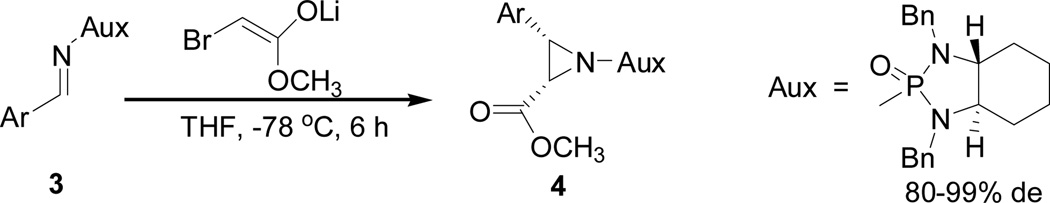
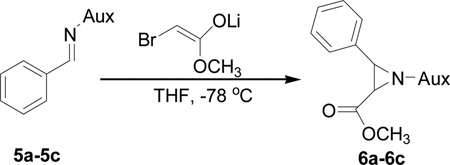
Abstract
Highly diastereoselective asymmetric synthesis of chiral aziridine-2-carboxylic esters is reported for 20 examples with good yields (51–87%) and excellent diastereoselectivities (>99:1 dr for most cases). The modified N-phosphonyl imines are proven to be superior to previous imine auxiliaries for the aza Darzens reaction by using secondary isopropyl to replace primary benzyl group for N,N-diamino protection. In the meanwhile, a special operation by slowly adding the pre-cooled imine solution at −78 °C into the preformed β-bromo lithium enolate mixture at this temperature in the presence of 4 Å molecular sieves was found to be crucial in terms of yields and diastereoselectivity. The present method can provide an easy and general access to β-hydroxy α-amino acids and other important amino building blocks.
Introduction
The asymmetric formation of nitrogen heterocycles from readily accessible starting materials has become an important topic for chemical and biomedical research.1 One of the privileged substructures among the nitrogen-containing heterocycles is represented by the aziridine ring which has been found in a variety of biologically active natural products including mitomycin, azicemycin, azinomycin and maduropeptin.2a,2b The structure-activity relationship (SAR) studies on mitosane derivatives revealed that the aziridine ring is crucial for their antitumor activity and selectivity.2c–2f This study has led to the finding that mitomycin C can act as the most potent candidate possessing antitumor activities against stomach, breast, oesophagus and bladder tumors.2b Many other aziridine-containing derivatives have also been found to exhibit various biological properties.2a,3a,3b For example, 2-(4-amino-4-carboxybutyl)aziridine-2-carboxylic acid 1 can serve as a potent irreversible inhibitor of the bacterial enzyme diaminopimelic acid epimerase while 2-(2- carboxyethyl)aziridine-2-carboxylic acid 2 as an irreversible inhibitor of glutamate racemase (Fig. 1).2a,3a,3b One of the major reasons for aziridines to play an important role in chemical and biomedical sciences is based on the fact that the strained three-membered rings can undergo regio- and stereoselective ring opening reactions.4a,4b For example, the aziridine-2-carboxylic esters can serve as versatile building blocks for making α- and β-amino acids, β-substituted α-amino acids, β-hydroxy α-amino acids, α-amino aldehydes and ketones.5,9a
Fig. 1.
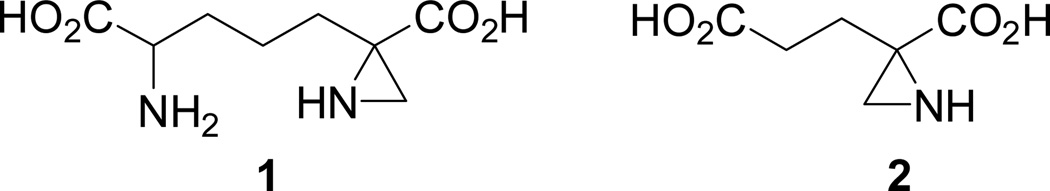
Biologically active synthetic aziridine-2-carboxylic acids
The asymmetric synthesis of aziridines has been pursued in several research groups for many years;6 typical known aziridination methods are represented by organocatalytic reactions of imines and enals, and transition metal catalyzed addition of amine or azide onto alkenes.7,8 Although a great progress has been made on this synthetic topic, it is still remained challenging to develop more efficient and facile approaches to a broad range of structurally diverse aziridines in enantio- and diastereoselective manner. In the past several years, we have designed and synthesized new chiral reagents of N-phosphonyl and N-phosphinyl amides and imines; we have successfully utilized them in various asymmetric reactions such as asymmetric aza-Darzen reaction,9a asymmetric aza-Henry reaction,9b asymmetric addition of allylmagnesium bromides,9c asymmetric synthesis of N-phosphonyl β-amino Weinreb amides,11a asymmetric synthesis of N-phosphonyl propargyl amines,12a and asymmetric synthesis of α-amino-1,3-dithianes.12c In our preliminary work on asymmetric aza-Darzens reaction, we utilized N,N-dibenzyl N- phosphonyl imines as the electrophiles to react with pre-generated β-bromo lithium enolate to give the aziridine products in good yields and diastereoselectivities.9a The important feature of these chiral N-phosphonyl imines is shown by the fact that the steric and electronic properties can be optimized by making modifications on different positions of the imine auxiliaries. In the continuing project on N-phosphonyl imines, herein we would like to report a more efficient asymmetric aza-Darzens reaction by using isopropyl to replace primary benzyl group for N,N-diamino protection on auxiliary and by modifying the synthetic operation.
Results and Discussion
In 1999, one of the great asymmetric methods for synthesizing aziridine-2-carboxylic esters was reported by Davis and coworkers through N-p-toluenesulfinyl imine-based Darzens reaction.13 The reaction showed the great success in regard to chemical yields and diastereoselectivity. Despite the above approach to these building blocks, we also made efforts on development of a facile method for the asymmetric synthesis of aziridine-2-carboxylic esters using N-phosphonyl imines as electrophiles. The advantages of using chiral N-phosphonyl imines would be showed by the following factors: higher thermolytic stability, modification and flexibility at their multiple sites, inertness of phosphonyl group to oxidative conditions, the 31P NMR determination of diastereoselectivity and easy recycling of diamine auxiliary precursors.9–12 Importantly, our N-phosphonyl and N-phosphinyl imine-based work has resulted in a new concept called GAP chemistry (Group-Assisted Purification chemistry)14 which can enable organic synthesis to be performed without using traditional purification techniques such as column chromatography and recrystallization, albeit a few non-GAP exceptions were encountered including the present aziridine formations in which there is no N-H moiety in the products.15 In our first aza-Darzens synthesis, we utilized N-benzyl as the protection group on phosphonyl imine auxiliary. Unfortunately, this auxiliary only showed a limitation on controlling diastereoselectivities in which only one example gave an excellent diastereoselectivity of 99% de (Scheme 1).9a
Scheme 1.
Synthesis of aziridine-2-carboxylic esters using N,N-dibenzylphosphonylimines
In order to achieve more efficient asymmetric synthesis of aziridine-2-carboxylic esters, we made an effort on the structural modifications on phosphonyl imine auxiliary by using other functional groups to replace the original benzyl and by changing their diamino core structures. We first synthesized the cyclohexane-based N,N-di-1-naphthylmethylphosphonyl benzaldimine (5a) and subjected it to the reaction with the pre-generated β-bromo lithium enolate. We found the reaction occurred to completion within a prolonged period of over 8.5 hours, but many impurities were formed with the diastereoselectivity of dr = 100:42 as revealed by the crude 31P NMR determination. This observation prove that the N,N-bisfunctional modifications can result in changes not only on solubility of products but also on the electrophilicity of N-phosphonyl imines. We next utilized another chiral imine (5b) which was available at that time in our labs and found that the reaction also proceeded at a slower rate with some starting material still remained even after 8.5 h. To make all chiral imine starting materials to be consumed, the reaction was then performed at room temperature. However, the diastereoselectivity was not improved much with dr = 100:15 with many impurities co-existing with the major isomeric product.
