Abstract
The study of consciousness is poised today at interesting crossroads. There has been a surge of research into various neurobiological underpinnings of consciousness in the past decade. The present article looks at the theories regarding this complex phenomenon, especially the ones that neurobiology, cognitive neuroscience and cognitive psychology have to offer. We will first discuss the origin and etymology of word consciousness and its usage. Neurobiological correlates of consciousness are discussed with structures like the ascending reticular activating system, the amygdala, the cerebellum, the thalamus, the frontoparietal circuits, the prefrontal cortex and the precuneus. The cellular and microlevel theories of consciousness and cerebral activity at the neuronal level contributing to consciousness are highlighted, along with the various theories posited in this area. The role of neuronal assemblies and circuits along with firing patterns and their ramifications for the understanding of consciousness are discussed. A section on the role of anaesthesia and its links to consciousness is presented, along with details of split-brain studies in consciousness and altered states of awareness, including the vegetative states. The article finally discusses the progress cognitive psychology has made in identifying and theorising various perspectives of consciousness, perceptual awareness and conscious processing. Both recent and past researches are highlighted. The importance and salient features of each theory are discussed along with the pitfalls, if present. A need for integration of various theories to understand consciousness from a holistic perspective is stressed, to enable one to reach a theory that explains the ultimate neurobiology of consciousness.
Keywords: Consciousness, Cognitive, Integrative, Neurobiology
Summary of Topics in the Article
-
Introduction and definition
-
1.1How to define consciousness
-
1.2Various references to the term consciousness
-
1.3Errors made in defining consciousness
-
1.4How to define the correlates of consciousness
-
1.5How to define conscious processes
-
1.1
-
Neuroanatomical models of consciousness
-
2.1Ascending Reticular Activating System and consciousness
-
2.2The role of the amygdala in consciousness
-
2.3The role of the cerebellum in consciousness
-
2.4The role of the thalamus in consciousness
-
2.5The role of frontoparietal circuits in consciousness
-
2.6The prefrontal cortex and consciousness
-
2.7The role of the precuneus in consciousness
-
2.8The role of neuroimaging studies in consciousness
-
2.9The role of fMRI and BOLD studies in consciousness
-
2.1
-
Neuronal models of consciousness
-
3.1Role of neuronal networks in consciousness
-
3.2Role of apical dendrite activity in consciousness
-
3.3NMDA activity and consciousness
-
3.4Baseline cerebral activity and consciousness
-
3.5The neural assembly model of consciousness
-
3.6The thalamocortical dialogue model of consciousness
-
3.1
-
Altered brain states and consciousness
-
4.1Altered brain states and consciousness
-
4.2Anaesthesia and consciousness
-
4.3Split-brain studies and consciousness
-
4.1
-
Cognitive psychology and consciousness
-
5.1The relation between attention and consciousness
-
5.2The global workspace model of consciousness
-
5.3The cognitive hierarchical model of consciousness
-
5.4Intuitive decision-making and consciousness
-
5.5Voluntary brain processing and consciousness
-
5.6Brain learning and consciousness
-
5.1
Introduction
The past decade has seen a rising interest in the meaning and scientific underpinnings of consciousness indicated by a spurt of new theories, numerous research publications and scientific debates or conferences on the same (Rose, 1998[179]; Velmans, 2000[228]; Singh et al., 2011[202], Singh and Singh, 2011[203]; Cohen and Dennett, 2011[43]; Sturm, 2012[209]). For example, relatively recently there has been an International Seminar on Brain, Mind and Consciousness held in January 2010, conducted by VPM's Joshi-Bedekar College, and co-sponsored by Mens Sana Monographs, World Psychiatric Association (Philosophy and Humanities Section) and the Indian Council of Philosophical Research. Various international scholars have debated in this conference on matters pertaining to various facets of the study of consciousness, and their articles have then been published as MSM 2011 [See http://msmonographs.org/showBackIssue. ssssssasp?issn = 0973-1229;year = 2011;volume = 9;issue = 1;month = January - December]. There have also been newer scientific reviews on moving towards an interdisciplinary perspective to study consciousness (Seth, 2010[198]; Singh and Singh, 2011[203]; Blanquet, 2011[20]).
The understanding of consciousness has been one of the most complex intellectual, philosophical and cognitive challenges faced by a spectrum of disciplines, ranging from quantum physics and psychology to neurosciences (Jasper et al., 1998[99]). In the last 20 years, advances in neuroimaging, neurophysiology, better experiments and the emergence of specific tools in neuropsychology have added to the excitement in the search for the correlates of consciousness (Hurley, 1998[96]). Authors agree that Brain, Mind and Consciousness are the research concerns of psychiatrists, psychologists, neurologists, cognitive neuroscientists and philosophers (Singh and Singh, 2011[203]). All of them are working in different and important ways to understand the workings of the brain, to understand the mysteries of the mind and to grasp that elusive concept called ‘consciousness’. Researchers from all fields are justified in forwarding their respective researches, but it is also necessary to integrate these diverse appearing understandings, to try and get a comprehensive perspective that is, hopefully, more than the sum of their parts (Singh and Singh, 2011[203]). There is also the need to understand each other's basic and fundamental ideological and foundational underpinnings, which must be followed by a comprehensive and critical dialogue between the respective disciplines (Singh and Singh, 2011[203]).
Consciousness research is thus interestingly poised. In fact the time is ripe with so many advances, to review the progress that has already been made, before we move ahead.
1.1 The etymology and meaning of the term ‘consciousness’
The word ‘conciousness’ is derived from Latin, having its roots in conscio formed by the coalescence of cum meaning ‘with’ and scio meaning ‘know’. In its original sense, to be conscious of something was to share the knowledge of it with someone else or with oneself (Zeman, 2001[243]; Koch, 2012[111]). All three senses, that is, knowledge shared with others, knowledge shared with the self and simply knowledge entered the English language as ‘conscience’, the first equivalent of conscientia (Zeeman, 2001[243]). The words conscious and consciousness first appeared in the seventeenth century followed by the term self-conscious and self-consciousness (Lewis, 1960[129]).
1.2 How to define consciousness
The Oxford English Dictionary (Simpson and Weiner, 2009[200]) has fifteen senses of ‘conscious’ and ten of ‘consciousness’. It is essential that we look at three principle concepts that have dominated the definitions of consciousness (Zeeman et al., 1997[244]).
In its first usage, which is predominantly neurological, consciousness is defined as a ‘waking state’. It is equated with the ability to perceive, interact and communicate with the environment and others in a manner that wakefulness normally implies. Consciousness in this sense lies on a spectrum from wakefulness to sleep and coma. These states can be defined using criteria like those in the Glasgow Coma Scale (Teasdale and Jennett, 1974[212]). Consciousness here may be lost and regained. To be conscious, is to be alert, active and vigilant (Boly, 2011[26]).
In its second usage, consciousness is defined as an experience or the content of experience from moment to moment. This may include what it feels to be a person or what one is feeling. This usage has a more inward connotation than the first. It highlights the qualitative and subjective dimension of experience. The term qualia has been used by philosophers to express this second usage of consciousness (Chalmers, 1996[35]; Balduzzi and Tononi, 2008[15]).
In its third usage, consciousness stemming from its Latin usage is looked at as a mental state. Here any mental state with propositional content may be said to be conscious. This may include anything we hope, believe, fear or expect. Consciousness here has been equated with the mind. It is noteworthy that most research into consciousness has focussed on the first two usages rather than this third version (Zeman, 2001[243]; Hill, 2009[93]).
Recent authors present four different forms of consciousness – for example, the Consciousness Tetrad (Singh and Singh, 2011[203]), namely:
Default Consciousness: Consciousness as a default state that separates the living from the dead. It also separates the living from the non-living.
Aware Consciousness: This looks at consciousness as a continuum of states that range from awake to sleep to drowsiness to semiconscious states like stupor and finally coma.
Operational Consciousness: This is consciousness as motor, sensory, cognitive, creative, emotive, aesthetic, ethical and other such abilities. It is also used with regard to an awareness of all mental operations.
Exalted Consciousness: This is an exalted state of consciousness of connecting with the divine, soul, inner self, God, special forms of creativity meditation and so on.
