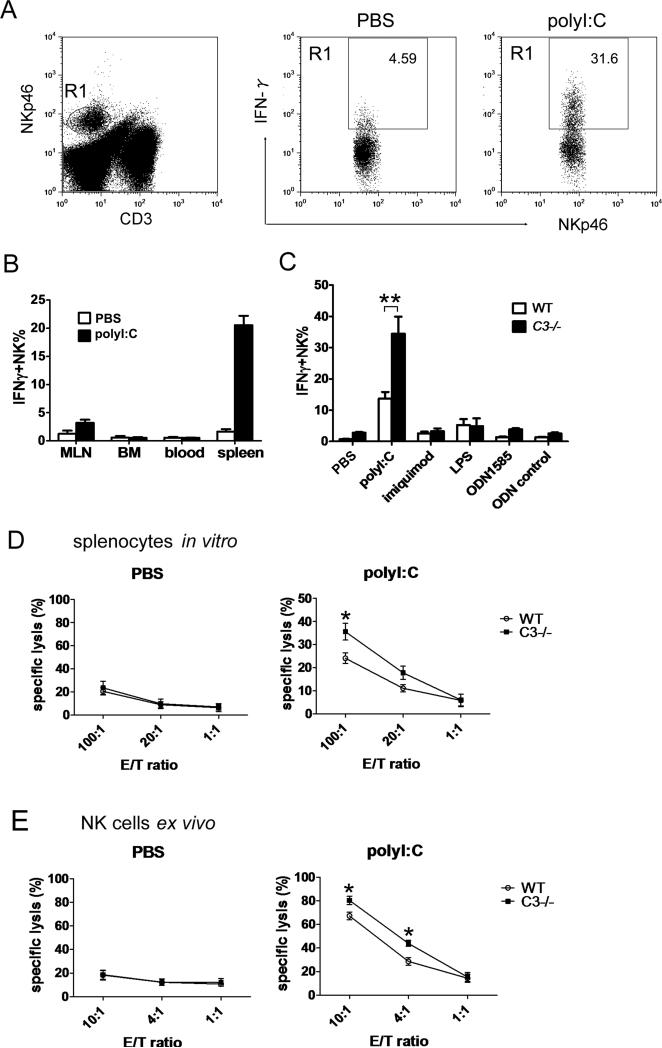
TLR stimulation is essential for priming and activation of NK cells. In initial experiments, we examined polyI:C induced NK-cell activation by stimulating unfractioned cells from spleen, blood, mesenteric lymph nodes (MLNs) and BM with polyI:C. As shown in Fig. 1A and B, polyI:C induced potent IFN-γ production by NK cells in the spleen, as early as 5 hours after stimulation, whereas minimal NK-cell activation was detected in the LNs , BM or blood, suggesting that the spleen is the primary site of early phase NK-cell activation. To determine if complement alters TLR-induced NK-cell activation in the spleen, we studied C3–/– mice which are unable to activate complement by any of the initiating pathways. There was a > 3-fold increase in polyI:C induced IFN-γ producing NK cells in C3–/– splenocytes compared with WT mice, while imiquimod (TLR7), LPS (TLR4) or CpG DNA ODN1585 (TLR9) did not cause significant induction of IFN-γ-producing NK cells in this 5 hour assay (Fig. 1C). Cytotoxic activity of splenic NK cells in response to in vitro polyI:C stimulation was also significantly augmented in C3–/– mice compared with WT mice using total splenocytes as effector cells and YAC-1 cells as target (Fig. 1D).
Figure 1.
Increased NK-cell activation in C3–/– mice in response to polyI:C. (A) Gating strategy. NK cells were gated as NKp46+CD3- cells (R1), and the percentage of IFN-γ+ NK cells was calculated by dividing IFN-γ+NK cells against total NK cells in the presence/absence of polyI:C stimulation. (B) MLNs, BM, blood and spleen cells were stimulated with polyI:C for 5 hours. The percentage of IFN-γ+ cells in NK cell population was analyzed by flow cytometry as in (A). Data are mean + S.D. n=5 mice per group and reprentative of 3 independent experiments. (C) Splenocytes from C3–/– or WT mice were stimulated with the indicated TLR agonists and the percentage of IFN-γ+ NK cells were determined. Data are mean ± SEM n=6 mice/group and reprentative of 3 independent experiments. (D) WT or C3–/– splenocytes were stimulated with polyI:C or left unstimulated before incubation with CFSE-label YAC-1 cells and lysis of YAC-1 cells was measured. E/T ratio: effector / target cell ratio. Data are means ± SD and are representative of 5 independent experiments each with n=3/group. (E) C3–/– or WT mice were i.p. injected with 100 μg polyI:C or PBS. Splenic NK cells were purified for cytotoxic assay against YAC-1 cells 24 hours later. Data are mean ± SEM n=6 mice/group and are representative of two independent experiments. * P<0.05, ** P<0.02 (Student t test).
Moreover, in vivo activation of NK cells upon polyI:C injection was significantly higher in C3–/– mice than in WT mice, as shown by their increased killing activity (Fig. 1E) and IFN-γ production, although CD69 and granzyme B expression in NK cells was similar between C3–/– and WT mice (Supporting Information Fig. 1A-C). Analyzing the percentage and absolute numbers of cDCs, NK cells, as well as the early activation marker CD69 on NK cells, revealed no significant difference between C3–/– and WT mice (Supporting Information Fig. 2A-D). These results indicate that complement C3 or a downstream product of the complement cascades plays an inhibitory role in polyI:C-induced NK-cell activation.