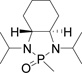
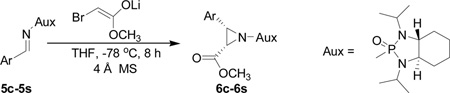
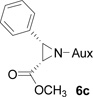
In several of our previous systems, the combination of N,N-diisopropyl and diaminocyclohexyl scaffolds have been proven to be superior to others in several nucleophilic addition reactions and syntheses, such as aza-Henry reaction,9b the addition reaction of allyl magnesium bromide,9c the synthesis of β-amino weinreb amides,11a α,β-diamino esters11d chiral propargyl amines,12a and umpolung reaction with lithium 1,3-dithianes.12c Therefore, this combination was considered to enhance the present synthesis of aziridine-2-carboxylic esters. We now found that (1R, 2R)-diaminocyclohexyl derived N,N-diisopropyl-N-phosphonyl benzaldimine (5c) can react with β-bromo lithium enolate smoothly to completion within 8 hours. Importantly, we found that by slowly adding the pre-cooled benzaldimine (5c) solution in THF at −78 °C into preformed β-bromo lithium enolate mixture at the same temperature in the presence of 4 Å molecular sieves is crucial in terms of yields and diastereoselectivity. With the optimized conditions in hand, we successfully synthesized aziridine-2-carboxylic esters with excellent diastereoselectivity (>99%, almost single diastereomer) as revealed by 31P NMR determination of crude products. The results were summarized in Table 1.
Table 1.
Optimizing the stereo effect of chiral N-phosphonyl iminesa
Reaction conditions: 0.57 mmol imine, 1.15 mmol methyl-2-bromo acetate, 1.20 mmol LiHMDS 14 mL solvent, −78 °C.
Diastereoselectivities were determined by 31P-NMR analysis of crude products.
>99:1 means only one isomer was observed by 31P NMR.
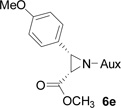
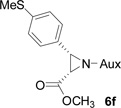
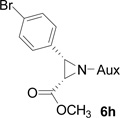
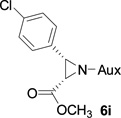
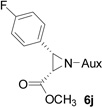
On the basis of the above optimal conditions, the substrate scope of this reaction was next examined. Besides N,N-diisopropylphosphonyl imines with both electron donating and electron withdrawing groups on the aromatic rings (entries 2–15, Table 2), we also examined substrate scope of two heterocyclic aldimines to give 85% and 76% yields and excellent diastereoselectivities (entries 16 and 17, Table 2). For all cases, isolated yields ranged from 51% to 87% and excellent diastereoselectivities from 96.2:3.8 to >99:1 were obtained. Similar to the previous system,9a the major isomeric products were confirmed to be formed in cis-geometry (Table 2).
Table 2.
Asymmetric addition of lithium enolate of methyl 2-bromoacetate onto N,N-diisopropylphosphonyl iminesa
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Yield(%)b | drc,d |
| 1 | 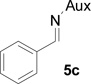 |
 |
78 | >99:1 |
| 2 |  |
 |
70 | 98.8:1.2 |
| 3 | 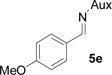 |
 |
84 | 98.7:1.7 |
| 4 | 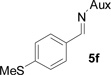 |
 |
71 | >99:1 |
| 5 | 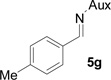 |
 |
87 | >99:1 |
| 6 | 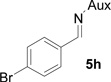 |
 |
69 | >99:1 |
| 7 | 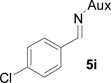 |
 |
70 | >98:2 |
| 8 | 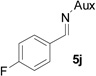 |
 |
79 | 99:1 |
| 9 | 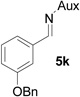 |
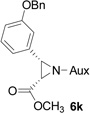 |
71 | >99:1 |
| 10 | 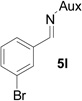 |
 |
75 | >99:1 |
| 11 | 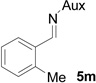 |
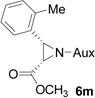 |
61 | >99:1 |
| 12 | 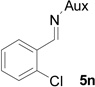 |
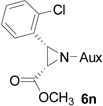 |
52 | >99:1 |
| 13 | 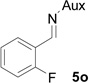 |
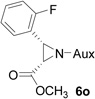 |
51 | >99:1 |
| 14 | 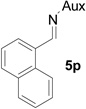 |
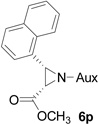 |
73 | >99:1 |
| 15 | 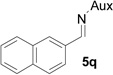 |
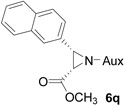 |
77 | >99:1 |
| 16 | 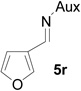 |
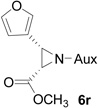 |
85 | >99:1 |
| 17 | 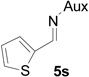 |
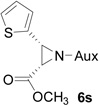 |
76 | 96.2:3.8 |
Reaction conditions: 0.57 mmol imine, 1.15 mmol methyl-2-bromo acetate, 1.20 mmol LiHMDS 14 mL solvent, −78 °C.
Isolated yield of the pure product.
Diastereoselectivities were determined by 31P-NMR analysis of crude products.
>99:1 means only diasteroisomer was observed in crude 31P-NMR spectrum.
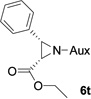
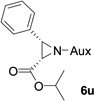
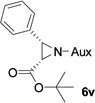
In addition to N,N-diisopropylphosphonyl imines, we also examined bromo lithium enolates derived from different bromo esters. As shown by Table 3, ethyl, isopropyl and tert-butyl group-derived enolates afforded good chemical yields and excellent diastereoselectivities (>99% dr). The steric effect of these ester groups did not affect cis-geometry and diastereoselectivity of the products.
Table 3.
Asymmetric addition of lithium enolates of different 2-bromoacetates onto N,N-diisopropylphosphonyl benzaldiminea
Reaction conditions: 0.57 mmol imine, 1.15 mmol methyl-2-bromo acetate, 1.20mmol LiHMDS 14 mL solvent, −78 °C.
Isolated yield of the pure product.
Diastereoselectivities were determined by 31P-NMR analysis of crude products.
>99:1 means only one isomer was observed by 31P NMR.
As compared to our previous aziridination, the present N,N-diisopropylphosphonyl imine-based synthesis resulted in much higher diastereoselectivity. In most cases, only one isomeric product was observed as proven by the 31P-NMR analysis of resulting crude products. The secondary alkyl group (isopropyl) is superior to its primary counterpart (benzyl) for cyclohexane diamino template in controlling asymmetric induction for this reaction. For many cases, not only diastereoselelectivity but also chemical yields were increased, particularly, for 4-methyl and 4-fluoro substrates, the yields were increased from 76 % and 64% to 87% and 79%, respectively (entries 3 and 8, Table 2). The present synthesis showed the synthesis of twenty (2S,3S) aziridine-2-carboxylic esters, which may indicate a broader substrate scope than the previous one.
