Abstract
Objective:
To review the discoveries underpinning the introduction of cerebral PET scanning and highlight its modern applications.
Background:
Important discoveries in neurophysiology, brain metabolism, and radiotracer development in the post–World War II period provided the necessary infrastructure for the first cerebral PET scan.
Methods:
A complete review of the literature was undertaken to search for primary and secondary sources on the history of PET imaging. Searches were performed in PubMed, Google Scholar, and select individual journal Web sites. Written autobiographies were obtained through the Society for Neuroscience Web site at www.sfn.org. A reference book on the history of radiology, Naked to the Bone, was reviewed to corroborate facts and to locate references. The references listed in all the articles and books obtained were reviewed.
Results:
The neurophysiologic sciences required to build cerebral PET imaging date back to 1878. The last 60 years have produced an evolution of technological advancements in brain metabolism and radiotracer development. These advancements facilitated the development of modern cerebral PET imaging. Several key scientists were involved in critical discoveries and among them were Angelo Mosso, Charles Roy, Charles Sherrington, John Fulton, Seymour Kety, Louis Sokoloff, David E. Kuhl, Gordon L. Brownell, Michael Ter-Pogossian, Michael Phelps, and Edward Hoffman.
Conclusions:
Neurophysiology, metabolism, and radiotracer development in the postwar era synergized the development of the technology necessary for cerebral PET scanning. Continued use of PET in clinical trials and current developments in PET-CT/MRI hybrids has led to advancement in diagnosis, management, and treatment of neurologic disorders.
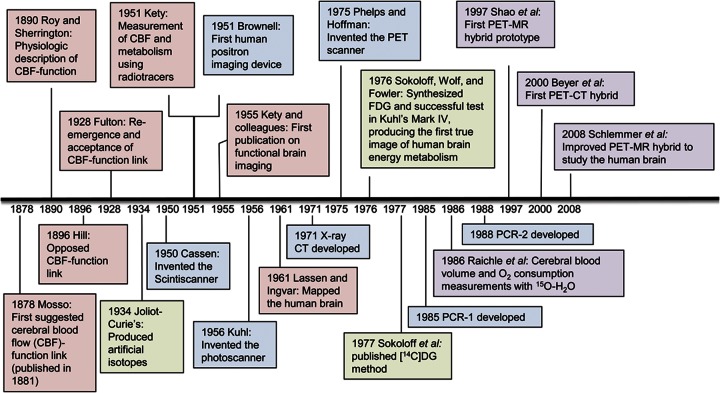
PET facilitates understanding of both normal and abnormal brain function. PET imaging was based on discoveries dating back to the late 1800s when the physiology of brain circulation first became appreciated.1 There were, however, critical challenges to the development of cerebral PET imaging, including overcoming previously existing and incorrect notions about physiologic aspects of brain circulation.1 The evolution from theory to medical practice did not occur until the 1950s, when modern radiotracers and technologically advanced scanning devices were introduced.2 Several key scientists devised novel methods and refined technology that enhanced detection of brain function. Here, we aim to review the physiology, brain metabolism, and radiotracer development that led to modern cerebral PET scanning. We also review the timeline of important discoveries that led to cerebral PET scanning (figure 1).
Figure 1. Timeline of important discoveries in cerebral PET imaging.
What is PET?
Radiotracers emit positrons, positive antiparticles of electrons, which then undergo radioactive decay. They collide with electrons to produce 2 photons, or gamma rays, which are emitted at 180-degree angles.2 PET scanners detect these photons and reconstruct an image of spatial density that highlights the functional data and reveals blood flow changes related to activity.2,3 PET scanners have proven particularly useful for studying normal vs abnormal brain activity. Combining PET with CT or MRI delineates both function and anatomic localization.3
METHODS
A complete review of the literature was undertaken for primary and secondary sources on the history of PET imaging. Searches were performed in PubMed, Google Scholar, and select journals. Primary neurophysiologic texts and secondary historical papers were also reviewed. Written autobiographies were obtained through the Society for Neuroscience Web site. A book on the history of radiology, Naked to the Bone, was reviewed to corroborate facts and to locate references.
RESULTS
Physiology and blood flow science.
