Abstract
Objective:
This study explores a large panel of cytokines in plasma and CSF of patients with Aicardi-Goutières syndrome (AGS) at different ages, in order to establish signatures of cytokines most predictive of AGS.
Methods:
Plasma from 22 subjects with known mutations were assayed for cytokines using the Milliplex MAP Immunobead system, and compared to results from 8 age-matched normal controls. CSF of 11 additional patients with mutation-proven AGS was tested in an identical manner and compared to results from age-matched controls. Samples were banked and analysis was carried out retrospectively.
Results:
Significant elevations were seen in FMS-related tyrosine kinase 3 ligand, IP-10, interleukin (IL)–12p40, IL-15, tumor necrosis factor α, and soluble IL 2 receptor α in both AGS patient plasma and CSF relative to controls. Additionally, this cytokine signature was able to correctly cluster 9 of 11 AGS cases based on CSF values. While most cytokines decreased exponentially with age, a subgroup including IP-10 demonstrated persistent elevation beyond early childhood.
Conclusion:
Patients with AGS exhibit plasma and CSF elevations of proinflammatory cytokines. Selected cytokines remain persistently elevated beyond the initial disease phase. This panel of proinflammatory cytokines may be considered for use as diagnostic and therapeutic markers of disease, and may permit improved understanding of disease pathogenesis.
Aicardi-Goutières syndrome (AGS) is a heritable disorder, characterized by basal ganglia and white matter calcifications, leukoencephalopathy, and elevated CSF interferon-α (IFN-α).1,2 AGS has long been understood as a neuroimmune disorder with characteristic presentations ranging from a syndrome that mimics in utero viral infections to lupus-like sterile pyrexias, skin, and joint manifestations.2 The disease is caused by mutations in any one of several genes encoding nucleases or other proteins involved in innate cellular immunity: TREX1,3 RNASEH2A, RNASEH2B, RNASEH2C,4 and SAMHD1.5 It is thought that failure of these enzymes in AGS results in accumulation of intracellular nucleic acid species, with subsequent activation of an innate immune response, and neurodegeneration. The exact mechanisms underlying activation of the innate cellular immune response and subsequent disease remains unclear; however, these observations indicate that the disease might be treatable by interrupting the accumulation of endogenous nucleic acids.6 Thus, identifying biomarkers, both to monitor disease progression and to better understand disease pathophysiology, is essential to the ongoing study of AGS.
METHODS
Subjects.
Plasma samples were obtained from 22 patients with AGS and mutations in TREX1 (n = 4), RNASEH2B (n = 13), or SAMHD1 (n = 5), and from 8 age-matched controls having venipuncture for reasons unrelated to neurologic disease. Additionally, we analyzed banked CSF samples from 11 patients with AGS with mutations in TREX1 (n = 3), RNASEHB (n = 7), or SAMHD1 (n = 1), and 8 age-matched patients undergoing lumbar puncture for reasons unrelated to leukodystrophy. Plasma samples were processed according to standard laboratory procedures and stored at −80°C until analyzed. CSF samples were centrifuged to remove cells and stored at −80°C until analyzed. Blood and CSF samples were unpaired, i.e., not obtained in the same patients or not obtained simultaneously in the blood and CSF from the same patient.
Standard protocol approvals, registrations, and patient consents.
Each patient’s family gave written informed consent to participate in the current research protocol, as approved by the Children's National Medical Center Institutional Review Board. CSF samples were excess clinical samples and not drawn for the express purpose of the study, but rather banked specimens.
Immunobead assay.
Milliplex MAP human cytokine/chemokine Immunobead assay (EMD Millipore, Billerica, MA) was used to quantify expression levels of 39 cytokines in plasma and CSF (table 1). When values were above or below the detectable range, the upper or lower detectable level for that cytokine on standard analysis curves was used to avoid missing values. Units for all analytes are in pg/mL of fluid.