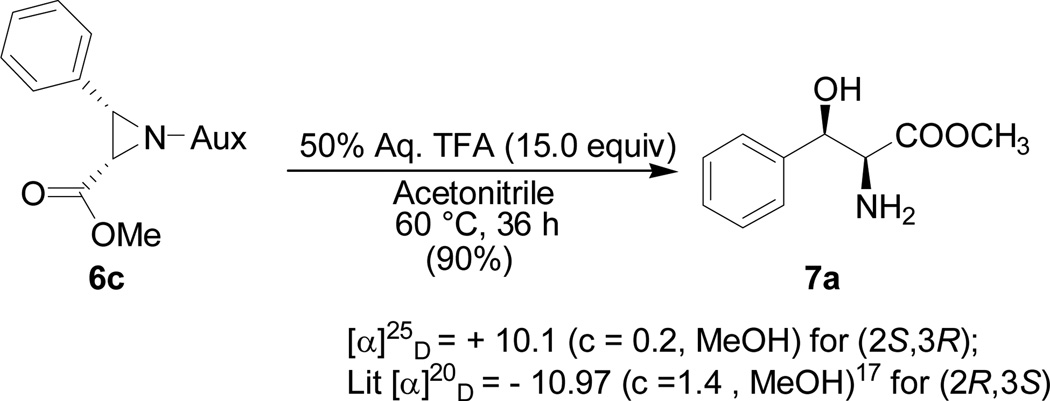
As anticipated the auxiliary can be successfully cleaved by treating the aziridine product (6c) with trifluoroacetic acid in acetone and water co-solvents at room temperature to give the ring opening product of (2S,3R)-β-hydroxy α-amino acid methyl ester (7a) as major diastereomer (Scheme 2). The cleavage reaction is used not only for the absolute structure determination, but also for providing an efficient approach to unnatural amino acids.16
Scheme 2.
Cleavage of the auxiliary and synthesis of (2S,3R)-β-hydroxy α-amino acid methyl ester
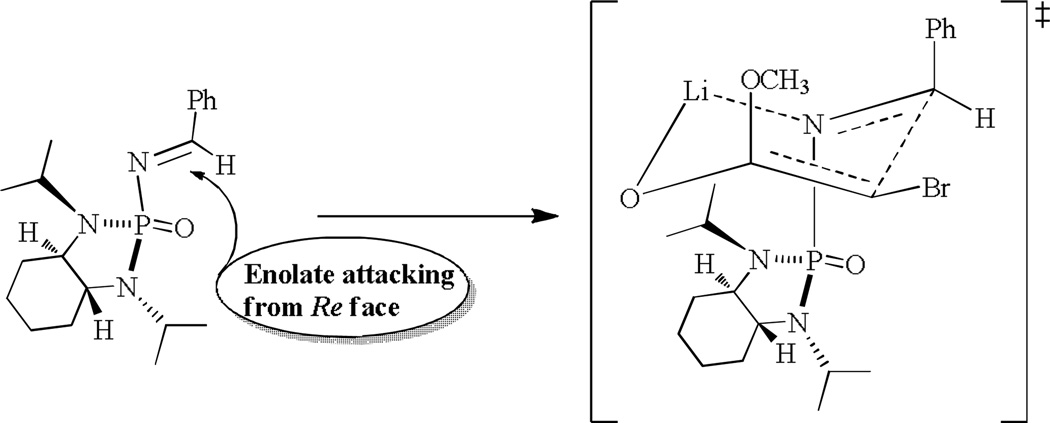
Similar to the previous system, a six-membered chair like transition state was proposed (Figure 3). The lithium metal cation serves as the Lewis acidic anchor to connect two reaction partners, imine electrophile and enolate nucleophile together, prior to aza Darzens reaction. This anchored reaction manner would be responsible for the excellent control of stereochemistry in which the enolate attacks imine's carbon center on its Re face. The asymmetric control is indirectly controlled by two nitrogens that are forced to be the original chirality of the nearby (2S,3S) vicinal centers on cyclohexane ring.
Fig. 3.
Proposed transition state model for the asymmetric aza-Darzens reaction.
Conclusions
In conclusion, we have developed an efficient protocol for the asymmetric synthesis of aziridine-2-carboxylic esters by using N,N-diisopropyl-N-phosphonyl imines in which the secondary alkyl is superior to its primary counterpart in providing asymmetric environment. Good yields and excellent diastereoselectivity have been achieved for a good spectrum of twenty substrates. A special operation manner by slowly adding the pre-cooled imine solution at −78 °C into preformed β-bromo lithium enolate mixture at the same temperature in the presence of 4 Å molecular sieves was found to be crucial for the outcomes.
Experimental Section
General experimental methods
All the reactions were carried out in oven-dried glassware under an atmosphere of dry argon, unless otherwise stated. All commercially available reagents were used without further purification. THF used for the reaction was distilled using benzophenone-sodium still under nitrogen prior to use. 4 Å molecular sieves were dried in oven prior to use. Solvents used for extraction and purification were used directly without further distillation. Reaction temperatures are reported as bath temperatures. NMR data was reported as follows: chemical shifts in ppm on the δ scale, multiplicity as (s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublet, m = multiplet), coupling constants in (Hz). 1H NMR spectra were recorded at ambient temperature at 500 MHz. 13C NMR spectra were recorded at ambient temperature at 125 MHz. 31P NMR spectra were recorded at ambient temperature at 202 MHz. 19F NMR were recorded at ambient temperature at 376 MHz. 1H NMR spectra were acquired in CDCl3, chemical shifts were reported as δ values in ppm and were calibrated according to internal CHCl3 (7.26 ppm). For 13C NMR spectra, chemical shifts are reported as δ values in ppm relative to CDCl3 (77.00 ppm). For 31P NMR spectra, chemical shifts are reported as δ values in ppm. For 19F NMR spectra, chemical shifts are reported as δ values in ppm. Infrared spectra (IR) were obtained on an FTIR spectrophotometer and are reported in wavenumbers (cm−1). HRMS data was collected at the University of Illinois at Urbana-Champagne instrumentation facility. Optical rotation values were taken using AUTOPOL IV automatic polarimeter. Analytical thin-layer chromatography (TLC) was performed using 0.25mm precoated silica gel plates and the compounds were visualized with UV light (λ = 254 nm) and/or immersing in iodine stain. Compounds were purified using flash column chromatography (forced flow) on silica gel (SiO2) 60 Å (32–63 µm) particle size.
General synthetic procedure for the asymmetric aza-Darzens reaction
Into an oven dried and argon flushed reaction vial, 4 Å molecular sieves were first taken followed by dry THF (7 mL) and methyl 2-bromoacetate (1.15 mmol, 2 equiv) and stirred. Reaction mixture was then cooled to −78 °C using dry ice-acetone mixture and lithium bis(trimethylsilylamide) (LiHMDS) (1 M solution in THF, 1.20 mmol, 2.1 equiv) was added slowly dropwise and the resulting reaction mixture was stirred for 45 min at −78 °C. N-phosphonyl imine (0.57 mmol, 1 equiv, dissolved in 7 mL of dry THF) properly sealed with a rubber septum in dry argon atmosphere, was cooled to −78 °C in the same dry ice-acetone bath for 45 min. After 45 min, the imine was added dropwise via a cannula at −78 °C and stirring was continued for 8h (reaction was monitored by TLC and/or crude NMR) at the same temperature. After confirming the consumption of imine, the reaction mixture was quenched with water (2 mL) and the organic layer was extracted with ethyl acetate. The organic layers were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified using flash column chromatography on silica gel (Rf = 0.25) using EtOAc/Hexanes (7:3) as the eluent to afford the aziridine product.
Compound 6c
White solid: mp 99–101 °C; IR (ATR) ν 2965, 2938, 1749, 1455, 1361, 1243, 1198, 1170, 1105, 1058, 1033, 1014, 939, 924, 876, 823, 787, 739, 723 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.44 (d, J = 7 Hz, 2H), 7.36-7.20 (m, 3H), 3.72 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.66-3.51 (m, 2H), 3.49 (s, 3H), 3.41 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.09-2.92 (m, 2H), 2.11 (d, J = 7.5 Hz, 1H), 2.05 (d, J = 10.5 Hz, 1H), 1.91-1.61 (m, 2H), 1.34 (d, J = 7 Hz, 6H), 1.31-1.24 (m, 1H), 1.21 (dd, J = 7.5 Hz and 3.5 Hz, 6H), 1.08 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.54, 134.09, 127.86, 127.78, 59.99, 58.92, 51.79, 45.53, 44.70, 42.70, 41.98, 31.55, 30.27, 24.23, 23.43, 22.65, 20.28, 20.18; 31P (202 MHz, CDCl3) δ 30.82; HRMS-(ESI) m/z [M+H]+ calcd for C22H35N3O3P, 420.2416; found, 420.2425; [α]D25 = −19.9 (c =1, CHCl3).