For a more detailed elaboration on this study the readers are referred to Singh and Singh (2011)[243] as moving beyond the above-mentioned points is not within the scope of this article.
1.3 Some references to the term ‘consciousness’
In some older writings, for example in the study of Descartes, ‘consciousness’ is not clearly differentiated from ‘mind’. Given the extensive, current evidence for preconscious and unconscious mental processing, this usage is too broad. How phenomenal consciousness relates to preconscious and unconscious mental processing is now a major topic for psychological research (Thiel, 2011[214]). To avoid confusion, and to enable such research, it is important to reserve the term ‘mind’ for psychological states and processes that may or may not be ‘conscious’.
Descartes also famously believed thought to epitomise the nature of consciousness, and consequently defined it as a ‘substance that thinks’ (res cogitans), which distinguishes it (in his view) from material substance that has an extension in space (res extensa). Modern psychology accepts that verbal thoughts (in the form of phonemic imagery or ‘inner speech’) are among the contents of consciousness. However, it does not accept that thoughts exemplify all conscious contents (Thiel, 2011[214]). Unlike thoughts, pains, tactile sensations, itches and other body experiences appear to have both spatial location and extension in different regions of the body, and the sights and sounds of the experienced external world (the phenomenal world) appear to have locations and extensions in a surrounding three-dimensional space. These interoceptive and exteroceptive experiences also differ widely from each other and many descriptive systems have been developed for investigating their phenomenology (in studies of visual and auditory perception, emotion, pain and so on). It must be evident that such developments in phenomenology are essentially the first steps in characterising what it is about consciousness that needs to be explained – and that restricting the phenomenology of ‘consciousness’ to the phenomenology of ‘thought’ is too narrow (Blanquet, 2011[20]).
In other, more modern writings, ‘consciousness’ is sometimes taken to be synonymous with ‘self-consciousness’. As one can be conscious of many things other than oneself (other people, the external world, etc.), this usage is also too narrow. To allow a clear distinction between consciousness of oneself and consciousness of things other than oneself, it makes more sense to reserve the term ‘self-consciousness’ for a special form of reflexive consciousness, in which the object of consciousness is the self or some aspect of the self (Aglioti and Candidi, 2011[1]).
Finally, ‘consciousness’ is sometimes used to mean ‘knowledge’, in the sense that if one is conscious of something one also has knowledge of it. This is an important feature of consciousness (that I do not have space to examine here). However, at any moment, a lot of knowledge is unconscious, or implicit (for example, the knowledge gained over a lifetime, stored in long-term memory). Thus, consciousness and knowledge cannot be co-extensive (Zeeman, 2001[243]).
The above-mentioned broad definitions and distinctions have been quite widely accepted in contemporary scientific literature (Farthing, 1992[69]; Velmans and Schneider, 2007[225]). Agreeing on definitions is important. Once a given reference for the term ‘consciousness’ is fixed in its phenomenology, the investigation of its nature and neurobiology can begin easily.
1.4 Errors in defining consciousness
Reductionists and non-reductionists adopt fundamentally differing assumptions about the ontology of consciousness, and there are many instances where these differing assumptions about ontology have intruded into how phenomenal consciousness has been defined. For example, it is common to take it for granted that an advanced form of brain science will ultimately demonstrate phenomenal consciousness to be nothing more than a state or function of the brain. However, most theories of consciousness that resist a reduction of conscious phenomenology to brain states and/or functions, fully accept that there is an intimate relationship between consciousness and the brain (Grey, 2006[84]). Recent writings mention that although the brain and mind are related they are not synonyms. They must not be used interchangeably. The Mind is the functional correlate of the brain, while the Brain is the structural correlate of the mind. One is an entity and the other is its operational correlate (see Brain-Mind Dyad, Singh and Singh, 2011[203]). The mind is a product of brain activities. The brain is an entity that exists, while the mind is a concept that we have formulated to understand the functions of the brain. The brain is the producer, while the mind is its product. Without the brain there is no mind (Singh and Singh, 2009[204]; Singh and Singh, 2011[203]).
The confusion lies in the nature of this intimate relationship. For example, many theories agree that, in humans, every distinct conscious experience is likely to be accompanied by distinct, correlated conditions in the brain (the neural correlates of consciousness or NCC), but naturalistic dualist and dual-aspect theories resist the reduction of phenomenal consciousness to brain states.
The dual-aspect theory suggests that conscious experiences and their correlated brain states are how the mind appears when viewed from the first and third person perspectives, respectively, and that these aspects of the mind are complementary and mutually irreducible (Velmans, 2009[227]; Pandya, 2011[159]; Singh et al., 2011[202]; Mehta, 2011[145]). If so, discovery of the neural correlates of the given experiences will not settle the fundamental differences among these theories. To achieve a genuine reduction, conscious experiences will have to be shown to be ontologically identical to their neural causes and/or correlates. Discovery of the neural causes and or correlates will not achieve this for the simple reason that causation, correlation and ontological identity are fundamentally different relationships (Grey, 2006[84]).
Some authors fully accept that conscious states have special phenomenal properties, for example, that they are intentional (about something), subjective and private (viewable only from a first-person perspective) – all characteristics that traditionally distinguish the mental from the physical (Lamme, 2010[123]). In an article by Chalmers, 1995[34], he simply declares such facts about consciousness to be ‘objective physical facts’ about the brain, thereby reducing the domain of the ‘mental’ to a subclass of what is ‘physical,’ by an act of redefinition – but leaving untouched, the problem of how objects such as brains can produce such intentional, subjective and private states (Chalmers, 1995[34]).
One researcher also entirely accepts the existence of phenomenal consciousness (with its special properties). However, he argues that there is another kind of consciousness, which he terms ‘access consciousness’ that enables ‘information access’ in the central nervous system, thereby giving consciousness a major role to play in the brain's activities (Block, 1995[21]). Even as this avoids reducing phenomenal consciousness to a function of the brain, this redefinition of information access as ‘access consciousness’ risks inflating a brain function to a conscious status that it does not possess. Information access and information availability have been widely recognised aspects of human information processing and it is true that information that enters phenomenal consciousness can be accessed, rehearsed, entered into long-term memory, used for the guidance of action and so on (Block, 2011[22]).
However, the processes that actually enable information access, rehearsal, transfer to long-term memory and guidance of action are not themselves conscious (if they were there would be no need to subject such processes to detailed investigation within the cognitive psychological research) (Velmans, 1991[226]). In short, ‘access consciousness’ is not actually a form of consciousness. The conscious part of ‘access consciousness’ is just phenomenal consciousness, and the processes that enable access to items in phenomenal consciousness are not conscious at all (Block, 2011[22]).
1.5 How do we define ‘conscious processes’
Psychological and philosophical literature confounds three distinct senses in which a process may be said to be ‘conscious’. It may be conscious
In the sense that one is conscious of the process
In the sense that the operation of the process is accompanied by consciousness
In the sense that consciousness enters into or causally influences the process (Mudrik et al., 2011[152]).
We do not have introspective access to how the preconscious cognitive processes that enable thinking, produce individual, conscious thoughts in the form of inner speech. However, the content of such thoughts and the sequence in which they appear do give some insight into the manner in which these cognitive processes (of which they are manifestations) operate over time in problem solving, thinking, planning and so on (Kunde et al., 2012[119]). Consequently, such cognitive processes are partly conscious in sense (a), but only in so far as their detailed operation is made explicit in conscious thoughts, thereby becoming accessible to introspection (Cohen and Dennett, 2011[43]).
Many psychological processes are conscious in sense (b), but not in sense (a) – that is, we are not conscious of how the processes operate, but we are conscious of their results. This applies to perception in all sense modalities. When consciously reading this sentence for example you become aware of the printed text on the page, accompanied, perhaps, by inner speech (phonemic imagery) and a feeling of understanding (or not), but you have no introspective access to the processes that enable you to read. Nor does one have introspective access to the details of most other forms of cognitive functioning, for example, to the detailed operations that enable conscious learning, remembering, engaging in conversations with others and so on (Velmans, 2007[225]).