Splenic cDCs from C3–/– and C5aR–/– mice are hyper-responsive to polyI:C
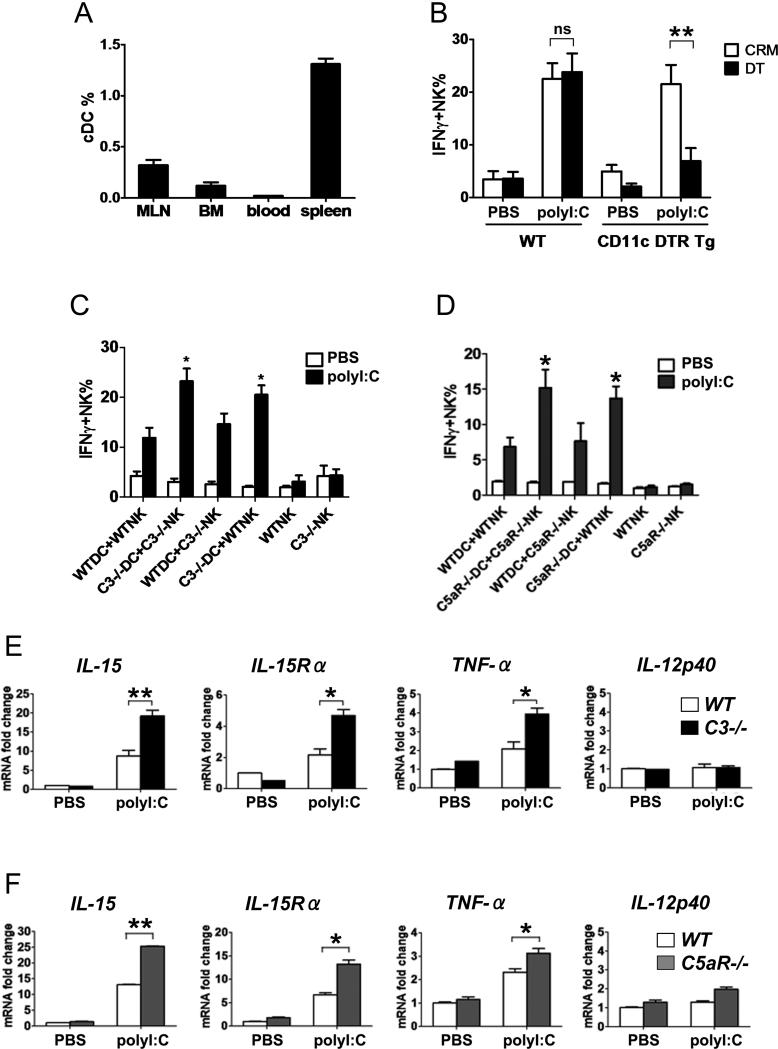
Previous studies indicate that cDCs, which preferentially express TLR3 [9], are essential for polyI:C induced NK-cell activation in vivo [2]. Therefore, we asked if polyI:C induced NK-cell activation in the spleen is mediated by cDCs. We first measured distribution of cDCs in lymphoid organs and found that spleen has the highest percentage of cDCs compared with LNs , blood and BM (Fig. 2A). Indeed, distribution of cDCs (gated as MHC classs II+CD11chigh cells Supporting Information Fig 2E) in the lymphoid organs mirrored their NK cell response to polyI:C (Fig. 1A). To confirm that polyI:C induced NK-cell activation in the spleen is mediated by cDCs, we used mice containing a diphtheria toxin receptor (DTR) transgene (tg) under the control of CD11c promoter (CD11c DTR tg mice), which allows for specific depletion of CD11chigh cells by a single injection of diphtheria toxin (DT) [22]. As shown in Fig. 2B, polyI:C induced NK-cell activation in the spleen of CD11c DTR tg mice was abolished 2 days after DT injection, a time when more than 70% of CD11chigh cells (Supporting Information Fig. 2E) but <5% of NK cells were depleted (data not shown). Hence, increased NK-cell activation in the spleen of C3–/– mice in response to polyI:C could be attributed to the impact of complement activation on either cDCs or NK cells.
Figure 2.
Enhanced activation of splenic cDCs in complement deficient mice. (A) The percentage of cDCs (CD11chighMHC class II+ cells) in the MLNs , BM, blood and spleen of C57BL/6 mice was measured by flow cytometry. Data are mean + SD n=5 mice/group. (B) WT or CD11c DTR tg mice were injected with DT or CRM as control. Two days later, splenocytes were isolated and stimulated with polyI:C for NK-cell activation. Data are mean ± SEM, n=4 mice/group. (C, D) Purified splenic cDCs and NK cells were isolated from WT and (C) C3–/– or (D) C5aR–/– mice and were co-cultured in the presence or absence of polyI:C. The percentage of IFN-γ+ cells in the NK cell population was determined by FACS. Data mean + SD n=3/group. Results are representative of 5 independent experiments in (A-D). (E, F) Splenic cDCs isolated from WT, (E) C3–/– or (F) C5aR–/– mice were stimulated with polyI:C. Expression of IL-15, IL-15Rα, TNF-α, and IL-12p40 was measured by quantitative real-time PCR. Data are mean + SD n=3/group and the results are representative of 6 independent experiments. * P<0.05, ** P< 0.02 (Student t test).
To determine which cells are the targets of complement activation, we isolated splenic cDCs and NK cells from WT, C3–/– or C5aR–/– mice, and performed mix-match co-culture experiments. In the absence of cDCs, NK cells did not respond to polyI:C (Fig. 2C, D). Splenic cDCs from C3–/– (Fig. 2C) and C5aR–/– (Fig. 2D) mice induced significantly higher IFN-γ production in NK cells than their corresponding WT cDCs (C57BL/6 as control for C3–/– mice and BALB/c for C5aR–/– mice). In contrast, NK cells from WT and C5aR–/– mice responded at similar levels to polyI:C in the presence of WT or C5aR–/– cDCs (Fig. 2D). In addition, in vivo activation of NK cells was also augmented in C5aR–/– mice compared with WT mice (Supporting Information Fig 1A-C). These data suggest that the impact of complement activation, specifically C5a, is mediated through cDCs, rather than directly on NK cells. Indeed, we were unable to detect C5aR expression on quiescent and activated NK cells in LPS- or polyI:C-treated mice (Supporting Information Fig. 3). Furthermore, the cDC-NK cell response was not altered in mice lacking C3aR or CR3 (data not shown), two down-stream receptors of C3, excluding their involvement in modulating cDC-NK cell activation.
To define the basis for increased cDC-NK cell activation in C3–/– and C5aR–/– mice, we examined production of pro-inflammatory cytokines/molecules in cDCs that are essential for NK-cell activation, including TNF-α, IL-15, IL-15 receptor α subunit (IL-15Rα), and IL-12 [23-25]. Upon polyI:C stimulation, splenic cDCs from C3–/– (Fig. 2E) and C5aR–/– mice (Fig. 2F) produced significantly higher levels of IL-15, IL-15Rα and TNF-α than WT cDCs, while minimal IL-12p40 was generated. Similar results were obtained upon polyI:C injection in vivo (Supporting Information Fig. 1D), suggesting that in the absence of complement, specifically C5a-C5aR interaction, splenic cDCs are hyper-responsive to polyI:C. Furthermore, MHC class II, CD80, CD86 (Supporting Information Fig. 1E-G), CD11b and TLR3 (data not shown) were similarly expressed in both naïve and activated C3–/– and C5aR–/– cDCs compared with WT cDCs, excluding the involvement of these factors in causing augmented NK-cell activation.