Compound 6d
Oily liquid; IR (thin film/NaCl) ν 2935, 2867, 1752, 1728, 1611, 1513, 1455, 1364, 1300, 1243, 1215, 1172, 1107, 1082, 1061, 1024, 929, 826 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.46-7.33 (m, 6H), 7.33-7.27 (m, 1H), 6.90 (d, J = 8.5 Hz, 2H), 5.00 (s, 2H), 3.67 (dd, J = 17.5 Hz and 6 Hz, 1H), 3.63-3.51 (m, 1H), 3.48 (s, 3H), 3.47-3.40 (m, 1H), 3.36 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.05-2.92 (m, 2H), 2.18-2.07 (m, 1H), 2.06-1.95 (m, 1H), 1.76 (s, 2H), 1.33 (d, J = 7 Hz, 6H), 1.30-1.23 (m, 1H), 1.2 (d, J = 6.5 Hz, 6H), 1.07 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.52, 158.39, 136.79, 128.86, 128.41, 127.83, 127.41, 126.22, 114.10, 69.81, 59.85, 58.81, 51.68, 45.40, 44.57, 42.35, 41.82, 31.43, 30.14, 24.11, 23.33, 22.52, 20.17, 20.05; 31P (202 MHz, CDCl3) δ 30.80; HRMS-(ESI) m/z [M+H]+ calcd for C29H41N3O4P, 526.2835; found, 526.2842; [α]D25 = −25.52 (c =0.76, CHCl3).
Compound 6e
White solid: mp 84–86 °C; IR (ATR) ν 2936, 2867, 1748, 1614, 1515, 1456, 1436, 1396, 1367, 1304, 1246, 1217, 1194, 1170, 1107, 1062, 1027, 1010, 995, 921, 879, 829, 764, 746, 725 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.37 (d, J = 8.5 Hz, 2H), 6.83 (d, J = 9 Hz, 2H), 3.78 (s, 3H), 3.67 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.64-3.53 (m, 1H), 3.51 (s, 3H), 3.49-3.41 (m, 1H), 3.37 (dd, J = 17.5 Hz and 6.5 Hz, 1H), 2.99 (s, 2H), 2.11 (d, J = 6Hz, 1H), 2.04 (d, J = 11 Hz, 1H), 1.78 (s, 2H), 1.34 (d, J = 7 Hz, 6H), 1.32-1.25 (m, 1H), 1.21 (d, J = 7 Hz, 6H), 1.08 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.68, 159.21, 128.95, 126.07, 113.30, 59.96, 58.93, 55.14, 51.81, 45.52, 44.70, 42.45, 41.94, 31.59, 30.28, 24.27, 23.48, 22.65, 20.27; 31P (202 MHz, CDCl3) δ 30.84; HRMS-(ESI) m/z [M+H]+ calcd for C23H37N3O4P, 450.2522; found, 450.2511; [α]D25 = −31.12 (c =0.75, CHCl3).
Compound 6f
White solid: mp 122–125 °C; IR (ATR) ν 2935, 2864, 1749, 1494, 1435, 1368, 1245, 1218, 1194, 1163, 1103, 1098, 1061, 1028, 997, 925, 883, 823, 746, 727 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.34 (d, J = 8 Hz, 2H), 7.13 (d, J = 8 Hz, 2H), 3.63 (dd, J = 15 Hz and 6 Hz, 1H), 3.59-3.50 (m, 1H), 3.47 (s, 3H), 3.45-3.39 (m, 1H), 3.35 (dd, J = 15 Hz and 6.5 Hz, 1H), 2.96 (s, 2H), 2.42 (s, 3H), 2.08 (s, 1H), 2.01 (d, J = 11.5 Hz, 1H), 1.75 (s, 2H), 1.31 (d, J = 7 Hz, 6H), 1.27-1.21 (m, 1H), 1.17 (t, J = 6.5 Hz, 6H), 1.04 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.38, 137.86, 130.73, 128.16, 125.62, 59.87, 58.81, 51.74, 45.40, 44.58, 42.33, 41.89, 31.44, 30.14, 24.12, 23.33, 22.52, 20.13, 15.40; 31P (202 MHz, CDCl3) δ 30.72; HRMS-(ESI) m/z [M+H]+ calcd for C23H37N3O3PS, 466.2293; found, 466.2305; [α]D25 = −18.13 (c =0.75, CHCl3).
Compound 6g
White solid: mp 120–122 °C; IR (ATR) ν 2937, 2867, 1749, 1396, 1366, 1249, 1217, 1193, 1164, 1107, 1062, 1026, 1010, 923, 881, 821, 759, 746, 726 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.33 (d, J = 8 Hz, 2H), 7.09 (d, J = 8 Hz, 2H), 3.68 (dd, J = 17.5 Hz and 6 Hz, 1H), 3.63-3.53 (m, 1H), 3.51 (s, 3H), 3.48-3.41 (m, 1H), 3.37 (dd, J = 15 Hz and 6.5 Hz, 1H), 2.99 (s, 2H), 2.31 (s, 3H), 2.11 (d, J = 6 Hz, 1H), 2.04 (d, J = 11 Hz, 1H), 1.78 (s, 2H), 1.34 (d, J = 7 Hz, 6H), 1.27 (m, 1H), 1.21 (dd, J = 7 Hz, 6H), 1.08 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.62, 137.41, 131.023, 128.59, 127.66, 59.96, 58.89, 51.79, 45.52, 44.69, 42.72, 41.89, 31.55, 30.29, 24.23, 23.46, 22.65, 21.19, 20.22; 31P (202 MHz, CDCl3) δ 30.87; HRMS-(ESI) m/z [M+H]+ calcd for C23H37N3O3P, 434.2573; found, 434.2575; [α]D25 = −29.73 (c =0.76, CHCl3).
Compound 6h
White solid: mp 139–141 °C; IR (ATR) ν 2936, 1752, 1487, 1444, 1389, 1362, 1242, 1216, 1192, 1163, 1103, 1063, 1026, 1010, 997, 924, 910, 884, 827, 744, 726 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.42 (d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.5 Hz, 2H), 3.66 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.62-3.52 (m, 1H), 3.51 (s, 3H), 3.50-3.43 (m, 1H), 3.40 (dd, J = 12.5 Hz and 6 Hz, 1H), 3.06-2.90 (m, 2H), 2.14 (d, J = 6.5 Hz, 1H), 2.03 (d, J = 11.5 Hz, 1H), 1.89-1.67 (m, 2H), 1.37 (d, J = 6.5 Hz, 6H), 1.30-1.23 (m, 1H), 1.18 (t, J = 7 Hz, 6H), 1.04 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.35, 133.20, 131.05, 129.55, 121.87, 60.01, 58.95, 51.93, 45.50, 44.69, 42.03, 31.51, 30.20, 24.22, 23.38, 22.62, 20.25; 31P (202 MHz, CDCl3) δ 30.59; HRMS-(ESI) m/z [M+H]+ calcd for C22H34BrN3O3P, 498.1521; found, 498.1512; [α]D25 = −19.63 (c =0.83, CHCl3).
