Abstract
Alpha-synuclein (SNCA) is central to the pathogenesis of Parkinson disease (PD), with 3 missense mutations reported to date. We report a novel mutation (p.H50Q) in a pathologically proven case.
α-Synuclein (SNCA) is central to the pathogenesis of Parkinson disease (PD), with 3 missense mutations reported to date. We report a novel mutation (p.H50Q) in a pathologically proven case.
Methods and results.
Standard protocol approvals, registrations, and patient consents.
Patients had given written informed consent for use of their brains in research. The study was approved by the local ethics committee.
Genetic analysis.
We amplified and sequenced SNCA exons (ENSEMBL transcript ID 394986) in DNA extracted from substantia nigra (SN) of 5 Queen Square PD Brain Bank cases (primers given in table e-1 on the Neurology® Web site at www.neurology.org). We detected a point mutation in exon 3 in 1 case (c.150T>G) (figure e-1), causing a nonconservative missense change of the basic histidine to the polar but uncharged glutamine (p.H50Q). We confirmed it by bidirectional sequencing, repeat PCR, and sequencing using 2 different primer pairs and Phusion high-fidelity polymerase, sequencing of DNA from the cerebellum of the same patient, restriction digestion (figure e-2), and TOPO cloning of PCR products followed by PCR and restriction digestion of 30 bacterial colonies; 14 of these had the mutation, which was confirmed by sequencing (figure e-1).
Clinical information.
The patient, a Caucasian English female, presented at age 71 years with right-sided tremor, responded to l-dopa, became forgetful at age 80, and died at age 83. There was no family history, but her parents' ages of death were not known. One of her 3 brothers had died of myocardial infarction, and the others, aged 74 and 64 years, and her daughter, were healthy. No relatives were available. Autopsy confirmed PD, with loss of pigmented cells in the SN, and Lewy bodies in the SN, midbrain pretectal area, and cerebral cortex. Plaques were noted in occipital and temporal cortex, with neurofibrillary tangles in the hippocampus, parahippocampal gyrus, and temporal cortex. Previous biochemical analysis demonstrated 44% reduction of mitochondrial complex I activity in the SN.1
Further evaluation and functional study of the mutation.
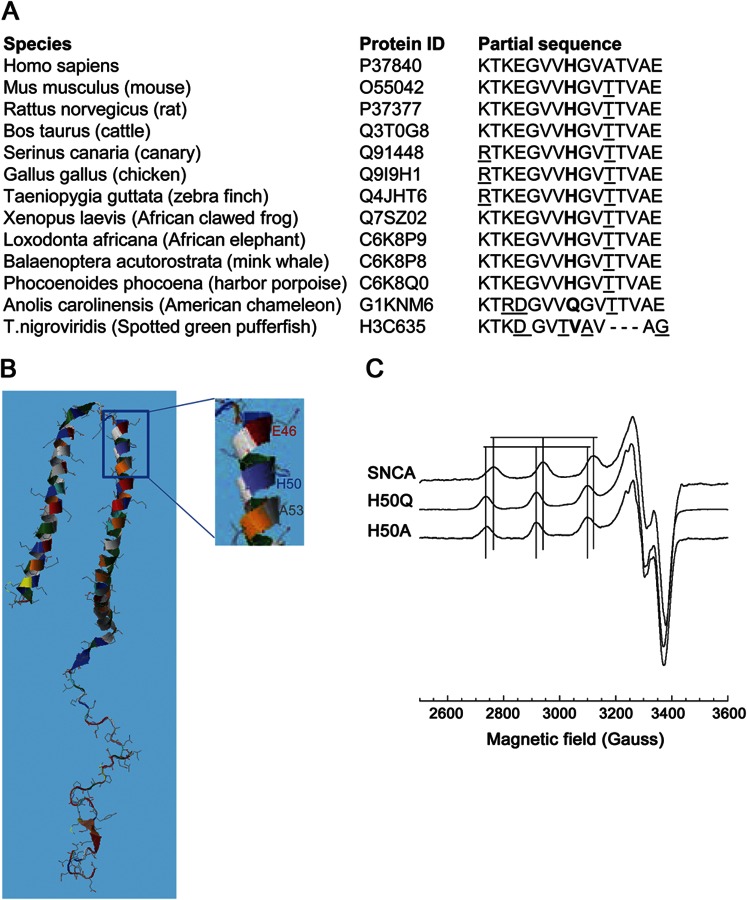
The mutation was absent in the database of single nucleotide polymorphisms and 1,000 genomes, and we excluded it by restriction digestion in 450 control DNA samples from the Wellcome Trust 1958 British birth cohort (99.8% Caucasoid). Multiple alignment using CLUSTAL-W revealed conservation of H50 in SNCA orthologs in higher vertebrates, although in the American chameleon Q occurs naturally (figure 1A). Bioinformatic analysis using Mutation Taster (http://www.mutationtaster.org) and SNPS&GO (http://snps-and-sgo.biocomp.unibo.it/snps-and-go/) predicted pathogenicity. Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/) predicted the mutation as possibly damaging (HumDiv score 0.797) or benign (HumVar score 0.452); notably, the A53T mutation is predicted as benign by Polyphen-2 (0 in both algorithms). Protein modeling using RaptorX (http://raptorx.uchicago.edu/) revealed that H50 is flanked within 1 turn of the α-helix by 2 known mutation sites, E46 and A53 (figure 1B).1
Figure 1. The H50Q mutation.