Enhanced TLR response of cDCs in C3–/– and C5aR–/– mice is mediated by Gr-1+ myeloid cells
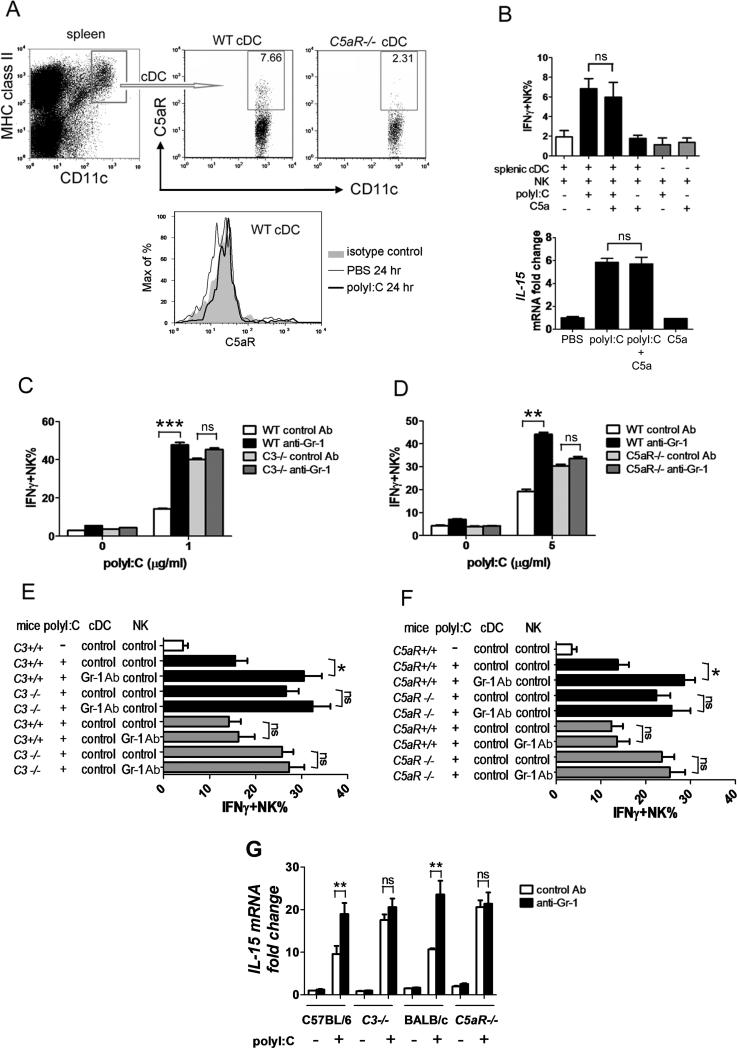
Analysis of C5aR expression revealed that only a very small portion (~5%) of splenic cDCs expressed C5aR (Fig. 3A). Moreover, polyI:C stimulation did not upregulate C5aR expression on cDCs (Fig. 3A). As expected, exogenous C5a had no direct impact on cDC-NK cell activation (Fig. 3B). Numerous studies have suggested that IL-15 is essential for DC-mediated NK-cell activation [2, 4, 24]. We found that IL-15 up-regulation in polyI:C stimulated cDCs was not affected by C5a pre-treatment (Fig. 3B). These results indicate that complement activation, specifically C5a, did not have a direct impact on splenic cDCs. Given that isolated cDCs from C3–/– and C5aR–/– mice are hyper-reactive to TLR3 stimulation in vitro, we predicted that complement activation may influence cDCs indirectly, possibly through C5aR-expressing cell population(s) that can negatively regulate cDC activity in vivo. Treg cells regulate development and activation of cDCs [5, 26]. However, Treg cells do not express C5aR, and depletion of Treg cells did not alter polyI:C triggered splenic cDC-NK-cell activation (Supporting Information Fig. 4).
Figure 3.
The impact of Gr-1+ cell depletion on cDC activity. (A) Fresh splenocytes and splenocytes either unstimulated for 24 hours or polyI:C treated for 24 hours were stained for cell surface expression of CD11c, MHC class II (I-A/I-E) and C5aR. Numbers in the plots indicate the percentage of gated cells. Data are representative of 6 independent experiments with 3 mice in each experiment. (B) Splenic cDCs from C57BL/6 mice were left untreated or pre-treated with C5a and then either stimulated with polyI:C or left unstimulated. IFN-γ producing NK cells were determined in co-cultured NK cells by flow cytometry. Expression of IL-15 was analyzed by real-time PCR in cDCs. Data are mean + SD, n=3 per group and are representative of 6 independent experiments. (C) C3–/– (D) C5aR–/– or WT mice were injected with anti-Gr-1 or control mAb. Three days after injection, splenocytes were isolated, stimulated with polyI:C, and IFN-γ production in NK cells was determined. Data are mean + SEM of n=6 mice/group and are representative of 5 independent experiments. (E) C3–/–, (F) C5aR–/– or (G) C3–/–, C5aR–/– and WT mice were injected with anti-Gr-1 or a control mAb. Splenic cDCs and NK cells were isolated 72 hours later. (E, F) cDCs and NK cells were co-cultured in the presence of polyI:C to determine IFN-γ production in NK cells. (G) cDCs were stimulated with polyI:C and IL-15 gene expression was measured by real-time PCR. Data are mean + SD, n=3 mice/group and are representative of 5 independent experiments. * P<0.05, ** P<0.02, *** P<0.01, ns, not significant (Student t test).
A second cell population we considered is myeloid Gr-1+CD11b+ cells, which have high levels of C5aR expression [27] (Supporting Information Fig. 5A) and are a major cell population that mediates C5a-induced effects. We found that injection of an anti-Gr-1 mAb resulted in ablation of Gr-1+CD11b+ cells (>90%), a small increase (~25%) in splenic cDCs and no significant change in NK cells, although some NK cells have low levels of Gr-1 expression [28]. Similar results were obtained in C3–/– and C5aR–/– mice compared with WT mice (Supporting Information Fig. 5B-D). Surprisingly, depletion of Gr-1+ cells led to a marked increase in polyI:C induced NK-cell activation using splenocytes from WT mice (2-3 fold), but minimal changes (~10%) with C3–/– or C5aR–/– cells (Fig. 3C, D). To address whether Gr-1+ cell depletion affects cDCs or NK cells, we isolated splenic cDCs and NK cells from anti-Gr-1 or control Ab treated WT or C5aR–/– mice, and co-cultured them with NK cells or cDCs from WT mice injected with control Ab, respectively. Upon polyI:C stimulation, cDCs from Gr-1+ cell depleted WT mice induced significantly higher IFN-γ production in co-cultured NK cells compared with cDCs from control Ab injected WT mice, whereas NK cells from Gr-1+ cell depleted WT mice responded similarly to those from control Ab injected WT mice (Fig. 3E, F). As expected, Gr-1+ cell depletion had no impact on cDCs or NK cells in C3–/– (Fig. 3E) or C5aR–/– mice (Fig. 3F). Consistently, cDCs isolated from WT, but not from C3–/– or C5aR–/– mice that are depleted of Gr-1+ cells, produced significantly higher IL-15 upon polyI:C stimulation compared with mice treated with isotype control Ab (Fig. 3G). These results indicate that cDCs are regulated by complement activation through Gr-1+ myeloid cells in vivo.