Compound 6i
White solid: mp 128–130 °C; IR (ATR) ν 2938, 2868, 1752, 1491, 1394, 1362, 1243, 1216, 1192, 1162, 1104, 1086, 1062, 1026, 1011, 998, 923, 909, 886, 825, 745, 727 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.40 (d, J = 8.5 Hz, 2H), 7.28 (d, J = 8 Hz, 2H), 3.68 (dd, J = 17.5 Hz and 6.5 Hz, 1H), 3.63-3.53 (m, 1H), 3.51 (s, 3H), 3.49-3.37 (m, 2H), 3.08-2.92 (m, 2H), 2.12 (s, 1H), 2.05 (d, J = 12 Hz, 1H), 1.80 (s, 2H), 1.46-1.31 (m, 6H), 1.31-1.24 (m, 1H), 1.21 (t, J = 7 Hz, 6H), 1.06 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.36, 133.63, 132.65, 129.20, 120.10, 60.00, 58.94, 51.90, 45.48, 44.68, 42.02, 31.51, 30.20, 24.22, 23.37, 22.61, 20.24; 31P (202 MHz, CDCl3) δ 30.63; HRMS-(ESI) m/z [M+H]+ calcd for C22H34ClN3O3P, 454.2026; found, 454.2024; [α]D25 = −35.60 (c =0.86, CHCl3)
Compound 6j
Oily liquid: IR (thin film/NaCl) ν 2937, 2869, 1752, 1732, 1605, 1511, 1438, 1396, 1364, 1298, 1212, 1175, 1106, 1061, 1026, 929, 881, 831, 770, 726 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.37 (t, J = 7.5 Hz, 2H), 6.92 (t, J = 9 Hz, 2H), 3.64 (dd, J = 15 Hz and 6 Hz, 1H), 3.57-3.46 (m, 1H), 3.43 (s, 3H), 3.42-3.37 (m, 1H), 3.33 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.02-2.84 (m, 2H), 2.06 (s, 1H), 1.99 (d, J = 11.5 Hz, 1H), 1.73 (s, 2H), 1.28 (d, J = 7 Hz, 6H), 1.25-1.18 (m, 1H), 1.15 (t, J = 6.5 Hz, 6H), 1.00 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.25, 163.24, 161.28, 129.62, 129.31, 114.66, 59.84, 58.79, 51.66, 45.32, 44.52, 41.81, 31.36, 30.03, 24.06, 23.18, 22.42, 20.06; 31P (202 MHz, CDCl3) δ 30.68; 19F NMR (376.17 MHz, CDCl3) δ −114.20; HRMS-(ESI) m/z [M+H]+ calcd for C22H34FN3O3P, 438.2322; found, 438.2326; [α]D25 = −30.86 (c =0.75, CHCl3).
Compound 6k
Oily liquid: IR (thin film/NaCl) ν 2936, 2868, 1754, 1730, 1584, 1488, 1449, 1395, 1364, 1246, 1209, 1175, 1106, 1081, 1060, 1026, 955, 928, 903, 878, 815, 791, 734 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.41 (d, J = 7 Hz, 2H), 7.35 (t, J = 7.5 Hz, 2H), 7.29 (d, J = 7 Hz, 1H), 7.18 (t, J = 8 Hz, 1H), 7.09 (s, 1H), 7.03 (d, J = 7.5 Hz, 1H), 6.86 (d, J = 8 Hz, 1H), 5.03 (s, 2H), 3.67 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.61-3.49 (m, 1H), 3.47 (s, 3H), 3.46-3.33 (m, 2H), 2.95 (s, 2H), 2.08 (s, 1H), 2.02 (d, J = 11.5 Hz, 1H), 1.76 (s, 2H), 1.31 (d, J = 7 Hz, 6H), 1.19 (d, J = 7 Hz, 7H), 1.07 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.29, 158.31, 136.86, 135.64, 128.79, 128.30, 127.71, 127.28, 120.37, 114.49, 113.77, 77.25, 77.00, 76.74, 69.70, 59.82, 58.75, 51.67, 45.40, 44.53, 42.59, 41.83, 31.38, 30.12, 24.06, 23.38, 22.47, 20.06; 31P (202 MHz, CDCl3) δ 30.69; HRMS-(ESI) m/z [M+H]+ calcd for C29H41N3O4P, 526.2835; found, 526.2829; [α]D25 = −21.01 (c =0.79, CHCl3).
Compound 6l
White solid: mp 130–132 °C; IR (ATR) ν 2981, 2932, 1757, 1400, 1361, 1238, 1219, 1198, 1175, 1140, 1105, 1062, 1042, 1018, 927, 909, 879, 843, 788, 758, 723 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.59 (s, 1H), 7.44-7.34 (m, 2H), 7.17 (t, J = 8 Hz, 1H), 3.66 (dd, J = 17.5 Hz and 6.5 Hz, 1H), 3.62-3.54 (m, 1H), 3.52 (s, 3H), 3.50-3.31 (m, 2H), 3.08-2.93 (m, 2H), 2.12 (s, 1H), 2.05 (d, J = 11.5 Hz, 1H), 1.79 (s, 2H), 1.36 (s, 2H), 1.33 (d, J = 7 Hz, 4H), 1.31-1.24 (m, 1H), 1.21 (dd, J = 6.5 Hz and 3 Hz, 6H), 1.08 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.30, 136.54, 130.93, 129.465, 126.52, 121.94, 60.06, 58.93, 51.91, 45.54, 44.68, 41.92, 31.48, 30.27, 24.22, 23.36, 22.71, 20.26; 31P (202 MHz, CDCl3) δ 30.50; HRMS-(ESI) m/z [M+H]+ calcd for C22H34BrN3O3P, 498.1521; found, 498.1522; [α]D25 = −26.00 (c =0.75, CHCl3).
Compound 6m
Oily liquid: IR (thin film/NaCl) ν 2936, 2867, 1756, 1728, 1460, 1396, 1364, 1201, 1177, 1129, 1106, 1083, 1060, 1028, 930, 830, 780, 742 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.55 (m, 1H), 7.18-7.07 (m, 2H), 7.04 (m, 1H), 3.71 (dd, J = 17.5 Hz and 6.5 Hz, 1H), 3.61-3.40 (m, 3H), 3.38 (s, 3H), 3.17-3.05 (m, 1H), 3.03-2.91 (m, 1H), 2.28 (s, 3H), 2.11 (d, J = 8 Hz, 1H), 2.04 (d, J = 8 Hz, 1H), 1.71 (s, 2H), 1.44-1.26 (m, 7H), 1.19 (dd, J = 15 Hz and 7 Hz, 6H), 1.04 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.57, 136.08, 132.09, 129.14, 128.09, 127.30, 125.14, 59.96, 58.72, 51.53, 45.28, 44.55, 41.25, 40.96, 31.34, 30.12, 24.11, 23.09, 22.58, 20.11, 18.57; 31P (202 MHz, CDCl3) δ 31.60; HRMS-(ESI) m/z [M+H]+ calcd for C23H37N3O3P, 434.2573; found, 434.2563; [α]D25 = −4.20 (c =0.77, CHCl3).
Compound 6n
White solid: mp 122–124 °C; IR (ATR) ν 2942, 2859, 1751, 1437, 1392, 1360, 1244, 1217, 1199, 1171, 1128, 1105, 1081, 1052, 1030, 1003, 928, 889, 831, 786, 764, 727 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 7.5 Hz, 1H), 7.30 (d, J = 8 Hz, 1H), 7.25-7.14 (m, 2H), 3.92 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.65-3.38 (m, 6H), 3.08 (t, J = 7.5 Hz, 1H), 3.00 (t, J = 9 Hz, 1H), 2.14 (s, 1H), 2.06 (d, J = 10.5 Hz, 1H), 1.80 (s, 2H), 1.37 (s, 3H), 1.33 (d, J = 7 Hz, 4H), 1.21 (d, J = 6.5 Hz, 6H), 1.09 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.60, 133.77, 132.15, 130.26, 128.86, 128.68, 126.12, 60.10, 58.93, 51.92, 45.49, 44.69, 41.29, 40.96, 31.50, 30.22, 24.25, 23.24, 22.67, 20.29; 31P (202 MHz, CDCl3) δ 31.13; HRMS-(ESI) m/z [M+H]+ calcd for C22H34ClN3O3P, 454.2026; found, 454.2018; [α]D25 = +5.60 (c =0.91, CHCl3).