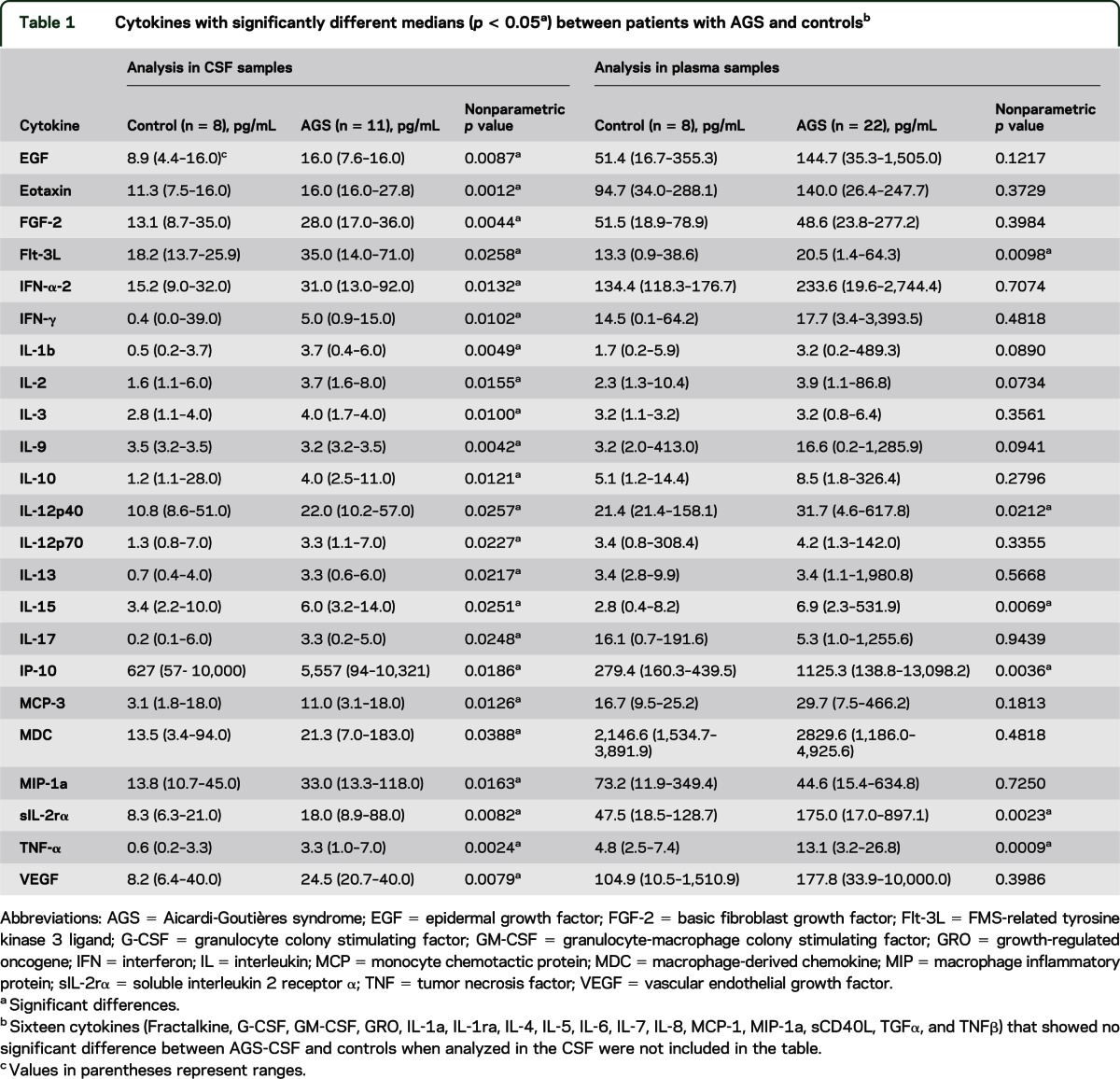
Table 1.
Cytokines with significantly different medians (p < 0.05a) between patients with AGS and controlsb
Statistical analysis.
Data from CSF and plasma samples were analyzed using the following approach. Values were compared between patients with AGS and controls as described above. The initial exploration of the data showed 2 characteristics: the variance was less in the CSF samples than in the plasma samples, and the cytokine values were not normally distributed. For these reasons, median cytokine levels between the 8 control and 11 AGS cases were compared in the CSF samples using a nonparametric Wilcoxon rank sum test first. The median (range) and p values were reported for cytokines that showed a significant difference (see table 1). Only cytokines that were identified to show a difference in CSF were then analyzed in plasma to identify those cytokines significant in both tissues. In addition, analysis was performed using nonparametric tests of the plasma samples first, followed by analysis for elevated cytokines in both plasma and CSF.