C5a induces TGF-β1 production in Gr-1+ myeloid cells
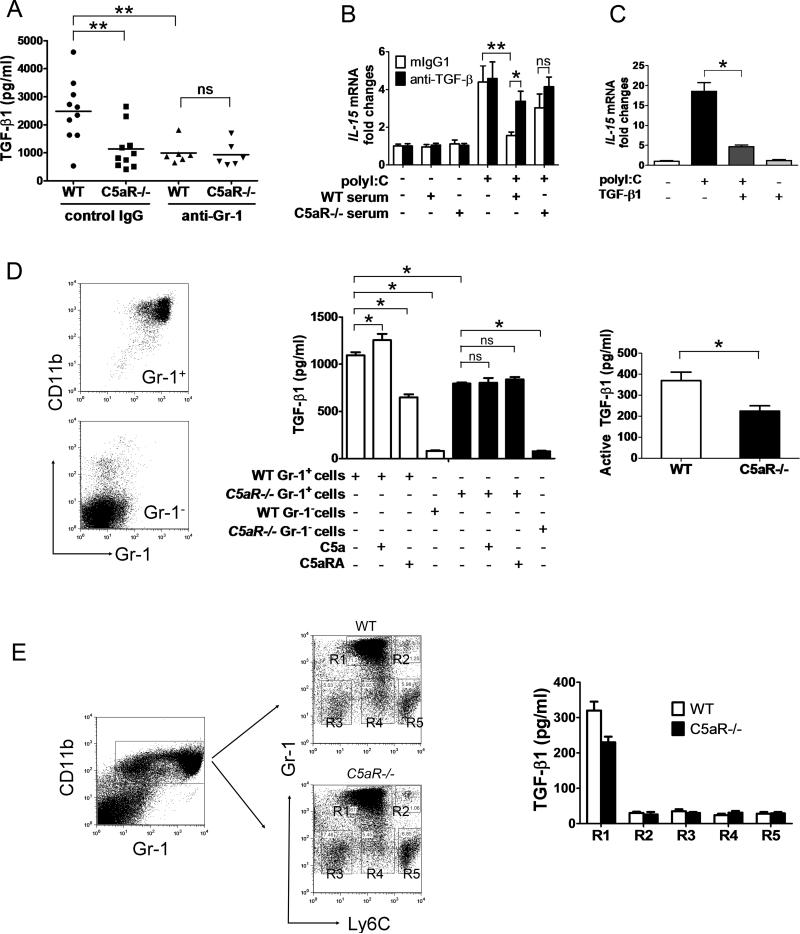
Next, we sought to determine how complement activation, specifically C5a, regulates cDC activity through Gr-1+ myeloid cells. We found that circulating TGF-β1 levels in the blood were significantly higher in WT mice than C5aR–/– mice (WT: 2481±354 pg/ml, n=10; C5aR–/– mice: 1139±241 pg/ml, n=10, P=0.0057, student t-test), and depletion of Gr-1+ cells abrogated this difference (WT mice anti-Gr-1: 995±171 pg/ml, n=6; C5aR–/– mice anti-Gr-1: 935±179 pg/ml, n=6, P=0.8139, student t-test), indicating that Gr-1+ cells are a main source of C5aR-associated TGF-β1 production in the circulation (Fig. 4A). Furthermore, pre-incubation of cDCs with serum from WT mice significantly inhibited polyI:C induced IL-15 upregulation, which was reversed by blocking TGF-β1. In contrast, serum from C5aR–/– mice only mildly suppressed activation of cDCs, and anti-TGF-β1 treatment had minimal effects (Fig. 4B). In addition, pre-treatment of cDCs with recombinant TGF-β1 inhibited polyI:C induced IL-15 expression (Fig. 4C). These findings suggest that steady-state cDCs may be conditioned by TGF-β1 in the circulation, which is generated, in part, by Gr-1+ myeloid cells in a C5aR-dependent manner.
Figure 4.
Modulation of cDC activity by C5a-induced TGF-β1 in Gr-1+ myeloid cells. (A) WT and C5aR–/– mice were injected with anti-Gr-1 or isotype control mAb. Plasma TGF-β1 levels were tested 2 days later. Each symbol represents data from an individual mouse and the horizontal bar represents the mean; n=6-10 mice/group. Data are representative of 2 independent experiments. Student t test ** P<0.02 (/Student t test). (B, C) Splenic cDCs (B) were pre-incubated with 10% of WT or C5aR–/– serum in the presence or absence of an anti-TGF-β1 mAb, or (C) were pre-treated with 10 ng/ml of TGF-β1 for 16 hr, then stimulated with polyI:C. IL-15 expression was measured by real-time PCR. (D) BM Gr-1+CD11b+ and Gr-1- cells isolated from WT or C5aR–/– mice were cultured with or without C5a (0.5 μg/ml) or C5aRA (250 nM) in serum-free OPT-MEM medium for 6 hours. Total (middle) and active (right) TGF-β1 production in the culture supernatant was measured by ELISA. (E) BM cells were sorted based on CD11b, Gr-1 and Ly6C expression. TGF-β1 was measured in the culture supernatant of sorted cells by ELISA. (B-E) Data are either representative (plots) or mean + SD (graphs) of n=3-5/mice per group and are representative of 4-6 independent experiments. * P<0.05, ** P<0.02 (Student t test).
We then measured in vitro production of TGF-β1 by Gr-1+CD11b+ cells. As shown in Fig. 4D, cultured BM Gr-1+CD11b+ cells (abbreviated as Gr-1+ cells in the graph), but not Gr-1- cells, spontaneously produce large amount of TGF-β1. Total TGF-β1 levels, measured by acid treatment of samples which activates latent form of TGF-β1 (Fig. 4D middle panel), as well as active TGF-β1 (Fig. 4D right panel), are both significantly higher in the culture supernatant of WT Gr-1+CD11b+ cells than C5aR–/– cells. Treatment of WT Gr-1+CD11b+ cells with C5a led to increased TGF-β1 production. Moreover, in the absence of exogenously added C5a, blocking C5aR signaling in Gr-1+CD11b+ cells with an antagonist for C5a-C5aR binding (C5aRA, A8δ71-73) [29] reduced TGF-β1 production in these cells, suggesting that C5a was generated by local complement activation in BM Gr-1+CD11b+ cells and acted in an autocrine manner (Fig. 4D middle panel). Although C5aRA, A8δ71-73, has been reported to block both C5aR and C5L2, an orphan receptor for C5a [29], there was no change in C5aR–/– cells treated with C5aRA, excluding the possibility that C5L2 plays a role in inducing TGF-β1. Because BM Gr-1+CD11b+ cells are a heterogeneous cell population, we examined TGF-β1 production in subsets of Gr-1+CD11b+ cells based on Ly6C expression. As shown in Fig. 4E, BM Gr-1+CD11b+ cells can be divided into 5 sub-populations, and there are no differences in their distribution between C5aR–/– and WT mice. Among these subsets, only Gr-1highLy6Cint cells produce significant amount of TGF-β1. Furthermore, arginase-1 and IL-10 have been implicated to be associated with regulatory activity of myeloid-derived suppressor cells (MDSC) and activated neutrophils [27, 30], both of which co-express Gr-1 and CD11b. However, we detected no difference in arginase-1 or IL-10 production between WT and C5aR–/– BM Gr-1+CD11b+ cells in normal mice (Supporting Information Fig. 6).