Compound 6o
White solid: mp 114–116 °C; IR (ATR) ν 2936, 2867, 1754, 1619, 1492, 1456, 1364, 1234, 1202, 1173, 1094, 1061, 1028, 930, 822, 779 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.61 (t, J = 7.5 Hz, 1H), 7.34-7.18 (m, 1H), 7.11 (t, J = 7.5 Hz, 1H), 6.99 (t, J = 7.5 Hz, 1H), 3.90 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.64-3.54 (m, 1H), 3.52 (s, 3H), 3.46 (dd, J = 16 Hz and 6.5 Hz, 2H), 3.11-2.92 (m, 2H), 2.12 (d, J = 6.5 Hz, 1H), 2.06 (d, J = 12.5 Hz, 1H), 1.89-1.66 (m, 2H), 1.44-1.29 (m, 7H), 1.22 (d, J = 7 Hz, 6H), 1.09 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.52, 162.69, 160.73, 129.98, 129.25, 123.44, 121.52, 114.64, 60.04, 58.94, 51.92, 45.52, 44.70, 43.66, 41.26, 37.38, 31.51, 30.23, 24.25, 23.32, 22.63, 20.26; 31P (202 MHz, CDCl3) δ 30.89; 19F NMR (376.17 MHz, CDCl3) δ −119.49; HRMS-(ESI) m/z [M+H]+ calcd for C22H34FN3O3P, 438.2322; found, 438.2317; [α]D25 = −20.39 (c =0.76, CHCl3)
Compound 6p
White solid: mp 145–147 °C; IR (ATR) ν 2968, 2944, 2862, 1729, 1440, 1366, 1333, 1729, 1440, 1366, 1333, 1285, 1246, 1232, 1216, 1198, 1173, 1129, 1060, 1030, 1001, 925, 884, 858, 808, 793, 765, 742 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 8.5 Hz, 1H), 7.84 (t, J = 7 Hz, 2H), 7.77 (d, J = 8.5 Hz, 1H), 7.58-7.41 (m, 3H), 4.3 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.73 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.66-3.43 (m, 2H), 3.27 (s, 3H), 3.23 (t, J = 9.5 Hz, 1H), 3.04 (t, J = 9.5 Hz, 1H), 2.18 (s, 1H), 2.11 (s, 1H), 1.83 (s, 2H), 1.38 (d, J = 7 Hz, 7H), 1.26 (d, J = 6.5 Hz, 3H), 1.22 (d, J = 7 Hz, 3H), 1.07 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.67, 133.12, 131.52, 129.77, 128.59, 128.01, 126.50, 126.18, 125.59, 125.17, 122.76, 60.18, 58.86, 51.69, 45.50, 44.75, 41.70, 40.76, 31.48, 30.28, 24.28, 23.22, 22.78, 20.31; 31P (202 MHz, CDCl3) δ 31.85; HRMS-(ESI) m/z [M+H]+ calcd for C26H37N3O3P, 470.2573; found, 470.2577; [α]D25 = +83.70 (c =0.75, CHCl3).
Compound 6q
White solid: mp 151–153 °C; IR (ATR) ν 2940, 2860, 1746, 1457, 1392, 1371,1245, 1198, 1166, 1125, 1106, 1080, 1062, 1018, 960, 927, 883, 866, 842, 822, 752, 723 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.92 (s, 1H), 7.88-7.71 (m, 3H), 7.59 (d, J = 8 Hz, 1H), 7.44 (p, J = 3.5 Hz, 2H), 3.88 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.72-3.55 (m, 1H), 3.54-3.34 (m, 5H), 3.14-2.87 (m, 2H), 2.11 (s, 1H), 2.05 (d, J = 10.5 Hz, 1H), 1.78 (s, 2H), 1.36 (d, J = 7 Hz, 7H), 1.22 (dd, J = 15 Hz and 7 Hz, 6H), 1.07 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.43, 133.01, 132.84, 131.65, 127.80, 127.58, 127.42, 127.05, 125.89, 125.80, 125.39, 59.95, 58.88, 51.74, 45.48, 44.64, 42.88, 42.04, 31.47, 30.22, 24.15, 23.36, 22.64, 20.18; 31P (202 MHz, CDCl3) δ 30.75; HRMS-(ESI) m/z [M+H]+ calcd for C26H37N3O3P, 470.2573; found, 470.2565; [α]D25 = −20.25 (c =0.77, CHCl3).
Compound 6r
White solid: mp 98–100 °C; IR (ATR) ν 2976, 2937, 2866, 1745, 1436, 1397, 1365, 1233, 1196, 1168, 1124, 1081, 1062, 1019, 981, 925, 873, 819, 801, 767, 750 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.48 (s, 1H), 7.31 (s, 1H), 6.44 (s, 1H), 3.62 (s, 3H), 3.60-3.37 (m, 3H), 3.30 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.03-2.83 (m, 2H), 2.14-1.96 (m, 2H), 1.77 (s, 2H), 1.33 (d, J = 7 Hz, 6H), 1.23 (d, J = 6.5 Hz, 4H), 1.18 (d, J = 6.5 Hz, 3H), 1.14 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.78, 142.65, 141.73, 119.90, 110.13, 59.91, 58.86, 51.96, 45.59, 44.68, 40.78, 36.35, 31.56, 30.23, 24.21, 23.52, 22.46, 20.16; 31P (202 MHz, CDCl3) δ 30.33; HRMS-(ESI) m/z [M+H]+ calcd for C20H33N3O4P, 410.2209; found, 410.2219; [α]D25 = −51.20 (c =0.80, CHCl3).
Compound 6s
White solid: mp 109–112 °C; IR (ATR) ν 2977, 2935, 2865, 1755, 1442, 1397, 1366, 1328, 1255, 1218, 1191, 1171, 1144, 1106, 1081, 1057, 1032, 1007, 928, 903, 877, 848, 803, 770, 746, 727 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.19 (d, J = 5 Hz, 1H), 7.09 (s, 1H), 6.93 (t, J = 4.5 Hz, 1H), 3.85 (dd, J = 15 Hz and 6 Hz, 1H), 3.59 (s, 3H), 3.57-3.26 (m, 3H). 2.98 (s, 2H), 2.11 (s, 1H), 2.07 (d, J = 10 Hz, 1H), 1.77 (s, 2H), 1.33 (d, J = 7 Hz, 7H), 1.21 (t, J = 6.5 Hz, 6H), 1.11 (d, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.38, 137.96, 126.59, 125.16, 59.95, 58.80, 51.97, 45.56, 44.74, 42.41, 39.15, 31.49, 30.19, 24.21, 23.42, 22.46, 20.23; 31P (202 MHz, CDCl3) δ 30.17; HRMS-(ESI) m/z [M+H]+ calcd for C20H33N3O3PS, 426.1980; found, 426.1974; [α]D25 = −25.46 (c =0.75, CHCl3).