Cytokines showing a significant elevation in both CSF alone and the CSF–plasma combination were then used in a hierarchical clustering analysis to assess whether they could separate AGS cases from controls. We used the average linkage cluster method with the squared Euclidian distance measure of dissimilarity. Both plasma and CSF samples were tested.
All analyses were performed using Stata V11 (College Station, TX). A nominal p value of 0.05 for significance was used for all statistical tests. Because this is an exploratory exercise, we were more interested in controlling type II error than the overall type I error rate, i.e., we preferred to retain a cytokine that may not differentiate between patients with AGS and controls, rather than to exclude a cytokine that does differentiate these states. Therefore, we kept the statistical significance criteria at the α = 0.05 level. The relationship of age vs cytokine level was assessed in a qualitative fashion using graphical analysis since sample sizes were too small to permit statistical testing of AGS vs control differences over the age range.
RESULTS
Twenty-three cytokines were found to be differentially elevated in AGS CSF vs controls using a nonparametric approach (table 1). In particular, 10 of these cytokines were highly elevated (p ≤ 0.01), including growth factors such as epidermal growth factor, basic fibroblast growth factor, and vascular endothelial growth factor; chemokines such as eotaxin; proinflammatory cytokines such as interleukin-1-β (IL-1β), IL-3, IL-9, and tumor necrosis factor–α (TNFα); IFNs such as IFN-γ; and markers of immune activation such as soluble IL-2 receptor α (sIL-2rα). When the 23 differentially elevated cytokines were analyzed in plasma, a subset of these cytokines were also found to be elevated in both CSF and plasma, including growth factors such as FMS-related tyrosine kinase 3 ligand (Flt-3L); chemokines such as IP-10 or C-X-C motif chemokine 10 (CXCL10); proinflammatory cytokines such as IL-12p40, IL-15, and TNFα; and markers of immune activation such as soluble sIL-2rα (table 1) (p < 0.05). A trio of cytokines, IP-10, sIL-2rα, and TNFα, were highly elevated, as defined more stringently by p < 0.005.
An analysis of plasma samples alone was also performed, and identified 11 cytokines, including 10 highly elevated cytokines with p ≤ 0.01: growth factors such as Flt-3L and granulocyte colony stimulating factor (G-CSF); proinflammatory cytokines such as IL-4, IL-12p40, IL-15, TNFα, and TNFβ; chemoattractant cytokines such as IP-10 and chemokine (C-C motif) ligand 2 (CCL2)/monocyte chemotactic protein–1 (MCP-1); and markers of immune activation such as sIL-2rα (table e-1 on the Neurology® Web site at www.neurology.org). Of note, when the same 11 cytokines that were elevated in the plasma were analyzed in the CSF, the same 6 cytokines were identified as when the analysis was started in the CSF (table e-1). Of impor-tance, the range of patient values for all the individual cytokines overlaps with the range of control values, with the exception of CSF IL-1b.
Younger patients with AGS appeared to show greater elevations of plasma and CSF cytokines than older patients with AGS, and the decrease in values of these cytokines occurred in an exponential fashion, approaching control values around 5 years of age for several cytokines (see figure 1 for examples of selected cytokines in plasma). However, 2 cytokines did not appear to decrease with age, namely IP-10 (figure 1) and sIL-2rα.
Figure 1. An example of age-related decrease in cytokines.