Taken together, the above data suggest that Gr-1+ myeloid cells are an important source of circulatory TGF-β1, the production of which is partially dependent on C5aR signaling, and that cDCs are conditioned by circulatory TGF-β1 in vivo in a complement-dependent manner through Gr-1+ myeloid cells.
Activation of cDCs and NK cells controls tumor growth in C3–/– mice
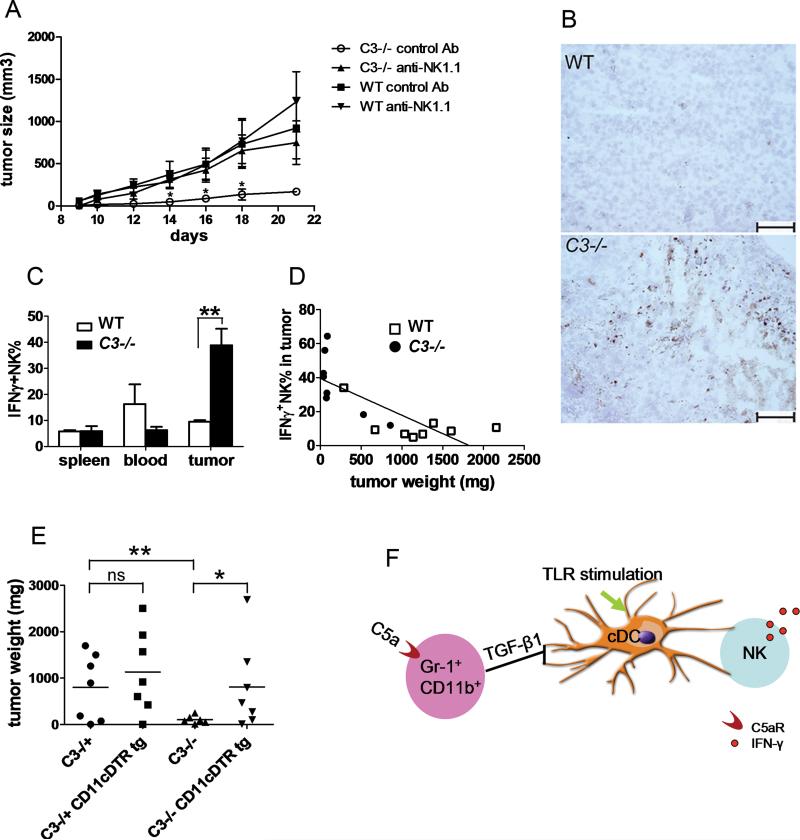
Given that complement-induced TGF-β1 production in Gr-1+ myeloid cells regulates capacity of cDCs to prime NK cells, and that NK cells play an important role in tumor immunity, we examined the possibility that augmented cDC-NK cell activity in the absence of C3 would lead to suppression of tumor growth in C3–/– mice. To address this, we employed B16 melanoma syngeneic tumor model. In agreement with a previous report using a different tumor model [27], tumor growth was dramatically delayed in C3–/– mice (Fig. 5A). Treatment with anti-NK1.1 mAb ablated >90% NK cells in both WT and C3–/– mice (Supporting Information Fig. 7). Upon NK cell depletion, tumor growth in C3–/– mice reverted to that of WT mice, whereas tumor growth in WT mice was not significantly altered by NK cell depletion (Fig. 5A). In C3–/– mice, there was increased NK cell infiltration in the tumor tissues compared with WT mice (Fig. 5B). Flow-cytometry analysis revealed a significant increase in activated IFN-γ-producing NK cells in the tumor tissues of C3–/– mice, while minimal IFN-γ-producing NK cells were found in the spleen or blood from both strains (Fig. 5C). In addition, tumor weight inversely correlated with IFN-γ-producing NK cells in the tumor (n=16 mice, r=-0.7661, P=0.003, Pearson correlation) (Fig. 5D). Thus, delayed tumor growth in C3–/– mice is dependent on enhanced NK-cell activation.
Figure 5.
Activation of cDCs and NK cells in tumor-bearing C3–/– mice. WT and C3–/– mice were injected with B16 melanoma cells subcutaneously. (A) NK cells were depleted with anti-NK1.1 or a control mAb on day -3, day 0 and day 7 of tumor inoculation. Data are means ± SEM of n=5-7 mice/group. *C3–/– mice receiving anti-NK1.1 vs. C3–/– mice receiving control Ab: p values on days after tumor inoculation: day 14 p=0.024, day 16 p=0.048, day 18 p=0.031, day 21 p=0.055, two-tailed unpaired Student t-test. (B) Infiltrating NK cells (brown) in the tumor tissues on day 21 after tumor cell injection. Scale bar: 60 μm. Images shown are representative of 5-7 mice per group. (C) IFN-γ-producing NK cells were measured on day 21 after tumor cell injection by flow cytometry. Date are mean + SEM of n=5-7 mice/group. (D) Correlation between tumor infiltrating IFN-γ+ NK cells with tumor weight. Each dot represents data from a single mouse. n=8 mice/group. (E) DT was injected to all groups of mice to deplete DCs on day 5 after tumor inoculation. Tumor weights were measured on day 23. Each symbol represents data from a single mouse (n=6-7 mice/group) and the horizontal line indicates the mean. . (A-E) Data are representative of 3 independent experiments. (C, E) * P<0.05, ** P<0.02, (Student t test). (F) Model of regulation of cDC-mediated NK-cell activation by complement. In normal hosts at steady state, complement activation, via C5aR signaling, promotes TGF-β1 production in Gr-1+ myeloid cells and conditions cDCs for their capacity to activate NK cells following, e.g. TLR stimulation.
Given the importance of splenic cDCs in priming NK cells, we predicted that activation of NK cells in tumor-bearing C3–/– mice is triggered by hyper-reactive cDCs in these mice. We were unable to detect cDCs in the tumor tissue from either C3–/– or WT mice, and there was no significant change in MHC class II or co-stimulatory molecule CD80 on splenic cDCs in these tumor-bearing mice (data not shown). To directly prove that cDCs are essential for controlling tumor growth in C3–/– mice, we bred CD11c DTR tg mice with C3–/– mice. Upon depletion of DCs with DT injection in CD11c DTR tg mice, C3 deficiency led to significantly increased tumor weights compared with C3–/– mice without CD11c DTR transgene. In contrast, DC depletion had no significant impact on tumor growth in C3+/- mice (Fig. 5G). Collectively, these results support our hypothesis that complement has a regulatory role in cDC-NK cell mediated anti-tumor immune response.
DISCUSSION
In the present study, we examined the influence of complement activation on cDC-NK cell interface, innate immunity elements essential in defense against viral infections and tumors. We showed for the first time that complement activation, specifically C5a, plays an inhibitory role for cDC-mediated NK-cell activation. Our finding that C5a-induced TGF-β1 production by Gr-1+ myeloid cells regulates cDC-NK-cell activation reveals a previously unidentified mechanism of immune regulation. We demonstrated the importance of these pathways in vivo by showing cDC and NK cell-dependent suppression of tumor growth in C3–/– mice. These results highlight the complexity of complement mediated regulation of DC function and elucidate new pathways of control (Fig. 5).