1.6 The correlates of consciousness
The expression ‘neural correlates of consciousness’ (NCC) had first been used to describe the neural models that tried to explain consciousness. It has been defined as a specific system in the brain, whose activity correlates directly with the states of conscious experience (Crick and Koch, 1990[48]). A major area when studying NCC is to investigate the difference between neural activities that are associated with awareness and those that are not. It is also stated that the NCC may involve a very small number of neurons with very distinctive properties (Crick and Koch, 1998[47]).
Authors have compiled a whole collection of proposed NCCs, wherein the list includes 40 Hz oscillations in the cerebral cortex (Crick and Koch, 1998[47]), the intralaminar nuclei of the thalamus (Bogen, 1995[24]), re-entrant loops in the thalamocortical systems (Edelman, 1989[65]), an extended reticular thalamic activation system (Newman and Baars, 1993[155]), neural assemblies bound by N-Methyl-D-Aspartate (NMDA) (Flohr, 1995[74]), the inferior temporal cortex (Sheinberg and Logothetis, 1997[199]), visual cortex connections to the prefrontal cortex (Crick and Koch, 1995[46]; Hirstein, 2011[94]) and visual processing within the visual stream (Milner and Goodale, 1995[146]). Studies in this area are constantly in evolution and with advances in neuroimaging and neuroanatomical understanding we are able to elicit newer neural correlates of consciousness, along with better relationships between the structures already established (Aru et al., 2012[7]; Jansen and Overgaard, 2011[101]).
Researchers now propose that a search for the NCC should be coupled with similar efforts aimed at detecting the behavioural correlates of consciousness (BCC) and the computational correlates of consciousness (CCC). The search for the CCC is a new field in consciousness studies (Cleermans, 2005[40]; Praharaj et al., 2009[172]). Researchers are pursuing the goal of building conscious machines (Holland, 2003[95]) through the development of computational models aimed at explaining consciousness (Aleksander, 2000[2]; Perruchet and Vinter, 2003[163]). These models also aim to provide the differences between conscious and unconscious cognition.
2. Neuroanatomical Models of Consciousness
2.1 Ascending reticular activating system and consciousness
The ascending reticular activating system (ARAS) located in the upper brainstem was one of the first areas to be suggested as the one that enhanced arousal (Moruzzi and Magoun, 1949[151]). Subsequent studies have demonstrated that the ARAS is not restricted to the classical reticular nuclei of the brainstem, but is a group of specialised nodes in a complex network that controls arousal. This includes the cholinergic nuclei in the upper brainstem and basal forebrain, noradrenergic nuclei, especially the locus coeruleus, the histamine projection from the posterior hypothalamus and probably dopamine and serotonin pathways that arise from the brainstem. Much of the activity exerted by these pathways is mediated by the thalamus, which can be regarded as the apex of the ARAS as well as a critical synaptic relay for most sensory and intracerebral pathways (Tononi and Koch, 2008[220]; Tononi, 2012[222]).
The role of the ARAS also involves coordination with the thalamus, which through extensive inhibitory axon collaterals, generates large scale thalamic burst discharges, which are responsible for gating specific reticular information that is transmitted back to the cortex, and this reverts the information back to the brainstem (Young and Pigott, 1999[239]). Positron emission tomography (PET) studies have demonstrated selective thalamic and ARAS hypometabolism during slow-wave sleep (Maquet, 2000[137]) and anaesthesia (Alkire, Haier and Fallon, 2000[3]). [See Figure 1]
Figure 1.
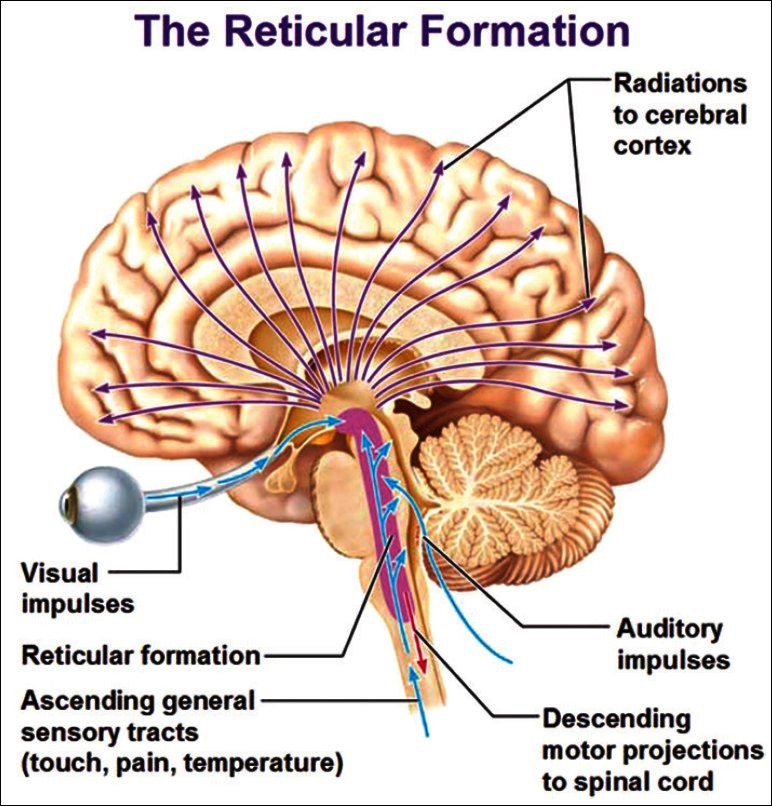
The ascending reticular activating system and its connections
[Author's note: Image taken from the website – www.antranik.org – May be subject to copyright. Author has written for permission, still to receive reply despite repeated emails.]
2.2 The role of the amygdala in consciousness
Neurobiological reviews clearly show that the amygdala plays a role in enhancing long-term memory (LTM), working memory (WM) and attention. The amygdala seems to mediate the effects of emotional contents on LTM and attention. It also seems to facilitate cognitive performance during challenging tasks and when motivationally relevant stimuli are detected (Arnsten and Li, 2005[6]). Reviewed findings sugge st that the relationship between the amygdala and cognitive systems is bi-directional, depending on corticolimbic functional links. Thus, noradrenaline and dopamine neurotransmitters may play a role in the relationship between the amygdala and higher cognition (Balleine and Killcross, 2006[16]; Kim et al., 2011[107]).
Furthermore, these results are consistent with extensive evidence indicating that noradrenaline and dopamine have a modulation effect on various higher cognitive functions (Davis and Whalen, 2001[53]). The evidence that the amygdala is also involved in higher cognition does not contradict the well-established link between the amygdala and emotional processing. They rather argue for the need to integrate, in a comprehensive model, the well-known involvement of the amygdala in emotion with its role in higher cognition (Phelps, 2006[166]). The amygdala serves a general vigilance function that is aimed at preparing the organism to cope with challenging, resource-demanding situations. The vigilance response of the amygdala can take the form of phasic neuromodulatory signals projected to cognitive, motor and/or autonomic nervous systems during a situation demanding an unusual addition of metabolic resources, such as, a situation of physical threat or a complex mental task (Schaefer and Gray, 2004[185]). More particularly, signals sent to the neocortical areas may enhance attentional processing of the environmental stimuli, which will have an obvious adaptive value during challenging situations (Zald, 2003[240]). As suggested by the reviewed evidence, this attentional enhancement may be specific to goal-relevant stimuli. It is noteworthy that goal processing is a fundamental component of both emotion and executive control (Ghashghaei and Barbas, 2002[80]).
Therefore, the vigilance response of the amygdala to motivationally relevant information may not only be the mechanism underlying the involvement of the amygdala in higher cognition, but it may also be the core basis of most emotional and stress responses, preparing the organism to cope with challenging situations (Koob, 1999[115]). The large variety of motivationally relevant stimuli that trigger the modulation of higher cognition by the amygdala imposes certain constraints on the type and origins of the inputs to the amygdala. On one hand, the amygdala is sensitive to stimuli that possess an intrinsic adaptive value (e.g. emotional stimuli, fearful faces, size of the white scleral field, etc.). On the other hand, the amygdala is sensitive to stimuli whose motivational value is arbitrarily determined by task goals or demands (Richardson, Strange and Dolan, 2004[177]). Therefore, this suggests that the amygdala needs to receive not only basic sensory inputs, but also inputs conveying high-level task goal and task demand information. The amygdala also has anatomical properties, making it particularly suitable to receive both low-level and higher-level inputs. More particularly, the amygdala not only has a remarkably rich pattern of direct and indirect connections with the subcortical and brainstem regions, but also with the neocortical regions involved in working memory (WM) and cognitive control, such as the prefrontal cortex (PFC) and the anterior cingulate cortex (ACC) (Maren, 2003[138]; LaBar and Cabeza, 2006[120]; Hirstein, 2011[94]).