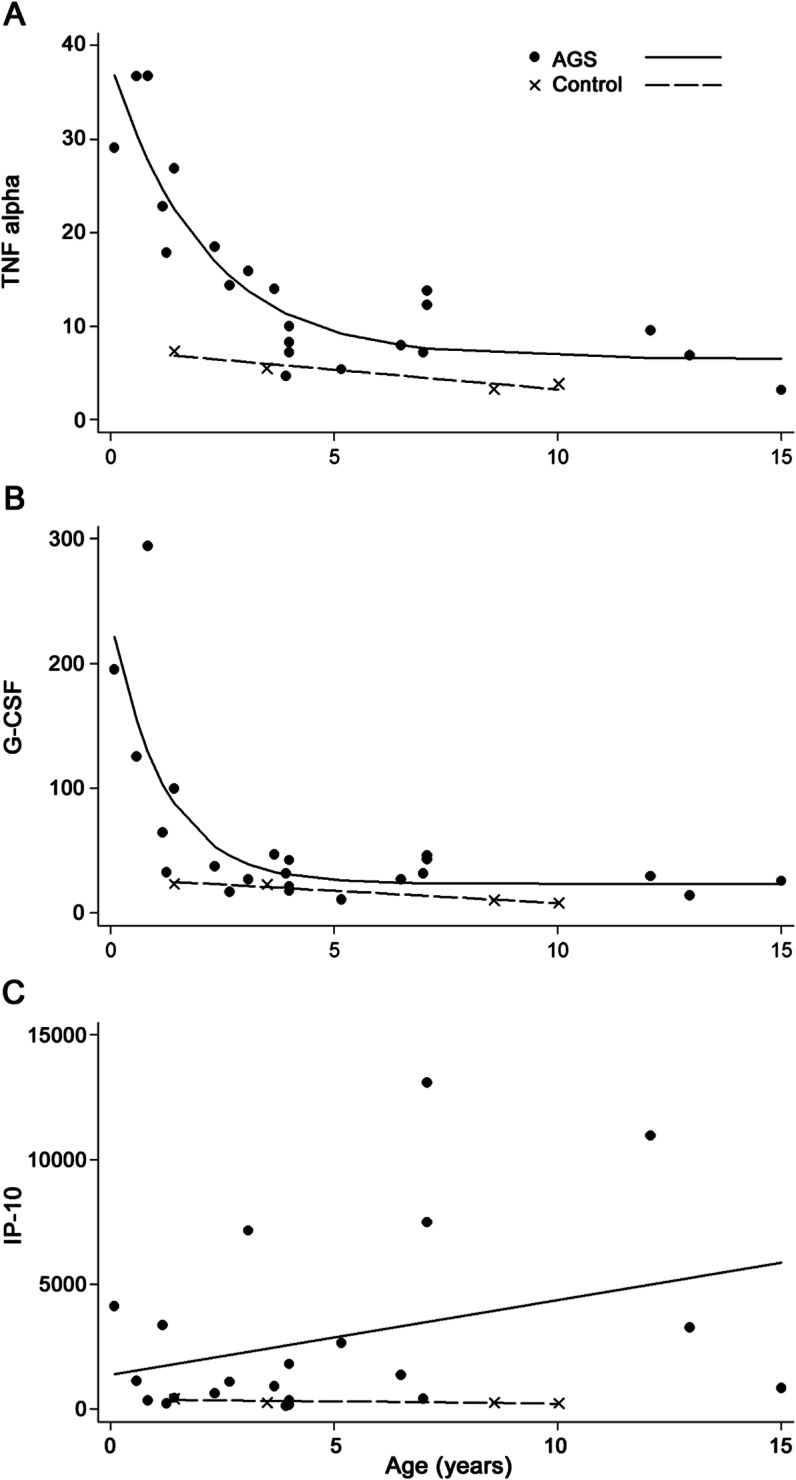
(A) Tumor necrosis factor (TNF) α in plasma expression levels vs age in patients with Aicardi-Goutières syndrome (AGS) and controls. (B) Granulocyte colony stimulating factor (G-CSF) in plasma expression levels vs age in patients with AGS and controls. (C) IP-10 in plasma expression levels vs age in patients with AGS and controls.