It is largely unknown how cDCs are regulated in vivo. Here we demonstrated that Gr-1+ myeloid cells inhibit cDCs in vivo. Both the development and activity of cDCs may be regulated by Gr-1+ myeloid cells. First, the presence of increased numbers of cDCs upon Gr-1+ cell depletion suggests that development of cDCs is affected by Gr-1+ myeloid cells, although complement may not be involved in this process because C3–/– and C5aR–/– mice have similar numbers of splenic cDCs compared with WT mice. Second, Gr-1+ cell depletion leads to augmented TLR3 response by cDCs in complement sufficient but not in C3–/– or C5aR–/– mice, indicating that steady-state cDCs may be conditioned by Gr-1+ myeloid cells and this is dependent upon C5a-induced TGF-β1 production. The suppression of cDC activity induced by Gr-1+myeloid cells is not related to changes in expression of canonical DC markers, including CD11c, MHC class II, CD11b, CD80, CD86 and TLR3. Myeloid Gr-1+CD11b+ cells consist of neutrophils, monocytes, some macrophages, and their progenitor or precursor cells. Treatment with anti-Gr-1 mAb depletes granulocytes, peripheral neutrophils, as well as MDSCs [31]. Regulatory activity has been reported in activated neutrophils and normal BM Gr-1+CD11b+ cells [30, 31]. Of note, MDSCs are the major TGF-β1 producing cells in tumor-bearing mice [32]. We recognize that the TGF-β1-producing Gr-1+ myeloid cells in our study could be related to MDSCs or regulatory neutrophils.
The mechanism we identify for Gr-1+ myeloid cell-mediated suppression of cDCs is novel. They are a significant source of circulating TGF-β1 at steady state, and their production of TGF-β1 is dependent upon C5aR signaling. Several other reports link complement activation to TGF-β1 in other cell types. C3a and C5a induce TGF-β1 production in human and mouse tubular cells, respectively [33, 34], while TGF-β1 upregulates local synthesis of C3 and factor B in human monocytes [35]. Our finding that TGF-β1 suppressed polyI:C induced IL-15 production in cDCs is in contrast to a previous report that TGF-β1 cannot inhibit TLR3-TRIF-dependent pathway in macrophages [36]. This difference may be due to the different cell types studied and experimental conditions. For example, TGF-β1 inhibition of polyI:C-stimulated cDCs occurred only with overnight incubation of the cells with TGF-β1 and was absent with 4 hour incubation (data not shown). Furthermore, increased cytokine production by cDCs from complement-deficient mice was demonstrated by ex vivo stimulation of cells in the absence of TGF-β1 suggesting a pre-conditional effect of TGF-β1 on TLR3 response in cDCs, rather than direct inhibition of MyD88-dependent pathway [36]. C5a-induced TGF-β1 may modulate biological function in other cell types, yet unlike cDCs, NK cell function, known to be regulated by soluble TGF-β1 [37], was not altered in C5aR–/– mice in spite of their reduced TGF-β1 levels. It is possible that sensitivity to TGF-β1 differs between cell types. Moreover, we showed that in BM Gr-1+CD11b+ cells TGF-β1 production is partially dependent on C5a, while plasma TGF-β1 levels in C5aR–/– mice are not higher than those in anti-Gr-1 mAb treated WT mice. This discrepancy may be explained by incomplete depletion of Gr-1+ cells. Nevertheless, the cross-talk between complement system and TGF-β1 in the immune system reveals a new pathway of regulation that may be important for proper function of immune cells.
Multiple studies have examined the role of complement components, particularly C5a, in modulating DC-mediated T-cell activation in various disease models but with no consensus [11-15]. Indeed, the findings are contradictory, perhaps because both cDCs and BMDCs have been targets. BMDCs, derived from monocytes and induced by GM-CSF in vitro, highly expressing C5aR, synthesize and activate complement components locally [11-13]. C5a has been shown to provide co-stimulatory signals for maturation of BMDCs and promote BMDC-mediated T-cell activation [12, 13]. In contrast, we and others showed that splenic cDCs had minimal C5aR expression at steady and activated states [18, 19], suggesting that C5a cannot influence cDCs directly. It is now known that cDCs arise independently from monocytes that give rise to BMDCs [5, 17]. Hence, we predict that the different effects of complement activation on cDCs and BMDCs reflects their distinct lineages and is related to the expression pattern of C5aR.
Given the importance of cDCs and NK cells in anti-tumor immunity, we focused on cDC-NK interactions rather than cDC-T cell interactions, and assessed tumor growth in complement C3 deficient mice. Delayed tumor growth has been noted in complement deficient and C5aR antagonist-treated mice and was shown to be a consequence of defective MDSC function and dependent upon activation of CD8+ T cells [27]. Our findings that NK cells and cDCs control tumor growth in C3–/– mice suggest a new mechanism for complement in tumor models, i.e., cDCs are conditioned by complement-induced TGF-β in tumor-free steady state. We focused on polyI:C stimulation in vitro because cDCs highly express TLR3 [9], although other stimuli could also influence cDC activity. However, the nature of the stimuli that activate cDCs in the tumor-bearing mice is unclear and may extend beyond those we studied. We speculate that cDCs are activated by tumor-derived antigens or endogenous TLR ligands, such as nucleic acids released from apoptotic and necrotic cells in tumor-bearing hosts. Based on our observation in polyI:C activated cDCs, it is possible that complement and Gr-1+ myeloid cells could also regulate activation of cDCs in response to stimuli other than TLR3 ligand in a tumor scenario. Activated cDCs could then prime NK cells, which may be required for cross-priming of tumor-specific CD8+ T cells [38]. In the tumor-bearing hosts, cDCs and NK cells might also be directly suppressed by tumor-associated MDSCs [39], the activity of which is enhanced by complement C5a [27].
In summary, our data suggest a novel model of complement-regulated cDC-NK-cell activation in which the effect of complement activation is mediated through the regulation of TGF-β1 production by Gr-1+ myeloid cells at steady state. We present evidence for another pathway for complement to control host responses to danger.
MATERIALS AND METHODS
Mice
Adult female mice (6-10 wk old) were used in all experiments. C3–/–, CR3–/–, CD11c DTR tg, C57BL/6 and BALB/c mice were purchased from The Jackson Laboratory. C3aR–/– and C5aR–/– mice on BALB/c background are kindly provided by Dr. Craig Gerald from Harvard Medical School. Mice were housed in the animal facilities of Hospital for Special Surgery in accordance with National Institutes of Health guidelines, and the studies were approved by the Institute of Animal Care and Use Committee.