Several avenues of future research are open. First, future studies should try to unveil the actual anatomical pathways underlying the modulation of higher cognitive processes by the human amygdala. Second, the human amygdala is not a monolithic structure. It is instead a compound of distinct substructures. In animals, there is extensive evidence that different amygdala nuclei play distinct functional roles (Sah et al., 2003[181]). Future studies should investigate whether the cognitive role of the human amygdala is specific to particular amygdala sections, such as the ventral and dorsal amygdalae (Walf and Frye, 2006[232]). Third, a promising line of research is also to investigate whether individual differences in cognitive abilities can be predicted from individual differences in amygdala activity and/or volume. A related important issue is the search for genetic markers of individual differences in amygdala activity and their relationships with cognition (Sara, 2000[183]). For more details on the role of the amygdala in consciousness readers are directed to bigger and more comprehensive reviews on the subject (Sah et al., 2003[184]; Whalen and Phelps, 2009[235]). [See Figure 2]
Figure 2.
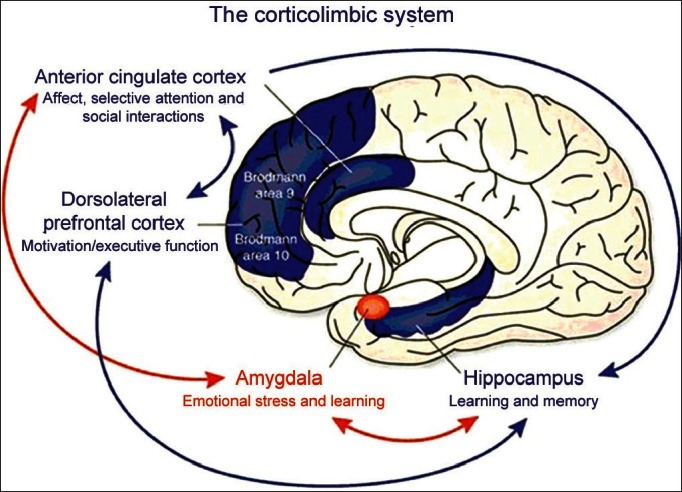
The role of the amygdala in consciousness
[Author's note: Image taken from Neuropsychopharmacology Reviews. May be subject to copyright. Author has written for permission, still to receive reply despite repeated emails.]
2.3 The role of the cerebellum in consciousness
For many years, functions related only to movement, gait, posture and balance were attributed to the cerebellum. However, some studies suggested a possible involvement of the cerebellum in cognition, emotion processing, consciousness and behaviour (Schmahmann, 2004[194]; Schmahmann, Weilburg, and Sherman, 2007[193]). From this perspective, the cerebellum would exert a regulatory function that would enhance and supplement other brain functions throughout the direct and indirect circuits (Timman et al., 2003[215]).
In order to appreciate the involvement of the cerebellum in cognition and consciousness, it is important to understand its afferent and efferent connections. The corticopontocerebellar pathway is the most important of the central afferent circuits originating from the motor and sensitive cortical areas. After connecting with the pontine nuclei, the pontocerebellar tracts connect with the contralateral cerebellar hemisphere in a somatotopic manner. The other cerebellar peripheral pathways originate from the brainstem: The olivocerebellar tract, with fibres originating from the inferior olive that receives excitatory impulses from the red nucleus, basal ganglia, reticular formation and spinal cord (spinal-olivar); the vestibulocerebellar tract, with fibres originating from the vestibular nuclei, which project to the fastigial and flocculonodular nuclei; and the reticulocerebellar tract, with fibres originating from the reticular formation, which project to the cerebellar vermis. The main outputs of the cerebellum are to the brainstem and to the cerebral motor cortex (via the red nucleus and ventrolateral nucleus of the thalamus) (Strata, 2011[208]).
There are four main cerebellar efferent pathways (Strata, 2011[208]):
Fibres that originate in the cerebellar vermis projecting to the fastigial nucleus and then to the pons, medulla oblongata and reticular formation
Fibres that originate in the intermediate zone of the cerebellar hemisphere projecting to the interpositus nucleus and then to the red nucleus and thalamus (ventrolateral and ventroposterior nucleus). After connecting to the thalamus, these fibres connect to the rubrospinal pathway and basal ganglia
Fibres that originate in the lateral zone of the cerebellar hemisphere and project to the dentate nucleus and then to thalamus. After the thalamic connection, those fibres are projected to the cerebral cortex (posterior parietal, superior temporal, prefrontal), reticular formation and then to the corticospinal and reticulospinal pathways.
The cerebellar nuclei project to the caudal ventrolateral contralateral thalamic nuclei and then to the frontal motor cortex. Cerebellar nuclei may also connect with the intralaminar and motor thalamic nuclei and then project to the association and limbic cortices (cingulate and parahippocampal gyri).
Many studies have also demonstrated crossed connections between the dentate nucleus and the dorsolateral prefrontal cortex in the cerebellum–thalamic–cortical direction. The same crossed pattern was observed in specific areas of the inferior posterior parietal cortex (Clower et al., 2001[41]). Through these extensive interconnections, with afferent (corticopontocerebellar) and efferent (cerebellothalamocortical) pathways, the cerebellum can receive information as well as influence cortical cerebral areas related to cognition (Schmahmann, 1997[195]) [Figure 3]
Figure 3.
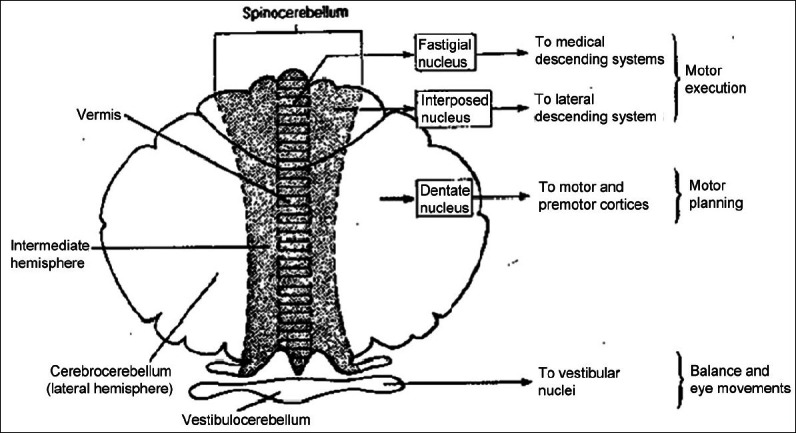
Cerebellum and its connections in consciousness
[Author's note: Image from www.benbest.com – free permission to copy image given provided the site is cited.]
Significant interconnections between the cerebellum and prefrontal cortex subdivisions related to executive functioning (working memory, attention, inhibition of behaviours and decision-making), verbal memory and language have been demonstrated (Schmahmann and Pandya, 1995[192]). Afferent projections from the parietal, temporal and occipital cortices and limbic system implicated in the integration of sensitive and sensorial information, visuospatial organisation, visual memory, consciousness and control of behaviour and motivation have also been proposed (Schmahmann and Pandya, 1992[191]).
More recently, a timekeeping or ‘clock’ function has been postulated for the cerebellar cortex and the inferior olive (the sole source of climbing fibre inputs to the Purkinje cells) based on their unique microstructure and intrinsic rhythmic oscillatory properties (Xu et al., 2006[237]). It is proposed that the primary role of the inferior olive and the climbing fibre system in timing is to mediate the encoding of temporal information independent of motor behaviour (Konarski et al., 2006[114]; Haines and Dietrichs, 2012[91]) [Figure 4].
Figure 4.