Compound 6t
Oily liquid: IR (thin film/NaCl) ν 2974, 2935, 2868, 1751, 1725, 1455, 1394, 1370, 1299, 1246, 1196, 1178, 1107, 1060, 1026, 955, 925, 809, 740 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.42 (d, J = 7 Hz, 2H), 7.32-7.16 (m, 3H), 3.98-3.82 (m, 2H), 3.70 (dd, J = 17.5 Hz and 6.5 Hz, 1H), 3.61-3.49 (m, 1H), 3.44 (h, J = 7 Hz, 1H), 3.37 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.08-2.90 (m, 2H), 2.10 (s, 1H), 2.02 (d, J = 13 Hz, 1H), 1.76 (s, 2H), 1.33 (d, J = 6.5 Hz, 6H), 1.19 (dd, J = 10 Hz and 7 Hz, 6H), 1.04 (d, J = 7 Hz, 3H), 0.94 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.01, 134.04, 127.71, 127.60, 77.25, 77.00, 76.74, 60.67, 59.85, 58.83, 45.40, 44.60, 42.43, 42.04, 31.46, 30.14, 24.14, 23.35, 22.50, 20.14, 13.80; 31P (202 MHz, CDCl3) δ 30.95; HRMS-(ESI) m/z [M+H]+ calcd for C23H37N3O3P, 434.2573; found, 434.2570; [α]D25 = −34.19 (c =0.75, CHCl3).
Compound 6u
White solid: mp 114–116 °C; IR (ATR) ν 2973, 2933, 2859, 1711, 1455, 1393, 1363, 1287, 1241, 1199, 1171, 1143, 1107, 1048, 1032, 978, 910, 883, 838, 785, 754, 735 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.43 (d, J = 7 Hz, 2H), 7.34-7.17 (m, 3H), 4.76 (p, J = 6.5 Hz, 1H), 3.72 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.64-3.28 (m, 3H), 3.18-2.87 (m, 2H), 2.31-1.99 (m, 2H), 1.78 (s, 2H), 1.36 (d, J = 7 Hz, 7H), 1.22 (dd, J = 15 Hz and 6.5 Hz, 6H), 1.06 (d, J = 7 Hz, 3H), 0.92 (t, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 166.66, 134.22, 127.83, 127.67, 127.55, 68.28, 59.89, 58.90, 45.41, 44.66, 42.38, 42.24, 31.54, 30.13, 24.20, 23.41, 22.47, 21.36, 21.25, 20.33, 20.12; 31P (202 MHz, CDCl3) δ 31.09; HRMS-(ESI) m/z [M+H]+ calcd for C24H39N3O3P, 448.2729; found, 448.2727; [α]D25 = −35.13 (c =0.76, CHCl3).
Compound 6v
White solid: mp 130–133 °C; IR (ATR) ν 2971, 2934, 2871, 1710, 1455, 1392, 1366, 1348, 1307, 1244, 1214, 1168, 1108, 1084, 1051, 1024, 974, 916, 882, 838, 800, 765, 751 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.44 (d, J = 7.5 Hz, 2H), 7.34-7.16 (m, 3H), 3.70 (dd, J = 16.5 Hz and 6.5 Hz, 1H), 3.64-3.51 (m, 1H), 3.51-3.39 (m, 1H), 3.31 (dd, J = 15 Hz and 6.5 Hz, 1H), 3.10-2.93 (m, 2H), 2.13 (s, 1H), 2.05 (d, J = 11.5 Hz, 1H), 1.79 (s, 2H), 1.45-1.28 (m, 7H), 1.24 (d, J = 6.5 Hz, 3H), 1.20 (d, J = 7 Hz, 3H), 1.12 (s, 9H), 1.07 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 166.25, 134.45, 127.92, 127.65, 127.48, 81.29, 59.94, 58.95, 45.43, 44.69, 42.79, 42.09, 31.57, 30.19, 27.58, 24.25, 23.42, 22.53, 20.34, 20.19; 31P (202 MHz, CDCl3) δ 31.17; HRMS-(ESI) m/z [M+H]+ calcd for C25H41N3O3P, 462.2886; found, 462.2881; [α]D25 = −33.03 (c =0.77, CHCl3).
Supplementary Material
Acknowledgements
We thank NIH, the National Institute on Drug Abuse and National Institutes of Health (NIDA, R21DA031860-01, GL). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA, NIH. We also thank the Robert Welch Foundation (D-1361, GL), the Fundamental Research Funds for the Key Universities (No. 1107020522, YP) and NSF (CHE-1048553) for NMR-instrument support.
Footnotes
Electronic Supplementary Information (ESI) available: Copies of 1H, 13C, 19F, 31P NMR spectra, IR spectra and copies of HRMS analysis for all the new compounds.
Notes and References
- 1.(a) Yudin AK. Aziridines and epoxides in organic synthesis. Weinheim, Germany: Wiley-VCH; 2006. [Google Scholar]; (b) Wolkenberg SE, Boger DL. Chem. Rev. 2002;102:2477. doi: 10.1021/cr010046q. [DOI] [PubMed] [Google Scholar]; (c) Hata T, Koga F, Sano Y, Kanamori K, Matsumae A, Sugawara R, Hoshi T, Shimi T, Ito S, Tomizawa S. J. Antibiot. 1954;7:107. [PubMed] [Google Scholar]; (d) Nagaoka K, Matsumoto M, Oono J, Yokoi K, Ishizeki S, Nakashima T. J. Antibiot. 1986;39:1527. doi: 10.7164/antibiotics.39.1527. [DOI] [PubMed] [Google Scholar]; (e) Kiyoto S, Shibata T, Yamashita M, Komori T, Okuhara M, Terano H, Kohsaka M, Aoki H, Imanaka HJ. J. Antibiot. 1987;40:594. doi: 10.7164/antibiotics.40.594. [DOI] [PubMed] [Google Scholar]; (f) Hanada M, Ohkuma H, Yonemoto T, Tomita K, Ohbayashi M, Kamei H, Miyaki T, Konishi M, Kawaguchi H, Forenza S. J. Antibiot. 1991;44:403. doi: 10.7164/antibiotics.44.403. [DOI] [PubMed] [Google Scholar]; (g) Tsuchida T, Inuma H, Kinoshita N, Ikeda T, Sawa T, Hamada M, Takeuchi T. J. Antibiot. 1995;48:217. doi: 10.7164/antibiotics.48.217. [DOI] [PubMed] [Google Scholar]
- 2.(a) Osborn HMI, Sweeny J. Tetrahedron: Asymmetry. 1997;8:1693. [Google Scholar]; (b) Andrez J–C. Beilstein J. Org. Chem. 2009;5(No. 33) doi: 10.3762/bjoc.5.33. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kasai M, Kono M. Synlett. 1992:778. [Google Scholar]; (d) Kinoshita S, Uzu K, Nakano K, Shimizu M, Takahashi T. J. Med. Chem. 1971;14:103. doi: 10.1021/jm00284a005. [DOI] [PubMed] [Google Scholar]; (e) Kunz KR, Iyengar BS, Dorr RT, Alberts DS, Remers WA. J. Med. Chem. 1991;34:2281. doi: 10.1021/jm00111a051. [DOI] [PubMed] [Google Scholar]; (f) Kuroda T, Hisamura K, Matsukuma I, Osawa Y, Sugaya T, Nishikawa H, Morimoto M, Ashizawa T, Nakamizo N, Otsuji Y. J. Heterocycl. Chem. 1994;31:113. [Google Scholar]
- 3.(a) Gerhart F, Higgins W, Tardif C, Ducep J. J. Med. Chem. 1990;33:2157. doi: 10.1021/jm00170a018. [DOI] [PubMed] [Google Scholar]; (b) Tanner ME, Miao S. Tetrahedron Lett. 1994;35:4073. [Google Scholar]; (c) Singh GS, D’hooghe M, Kimpe ND. Chem. Rev. 2007;107:2080. doi: 10.1021/cr0680033. [DOI] [PubMed] [Google Scholar]