Hierarchical clustering analysis of the levels of cytokines in CSF demonstrated that all but 3 samples were correctly assigned using the 23 elevated cytokines identified in the CSF. A similar cluster analysis in the CSF samples performed using the 6 cytokines significant both in CSF and plasma were the same as when the 23 significant cytokines were used (figure 2). Case C282-control was clustered in the AGS group, while cases 32-AGS and 38-AGS were clustered in the control group. Of note, data relating to case C282-control was then reviewed and highlighted that this individual had presented with fever and emesis, abnormalities on routine CSF analysis (including 14 leukocytes/mm3), and a clinical diagnosis of presumed viral encephalitis. No further clinical characteristics were available for cases 32-AGS and 38-AGS to explain why they did not cluster with other patients with AGS; their ages of 1.25 and 4 years respectively makes it unlikely that these findings are reflective of a possible age-related decrease in cytokine levels. Additional cluster analysis was performed in the plasma samples using the 6 cytokines identified to be elevated both in CSF and plasma samples. All controls were clustered together, though with fairly weak discrimination. However, 6 patients with AGS were clustered into the control group rather than the AGS group. Even when case C282-control was excluded from the analysis, the same cytokines were identified as significant, with the additional finding of elevations in G-CSF (p < 0.01), also increased in plasma, and macrophage inflammatory protein–1 (MIP-1b, p < 0.05), not increased in plasma (table e-2). Cluster analysis excluding control-282 did not change clustering of AGS samples 32 and 38 (figure e-1).
Figure 2. Cluster analysis using cytokines.
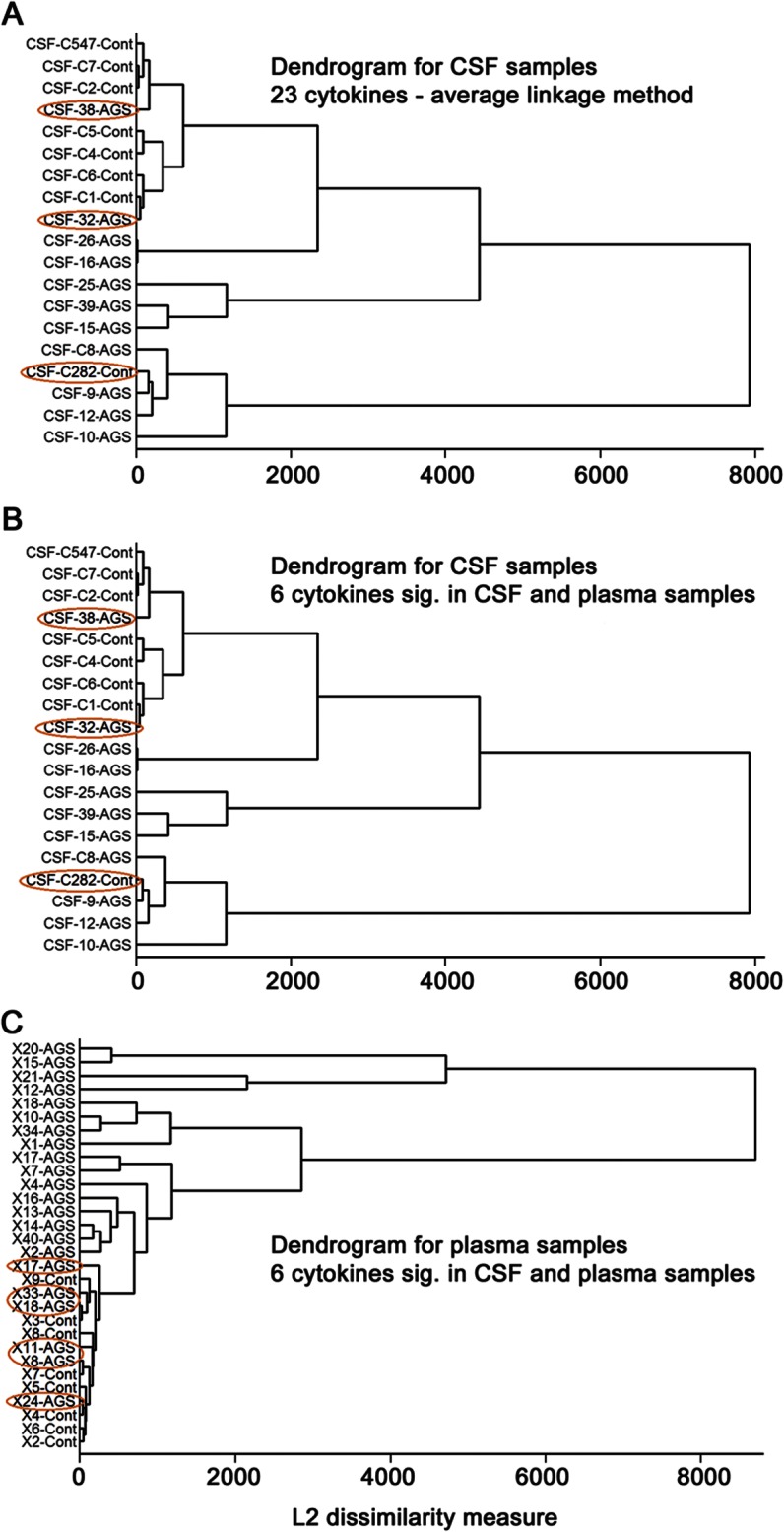
(A) Cluster analysis using 23 cytokines significant in CSF samples; applied to CSF samples. (B) Cluster analysis using 6 cytokines significantly elevated in both CSF and plasma samples; applied to CSF samples. (C) Cluster analysis using 6 cytokines significantly elevated in both CSF and plasma samples; applied to plasma samples.
