Abstract
Objective:
To evaluate total antioxidant capacity of the diet, measured by the ferric-reducing antioxidant power (FRAP) assay, in relation to risks of dementia and stroke, as well as key structural brain volumes, in the elderly.
Methods:
We prospectively studied 5,395 participants in the Rotterdam Study, aged 55 years and older, who were dementia free and provided dietary information at study baseline; 5,285 individuals were also stroke free at baseline, and 462 were dementia and stroke free at the time of an MRI brain scan 5 years after baseline. Dietary data were ascertained using a semiquantitative food-frequency questionnaire, and combined with food-specific FRAP measurements from published tables; this information was aggregated across the diet to obtain “dietary FRAP scores.” Multivariable-adjusted Cox proportional hazard models were used to estimate relative risks of dementia and stroke, and multivariable-adjusted linear regression was used to estimate mean differences in structural brain volumes, across tertiles of dietary FRAP scores.
Results:
During a median 13.8 years of follow-up, we identified approximately 600 cases each of dementia and stroke. In multivariable-adjusted models, we observed no associations between dietary FRAP scores and risk of dementia (p trend = 0.3; relative risk = 1.12, 95% confidence interval = 0.91–1.38, comparing the highest vs lowest FRAP tertiles) or risk of stroke (p trend = 0.3; relative risk = 0.91, 95% confidence interval = 0.75–1.11, comparing extreme FRAP tertiles); results were similar across subtypes of these outcomes. Dietary FRAP scores were unrelated to brain tissue volumes as well.
Conclusions:
Total antioxidant capacity of the diet, measured by dietary FRAP scores, does not seem to predict risks of major neurologic diseases.
Oxidative stress is an important contributor to pathogenesis of dementia and stroke, although epidemiologic studies, including clinical trials, of antioxidant vitamins have yielded mixed results for these outcomes.1–4 However, most studies have focused on single antioxidant supplements, despite growing evidence that wider varieties of antioxidant nutrients available from foods could reduce various chronic disease risks.5 Indeed, increased attention has focused on measurement of “total antioxidant capacity of the diet,” which might be a useful metric for capturing overall effects of antioxidant nutrients across dietary sources.6,7 In the Rotterdam Study, we previously found that higher intakes of dietary antioxidants were related to lower risks of dementia and stroke, but these associations might be attributable to individual antioxidant nutrients per se, or alternatively, to overall antioxidant capacity of the diet.8,9 In the present study, we examined total antioxidant capacity of the diet in relation to risks of dementia and stroke in the Rotterdam Study. We also evaluated the association between total antioxidant capacity of the diet and structural brain volumes in the Rotterdam Scan Study because such measurements are strong preclinical markers of dementia.10–14
METHODS
The Rotterdam Study is a population-based cohort study designed to examine the epidemiology of chronic diseases in the elderly.15 It began in 1990, when 7,983 residents of Rotterdam, aged 55 years and older, participated in a home interview and clinical examination (78% response rate). Follow-up examinations were performed in 1993–1994, 1997–1999, and 2002–2004. The cohort is continuously monitored for morbidity and mortality through computerized linkage to participant medical records. In 1995, the Rotterdam Scan Study was established to investigate brain abnormalities in the elderly; 563 dementia-free participants of the Rotterdam Study consented for MRI, and complete data were obtained on 490 individuals.16
Standard protocol approvals.
All participants granted written informed consent, and the medical ethics committee of Erasmus Medical Center (Rotterdam, the Netherlands) approved the study.
Population for analysis.
For dementia analyses, 7,046 participants (88% of individuals in the Rotterdam Study) were dementia free at baseline; of these, 125 people were excluded because of questionable cognitive status, and 477 were excluded because they lived in a nursing home. An additional 1,049 individuals provided no dietary information at baseline, leaving 5,395 participants for analyses of dietary ferric-reducing antioxidant power (FRAP) scores and dementia risk. For stroke analyses, we applied the same criteria but additionally excluded 110 participants with a prior stroke at baseline, leaving 5,285 individuals for analyses of dietary FRAP and stroke risk. Finally, for analyses involving brain volumes, we analyzed 462 participants with complete dietary and MRI data, who had no prior stroke or dementia at MRI.
Dietary assessment.
Participants completed a meal-based checklist of foods during their home interview, and a detailed semiquantitative food-frequency questionnaire at clinical examination.17 The food-frequency questionnaire asked participants about their “usual” frequency and amount of consumption of 170 food items over the previous year. To calculate each food's contribution to FRAP, we used the Antioxidant Food Table published by the Institute of Nutrition Research, University of Oslo, which includes measurements of >3,000 foods worldwide based on the FRAP assay.18 We consulted nutritional experts at Wageningen University (the Netherlands) to determine FRAP assignments for Dutch foods. For each participant, we multiplied the consumption frequency of each food by the corresponding FRAP value, and summed these values across all dietary sources.
In this cohort, most variation in dietary FRAP scores was explained by intakes of coffee (65%) and tea (21%), which contain high levels of nontraditional antioxidants (e.g., flavonoids and other polyphenols); oranges, red wine, and chocolate each contributed an additional 1% to 2% to this variation.
Diagnosis of dementia and stroke.