- 4.(a) Davis FA, Wu Y, Yan H, McCoull W, Prasad KR. J. Org Chem. 2003;68:2410. doi: 10.1021/jo020707z. [DOI] [PubMed] [Google Scholar]; (b) Stankovic S, D’hooghe M, Catak S, Eum H, Waroquier M, Speybroeck VV, Kimpe ND, Ha H-J. Chem. Soc. Rev. 2012;41:643. doi: 10.1039/c1cs15140a. [DOI] [PubMed] [Google Scholar]
- (a) Davis FA, Zhou P, Chen BC. Chem. Soc. Rev. 1998;27:13. and references cited there in; [Google Scholar]; (b) Davis FA. J. Org. Chem. 2006;71:8993. doi: 10.1021/jo061027p. [DOI] [PubMed] [Google Scholar]; (c) Ishikawa T. Heterocycles. 2012;85:2837. [Google Scholar]; (d) Cardillo G, Gentilucci L, Tolomelli A. Aldrichimica Acta. 2003;36:39. [Google Scholar]; (e) Lee WK, Ha H-J. Aldrichimica Acta. 2003;36:57. [Google Scholar]
- 6.(a) Wenker H. J. Am. Chem. Soc. 1935;57:2328. [Google Scholar]; (b) Gabriel S. Chem. Ber. 1888;21:1049. [Google Scholar]; (c) Gabriel S. Chem. Ber. 1888;21:2664. [Google Scholar]; (d) Minoura Y, Takebayashi M, Price CC. J. Am. Chem. Soc. 1959;81:4689. [Google Scholar]; (e) Berry MB, Craig D. Syn. Lett. 1992:41. [Google Scholar]; (f) Maruoka K, Sano H, Yamamoto H. Chem. Lett. 1985:599. [Google Scholar]; (g) Blandy C, Choukroun R, Gervais D. Tetrahedron Lett. 1983;24:4189. [Google Scholar]; (h) Sinou D, Emziane M. Tetrahedron Lett. 1986;27:4423. [Google Scholar]; (i) Legters J, Thijs L, Zwanenburg B. Recl. Trav. Chim. Pays-Bas. 1992;111:1. [Google Scholar]; (j) Legters J, Thijs L, Zwanenberg B. Tetrahedron Lett. 1989;30:4881. [Google Scholar]; (k) Tanner D, Birgesson C, Dhaliwal HK. Tetrahedron Lett. 1990;31:1903. [Google Scholar]; (l) Legters J, Thijs L, Zwanenburg B. Tetrahedron. 1991;47:5287. [Google Scholar]; (m) Toshimitsu A, Abe H, Hirosawa C, Tamao K. J. Chem Soc. Perkin Trans I. 1994;23:3465. [Google Scholar]; (n) Colpaert F, Mangelinckx S, Leemans E, Denolf B, Kimpe ND. Org. Biomol. Chem. 2010;8:3251. doi: 10.1039/c001471k. [DOI] [PubMed] [Google Scholar]; (o) Denolf B, Mangelinckx S, Tornroos KW, Kimpe ND. Org. Lett. 2006;8:3129. doi: 10.1021/ol0611245. [DOI] [PubMed] [Google Scholar]; (p) Denolf B, Leemans E, Kimpe ND. J. Org Chem. 2007;72:3211. doi: 10.1021/jo0624795. [DOI] [PubMed] [Google Scholar]; (q) Leemans E, Mangelinckx S, Kimpe ND. Synlett. 2009:1265. [Google Scholar]; (r) Colpaert F, Mangelinckx S, Brabandere SD, Kimpe ND. J. Org Chem. 2011;76:2204. doi: 10.1021/jo200082w. [DOI] [PubMed] [Google Scholar]; (s) Kiss L, Mangelinckx S, Sillanpaa R, Fulop F, Kimpe ND. J. Org Chem. 2007;72:7199. doi: 10.1021/jo0710634. [DOI] [PubMed] [Google Scholar]; (t) Callebaut G, Mangelinckx S, Kiss L, Sillanpaa R, Fulop F, Kimpe ND. Org. Biomol. Chem. 2012;10:2326. doi: 10.1039/c2ob06637h. [DOI] [PubMed] [Google Scholar]; (u) Callebaut G, Mangelinckx S, Veken PV, Tornroos KW, Augustyns K, Kimpe ND. Beilstein J. Org. Chem. 2012;8:2124. doi: 10.3762/bjoc.8.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Desai AA, Moran-Ramallal R, Wulff WD. Org. Synth. 2011;88:224. [Google Scholar]; (b) Desmarchelier A, Pereira de Sant’Ana D, Terrasson V, Campagne JM, Moreau X, Greck C, Marcia de Figueiredo R. Eur. J. Org. Chem. 2011:4046. [Google Scholar]
- 8.(a) Hayashi S, Yorimitsu H, Oshima K. Angew. Chem. Int. Ed. 2009;48:7224. doi: 10.1002/anie.200903178. [DOI] [PubMed] [Google Scholar]; (b) Cramer SA, Jenkins DM. J. Am. Chem. Soc. 2011;133:19342. doi: 10.1021/ja2090965. [DOI] [PubMed] [Google Scholar]
- 9.(a) Kattuboina A, Li G. Tetrahedron. Lett. 2008;49:1573. [Google Scholar]; (b) Kattuboina A, Kaur P, Ai T, Li G. Chem. Biol. Drug. Des. 2008;71:216. doi: 10.1111/j.1747-0285.2008.00633.x. [DOI] [PubMed] [Google Scholar]; (c) Kattuboina A, Kaur P, Nguyen T, Li G. Tetrahedron Lett. 2008;49:3722. [Google Scholar]
- 10.(a) Han J, Ai T, Nguyen T, Li G. Chem. Biol. Drug. Des. 2008;72:120. doi: 10.1111/j.1747-0285.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Han J, Ai T, Li G. Synthesis. 2008;16:2519. [Google Scholar]
- 11.(a) Kaur P, Nguyen T, Li G. Eur. J. Org. Chem. 2009:912. [Google Scholar]; (b) Ai T, Han J, Chen Z–X, Li G. Chem. Biol. Drug. Des. 2009;73:203. doi: 10.1111/j.1747-0285.2008.00771.x. [DOI] [PubMed] [Google Scholar]; (c) Chen Z–X, Ai T, Kaur P, Li G. Tetrahedron Lett. 2009;50:1079. [Google Scholar]; (d) Ai T, Li G. Bioorg. Med. Chem. Lett. 2009;19:3967. doi: 10.1016/j.bmcl.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 12.(a) Kaur P, Shakya G, Sun H, Pan Y, Li G. Org. Biomol. Chem. 2010;8:1091. doi: 10.1039/b923914f. [DOI] [PubMed] [Google Scholar]; (b) Ai T, Pindi S, Kattamuri PV, Li G. Sci. China Series B: Chem. 2010;53:125. [Google Scholar]; (c) Kattamuri PV, Ai T, Pindi S, Sun Y, Gu P, Shi M, Li G. J. Org. Chem. 2011;76:2792. doi: 10.1021/jo200070d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis FA, Liu H, Zhou P, Fang T, Reddy GV, Zhang Y. J. Org. Chem. 1999;64:7559. doi: 10.1021/jo991389f. [DOI] [PubMed] [Google Scholar]
- 14.The term “GAP chemistry” is greater to be defined as “Group-Assisted Purification chemistry” instead of “Group-Assistant Purification chemistry”.
- 15.Sometimes the GAP aziridination can be achieved by evaporating off solvents of crude solution and then put the mixture in the refrigerator followed by washing.
- 16.(a) Li G, Russel KC, Jarosinski MA, Hruby VJ. Tetradhedron Lett. 1993;34:2565. [Google Scholar]; (b) Hruby VJ, Li G, HaskellLuevano c, Shenderovich M. Biopolymer. 1997;43:219. doi: 10.1002/(SICI)1097-0282(1997)43:3<219::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Beulshausen T, Groth U, Schollkopf U. Liebigs Ann. Chem. 1991:1207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.