Discovery of the concept that blood flow relates to brain function can be traced to the Italian physiologist Angelo Mosso.3 In 1878, Mosso measured an increase in brain pulsations from the right prefrontal cortex during an arithmetic task performed by a subject with a bony skull defect.4 This method was cited in 1890 as a credible means for understanding cerebral circulation by 2 scientists, Charles Roy and Charles Sherrington.5 The men both concluded that there were 2 key mechanisms controlling cerebral circulation. An intrinsic mechanism occurs in which “chemical products of cerebral metabolism … can cause variations of the caliber of the cerebral vessels” and “its vascular supply can be varied locally in correspondence with local variations of functional activity.”5 An extrinsic mechanism functions during periods of increased cerebral activity or interference to brain circulation, which depends on blood pressure and redirects blood flow away from other organs toward the brain.5 Their work is considered the first accurate description of a physiologic relationship between brain function and blood flow.3
Sir Leonard Hill, 7 an eminent British physiologist, opposed the relationship between blood flow and function. In his 1896 book, The Physiology and Pathology of Cerebral Circulation: An Experimental Research, Hill rejected Roy and Sherrington's claims as false.3,6,7 Hill7 conducted his own experiments that he claimed were more accurate. Although his technique and conclusions were criticized, Hill's work stood for many years.8 In 1928, neurosurgeon John Fulton reported a case that refocused discussions of brain circulation. Fulton's patient had a vascular malformation in the occipital lobe, and when the patient used his eyes, the intensity of the audible bruit increased.3 Fulton became well-known for localizing cerebral function in primates. He later authored several important articles in the 1930s.9,10 Fulton was generous in crediting his fellow scientists and viewed himself as belonging to a club.9 With Fulton's work and reputation, the blood flow–function theory re-emerged and gained momentum.
Radiotracer development.
Following World War II, nuclear research transitioned from the Manhattan Project to scientific particle pursuits.2 This led to the development of safe radioisotopes, bringing PET imaging one step closer to fruition. As early as 1911, however, Hungarian physicist George Hevesy tagged a hostel's food with radioactive lead, and was able to demonstrate that leftover meat had been reprocessed into 2 different dinner meals.2 Following World War II, Hevesy traced radioactive plants tagged with lead isotopes. In 1943, Hevesy was awarded the Nobel Prize in Chemistry. Irene and Frederic Joliot-Curie also added a critical discovery when in 1934 they produced an artificial isotope. This finding suggested that new elements could be formed and made safe for humans.2,11
Seymour Kety,12 a physiologist at the University of Pennsylvania and NIH, measured cerebral blood flow through the exchange of inert gas tracers beginning in 1948. Kety and his colleagues then measured increases in local blood perfusion in cats following visual stimulation. The group used autoradiography (x-ray images of radiolabeled tissue) to visualize the distribution and the rates of local blood flow.13 The research provided a direct line of evidence correlating brain circulation and function.2,12 In 1961, Niels Lassen and David Ingvar utilized radiotracer 133Xe to localize sensory, motor, and mental functions in a human brain. Color-coded patterns tracked brain blood flow as it related to function, becoming the standard visual representation of cerebral PET images.2,14
The development of human brain radioisotopes was successfully completed by Kety's student, Louis Sokoloff. Sokoloff13 studied the compound 2-deoxyglucose (DG), a reversible competitive inhibitor of glucose-6-phosphate, an important part of the glycolytic pathway. In 1969, he began research with [14C]DG, a safer and longer-lasting radiotracer that could be used to measure cerebral glucose utilization (because it accumulated in the brain). Sokoloff and colleagues created the [14C]DG method, published in 1977.13 The method facilitated direct mapping of neuroanatomical and functional pathways.13 In 1978, at the Society for Neuroscience meeting, Charlene Jarvis presented the newly minted method revealing a metabolic map of a primate brain. Visual deprivation in one hemisphere illustrated differential rates of glucose utilization.13 The Chemical & Engineering News reported that the scientific world was astounded by this discovery.