We used a 3-step protocol at baseline and follow-up examinations to ascertain dementia: 1) brief screening tests for all participants (Mini-Mental State Examination and Geriatric Mental State—Organic Level); 2) additional neuropsychological testing for those who screened poorly (Cambridge Assessment Mental Disorders in the Elderly); and 3) further evaluation by a neurologist or neuropsychologist, if necessary.19 Dementia was diagnosed with internationally accepted criteria for dementia (DSM-III-R),20 Alzheimer disease (National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association),21 and vascular dementia (National Institute of Neurological Disorders and Stroke–AIREN [Association Internationale pour la Recherche et l’Enseignement en Neurosciences]).22 To ascertain stroke cases, we used medical records to verify self-reported strokes at baseline; cases were then classified as ischemic, hemorrhagic, or unspecified origin.23 In addition, participants are continuously monitored for incident dementia or stroke by computerized linkage to participants' digitalized medical records.
Measurement of brain tissue volumes and white matter lesions.
A 1.5-tesla VISION scanner (Siemens, Erlangen, Germany) was used to obtain 3 axial brain scans (T1 weighted, proton density weighted, and T2 weighted) and a custom-made, inversion-recovery double-contrast, 3-dimensional half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence.16 The proton density–weighted, T2-weighted, and HASTE scans were used to perform an automated classification of brain tissue. We transferred scans offline to a Linux workstation and conducted preprocessing and tissue classification steps using methods previously described. In short, the k-nearest neighbor classification method was utilized to differentiate CSF, gray matter, normal white matter, and white matter lesions. After segmentation, we visually inspected and manually corrected scans as necessary, and applied a nonrigid registration to remove noncerebral tissue with a template scan. We summed all voxels assigned to each tissue class to calculate brain volumes; correlations between automated and manual classification of brain tissue are excellent in our study.11
Hippocampal segmentation.
The hippocampus was segmented by an automated method that utilized both a statistical intensity model and a spatial probability map.24 The statistical intensity model contains information about typical intensities of the hippocampus and background, and was acquired from a training set of 20 manually segmented scans. The spatial probability map contains information on the probability that each voxel is part of the hippocampus, and was obtained by nonrigidly registering labeled images from the training set to unlabeled target images, then deforming the manual segmentation and averaging them. All automatically generated segmentations were visually inspected and manually edited, if necessary.
Covariates.
Detailed information on health and lifestyle was ascertained during the home interview and clinical examination.
Statistical analysis.
We used age- and multivariable-adjusted Cox proportional hazard models to obtain hazard ratios (HRs), estimated by relative risks, of dementia (all dementia, Alzheimer disease, and vascular dementia) across sex-specific tertiles of energy-adjusted dietary FRAP scores.25 Participants were censored at time of dementia, death, or loss to follow-up. We considered the following potential confounders: age (continuous), education (low, intermediate, high), APOE ε4 genotype (at least 1 ε4 allele vs no ε4 allele), total energy intake (continuous in kilojoules per day), smoking habits (current, former, never), body mass index (continuous), and supplement use (yes vs no). There were 226 participants without APOE genotype who were excluded from models that adjusted for this variable. Similar models were used to examine the relation of dietary FRAP scores and risk of stroke (all stroke, ischemic, hemorrhagic, and unspecified), in which participants were censored at time of stroke, death, or loss to follow-up. For these analyses, we considered possible confounding by age, total energy intake, smoking habits, supplement use, and history of blood pressure, diabetes, and myocardial infarction (yes vs no). In addition, we used age- and multivariable-adjusted linear regression to estimate mean differences in structural brain volumes across tertiles of dietary FRAP scores; we examined the same potential confounding variables as described for dementia analyses.
In secondary analyses, we evaluated whether associations of dietary FRAP scores with dementia, stroke, and brain tissue volumes might be modified by smoking status. Interaction terms were included in models with smoking (never, former, current) and FRAP (in tertiles) as ordinal variables. We repeated all analyses excluding supplement users at baseline, and using quintiles of FRAP scores and cubic spline analyses to further evaluate nonlinear associations.
Data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL).