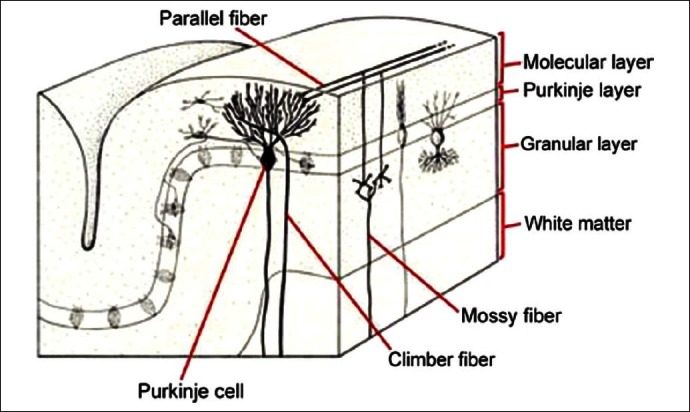
Cerebellar cellular makeup
[Author's note: Original picture at www.astralgia.com suitably modified by present author. May be subject to copyright. Author has written for permission, still to receive reply despite repeated emails.]
The exact nature of cerebellar involvement in cognitive processes, is so far, less understood. Functional and structural neuroimaging techniques also provide a valuable tool to study cerebellar contribution to different cognitive abilities and its role in psychiatric disorders. Abnormalities in cerebellar structure and function have been reported in some psychiatric disorders (Konarski et al., 2006[114]). Moreover, pharmacological and psychosocial therapeutic interventions for patients with those disorders have been associated with changes in cerebellar function, suggesting an important role of the cerebellum in different mental processes, which are disturbed in some psychiatric disorders. Future studies examining cerebellum functions in normal subjects and patients with psychiatric disorders may extend the current knowledge on this issue. Analyses of convergent data from genetic, neuropsychological and structural and functional brain imaging studies may provide a unique opportunity to explore the role of the cerebellum in the pathophysiology of psychiatric disorders and also in developing an integrated neurobiology of consciousness (Strata, 2011[208]).
2.4 The role of the thalamus in consciousness
Many neurons within the central thalamus (defined here as the anterior and posterior intralaminar nuclei, the paralaminar portions of related thalamic association nuclei – median dorsalis, ventral anterior, ventral lateral and inferior pulvinar) share specific anatomical and physiological specialisations that support their key role in the general functions of sustained attention, working memory and motor preparation. These neuronal populations are interposed between the brainstem/basal forebrain arousal systems, which control the overall levels of thalamic and cortical activity, and the supervisory frontal systems) that organise both premotor shifts of attention and adjustments of vigilance level or alertness (Parsuraman, 1999[160]). In parallel, individual neurons within these cellular aggregates are targets of a variety of dedicated brainstem sensory relays that have evolved, to quickly capture attention and redirect behaviour (Krout, Belzer and Loewy, 2002[117]). Collectively, these anatomical specialisations suggest that many neurons within the central thalamus may serve the general function of supporting large-scale cerebral dynamics associated with goal-directed behaviours and consciousness.
The central thalamus receives broad innervation from both the brainstem and basal forebrain arousal systems (Praff, 2006[164]). Ascending inputs to the anterior intralaminar nuclei (CL, Pc) and adjacent paralaminar regions of the thalamic association nuclei make them particularly well-positioned to play a role in arousal regulation. These regions receive the heaviest thalamic innervation from the brainstem and basal forebrain cholinergic neuronal populations (Kolmac and Mitrofanis, 1999[113]) and heavy innervation from the noradrenergic afferents of the locus ceruleus and serotoninergic afferents from the medial raphe (McCormick and Bal, 1997[143]).
It is well-established that the central thalamus plays an important role in neurological disorders of consciousness (Schiff and Plum, 2000[187]). The brief review below links this role of the central thalamus to the pathophysiological mechanisms that produce both persistent and paroxysmal impairments of arousal regulation after severe brain injury. An interpretative framework is then proposed to explain the underlying mechanisms, for interventions that improve neurological function in patients with disorders of consciousness (Saalmann and Kastner, 2011[180]).
Direct injuries to the central thalamus can alone produce global disturbances of consciousness (Parvizi and Damasio, 2003[161]). If unilateral, these lesions may produce hemispatial unawareness or altered states of consciousness similar to acute mania or delirium. Restricted bilateral injuries to the central thalamus may produce acute coma, indicating the important role these structures play in a normal, alert, wakeful state. Recovery of cyclical arousal patterns, with periods of eye opening, occurs rapidly with these lesions and is usually present within 48 hours, again emphasising that these neurons are not the primary sources of ascending control of the arousal state per se. However, unlike brainstem lesions that produce coma following focal injuries, the recovery of consciousness in terms of goal-directed behaviour and communication skills is very slow following bilateral central thalamic injuries and also uncertain (Schiff, 2008[188]).
These clinical observations are consistent with the experimental studies, which demonstrate that neither the central thalamus nor the basal forebrain are indispensable for maintaining the general arousal associated with some recovery of electroencephalograph (EEG) cycling and wakefulness (Gold and Lauritzen, 2002[82]), suggesting that the parallel basal forebrain and thalamic mechanisms and direct brainstem to the cortex pathways are pleuripotent for maintaining cerebral arousal.
Understanding the contributions of the central thalamus to arousal regulation may aid our rethinking and reformulating of how to approach the problems posed by disorders of consciousness. Based on their unique anatomical and physiological properties, it is suggested that neurons within the central thalamus are targets of interventions aimed at restoring function at the circuit level, for patients with disorders of consciousness. The observations of individual patient responses to deep brain stimulation (DBS), sedatives and other drugs reviewed earlier in the article emphasise the key role of the central thalamus in circuit disturbances affecting large-scale forebrain dynamics (Schiff, 2008[188]). [Figure 5]
Figure 5.
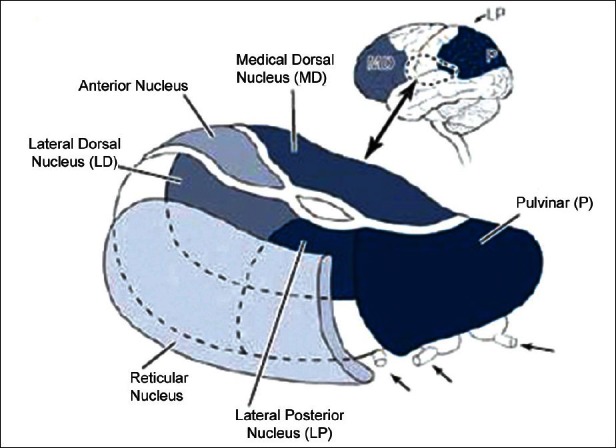
The main structures of the thalamus
[Author's note: Figure from www.dana.org. May be subject to copyright. Author has written for permission, still to receive reply despite repeated emails.]
2.5 The role of frontoparietal circuits in consciousness
Even as phenomenal consciousness refers to the subjective aspect of experience, access consciousness refers to the direct control of experience through reasoning, reporting, or action. Visual awareness is the most studied type of conscious perception in normal and abnormal humans, as well as animals. Some neuroimaging and neurophysiological studies of visual awareness (Logothetis, 1998[133]; Tong et al., 1998[218]) have concentrated on the role of the ventral stream and have not typically considered the potential role of frontoparietal activations. However, more recent studies suggest that joint activation of the category-specific regions in the ventral stream and activity in the parietal and prefrontal areas may be crucial for visual awareness (Rees et al., 2002[176]).
Neuroimaging studies suggest that frontoparietal activity makes an important contribution to conscious perception. Although activity in the ventral visual cortex is a consistent neural correlate of visual perception, it may be insufficient to produce awareness without an additional contribution from the parietal and prefrontal areas (Crick and Koch, 1995[46]; Rees et al., 2002[176]). They have suggested that the correlates of consciousness are divided into primary and secondary network nodes, where early activity in the occipital lobe correlates with the perceptual processes, and later, the activity in the frontoparietal areas correlates with the secondary processes contingent, on the outcome of the earlier perceptual processing.
The distinction between phenomenal and access consciousness (Block, 1997[23]) would be of relevance for understanding the putative role of frontoparietal areas in conscious perception. Even as phenomenal consciousness might be mainly associated with activation of sensory regions, access consciousness might need involvement of the frontoparietal areas. The specific pattern of frontoparietal activations during conscious perception might further be differentiated depending on the relevant stages and/or types of processing, for instance, perceptual transitions versus sustained perception.