A reliable method for studying the function of the awake human brain requires a radioisotope with a longer half-life than [14C]DG.13 In 1976, Sokoloff was joined by radiochemists Dr. Alfred Wolf and Joanna Fowler. Together, they synthesized 2-[18F]fluoro-2-deoxy-d-glucose (FDG).13 The compound is one of the most widely used radiotracers today.2 FDG was tested in the Mark IV scanner, establishing its safety and efficiency, and paving the way for imaging brain metabolism in humans.12,15
Technological innovation.
Benedict Cassen, a physicist at the University of California in 1950, developed the first true radioisotope imaging system, the scintiscanner.2,16 Cassen combined the Geiger counter, the only detector of radioactivity at the time, with crystal components of the newly developed photomultiplier tube, which facilitated amplification and detection of gamma ray emissions.2,16,17 Later, in 1956, Dr. David Kuhl, then a resident at the University of Pennsylvania, modified Cassen's device and developed the photoscanner. In his design, a radioisotope emission-activated glow lamp provided grayscale images with a greater sensitivity and resolution than ever before.16 Kuhl developed several SPECT devices known as Mark II, Mark III, and Mark IV in 1964, 1970, and 1976, respectively. These early devices predated x-ray CT, developed in 1971 by Godfrey Hounsfield.2,15 He improved the machines to measure in 3D physiologic function and to develop cross-sectional reconstructions.2,15,17 The machines are considered the forerunners of SPECT, PET, and CT technology. Kuhl has been referred to as “the father of emission tomography.”15
The first large-scale use of a human positron imaging device was developed by physicist Gordon Brownell and neurosurgeon William Sweet at the Massachusetts General Hospital in the 1950s. Their machine was used to detect brain tumors with sodium iodide.18 Refinements led to increased sensitivity and to multiple detectors. PC-I, one of the first PET imaging devices, was unveiled in 1972 at the Meeting on Tomographic Imaging in Nuclear Medicine.18 While lecturing on PC-I and the resulting PET images at Washington University in St. Louis in 1974, Brownell discussed the possibility of a hexagonal arrangement of detectors with nuclear physicist Michael Ter-Pogossian.18 Michael Phelps and Edward Hoffman, then assistant professors in the Ter-Pogossian laboratory, constructed and in 1975 introduced an improved PET scanner with hexagonal detectors.2 A ring-shaped PCR-I (1985) and a cylindrical shaped PCR-II (1988) detector provided even better resolution and sensitivity.18
Modern era.
Cerebral PET imaging and radiotracer development improves diagnosis, management, and treatment of neurologic disorders, including but not limited to Parkinson disease (PD), dementias, and epilepsy. Furthermore, technology and computer-based algorithms have enhanced image resolution (figure 2) and greatly improved the use of PET as a clinical tool.
Figure 2. Comparison of PET images showing advances in resolution.