RESULTS
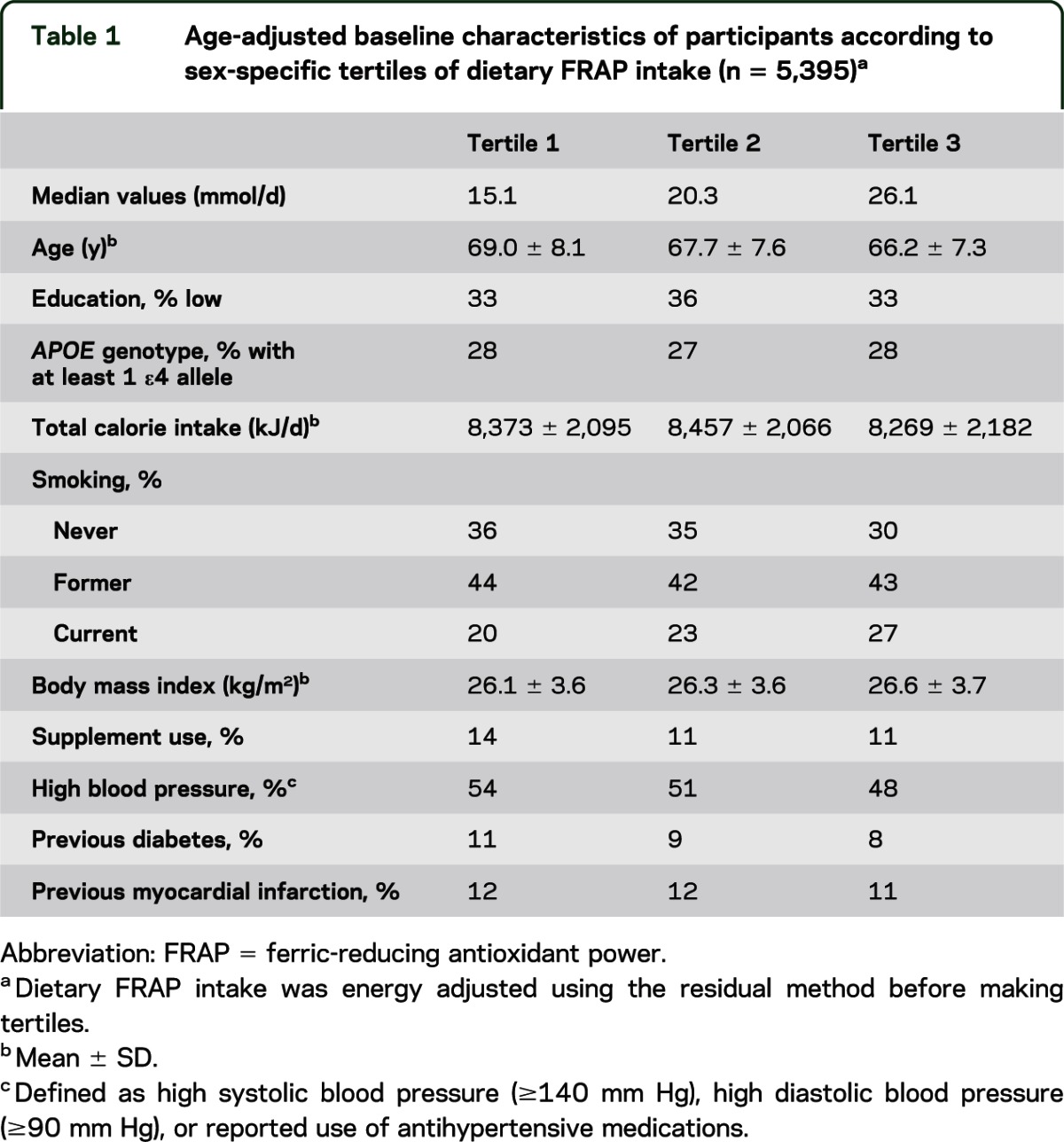
Table 1 indicates that key participant characteristics were similar across tertiles of dietary FRAP scores, except that participants were slightly younger and more often current smokers over increasing tertiles. During a median follow-up period of 13.8 years (interquartile range = 9.2–14.8), we identified 599 cases of dementia that included 484 cases of Alzheimer disease, 59 cases of vascular dementia, and 56 cases of dementia of unknown origin. We also documented 601 cases of stroke during this period, including 381 cases of ischemic stroke, 64 cases of hemorrhagic stroke, and 156 cases of unspecified origin.
Table 1.
Age-adjusted baseline characteristics of participants according to sex-specific tertiles of dietary FRAP intake (n = 5,395)a
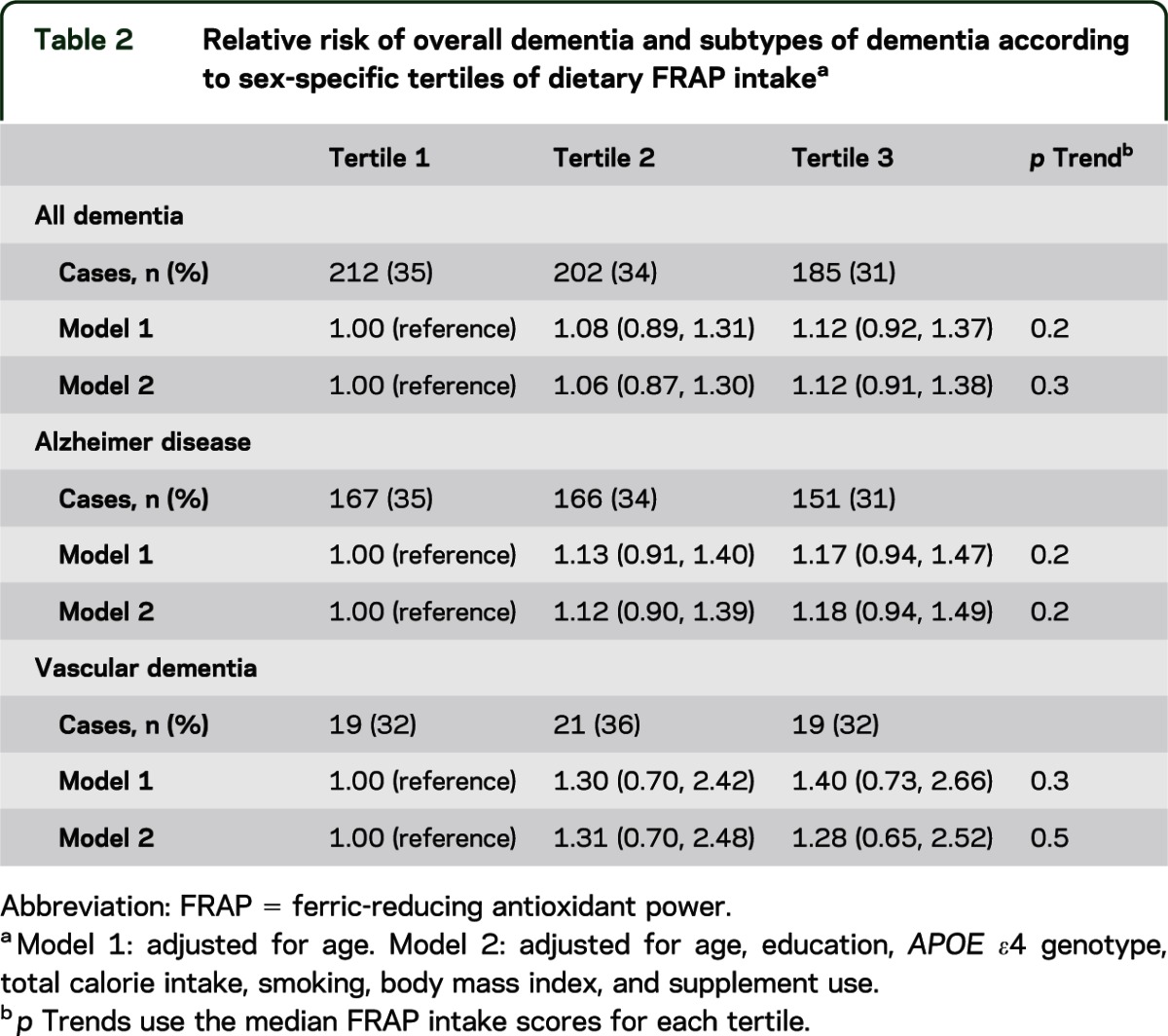
We found no association of dietary FRAP scores and dementia risk in age-adjusted models (p trend = 0.2) or multivariable models controlling for age, education, APOE ε4 genotype, total calorie intake, smoking, body mass index, and supplement use (p trend = 0.3) (table 2). Specifically, in fully adjusted models, participants in the highest tertile of dietary FRAP scores had a similar dementia risk compared with those in the lowest tertile of FRAP scores (HR = 1.12; 95% confidence interval [CI] = 0.91–1.38, comparing top vs bottom FRAP tertiles). When we examined specific types of dementia, there was no relation between dietary