In vivo depletion of DCs, Treg and Gr-1+ myeloid cells
To deplete CD11chigh cells in vivo, CD11c DTR tg mice were i.p. injected with 100 ng DT or a mutant form CRM as control (VWR). Two days later, CD11c expression was analyzed in the spleen with more than 70% reduction in CD11chigh cells and less than 10% reduction in CD11clow cells. To deplete Gr-1+ myeloid cells, mice were i.p. injected with 50 μg anti-Gr-1 mAb (clone RB6-8C5) or isotype control mAb (BD Pharmingen). Three days later, more than 90% CD11b+Gr-1+ cells were depleted in the blood and spleen. To deplete Treg cells, mice were i.p. injected with 100 μg anti-CD25 mAb (clone PC61, BD Pharmingen). Three days later, more than 80% of CD4+CD25+ Treg cells were ablated.
Reagents
Recombinant human C5a was purchased from Calbiochem (EMD chemicals, Gibbstown, NJ). C5aR antagonist (A8δ71-73) was a gift from Dr. Jörg Köhl (Medical School Hanover, Germany). Recombinant TGF-β1 and anti-TGF-β1 mAb (clone 1D11) were purchased from R&D Systems. TLR agonists, including polyI:C, LPS, imiquimod, CpG DNA ODN1585 and control ODN were purchased from Invivogene (San Diego, CA). 5-(and-6)-carboxyfluorescein diacetate succinimydyl ester (CFSE) was purchased from Invitrogen.
Flow cytometry
Cells were blocked for Fc receptors with anti-mouse CD16/CD32 mAb (clone 2.4G2, BD Pharmingen). Fluorochrome conjugated anti-mouse antibodies for flowcytometry were purchased from eBioscience, BD Pharmingen, Cedarline or Abcam, including: anti-NKp46 (29A1.4), anti-CD3ε (145-2C11), anti-CD11c (N418), anti-MHC class II I-A/I-E (M5/114.15.2), anti-CD11b (M1/70), anti-Gr-1 (RB6-8C5), anti-Ly6C (HK1.4), anti-C5aR (20/70), and anti-CD25 mAb(3C7). For intracellular staining of IFN-γ, cells were fixed and permeabilized with cytofix/cytoperm and perm/wash buffer (BD Bioscience). PerCP-Cy5.5 anti-mouse IFN-γ (XMG1.2) was used to stain the cells. Data were acquired on a FACScan or FACS Calibur cytometer (Becton Dickinson). FACS data were analyzed using Flowjo software (Tree Star).
Cell isolation and stimulation
Mouse NK cells were purified from spleen with NK cell isolation kit by negative selection (Miltenyi Biotech). The purity of NK cells are generally >85%. For isolation of cDCs from spleen, in order to avoid NK cell contamination, mice were first depleted of NK cells by injecting anti-NK1.1 (C57BL/6 background) or anti-Asio-GM1 Ab (BALB/c background) 24 hours before DC isolation. Splenic cDCs were then enriched by positive selection of CD11c+ cells using magnetic microbeads (Miltenyi Biotech) and CD11chighMHC class II+ cells were sorted on a Becton-Dickinson Vantage cell sorter (Becton Dickinson), with >95% purity. To isolate Gr-1+CD11b+ cells, BM cells were enriched with biotin-anti-Gr-1 mAb (RB6-8C5) and anti-biotin-magnetic beads (Miltenyi Biotech) and Gr-1+CD11b+ cells were then sorted with >95% purity.
Total splenocytes (2×106 cells), or purified NK (1×105 cells) co-cultured with 2×105 splenic cDCs (DC:NK ratio 2:1), were stimulated with: polyI:C (0.5-20 μg/ml) for 5 hours at 37°C in serum-free OPTI-MEM culture medium (Gibco) in 96-well plate. In some experiments, cDCs were pre-incubated with recombinant hC5a (Sigma-Aldrich) 1 μg/ml for 10 min before polyI:C stimulation.
Cytotoxic assay of NK cells
Total splenocytes or purified NK cells were used as effector cells to kill target YAC-1 cells in a fluorescence-based cytotoxic assay [40]. Briefly, YAC-1 cells were labeled with 200 nM CFSE at 37°C for 15 min, then cultured with purifed NK cells or splenocytes that have been adjusted to contain the same number of NK cells prior to the cytotoxic assay. After 5 hours of incubation at 37°C, cells were stained with 7’-AAD for dead cells. Specific lysis was determined by calculating the percentage of 7’-AAD+ cells in the CFSE+ YAC-1 population.
Quantitative real-time PCR
Total RNA was extracted with RNeasy isolation kit (Qiagen), and reverse-transcribed into cDNA by Superscript RT III (Qiagen). Real-time PCR was performed in triplicate with SYBR-green method on the ABI PRISM 7900HT Sequence Detection system (Applied Biosystem). The expression of each gene (fold change) was calculated by two rounds of normalization (2δδCt method), first, normalizing Ct value of the target gene against the average Ct value of 2 control genes (Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Cyclin I (CCNI)), then, normalizing an experimental condition against untreated control. The primer sequences were: TNF-α forward 5’-CCACCACGCTCTTCTGTCTA-3’, reverse 5’-AGGGTCTGGGCCATAGAACT-3’; IL-12p40 forward 5’-AGGAGACAGAGGAGGGGTGT-3’, reverse 5’-AATAGCGATCCTGAGCTTGC-3’; IL-15 forward 5’-CGTGCTCTACCTTGCAAACA-3’, reverse 5’-TCTCCTCCAGCTCCTCACAT-3’; IL-15Rα forward 5’-TGGCCTGGTACATCAAATCA-3’, reverse 5’-TCTTCATCCTCCTTGCTGCT-3’; CCNI forward 5’-GAAATGGAGAAACTCATTCCTGATT-3’, reverse 5’-CCCGACAGTGGATCAAC-3’; GAPDH forward 5’-ACCCAGAAGACTGTGGATGG-3’, reverse ‘5-GGATGCAGGGATGATGTTCT-3’.
ELISA
Cell culture supernatant or plasma collected in EDTA was measured for TGF-β1 with ELISA kit from eBioscience following the manufacturer's instruction. Except where measurement of active TGF-β1 was specified, total TGF-β1 was measured by treating samples with 1N HCL for 10 min and neutralizing with 1N NaOH in order to activate all latent form of TGF-β1.
Mouse tumor model
Mouse B16 tumor model was established as described before [37]. Briefly, 6-8 wks old WT C57BL/6 and C3–/– female mice were s.c. injected with 5x105 B16 melanoma cells on the right flank. NK cells were depleted by i.p. injection of 100 μg anti-NK1.1 mAb (PK136) or isotype control mAb on day-3, day0 and day7 of tumor inoculation. To deplete DC, CD11c DTR tg and control mice were injected with 100 ng DT i.p. on day5 of tumor inoculation. Tumor growth was monitored by measuring tumor size (mm3=length × width × depth × 0.4). To isolate lymphocytes from tumors, tumor tissues were minced, digested with collagenase and DNase, and lymphocytes were enriched by centrifuging cells in 30% percoll.