What is the functional significance of overlapping frontoparietal activations? Possibly, these activations reflect shared cognitive processes. Several studies converge on attentional and/or working memory processes as the explanation for overlapping frontoparietal activity patterns. It has been proposed that prefrontal activation during episodic retrieval may be seen as reflecting specific working memory contributions to episodic memory (Wagner, 1999[231]). He has argued that material-independent working memory functions contributing to episodic retrieval are mediated by the dorsolateral and anterior prefrontal regions. The frontal and parietal mechanisms involved in spatial working memory represent an attentional circuit that operates in the service of memory (Awh and Jonides, 2001[8]). They have postulated that spatial attention mechanisms are recruited in the service of a rehearsal-like function to maintain information in a working memory. The dorsolateral prefrontal cortex aids in the maintenance of information by directing attention to the internal representations of sensory stimuli and motor plans that are stored in more posterior regions (Curtis and D’Esposito, 2003[50]). Researchers have also suggested that the common activity in a frontoparietal–cingulate–thalamic network, during episodic retrieval and visual attention, reflects general attentional processes (Cabeza and Nyberg, 2000[30]) [Figure 6].
Figure 6.
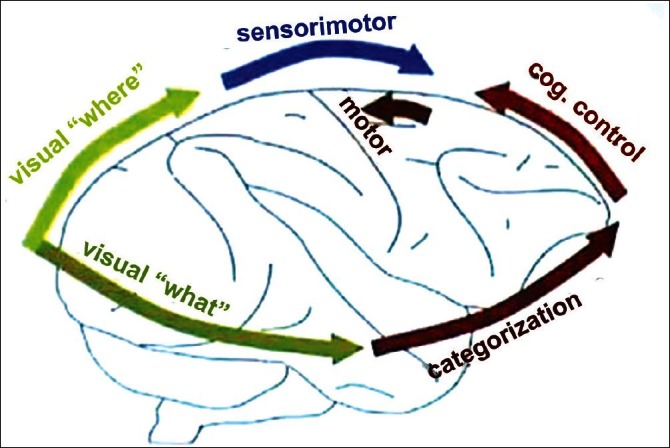
The frontoparietal network
[Author's note: Source not traceable. May be subject to copyright. Will acknowledge if brought to notice.]
2.6 The prefrontal cortex and consciousness
Following up on the seminal proposal of Crick and Koch, 1995[46], many theoretical models and neuroimaging experiments argue for an essential role of distributed long-distance brain networks linking higher visual areas to the prefrontal cortex (PFC) and parietal cortex (Beck et al., 2001[18]; Dehaene and Naccache, 2001[61]; Dehaene et al., 2001[60], 2003[62], 2006[57]; Marois et al., 2004[134]). Alternative theories, however, associate conscious perception either to the early activation of specialised visual areas (Tong, 2003[219]; Zeki, 2003[241]) or to the reverberating loops linking the occipital and temporal regions (Lamme, 2006[124]). Advocates of the latter theories emphasise that frontal lesions typically do not yield impairments in conscious perception (Pollen, 1999[170]), and that the prefrontal cortex (PFC) activation during conscious perception tasks may reflect additional reporting or working memory processes unneeded for conscious experience.
Unfortunately, very few experimental studies have directly probed conscious perception in patients with frontal lesions. Frontal lesions lead to neuropsychological deficits that can arguably be related to impaired conscious access and control, such as, hemineglect, abulia, akinetic mutism, anosognosia, impaired autonoetic memory, loss of intentional control and a surge of automatic activities, such as, utilisation and imitation behaviours (Passingham, 1993[162]; Husain and Kennard, 1997[97]). Some PFC and anterior cingulated patients show a preserved behavioural adjustment to motor or cognitive conflict, but a drastic impairment in their awareness of the conflict.
Most research findings are compatible with an intervention of prefrontal areas at a level of cognitive architecture concerned with conscious executive monitoring. Yet, whether such high-level processing is necessary for conscious perception itself remains controversial, especially in the light of recent evidence that these executive processes can be partially triggered by non-conscious, masked stimuli (Lau and Passingham, 2007[126]).
At present, the results fit in with the classical view (Fuster, 1989[76]) that PFC plays a greater role in conscious monitoring than in automatic or non-conscious evidence accumulation. The suggestion is that the higher level conscious route corresponds to parieto-prefrontal networks and associated higher cortical areas (Hirstein, 2011[94]) [Figure 7].
Figure 7.
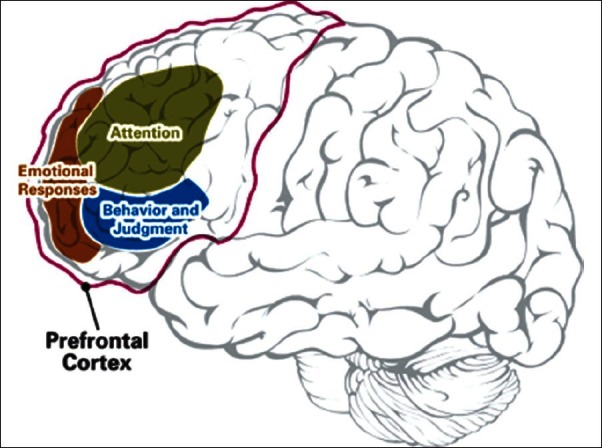
The prefrontal cortex and consciousness
[Author's note: Taken from website – www.adhdandyou.com. May be subject to copyright. No privacy policy on usage.]
2.7 The role of the precuneus in conciousness
Over the last few years, functional neuroimaging studies have started unravelling unexpected functional attributes for the precuneus, a cortical region located in the posteromedial portion of the parietal lobe, which has widespread connections with both associative areas and the subcortical structures (Cavanna and Trimble, 2006[32]). The posteromedial parietal areas are among the brain structures displaying the highest resting metabolic rates (hot spots) and are characterised by transient decreases in the tonic activity during engagement in non-self-referential, goal-directed tasks (default mode of brain function) (Gusnard and Raichle, 2001[90]). On the other hand, precuneus activation has been documented in healthy subjects engaged in self-related mental representation and episodic/autobiographical memory retrieval (Vogeley and Fink, 2003[229]).
Moreover, selective precuneal hypometabolism has been reported in a wide range of altered conscious states, such as, sleep and hypnosis (Rainville et al., 1999[174]), drug-induced anaesthesia, vegetative state (Laureys, Owen and Schiff, 2004[127]) and in neuropsychiatric conditions characterised by impaired consciousness (e.g. Alzheimer's disease, epilepsy, schizophrenia) (Sass and Parnas, 2003[184]; Grecious et al., 2004[88]; Monaco et al., 2005[149]).
It has been suggested that precuneus activity during conscious resting states supports conceptual processing operating on the internal stores of information (endogenous signals) rather than ‘perceptual’ functions (concerned with sources of information external to the brain). In other words, this area seems to contribute to the self-referential ‘thought’ processing that humans experience during resting consciousness (Cavanna, 2007[33]).
Finally, another line of evidence points towards a central role for the precuneus in the internal mentation processes of self-consciousness. An interaction between the precuneus and prefrontal cortex has been postulated in states of consciousness characterised by a high level of reflective self-awareness. The role of global changes and interactions between the precuneus and the frontopolar regions is not yet clear. However, the functional linkage between these systems and the thalamic and reticular-activating system points towards a pivotal role for the precuneus in self-consciousness, especially when viewed in relation to the high level of resting metabolism displayed by this area (Kjaer et al., 2002[109]; Cavanna and Trimble, 2006[32]).
Taken together, these findings provide strong, albeit preliminary, evidence that this richly connected multimodal associative area belongs to the neural network subserving awareness and producing a conscious self-percept, a process that possibly runs in the background (by default) during silent rest (Trimble, 2007[223]) [Figure 8].
Figure 8.