FRAP scores and risk of Alzheimer disease (e.g., in fully adjusted models, p trend = 0.2 and HR = 1.18, 95% CI = 0.94–1.49, comparing extreme FRAP tertiles) or risk of vascular dementia (e.g., in fully adjusted models, p trend = 0.5 and HR = 1.28, 95% CI = 0.65–2.52, comparing extreme FRAP tertiles).
Table 2.
Relative risk of overall dementia and subtypes of dementia according to sex-specific tertiles of dietary FRAP intakea
In addition, dietary FRAP scores were not related to overall risk of stroke in models adjusted for age alone (p trend = 0.4), or models additionally adjusted for total calorie intake, smoking, high blood pressure, diabetes, myocardial infarction, and supplement use (p trend = 0.3) (table 3). In particular, stroke risk was similar for participants in the highest vs lowest tertiles of FRAP scores (e.g., in fully adjusted models, HR = 0.91, 95% CI = 0.75–1.11, comparing extreme FRAP tertiles). When we examined different types of stroke, no associations were observed for ischemic stroke, hemorrhagic stroke, or stroke of unknown origin (e.g., p trends = 0.9, 0.3, and 0.3, respectively, in models adjusted for multiple potential confounders) and participants had similar risks of each stroke type when highest vs lowest tertiles of FRAP scores were compared (e.g., for ischemic stroke: HR = 0.99, 95% CI = 0.77–1.26; for hemorrhagic stroke: HR = 0.73, 95% CI = 0.39–1.36; for stroke of unknown origin: HR = 0.79, 95% CI = 0.52–1.19).
Table 3.