DISCUSSION
Our results confirm prior findings of proinflammatory cytokine and chemokine elevations in the plasma and CSF of patients with AGS.1,7,8 In the CSF, we reproduced data from previous publications identifying elevations in IFN-α and IP-10/CXCL10 in patients with AGS (p < 0.05).1,7,9 Additionally, we confirmed elevations of IP-10/CXCL10 in the plasma of patients with AGS (p < 0.005),7 without elevations in IFN-α. It should be noted that the technique used in this analysis shows variable sensitivity for measuring IFN-α10 and, additionally, that only IFN-α-2 is assayed in this system.7 Although MCP-1 or CCL2 was increased in AGS plasma (p < 0.005), we did not replicate CSF elevations previously recorded in patients with AGS.7
In this study, we have also identified significant alterations in a broader panel of cytokines in our CSF and plasma samples than in previous studies. In some cases, this may be because samples were not previously tested for these cytokines (Flt-3L, IL-3, IL-9, MCP-3, MDC, sIL-2rα) or, alternately, because AGS series were compared to controls demonstrating an inflammatory phenotype, in order to try to discriminate the genetic disease AGS from virus-mediated disorders.7 A panel of 6 cytokines (Flt-3L, IP-10, IL-12p40, IL-15, TNFα, and sIL-2rα) was found to correctly cluster 9 out of 11 AGS cases in the CSF samples. As expected, given the variance in plasma, only 16 out of 22 AGS cases clustered correctly in the plasma samples. The utility of this panel of cytokines in identifying cases of AGS remains to be tested in a prospective manner.
Cytokine and chemokine analysis has a potential role in the diagnosis of AGS, in disease monitoring, and in the response to treatments. IFN-α measurement in the CSF has long been used as a tool for the clinical diagnosis of AGS. However, this and other disease markers such as CSF pleocytosis and CSF pterins have previously been considered to be age-dependent (with levels falling over time), making them potentially unsuited to disease monitoring, or even diagnosis, in the older child. Our data confirm previous findings of a general decrease in cytokines in patients with AGS after 4 to 5 years of age,7,11,12 but also highlight a persistent increase in IP-10.7 In some patients diagnosed later in life, persistent increases of CSF IFN-α have been observed,13 in particular in serum from SAMHD1 mutation-positive patients (Dr. Pierre Lebon, personal communication, 2012). Because of restricted sample size, we were unable to adequately test significance in our analysis of cytokine levels across age and diagnosis. Despite remaining questions, the persistent elevations in plasma above normal of IP-10 in our older subjects identify cytokine measures as a candidate screening tool even in older AGS-suspected subjects. It also suggests ongoing disease activity in the older patient, with the potential for therapeutic intervention. Further study in a larger sample size is required to more thoroughly explore this relationship.
An additional challenge in the use of cytokines as biomarkers in AGS is the possible difference in the utility of CSF and blood samples. In AGS, primary cytokine synthesis within the CNS has been hypothesized, as demonstrated for IP-10 and IFN-α.7 Additionally, previous evidence showing higher cytokine values in CSF than in plasma, and the increased variance in the plasma samples in our study, are suggestive of possible CSF synthesis of many of the elevated cytokines. This would indicate the continued need for CSF sampling in disease diagnosis and monitoring. Alternatively, it is possible that the blood and CSF compartments have discrete cytokine signatures, representing the involvement of different cell types in these 2 tissues. Certain cytokines are of particular interest for possible plasma monitoring. These include Flt3, a dendritic cell maturation marker14; IP-10, known to be increased in plasma in autoimmune disorders such as neuropsychiatric lupus and persistently elevated in plasma; sIL-2rα, associated with B- and T-cell activation and used as a monitoring tool in a number of autoimmune,15–17 infectious,18 and oncologic19 disorders; and TNFα, a proinflammatory cytokine with widespread effects.