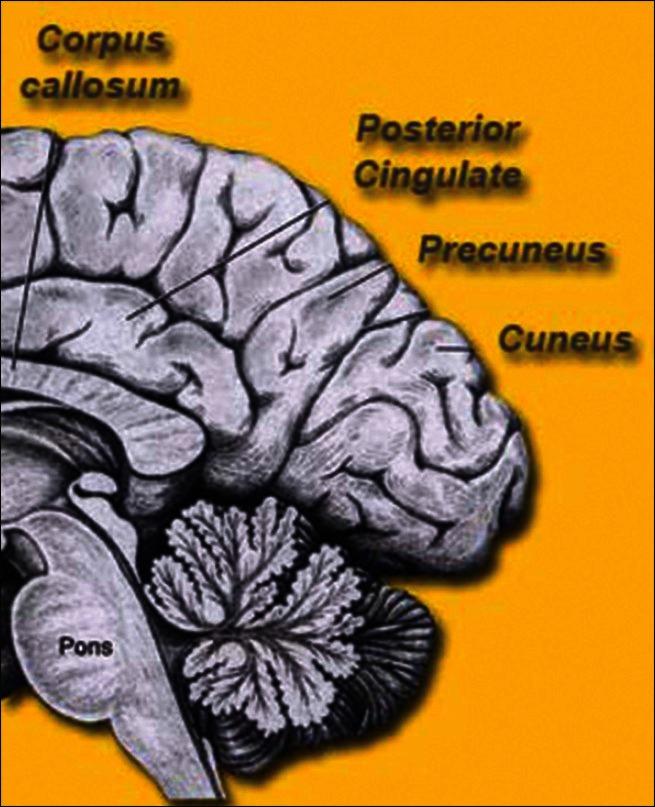
The precuneus
[Author's note: Taken from the website www.brain-maps.com. May be subject to copyright. Author has written for permission, still to receive reply despite repeated emails.]
2.8 The relevance of neuroimaging to consciousness
Clinical audits have highlighted the many challenges and dilemmas faced by clinicians assessing persons with disorders of consciousness (vegetative state and minimally conscious state). The diagnostic decision-making process is highly subjective, dependent upon the skills of the examiner and invariably dictated by the patients’ ability to move or speak (Bruno et al., 2011[28]). Although a considerable amount has been learnt, the assessment process remains largely unchanged; conducted at the bedside, using behavioural assessment tools, which are susceptible to environmental and physiological factors. This has created a situation where the rate of misdiagnosis is unacceptably high (up to 43%) (Northoff et al., 2011[156]). In order to address these problems, various functional brain imaging paradigms, which do not rely upon the patient's ability to move or speak, have been proposed, as a source of additional information, to help in the diagnostic decision-making process (Coleman et al., 2009[44]).
Although accumulated evidence from brain imaging, particularly functional magnetic resonance imaging (fMRI), has been encouraging, the empirical evidence is still based on a relatively small number of patients. It remains unclear whether brain imaging is capable of informing the diagnosis beyond the behavioural assessment and whether brain imaging has any prognostic utility (Jennett, 2002[100]).
Despite the hopes for the evolution of technology and expectations as fostered by the media characterisation of imaging and consciousness in brain-injured patients (Singh et al., 2007[205]), the translation of neuroimaging research to utility as a clinical tool is still in its infancy. The major challenges are patient selection, study design and standardisation of the technology, including stimulus selection and experimental protocols (Fins et al., 2008[71]).
The current data pool derives from a small number of subjects and an equally limited number of investigative teams, which is reflective of the pervasive neglect of this population and the nihilistic assumption that all cases of severe brain injury will lead to fixed, inevitable and catastrophic outcomes (Fins, 2003[72]).
2.9 The significance of functional magnetic resonance imaging studies in consciousness
An accurate and reliable evaluation of the level and content of cognitive processing is of paramount importance for the appropriate management of severely brain-damaged patients, in altered states of consciousness (Bernat, 2006[19]). Objective behavioural assessment of residual cognitive function can be extremely challenging in these patients, because no cognitive output is possible. This difficulty leads to errors and a potentially high-level of misdiagnosis in a vegetative state (Childs, Mercer and Childs, 1993[37]), minimally conscious state (MCS) and locked-in syndrome (Schiff, 2006[189]). Recent advances in functional neuroimaging suggest a novel solution to this problem; the so-called ‘activation’ studies can be used to assess cognitive functions in altered states of consciousness without the need for any overt response on the part of the patient.
In several recent cases, this approach has been used to identify residual cognitive function and even conscious awareness, in patients who behaviourally meet the criteria defining the vegetative state yet retain cognitive abilities, which have evaded detection when using the standard clinical methods. Similarly, in other studies, the cognitive capabilities of patients diagnosed as MCS have been explored using functional neuroimaging. Such studies suggest that the future integration of emerging functional neuroimaging techniques (Owen and Coleman, 2007[158]) with the existing clinical and behavioural methods of assessment will be essential for improving our ability to reduce diagnostic errors between these related conditions.
Moreover, such efforts may provide important new prognostic indicators, helping to disentangle differences in outcome on the basis of a greater understanding of the underlying mechanisms responsible, and thus, improve the therapeutic choices in these challenging populations (Schiff, 2006[189]). More generally, the acquisition, analysis and interpretation of ( fMRI) data from patients with severe brain damage are also complex (Poldrack, 2006[168]). For example, in patients with brain damage, the coupling of neuronal activity and local hemodynamics, essential for fMRI activation measurements, is likely to be different from that in healthy controls (Christoff and Owen, 2006[38]), making interpretation of such data sets extremely difficult.
Disorders of consciousness present unique problems for diagnosis, prognosis, treatment and everyday management. At the patient's bedside, the evaluation of a possible cognitive function in these patients is difficult because voluntary movements may be very small, inconsistent and easily exhausted. The fMRI appears to offer a complementary approach to the clinical assessment of patients in a vegetative state and other altered states of consciousness, and can objectively describe (using population norms) the regional distribution of cerebral activity under various conditions of stimulation. Indeed, in some rare cases, fMRI has demonstrated preserved cognitive function and even conscious awareness, in patients who are assumed to be vegetative, yet retain cognitive abilities that have evaded detection when using standard clinical methods. The future use of fMRI will substantially increase our understanding of severely brain-injured patients (Laureys, Owen and Schiff, 2004[127]).
3. Neuronal Models of Consciousness
3.1 The need for micro-consciousness at the neuronal level
Just as we have looked at the macrostructure of consciousness, it is prudent that by considering a top down approach, we also look at the microstructure of consciousness that stems from a cellular level. We do not doubt that attention, recurrent computations and complexity are important aspects to understand consciousness. However, we propose that these aspects are often trivially necessary rather than sufficient. For example, often it is assumed that consciousness emerges not before several hundred milliseconds after stimulus onset (Grossberg, 1999[89]; Scharnowski et al., 2007[186]). Hence, given the short time constants of the membranes of neurons, recurrent connections are obviously necessary to store and process the stimulus before consciousness is reached.
Complexity is surely of primary importance for consciousness, because networks with the same number of neurons can create trivial as well as complex behaviours, depending on their connectivity. Therefore, the important question is which kind of connectivity or which exact degree of complexity, determined with which mathematical norm, is sufficient for consciousness and why (Moody, 2003[150]). A rather different way to cope with the small network argument is to claim that, indeed, each small network has some kind of (almost vanishing) consciousness (Lamme, 2006[124]; Lamme, 2010[123]).
It is evident that such kind of panpsychism encounters serious problems as well. Consider, for example, two non-connected systems with three neurons each and each system having its own consciousness. What happens when these two systems are connected? Does one unified consciousness emerge, do the two consciousnesses stay separate, independent of each other (as proposed in split brain patients), or are there new coalitions of neurons making up new (micro) consciousnesses (Zeki, 2003[241]). In the later cases, there can be as many consciousnesses at a given time as there are, for example, recurrent connections, synchronisations, or winner-take-all competitions. In short, it is by far not obvious why an increase in ‘conscious’ elements yields one unity consciousness we experience and not many separate consciousnesses.
The activities of everyday cognition can be classified as brief events or as prolonged events. Brief cognitive events take place in a matter of tens of milliseconds and prolonged events persist over durations of seconds or more. Examples of brief cognitive events are identifications of familiar words in fluent reading, orienting to the location of a sudden sound, and the scanning of objects on a table in search of a pencil. Examples of prolonged cognitive events are holding a telephone number in working memory, waiting for the next person in line to move, imaging an upcoming green traffic light and savouring the sound of a one-ton temple bell (Wang, 2001[233]).