Relative risk of overall stroke and subtypes of stroke according to sex-specific tertiles of dietary FRAP intakea
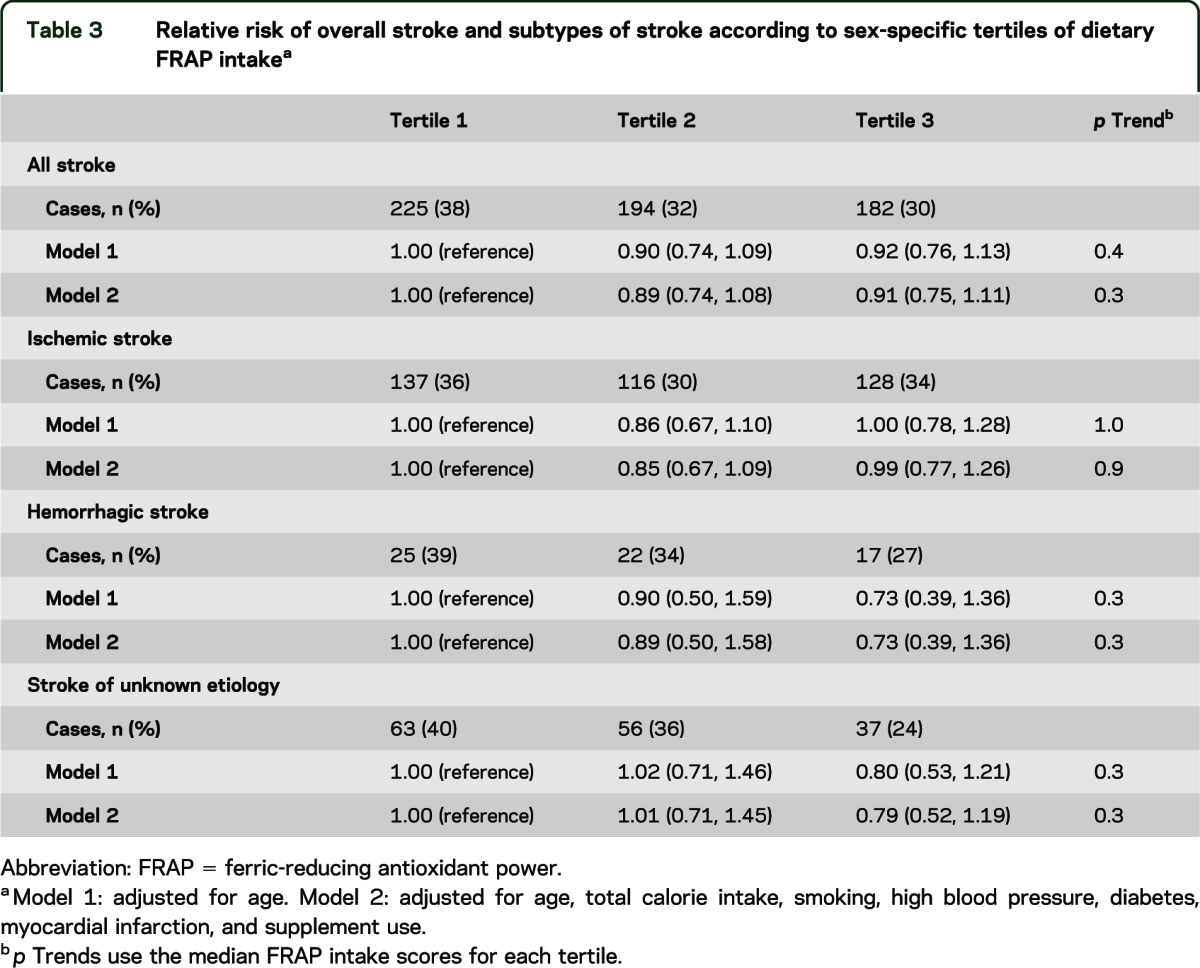
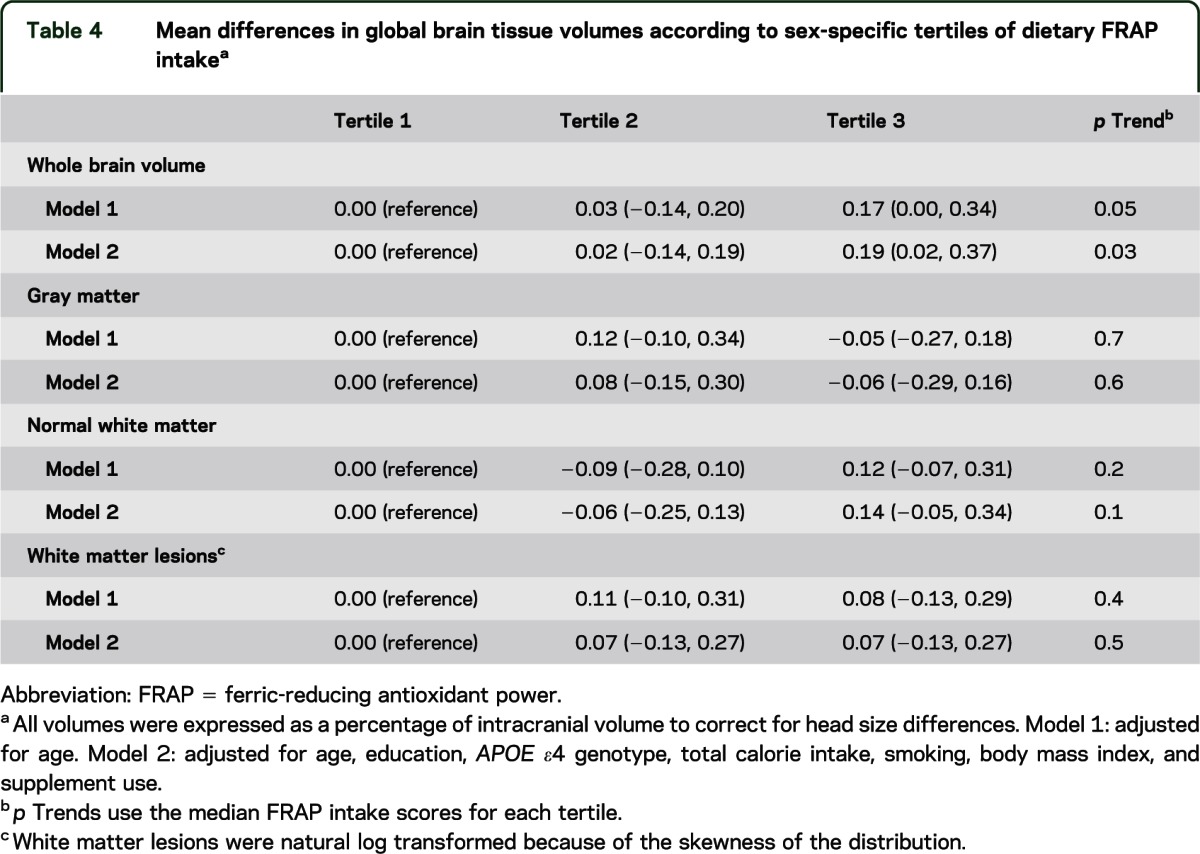
Table 4 shows the associations of dietary FRAP scores and brain tissue volumes, including whole brain volume (the sum of gray matter and white matter), gray matter, normal white matter, and white matter lesions in models adjusted for age and those additionally adjusted for education, APOE ε4 genotype, total calorie intake, smoking, body mass index, and supplement use. We observed that higher scores for dietary FRAP were related to greater whole brain volumes in models adjusted for multiple potential confounders (e.g., p trend = 0.03 and mean difference = 0.19; 95% CI = 0.02–0.37, comparing extreme tertiles of FRAP scores). However, dietary FRAP scores were not associated with volumes of gray matter, normal white matter, and white matter lesions in fully adjusted models (p trends = 0.6, 0.1, and 0.5, respectively). We also found no association between dietary FRAP scores and either left or right hippocampal volume (in multivariable models, p trends = 1.0 and 0.7, respectively) (results not shown in tables).
Table 4.
Mean differences in global brain tissue volumes according to sex-specific tertiles of dietary FRAP intakea
In additional analyses, there was no effect modification by smoking of associations between dietary FRAP scores and neurologic outcomes of interest (e.g., p interactions = 0.5 for dementia, 0.4 for stroke, and 0.6 for whole brain volume). Results were virtually identical when we excluded participants reporting supplement use at baseline, and when we conducted analyses of dietary FRAP in quintiles (e.g., see figure e-1 for dementia and stroke on the Neurology® Web site at www.neurology.org). Cubic spline analyses produced results that were generally similar as well, although there was a suggestion that extremely high FRAP scores might be related to higher stroke risk (p value for nonlinearity = 0.02; see figure e-2 for stroke, as well as for dementia). However, this result was contrary to our a priori hypothesis, and the CI in this extreme part of the distribution was very wide; therefore, it should not be overinterpreted.
DISCUSSION
Total antioxidant capacity of the diet, measured by dietary FRAP scores, was not related to a reduced risk of dementia or stroke in this cohort. In addition, no associations of dietary FRAP scores and structural brain volumes were identified, except that higher scores for dietary FRAP were associated with larger whole brain volume. Although this finding might represent a causal association, this result should be interpreted with caution given that no relations were observed for the constituents of whole brain volumes (i.e., gray and white matter).