These data do not allow us to draw conclusions about the relationship between serum and CSF proinflammatory cytokine levels, as blood draw and lumbar punctures were not conducted simultaneously in these patients. Future studies are required to characterize the correlation of CSF and plasma cytokine levels within subjects. Additionally, correlation of genotype with cytokine levels and clinical phenotype was not feasible with our restricted sample size, but merits future investigations with larger patient cohorts. Finally, age-related correlations were also limited by sample size, and will require larger numbers of patients with widely distributed ages in order to establish trends in individual cytokines.
Panels of proinflammatory cytokines may prove valuable as diagnostic markers in AGS, serving as a potential diagnostic link between clinical and neuroradiologic phenotypes in AGS and targeted genetic testing for confirmatory mutations. Although cytokine values are more likely to be elevated in CSF, selected cytokines are elevated in plasma as well as CSF, such as IP-10, sIL-2rα, and TNFα, and may be targets for future studies as diagnostic markers in blood samples. Certain markers, such as IP-10, may also persist after early childhood. Thus, these cytokines may be useful both as markers of disease activity and for disease monitoring in the treatment of this devastating disorder.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Karen F. Gold for the design of the analysis. Y.J.C. thanks the Manchester NIHR Biomedical Research Centre, the European Union's Seventh Framework Program (FP7/2007-2013) grant agreement number 241779 (NIMBL: Nuclease Immune Mediated Brain and Lupus-like disorders), and the European Leukodystrophy Association for support. A.V. thanks the National Institute of Neurological Disorders and Stroke, K08 award mechanisms (1K08NS060695), the Koos family fund, and the American Academy of Neurology Foundation for support. A.V. and J.S. thank the Seventh Framework Program (FP7-NIMBL) for support. M.P. thanks the Delman family fund for support.
GLOSSARY
- AGS
Aicardi-Goutières syndrome
- CCL2
chemokine (C-C motif) ligand 2
- CXCL10
C-X-C motif chemokine 10
- Flt-3L
FMS-related tyrosine kinase 3 ligand
- G-CSF
granulocyte colony stimulating factor
- IFN
interferon
- IL
interleukin
- MCP-1
monocyte chemotactic protein–1
- sIL-2rα
soluble interleukin 2 receptor α
- TNF
tumor necrosis factor
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Asako Takanohashi: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. Morgan J. Prust: drafting/revising the manuscript, analysis or interpretation of data. Jichuan Wang: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. Heather Gordish-Dressman: analysis or interpretation of data, statistical analysis. Miriam Bloom: study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patients, acquisition of data. Gillian I. Rice: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Johanna L. Schmidt: drafting/revising the manuscript. Yanick J. Crow: drafting/revising the manuscript, contribution of vital reagents/tools/patients. Pierre Lebon: analysis or interpretation of data, acquisition of data. Taco W. Kuijpers: drafting/revising the manuscript, analysis or interpretation of data. Kanneboyina Nagaraju: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. Adeline Vanderver: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patients, acquisition of data, study supervision.
STUDY FUNDING
Work in the Crow Laboratory (Dr. Yanick, J. Crow, Dr. Rice) is supported by funding from the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement number 241779, and the European Leukodystrophy Association. K.N. is supported in part by Department of Defense grants W81XWH-11-1-0782, W81XWH-05-1-0616, and W81XWH-11-1-0809, and NIH grants R01-AR050478; K26OD011171 and 1U54HD053177-01A1 and 2R24HD050846-06.