Immunohistochemistry
To stain for infiltrated NK cells, frozen tumor sections were fixed in ice-cold acetone for 10 min and blocked with 10% goat serum, 2% BSA/PBS and peroxidase blocking reagent (Dako). Tissues were incubated with biotin-anti-NKp46 Ab (R&D System) at RT for 2 hr, followed with HRP-conjugated secondary Ab, and developed with 3,3’-diaminobenzidine tablets (Sigma). Mayer's hematoxylin (Sigma) was applied for counter-staining.
Statistical analysis
Data were expressed as mean ± s.d. or mean ± s.e.m. Normal distribution of data was tested by Kolmogorov–Smirnov test. Student's t-test was used for statistic analysis of FACS, real-time PCR and tumor growth data. Pearson test was used for correlation analysis. P<0.05 was assigned to reject null hypothesis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Alessandra Pernis, Theresa Lu, and Xiaoyu Hu (Hospital for Special Surgery, New York), and Zeguo Zhao (Memorial Sloan-Kettering Cancer Center, New York) for critical reading of the manuscript; Craig Gerald (Harvard Medical School, Boston) for providing C3aR–/– and C5aR–/– mice; Alan Houghton (Memorial Sloan-Kettering Cancer Center, New York) for providing B16 melanoma cell line; and Jörg Köhl, (Medical School Hanover, Germany) for providing C5aR antagonist.
This work is supported in part by NIH grants RO1 AR 38889 (JES) and the Mary Kirkland Center for Lupus Research at Hospital for Special Surgery.
Abbreviations used
- BMDC
bone marrow-derived dendritic cell
- C5aR
C5a receptor
- cDC
conventional DC
- DTR
diphtheria toxin receptor
- MDSC
myeloid derived suppressor cell
Footnotes
Conflict of interest
The authors declare no commercial or financial conflicts of interest.
REFERENCE
- 1.Brilot F, Strowig T, Munz C. NK cells interactions with dendritic cells shape innate and adaptive immunity. Front Biosci. 2008;13:6443–6454. doi: 10.2741/3165. [DOI] [PubMed] [Google Scholar]
- 2.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyake T, Kumagai Y, Kato H, Guo Z, Matsushita K, Satoh T, Kawagoe T, et al. Poly I:C-induced activation of NK cells by CD8 alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J.Immunol. 2009;183:2522–2528. doi: 10.4049/jimmunol.0901500. [DOI] [PubMed] [Google Scholar]
- 4.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J.Exp.Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat.Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 7.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J.Exp.Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur.J.Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 9.Luber CA, Cox J, Lauterbach H, Fancke B, Selbach M, Tschopp J, Akira S, et al. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity. 2010;32:279–289. doi: 10.1016/j.immuni.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat.Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng Q, Li K, Wang N, Li Q, Asgari E, Lu B, Woodruff TM, et al. Dendritic cell function in allostimulation is modulated by C5aR signaling. J.Immunol. 2009;183:6058–6068. doi: 10.4049/jimmunol.0804186. [DOI] [PubMed] [Google Scholar]
- 13.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J.Clin.Invest. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver DJ, Jr., Reis ES, Pandey MK, Kohl G, Harris N, Gerard C, Kohl J. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur.J.Immunol. 2010;40:710–721. doi: 10.1002/eji.200939333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 17.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol.Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto M, Hirota K, Yoshitomi H, Maeda S, Teradaira S, Akizuki S, Prieto-Martin P, et al. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J.Exp.Med. 2010;207:1135–1143. doi: 10.1084/jem.20092301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soruri A, Kim S, Kiafard Z, Zwirner J. Characterization of C5aR expression on murine myeloid and lymphoid cells by the use of a novel monoclonal antibody. Immunol.Lett. 2003;88:47–52. doi: 10.1016/s0165-2478(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 20.Charriaut C, Senik A, Kolb JP, Barel M, Frade R. Inhibition of in vitro natural killer activity by the third component of complement: role for the C3a fragment. Proc.Natl.Acad.Sci.U.S.A. 1982;79:6003–6007. doi: 10.1073/pnas.79.19.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SY, Racila E, Taylor RP, Weiner GJ. NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood. 2008;111:1456–1463. doi: 10.1182/blood-2007-02-074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung S, Unutmaz D, Wong P, Sano G, De los SK, Sparwasser T, Wu S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J.Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Q, Egelston C, Gagnon S, Sui Y, Belyakov IM, Klinman DM, Berzofsky JA. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J.Clin.Invest. 2010;120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc.Natl.Acad.Sci.U.S.A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, et al. Modulation of the antitumor immune response by complement. Nat.Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlueter AJ, Malek TR, Hostetler CN, Smith PA, deVries P, Waldschmidt TJ. Distribution of Ly-6C on lymphocyte subsets: I. Influence of allotype on T lymphocyte expression. J.Immunol. 1997;158:4211–4222. [PubMed] [Google Scholar]
- 29.Otto M, Hawlisch H, Monk PN, Muller M, Klos A, Karp CL, Kohl J. C5a mutants are potent antagonists of the C5a receptor (CD88) and of C5L2: position 69 is the locus that determines agonism or antagonism. J.Biol.Chem. 2004;279:142–151. doi: 10.1074/jbc.M310078200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31:761–771. doi: 10.1016/j.immuni.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Ribechini E, Leenen PJ, Lutz MB. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur.J.Immunol. 2009;39:3538–3551. doi: 10.1002/eji.200939530. [DOI] [PubMed] [Google Scholar]
- 32.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J.Exp.Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boor P, Konieczny A, Villa L, Schult AL, Bucher E, Rong S, Kunter U, et al. Complement C5 mediates experimental tubulointerstitial fibrosis. J.Am.Soc.Nephrol. 2007;18:1508–1515. doi: 10.1681/ASN.2006121343. [DOI] [PubMed] [Google Scholar]
- 34.Peake PW, O'Grady S, Pussell BA, Charlesworth JA. C3a is made by proximal tubular HK-2 cells and activates them via the C3a receptor. Kidney Int. 1999;56:1729–1736. doi: 10.1046/j.1523-1755.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- 35.Hogasen AK, Hestdal K, Hogasen K, Abrahamsen TG. Transforming growth factor beta modulates C3 and factor B biosynthesis and complement receptor 3 expression in cultured human monocytes. J.Leukoc.Biol. 1995;57:287–296. doi: 10.1002/jlb.57.2.287. [DOI] [PubMed] [Google Scholar]
- 36.Naiki Y, Michelsen KS, Zhang W, Chen S, Doherty TM, Arditi M. Transforming growth factor-beta differentially inhibits MyD88-dependent, but not TRAM- and TRIF-dependent, lipopolysaccharide-induced TLR4 signaling. J.Biol.Chem. 2005;280:5491–5495. doi: 10.1074/jbc.C400503200. [DOI] [PubMed] [Google Scholar]
- 37.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat.Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Lou Y, Lizee G, Qin H, Liu S, Rabinovich B, Kim GJ, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J.Clin.Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J.Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 40.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.