We evaluated total antioxidant capacity of the diet because it might be a useful metric for understanding the relation between dietary antioxidants and major neurologic diseases of the elderly. First, it captures antioxidant effects of a wide variety of dietary nutrients, including micronutrients that are not well characterized or well measured (e.g., flavonoids). Second, dietary FRAP scores estimate total antioxidant capacity of the diet based on food-specific measurements, and thus inherently capture interactions among antioxidant nutrients in these foods; analyses of single antioxidant nutrients do not capture such interactions. Finally, dietary antioxidant scores are useful for eliminating issues of multiple comparisons that are inherent in analyses of multiple, individual antioxidant nutrients. Although this approach has several important advantages compared with studying individual antioxidant nutrients, our findings suggest that this metric does not capture as much biologically relevant information as individual antioxidant nutrients for predicting key neurologic outcomes in older adults.
Previous literature is sparse regarding the associations of total antioxidant capacity of the diet with major neurologic outcomes.6,7 However, our findings are generally consistent with a large, prospective analysis of 16,010 participants in the Nurses' Health Study, which did not identify a relation between dietary FRAP scores and cognitive decline (a strong marker of impending dementia) in older women (e.g., for a global composite score, p trend = 0.5 and mean difference = 0.00, 95% CI = 0.00, 0.01, comparing extreme quintiles of FRAP scores).7 A second study, conducted in a large cohort of 41,620 Italian participants, found that higher antioxidant capacity of the diet was associated with a reduced risk of ischemic stroke (p trend = 0.003 and HR = 0.41, 95% CI = 0.23–0.74, comparing extreme tertiles of dietary antioxidant scores).6 Although we identified 3 times as many stroke cases in our cohort (601 vs 194 cases), we did not detect a similar inverse association between dietary antioxidant capacity and ischemic stroke risk. However, participants in the Italian cohort were younger than our participants (approximately 50 vs 68 years old), and therefore discrepant findings may reflect a true difference in the biology of this association—total antioxidant capacity of the diet could help alleviate ischemic stroke pathology at midlife, but not at older ages. Another important difference between our study and the Italian study is that almost 90% of variation in FRAP scores in our cohort was attributable to coffee and tea intake, whereas the Italian study reported smaller relative contributions of coffee and tea (31%) and larger relative contributions of alcoholic beverages (28%), fruits (10%), and vegetables (7%) to total antioxidant capacity of the diet.26 Growing epidemiologic evidence does suggest that higher consumption of fruits, vegetables, and alcohol may be associated with reduced risk of stroke27,28; thus, these findings could reflect that specific antioxidant-rich food and beverage intakes are more important for lowering stroke risk than overall antioxidant capacity of the diet.