(A) Multiple alignment of α-synuclein (SNCA) indicates conservation of H50 (in bold) in higher vertebrates. Other nonconserved residues in different species in the region shown are underlined. (B) Schematic representation of SNCA secondary structure, highlighting the positions of the new and known mutations in exon 3. (C) X-band electron paramagnetic resonance spectra of wild-type (wt) SNCA, H50Q, and H50A, with 1.0 Eq Cu2+. Vertical lines correspond to parallel hyperfine features of both wt and mutant forms.
The H50 imidazole side chain is an avid copper (Cu2+) binding group, and our previous work confirmed its participation in copper binding.2 We investigated the effect of H50Q by electron paramagnetic resonance (EPR) using recombinantly expressed wild-type (wt) SNCA, H50Q, and the previously studied H50A, as described.2 Comparison of EPR spectra from all, each with a single equivalent of Cu2+, revealed greater splitting (larger All) and a downfield shift (larger gII) of the parallel hyperfine region for H50Q relative to wt, similar to H50A, and consistent with replacement of an equatorial nitrogen with an oxygen (figure 1C, table e-2). Moreover, the H50Q spectrum did not overlap with that of wt. These data demonstrate that, in contrast to wt SNCA, H50Q takes up Cu2+ exclusively at its N terminus.
Discussion.
We have detected a novel SNCA missense change that affects copper coordination in 1 apparently sporadic PD case. H50Q SNCA does bind Cu2+, but there is no participation in metal coordination from other portions of the protein. This contrasts with wt, where H50 wraps around to participate in the Cu2+ coordination environment, in a structure incompatible with an α-helix.2 H50Q may therefore enhance the stability of an extended α-helix. Moreover, H50Q in solution is less conformationally restricted than wt in the presence of Cu2+. Copper is elevated in the CSF of patients with PD,3 it enhances SNCA fibril formation4 and aggregation in cell cultures,5 and dopaminergic neurons are vulnerable to Cu2+ in rats6 and Drosophila.7
Based on the conservation of H50, the absence of the mutation in controls, and the predicted and observed functional effects, we propose c.150T>G (p.H50Q) as a pathogenic mutation, possibly with reduced penetrance, although we cannot exclude the possibility that it is a rare nonpathogenic variant. In vivo work, and identification of this mutation in other cases, will help confirm pathogenicity. The relevance of Cu2+ to PD merits further study.
Note added in proof:
The same mutation has been reported in a Canadian patient in abstract form (Appel-Cresswell et al., Mov Disord 2012;27:S597).
Supplementary Material
Footnotes
Author contributions: Dr. Proukakis: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; acquisition of data; study supervision or coordination; obtaining funding. Dr. Dudzik: drafting/revising the manuscript for content, including medical writing for content; analysis or interpretation of data; acquisition of data. Dr. Brier: analysis or interpretation of data; acquisition of data. Dr. MacKay: drafting/revising the manuscript for content, including medical writing for content; analysis or interpretation of data. Dr. Cooper: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. Dr. Millhauser: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; study supervision or coordination; obtaining funding. Dr. Houlden: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. Dr. Schapira: drafting/revising the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; study supervision or coordination; obtaining funding.
Acknowledgment: The authors are grateful to the Queen Square Brain Bank for providing brain samples. This work made use of samples generated by the 1958 Birth Cohort (http://www2.le.ac.uk/projects/birthcohort; http://www.bristol.ac.uk/alspac/; http://www.cls.ioe.ac.uk/ncds; http://www.esds.ac.uk/findingData/ncds.asp) under grant G0000934 from the Medical Research Council, and grant 068545/Z/02 from the Wellcome Trust.
Study funding: Supported by the Royal Free Peter Samuel fund, the Wellcome Trust/MRC joint call in Neurodegeneration award (WT089698), the Kattan Trust, the NIH (grant GM065790), and Parkinson's UK (innovation grant K-1111).
Disclosure: C. Proukakis has received research funding from the Royal Free Peter Samuel Research Fund, and Parkinson's UK. C.G. Dudzik, T. Brier, and D.S. MacKay report no disclosures. J.M. Cooper has received research funding from Parkinson's UK, the Medical Research Council (UK), and the Wellcome Trust. G.L. Millhauser has received funding from the NIH. H. Houlden has received research funding from the Medical Research Council (UK). A.H. Schapira has received research funding Parkinson's UK, the Medical Research Council (UK), the Wellcome Trust, and the Kattan Trust. Go to Neurology.org for full disclosures.
Supplemental data at www.neurology.org
References
- 1.Gu M, Owen AD, Toffa SE, et al. Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J Neurol Sci 1998;158:24–29 [DOI] [PubMed] [Google Scholar]
- 2.Dudzik CG, Walter ED, Millhauser GL. Coordination features and affinity of the Cu2+ site in the α-synuclein protein of Parkinson’s disease. Biochemistry 2011;50:1771–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pall HS, Blake DR, Gutteridge JM, et al. Raised cerebrospinal fluid copper concentration in Parkinson's disease. Lancet 1987;330:238–241 [DOI] [PubMed] [Google Scholar]
- 4.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. J Biol Chem 2001;276:44284–44296 [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Moualla D, Wright JA, Brown DR. Copper binding regulates intracellular alpha-synuclein localisation, aggregation and toxicity. J Neurochem 2010;113:704–714 [DOI] [PubMed] [Google Scholar]
- 6.Yu WR, Jiang H, Wang J, Xie JX. Copper induces degeneration of dopaminergic neurons in the nigrostriatal system of rats. Neurosci Bull 2008;24:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonilla-Ramirez L, Jimenez-Del-Rio M, Velez-Pardo C. Acute and chronic metal exposure impairs locomotion activity in Drosophila melanogaster: a model to study parkinsonism. Biometals 2011;24:1045–1057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.