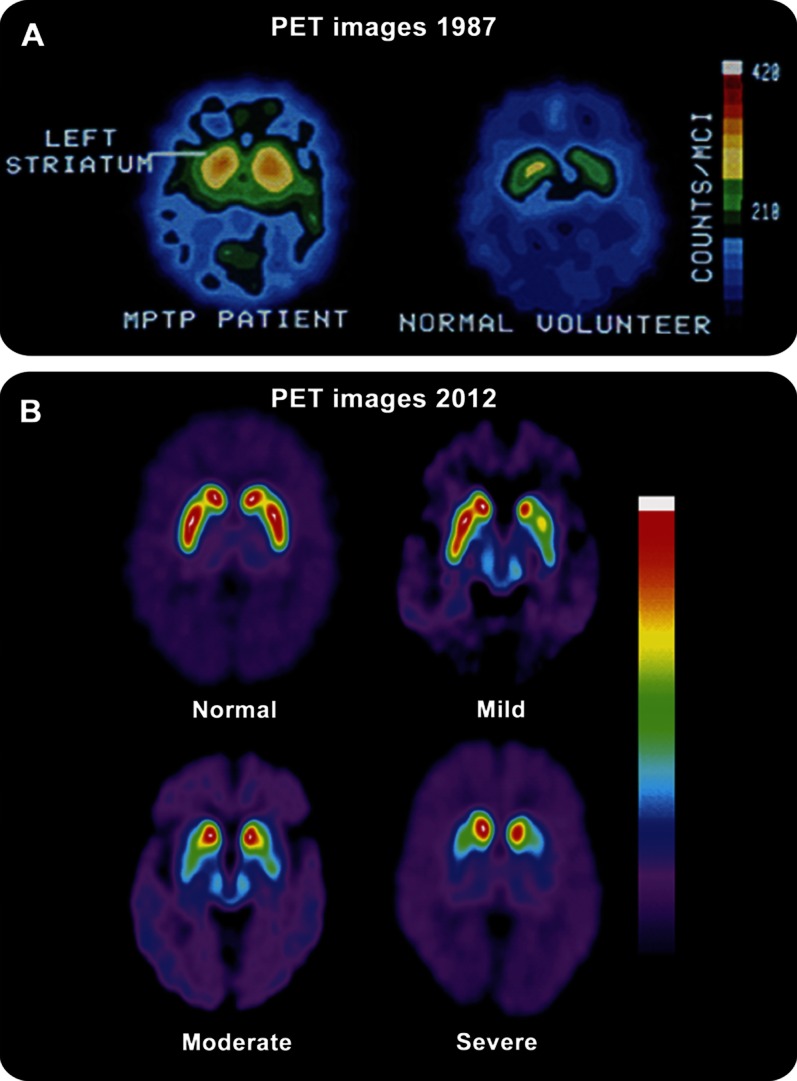
(A) Cerebral PET images from a PET 6 with 16-mm resolution comparing an untreated symptomatic subject with MPTP-induced parkinsonism using an older radioligand 18F-spiperone, a D2-like antagonist. (B) 18F-FPCIT cerebral PET images from a GE Advance scanner with 4.25 mm resolution at different stages of disease (normal, mild, moderate, and severe, as indicated). These images clearly show increased resolution and definition between the caudate nuclei and putamen in comparison to (A). (Figure 2B reprinted from Dhawan V, Eidelberg D. PET imaging in Parkinson's disease and other neurodegenerative disorders. Neurobiology of Disease 2007;821–828, with permission from Elsevier.30)
Recent advances in the differential diagnosis and assessment of treatment outcomes for PD include the use of FDG PET multivariate pattern analysis.19 Tang et al.20 developed an image-based classification algorithm to differentiate among PD, multiple system atrophy, and progressive supranuclear palsy. The initial image-based diagnoses were compared with gold-standard clinical diagnoses given by specialists.20 Image-based classification algorithms improved diagnostic accuracy of PD by 20% and were accurate earlier than with clinical diagnosis alone.20 The algorithm demonstrated high sensitivity and specificity in classifying atypical parkinsonian syndromes, providing the potential advantage of earlier diagnosis and assessment of candidates for clinical drug trials.20 Additionally, striatal dopamine deficiency as measured by 18F-dopa uptake and presynaptic dopamine transporter binding correlate directly with severity of bradykinesia and rigidity.21 Regarding PD treatment, Feigin et al.22 demonstrated that novel unilateral subthalamic nucleus gene therapy resulted in improved motor function. Reduced glucose metabolism was found in the thalamus with concurrent metabolic increases in the ipsilateral primary motor and premotor cortex.22 Overall, FDG PET scans have recently provided objective quantifiable network analysis data in PD that correlates with clinically observed outcomes in diagnosis, disease severity, and treatment.
Advances in the early and differential diagnosis of Alzheimer disease (AD) include the use of radiolabeled β-amyloid peptide. Studies using the 11C-Pittsburgh compound B (11C-PiB) have shown that amyloid deposition occurs years before clinical dementia and is related to disease progression.23 Villemagne et al.24 published a recent review of 109 patients with 18F-florbetaben. Radioactivity retention was far more pronounced in mild cognitive impairment (60%) and AD (96%) as compared to the low-binding frontotemporal lobar degeneration, dementia with Lewy bodies, and PD groups. Thus the longer half-life 18F-compound is comparable to 11C-PiB in accuracy and can be used more widely in routine clinical practice.24 Continuing research with β-amyloid peptide and other biomarkers may establish improved in vivo diagnoses for AD.