DISCLOSURE
A. Takanohashi, M. Prust, J. Wang, H. Gordish-Dressman, M. Bloom, and P. Lebon report no disclosures. T.W. Kuijpers is supported in part by funding from the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement number 241779. K. Nagaraju is supported in part by Department of Defense grants W81XWH-11-1-0782, W81XWH-05-1-0616, and W81XWH-11-1-0809 and NIH grants R01-AR050478; K26OD011171 and 1U54HD053177-01A1 and 2R24HD050846-06. A. Vanderver is supported by a grant from the National Institute of Neurological Disorders and Stroke (K08) and the American Academy of Neurology Foundation. She has a patent pending for the use of CSF asialotransferrin in the diagnosis of vanishing white matter disease. She has spoken at the American College of Medical Genetics. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lebon P, Badoual J, Ponsot G, Goutieres F, Hemeury-Cukier F, Aicardi J. Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J Neurol Sci 1988;84:201–208 [DOI] [PubMed] [Google Scholar]
- 2.Crow YJ, Livingston JH. Aicardi-Goutieres syndrome: an important Mendelian mimic of congenital infection. Dev Med Child Neurol 2008;50:410–416 [DOI] [PubMed] [Google Scholar]
- 3.Crow YJ, Hayward BE, Parmar R, et al. Mutations in the gene encoding the 3'-5' DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet 2006;38:917–920 [DOI] [PubMed] [Google Scholar]
- 4.Crow YJ, Leitch A, Hayward BE, et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nat Genet 2006;38:910–916 [DOI] [PubMed] [Google Scholar]
- 5.Rice GI, Bond J, Asipu A, et al. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet 2009;41:829–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck-Engeser GB, Eilat D, Wabl M. An autoimmune disease prevented by anti-retroviral drugs. Retrovirology 2011;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Heteren JT, Rozenberg F, Aronica E, Troost D, Lebon P, Kuijpers TW. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutieres syndrome. Glia 2008;56:568–578 [DOI] [PubMed] [Google Scholar]
- 8.Goutieres F, Aicardi J, Barth PG, Lebon P. Aicardi-Goutieres syndrome: an update and results of interferon-alpha studies. Ann Neurol 1998;44:900–907 [DOI] [PubMed] [Google Scholar]
- 9.Rice G, Patrick T, Parmar R, et al. Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Am J Hum Genet 2007;81:713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum 2006;54:1906–1916 [DOI] [PubMed] [Google Scholar]
- 11.Lanzi G, Fazzi E, D'Arrigo S, et al. The natural history of Aicardi-Goutieres syndrome: follow-up of 11 Italian patients. Neurology 2005;64:1621–1624 [DOI] [PubMed] [Google Scholar]
- 12.Lebon P, Meritet JF, Krivine A, Rozenberg F. Interferon and Aicardi-Goutieres syndrome. Eur J Paediatr Neurol 2002;(6 suppl A):A47–A53; discussion A55–48, A77–86. [DOI] [PubMed] [Google Scholar]
- 13.Dimova PS, Mikova OA. Case of Aicardi-Goutieres syndrome with long-lasting increase of cerebrospinal interferon-alpha. J Child Neurol 2005;20:915–919 [DOI] [PubMed] [Google Scholar]
- 14.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000;95:3489–3497 [PubMed] [Google Scholar]
- 15.Gilad R, Lampl Y, Eshel Y, Barak V, Sarova-Pinhas I. Cerebrospinal fluid soluble interleukin-2 receptor in cerebral lupus. Br J Rheumatol 1997;36:190–193 [DOI] [PubMed] [Google Scholar]
- 16.Zaheer A, Zaheer S, Sahu SK, et al. A novel role of glia maturation factor: induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J Neurochem 2007;101:364–376 [DOI] [PubMed] [Google Scholar]
- 17.Caruso C, Candore G, Cigna D, Colucci AT, Modica MA. Biological significance of soluble IL-2 receptor. Mediators Inflamm 1993;2:3–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aziz N, Nishanian P, Fahey JL. Levels of cytokines and immune activation markers in plasma in human immunodeficiency virus infection: quality control procedures. Clin Diagn Lab Immunol 1998;5:755–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bien E, Rapala M, Krawczyk M, Balcerska A. The serum levels of soluble interleukin-2 receptor alpha and lactate dehydrogenase but not of B2-microglobulin correlate with selected clinico-pathological prognostic factors and response to therapy in childhood soft tissue sarcomas. J Cancer Res Clin Oncol 2010;136:293–305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