Alternatively, the discrepancy in stroke findings might be explained by the different assays used to estimate dietary antioxidant scores. In the Rotterdam Study, we assessed total antioxidant capacity of the diet using the FRAP assay, whereas the Italian study used the Trolox equivalent antioxidant capacity (TEAC) assay, and these assays measure different antioxidant mechanisms. Because there is no “gold standard” for assessing the total antioxidant capacity of foods, it is difficult to determine which assay is more relevant for measuring the exposure of interest. However, the FRAP assay does not detect glutathione or protein thiols, which are largely degraded in the intestine and poorly absorbed; thus, FRAP is thought to have an advantage over TEAC in the context of studying human disease.5 Still, previous studies have found that FRAP- and TEAC-based antioxidant scores predict important health-related end points,29–31 such that both seem to capture biologically relevant information for disease prediction. For this reason, the difference in antioxidant assays utilized in the Rotterdam vs Italian studies is unlikely to fully explain the discrepant results in these studies.
Previous analyses in the Rotterdam Study indicate that greater intakes of vitamin C might be related to lower risk of stroke,9 and higher consumption of vitamin E may be associated with a reduced risk of dementia.8 Although these results were based on shorter follow-up time in this cohort, we have confirmed these results in the present dataset with longer follow-up and more incident cases of stroke and dementia. Taken together with the current findings for dietary FRAP scores, these results suggest that individual antioxidants, or major food contributors to those antioxidants—not overall antioxidant capacity of the diet—probably contribute to lower risks of dementia and stroke in the Rotterdam Study.
Certain limitations of this study should be considered. First, dietary FRAP scores were only modestly associated with plasma FRAP measurements in 2 previous studies. However, most feeding studies have demonstrated that consumption of antioxidant-rich foods is significantly related to plasma FRAP measurements taken immediately after ingestion.26,32,33 In addition, plasma FRAP measurements may not provide an appropriate gold standard for dietary FRAP scores when they are based on long-term diet, which might explain the modest associations previously observed between plasma- and diet-based measures. Similar modest associations have been identified for other antioxidant assay–based diet scores, including TEAC, which supports the notion that plasma antioxidant measurements may be insufficient markers of long-term antioxidant intake.26 Furthermore, significant associations of higher dietary FRAP scores with lower levels of components of the metabolic syndrome, higher concentrations of adiponectin, and lower risk of mortality have been reported.29–31 An additional limitation of this study is that our self-reported food-frequency data contain some random measurement error, which would tend to bias our results toward the null.34 However, we used a validated assessment for dietary intake, and relations between dietary antioxidants and multiple chronic diseases have been previously identified in the Rotterdam Study, establishing our ability to detect these relations in this cohort.8,9,35–38
Overall, we found little association between total antioxidant capacity of the diet, measured by FRAP, and major neurologic diseases of the elderly in the Rotterdam Study.
Supplementary Material
GLOSSARY
- CI
confidence interval
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- FRAP
ferric-reducing antioxidant power
- HASTE
half-Fourier acquisition single-shot turbo spin-echo
- HR
hazard ratio
- TEAC
Trolox equivalent antioxidant capacity
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Elizabeth E. Devore contributed to the analysis and interpretation of the data, as well as drafting and revision of the manuscript for intellectual content. Edith Feskens contributed to the analysis and interpretation of the data, and to revisions of the manuscript for intellectual content. M. Arfan Ikram, Tom Den Heijer, Meike Vernooij, and Fedde van der Lijn contributed to revisions of the manuscript for intellectual content. Albert Hofman contributed to the design and conceptualization of the Rotterdam Study and Rotterdam Scan Study, as well as revision of the manuscript for intellectual content. Wiro J. Niessen contributed to revisions of the manuscript for intellectual content. Monique M.B. Breteler contributed to the design and conceptualization of the Rotterdam Study and Rotterdam Scan Study, to the analysis and interpretation of the data, and revision of the manuscript for intellectual content.