In epilepsy management and treatment, FDG PET patterns can show a single hypometabolic focus following a partial seizure.25 Takahashi et al.26 used FDG PET to characterize surgical treatment outcomes for 28 mesial temporal lobe epilepsy patients. Patients who were postoperatively seizure-free had specific hypometabolic foci compared to controls and non-seizure-free patients, which may facilitate predicting seizure outcomes after TLE surgery for future patients.26
PET-CT/MRI hybrid technology.
Today, PET scanners are combined with CT and MRI to form a functional-anatomic hybrid.27 In 2000, Beyer et al.27 described the novel PET/CT hybrid scan. Various oncologic tumors were diagnosed and staged using FDG and a treatment response was tracked.27 In 1997, Shao et al.28 reported the first PET-MRI hybrid prototype. The group used novel PET detectors that utilized lutetium oxyorthosilicate (LSO) scintillation crystals and avalanche photodiodes (APD) to prevent sensitivity to the MRI magnetic field.28 In 2008, Schlemmer et al.29 reported the use of an improved PET-MRI system. A 3.0-Tesla MRI system yielded improved soft-tissue diagnostic accuracy, while LSO-APD PET detectors with FDG allowed a better characterization of brain function.29 Advantages to the hybrid system included improved sensitivity, minimal radiation doses, decreased scanning time, and reduced motion artifact. The ability to study the distribution of radiotracers and drugs may ultimately prove useful in monitoring new therapies.29
Continued advancements in both PET/CT and PET-MRI hybrids will improve our understanding of cerebral processes, and may help us to track treatment outcomes and disease-modifying therapies. The combination of understanding physiology and brain metabolism and radiotracer and technological development spurred the current revolution in functional PET cerebral imaging.
ACKNOWLEDGMENT
The authors thank David Eidelberg, MD, Director, and Vijay Dhawan, PhD, Senior Investigator, Center for Neurosciences, The Feinstein Institute for Medical Research, Manhasset, NY; and Joel Perlmutter, MD, Washington University, for providing guidance, references, advice, and images.
GLOSSARY
- AD
Alzheimer disease
- APD
avalanche photodiodes
- DG
deoxyglucose
- FDG
[18F]fluoro-2-deoxy-d-glucose
- LSO
lutetium oxyorthosilicate
- PD
Parkinson disease
- PiB
Pittsburgh compound B
AUTHOR CONTRIBUTIONS
Leah H. Portnow: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data. Michael S. Okun: drafting/revising the manuscript, study concept or design, acquisition of data, study supervision. David E. Vaillancourt: drafting/revising the manuscript, study concept or design, obtaining funding.
STUDY FUNDING
Study sponsorship is by the Center for Movement Disorders & Neurorestoration, University of Florida College of Medicine, Gainesville. Supported by NIH (R01 NS052318 and R01 NS075012).
DISCLOSURE
L.H. Portnow reports no disclosures. D.E. Vaillancourt receives grant support from NIH, Michael J. Fox Foundation, and consults for projects at the DoD and Great Lakes NeuroTechnologies. M.S. Okun consults for the National Parkinson Foundation, Journal Watch, and receives grant support from NIH, NPF, and Michael J. Fox Foundation. He has received no industry-related honoraria for >36 months. He has participated in CME activities for PeerView, Prime, USF CME, and Vanderbilt CME. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Vanzetta I, Grinvald A. Coupling between neuronal activity and microcirculation: implications for functional brain imaging. HFSP J 2008;2:79–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kevles BH. Naked to the Bone: Medical Imaging in the Twentieth Century. New Brunswick, NJ: Rutgers University Press; 1997 [Google Scholar]
- 3.Raichle ME. A brief history of human brain mapping. Trends Neurosci 2009;32:118–126 [DOI] [PubMed] [Google Scholar]
- 4.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci 2006;29:449–476 [DOI] [PubMed] [Google Scholar]
- 5.Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol 1889;11:85–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill AB, Hill B. The life of Sir Leonard Erskine Hill FRS (1866–1952). Proc R Soc Med 1968;61:307–316 [PMC free article] [PubMed] [Google Scholar]
- 7.Hill L. The Physiology and Pathology of the Cerebral Circulation: An Experimental Research. London: J & A Churchill; 1896 [Google Scholar]
- 8.Partin C. Profiles in cardiology: Sir Leonard Erskine Hill. Clin Cardiol 2001;24:169–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz H. John F. Fulton (1899–1960). Neurosurgery 1998;43:178–1849657209 [Google Scholar]
- 10.Davey LM. John Farquhar Fulton. Neurosurgery 1998;43:185–187 [DOI] [PubMed] [Google Scholar]
- 11.Joliot F, Curie I. Artificial production of a new kind of radio-element. Nature 1934;204 In: Brucer M, Harris CC, MacIntyre WJ, Taplin GV. The Heritage of Nuclear Medicine. Reston, VA: Society of Nuclear Medicine; 1979 [Google Scholar]
- 12.Kety S. Seymour Kety. In: Squire LR, ed. The History of Neuroscience in Autobiography. Vol 1 Washington, DC: Society for Neuroscience; 1996:382–413 [Google Scholar]
- 13.Sokoloff L. Louis Sokoloff. In: Squire LR, ed. The History of Neuroscience in Autobiography. Vol 1 Washington, DC: Society for Neuroscience; 1996:454–497 [Google Scholar]
- 14.Lassen NA, Ingvar DH, Skinhoj E. Brain function and blood flow. Sci Am 1978;239:62–71 [DOI] [PubMed] [Google Scholar]
- 15.Kuhl DE. Citation for 2009 Japan Prize. Presented at The Science and Technology Foundation of Japan; Tokyo, April 21, 2009
- 16.Blahd WH. Benedict Cassen: the father of body organ imaging. Cancer Biother Radiopharm 2000;15:423–429 [DOI] [PubMed] [Google Scholar]
- 17.Allen H, Libby R, Cassen B. The scintillation counter in clinical studies of human thyroid physiology using I-131. J Clin Endocrinol Metab 1951;11:491–511 [DOI] [PubMed] [Google Scholar]
- 18.Brownell GL. A history of positron imaging. Presented at the Celebration of the 50th Year of Services to the Massachusetts General Hospital, Cambridge, MA, October 1999
- 19.Poston KL, Eidelberg D. 18F-Fluorodeoxyglucose PET in the evaluation of Parkinson disease. PET Clin 2010;5:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang CC, Poston KL, Eckert T, et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol 2010;9:149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks DJ. Imaging approaches to Parkinson disease. J Nucl Med 2010;51:596–609 [DOI] [PubMed] [Google Scholar]
- 22.Feigin A, Kaplitt MG, Tang C, et al. Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson's disease. Proc Natl Acad Sci USA 2007;104:19559–19564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira LK, Busatto GF. Neuroimaging in Alzheimer's disease: current role in clinical practice and potential future applications. Clinics 2011;66:19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villemagne VL, Ong K, Mulligan RS, et al. Amyloid imaging with 18F-florbetaben in Alzheimer disease and other dementias. J Nucl Med 2011;52:1210–1217 [DOI] [PubMed] [Google Scholar]
- 25.Alavi A, Yakir S, Newberg AB. Positron emission tomography in seizure disorders. Ann NY Acad Sci 2011;1228:E1–E12 [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M, Soma T, Kawai K, et al. Voxel-based comparison of preoperative FDG-PET between mesial temporal lobe epilepsy patients with and without postoperative seizure-free outcomes. Ann Nucl Med 2012:E1–E9 [DOI] [PubMed] [Google Scholar]
- 27.Beyer T, Townsend DW, Brun T, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med 2000;41:1369–1379 [PubMed] [Google Scholar]
- 28.Shao Y, Cherry SR, Farahani K, et al. Simultaneous PET and MR imaging. Phys Med Biol 1997;42:1965–1970 [DOI] [PubMed] [Google Scholar]
- 29.Schlemmer HPW, Pichler BJ, Schmand M, et al. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology 2008;248:1028–1035 [DOI] [PubMed] [Google Scholar]
- 30.Dhawan V, Eidelberg D. PET imaging in Parkinson's disease and other neurodegenerative disorders. In: Gilman S, ed. Neurobiology of Disease. San Diego: Academic Press; 2007:821–828 [Google Scholar]