STUDY FUNDING
The Netherlands Organization for Scientific Research (NOW, 918-46-615) supported this research, and NIH (F32 AG031633) provided salary support for Dr. Devore to conduct this research.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 2009;4:461–470 [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature 2004;430:631–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington M, Grodstein F. Antioxidant vitamins and Alzheimer's disease: a review of the epidemiological literature. Aging Health 2007;3:23–32 [Google Scholar]
- 4.Schurks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 2010;341:c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blomhoff R. Dietary antioxidants and cardiovascular disease. Curr Opin Lipidol 2005;16:47–54 [DOI] [PubMed] [Google Scholar]
- 6.Del Rio D, Agnoli C, Pellegrini N, et al. Total antioxidant capacity of the diet is associated with lower risk of ischemic stroke in a large Italian cohort. J Nutr 2011;141:118–123 [DOI] [PubMed] [Google Scholar]
- 7.Devore EE, Kang JH, Stampfer MJ, Grodstein F. Total antioxidant capacity of diet in relation to cognitive function and decline. Am J Clin Nutr 2010;92:1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devore EE, Grodstein F, van Rooij FJ, et al. Dietary antioxidants and long-term risk of dementia. Arch Neurol 2010;67:819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voko Z, Hollander M, Hofman A, Koudstaal PJ, Breteler MM. Dietary antioxidants and the risk of ischemic stroke: the Rotterdam Study. Neurology 2003;61:1273–1275 [DOI] [PubMed] [Google Scholar]
- 10.Kern A, Behl C. The unsolved relationship of brain aging and late-onset Alzheimer disease. Biochim Biophys Acta 2009;1790:1124–1132 [DOI] [PubMed] [Google Scholar]
- 11.Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiol Aging 2010;31:378–386 [DOI] [PubMed] [Google Scholar]
- 12.den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry 2006;63:57–62 [DOI] [PubMed] [Google Scholar]
- 13.Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease: a longitudinal MRI study. Brain 1996;119:2001–2007 [DOI] [PubMed] [Google Scholar]
- 14.van de Pol LA, van der Flier WM, Korf ES, Fox NC, Barkhof F, Scheltens P. Baseline predictors of rates of hippocampal atrophy in mild cognitive impairment. Neurology 2007;69:1491–1497 [DOI] [PubMed] [Google Scholar]
- 15.Hofman A, Breteler MM, van Duijn CM, et al. The Rotterdam Study: 2010 objectives and design update. Eur J Epidemiol 2009;24:553–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikram MA, van der Lugt A, Niessen WJ, et al. The Rotterdam Scan Study: design and update up to 2012. Eur J Epidemiol 2011;26:811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, et al. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr 1998;52:588–596 [DOI] [PubMed] [Google Scholar]
- 18.Carlsen MH, Halvorsen BL, Holte K, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J 2010;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 2012;78:1456–1463 [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington, DC: American Psychiatry Association; 1987 [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 22.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260 [DOI] [PubMed] [Google Scholar]
- 23.Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MM. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol 2012;27:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Lijn F, den Heijer T, Breteler MM, Niessen WJ. Hippocampus segmentation in MR images using atlas registration, voxel classification, and graph cuts. Neuroimage 2008;43:708–720 [DOI] [PubMed] [Google Scholar]
- 25.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–1228S [DOI] [PubMed] [Google Scholar]
- 26.Pellegrini N, Salvatore S, Valtuena S, et al. Development and validation of a food frequency questionnaire for the assessment of dietary total antioxidant capacity. J Nutr 2007;137:93–98 [DOI] [PubMed] [Google Scholar]
- 27.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet 2006;367:320–326 [DOI] [PubMed] [Google Scholar]
- 28.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puchau B, Zulet MA, Gonzalez de Echavarri A, Hermsdorff HH, Martinez JA. Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young adults. Nutrition 2010;26:534–541 [DOI] [PubMed] [Google Scholar]
- 30.Detopoulou P, Panagiotakos DB, Chrysohoou C, et al. Dietary antioxidant capacity and concentration of adiponectin in apparently healthy adults: the ATTICA study. Eur J Clin Nutr 2010;64:161–168 [DOI] [PubMed] [Google Scholar]
- 31.Agudo A, Cabrera L, Amiano P, et al. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Am J Clin Nutr 2007;85:1634–1642 [DOI] [PubMed] [Google Scholar]
- 32.Rautiainen S, Serafini M, Morgenstern R, Prior RL, Wolk A. The validity and reproducibility of food-frequency questionnaire-based total antioxidant capacity estimates in Swedish women. Am J Clin Nutr 2008;87:1247–1253 [DOI] [PubMed] [Google Scholar]
- 33.Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: is the Total Antioxidant Capacity the right tool? Redox Rep 2004;9:145–152 [DOI] [PubMed] [Google Scholar]
- 34.Willett WC. Nutritional Epidemiology, 2nd ed New York: Oxford University Press; 1998 [Google Scholar]
- 35.Klipstein-Grobusch K, den Breeijen JH, Grobbee DE, Boeing H, Hofman A, Witteman JC. Dietary antioxidants and peripheral arterial disease: the Rotterdam Study. Am J Epidemiol 2001;154:145–149 [DOI] [PubMed] [Google Scholar]
- 36.Klipstein-Grobusch K, Geleijnse JM, den Breeijen JH, et al. Dietary antioxidants and risk of myocardial infarction in the elderly: the Rotterdam Study. Am J Clin Nutr 1999;69:261–266 [DOI] [PubMed] [Google Scholar]
- 37.de Rijk MC, Breteler MM, den Breeijen JH, et al. Dietary antioxidants and Parkinson disease: the Rotterdam Study. Arch Neurol 1997;54:762–765 [DOI] [PubMed] [Google Scholar]
- 38.van Leeuwen R, Boekhoorn S, Vingerling JR, et al. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 2005;294:3101–3107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