The activities of everyday consciousness, however, are almost always of a prolonged duration and fill the hours in which we are awake. Consciousness provides a sensory background for the cognitive activities just described. Conscious activities include the ambient registration of indoor and outdoor visual scenes, the continued sounds of a ticking clock and the ongoing traffic noise of the city streets. A particularly persistent source of background conscious activity is the ongoing feeling of our bodies. Brief events of cognition are typically described at the neural level in terms of rapid input–output processing. The single neuron, with its dendrite–soma–axon structure, provides a very simple basis for rapid input–output processing, and chains of neurons serve as a framework for a variety of stimulus–response accounts of behaviour. However, neuronal accounts of prolonged events, particularly the persistence of working memory over time, are somewhat more complicated, and typically involve several neurons arranged in a recurrent circuit (Wang, 2001[233]; Tegner, Compte, and Wang, 2002[213]). Readers are also directed to more recent attempts to explain these mental operations called, ‘The Lattice of Mental Operation’ in a recent review (Singh and Singh, 2011[203]).
3.2 Apical dendrite activity and consciousness
Mammals other than humans are presumed to have conscious states because their brains also contain thalamocortical circuits of the appropriate configurations. The apical dendrite activity theory takes the additional step of implicating the apical dendrite part of thalamocortical circuits to be the generator of consciousness. It suggests that the length of the apical dendrite increases the stability of the thalamocortical circuit activity, and thereby, increases the stability of the apical dendrite wave activity itself (LaBerge, 2006[121]). It is conjectured here that the stability of the apical dendrite activity contributes to the steadiness of the ongoing conscious impressions in the primary sensory areas, as well as influences the duration of sustained attentional operations in the higher-order cortical areas.
The role of the frontal cortex in consciousness is emphasised in the account of consciousness given by Crick and Koch (1995)[46], who assume that neural activity must reach the frontal lobe directly for awareness to occur. The present theory contrasts with their account in assuming that apical dendrite activity in area V1 itself is the basis for visual consciousness.
Synchronous activity in clusters of apical dendrites produces electromagnetic (EM) fields that can radiate outward, and if they are strong enough to reach the surface of the scalp they can be measured as EEGs. The electromagnetic field has been proposed as the physical substrate of consciousness (McFadden, 2000[144]; Pockett, 2000[167]). The fluctuating currents that produce EM fields are produced by many sources, among which are, the membrane activity of axons, dendrites and the somas in the neurons that are located in subcortical as well as cortical regions of the brain. According to these investigators it is the overall field pattern within the brain formed by all of these individual fields that constitutes momentary consciousness.
3.3 N-Methyl-D-aspartate activity and consciousness
It has been hypothesised that some of the rapid transient cell assemblies that are the building blocks for mental representations are conscious. The ascending reticular activating system determines how likely it is that a cell assembly forms as well as aids in binding together several simple assemblies into more complex representational states (Flohr, 1995[74]). The occurrence of states of consciousness critically depends on a specific class of computational processes that are mediated by the NMDA synapse. Arguments in favour of the hypothesis are based on the idea that NMDA receptor activity is capable of forming representational states in the brain and all general anaesthetics ultimately inhibit NMDA receptor activity. Hence, NMDA receptor activity is essential for consciousness (Gambrill et al., 2011[77]).
Autoradiographic techniques (Flohr, Glade and Motzko, 1998[73]) have been used to visualise the activation state of the cortical NMDA synapses directly and the difference between the anaesthetised and the conscious brain is the presence or absence of NMDA-dependent computational processes (Yashiro et al., 2008[238]; Zhao et al., 2008[245]).
3.4 The neural assembly model of consciousness
A neuronal cell assembly is defined as a diffuse structure comprising of cells in the cortex and the diencephalons, capable of acting briefly as a closed system and delivering facilitation to other such systems (Hebb, 1949[92]). Such assemblies, where 10 million neurons can synchronise activity in over merely 230 ms have been established. They have been postulated to be essential for the production of conscious states.
According to the neural ‘time on’ theory (Libet, 1993[130]), consciousness is associated with neuronal activities that persist for a long enough time with a minimal duration of 500 ms. The binding is achieved by the synchronised activity of a set of neurons over the short time window (Von der Malsburg and Schneider, 1986[230]). In the synapses of neural assemblies, known as Malsburg synapses, the strength of the synapse temporarily increases when there is a strong correlation between pre- and postsynaptic activity (Crick, 1984[49]). These three-dimensional configurations of large scale assemblies in the brain, which need not respect conventional anatomical boundaries, will correlate with different degrees of consciousness at any moment (Greenfield, 1997[86]). A recent study by some researchers has added support to this neuronal assembly model (Koch, 2005[112]; Overgaard and Morgensen, 2011[157]; Jansen and Overgaard, 2011[101]).
These assemblies vary in size from one moment to the next based on (a) the strength of the trigger that initiates their transient synchrony, (b) the ease with which the neurons will be synchronised, which is in turn dependent on the availability of the facilitating modulatory chemicals. It is this two-way iteration of chemicals that is viewed as ‘consciousness’, the assembly size of which is a mere index. Imaging these transient assemblies will provide a net index of consciousness (Greenfield, 2000[87]). Perhaps in the future, a unit can be formulated that determines an assemblage, that is, a quantifiable measure reflecting the combined temporal and spatial dynamics of constantly changing neuronal assemblies (Greenfield and Collins, 2005[85]).
3.5 The thalamocortical dialogue model of consciousness
The physiological candidate that might play a key role in consciousness is neural activity synchronised in the gamma frequency range (25 – 50 Hz), mostly close to 40 Hz between the thalamic and cortical structures (Llinas and Pare, 1991[132]; Cavanna et al., 2011[31]). These are known as the 40 Hz thalamocortical oscillations and may be likely candidates for the binding problem (Singer and Gray, 1995[201]). These oscillations are generated when the thalamic neurons are depolarised beyond – 45 mV, which depends on the activation of the voltage-gated calcium channels. The thalamocortical system functions on the basis of temporal coherence embodied by the simultaneity of neuronal firing, based on passive and active dendritic conduction along the apical dendritic core conductors. This results in the thalamocortical resonant column, which is compromised of the basic functional unit of consciousness (Lopes da Silva, 2004[52]).
Several neural-based theories have implicated thalamocortical circuitry in the generation of consciousness (Llinas et al., 1998[131]). Researchers regard the interaction between two thalamocortical circuits as being the generator of the states of consciousness. One circuit involves the thalamic neurons in the intralaminar nuclei of the thalamus, which project preferentially to layer 1 across the broad expanses of the cortex. Intralaminar neurons are innervated in part by sources in the brain stem that participate in the arousal states, and current arousal level activity is apparently relayed by these neurons in a diffuse manner to the tufts of pyramids in layer 1, with pulse frequencies near 1000 Hz. In contrast to these non-specific thalamocortical circuits, the specific circuits involve neurons in the dorsal thalamus, which project to the midlayers of relatively small clusters of cortical columns that code for a particular cognitive content. On this view, consciousness is generated when both the specific and the non-specific thalamocortical circuits are simultaneously active. The binding of different cognitive contents into a unified conscious event is presumed to take place through coincidence detection, by coactivation of the specific and non-specific thalamic nuclei (Jones, 2007[103]).
The theory of consciousness described centres on the thalamocortical circuit that involve the intralaminar nuclei, which contain cells that relay brainstem activity of general states of waking and alertness to widely distributed areas of the cortex (Bogen, 1995[24]). Others have proposed that thalamic and cortical structures (along with their relation to brainstem reticular nuclei) are both necessary and sufficient conditions for the existence of a state of wakeful consciousness. The recurrent or ‘re-entrant’ property of the thalamocortical circuit is regarded as crucial for generating conscious states, in several articles (Baars et al., 2003[9]; Seth et al., 2005[197]).
3.6 Baseline cerebral neuronal activity and consciousness
In perceptual experiments, within-subject variability in perception is commonly observed across multiple presentations of the same stimuli (Sergent, Baillet, and Dehaene, 2005[196]). In parallel, trial-to-trial variability in event-related BOLD responses magnitude has been shown to be relevant to human perception and behaviour (Fox et al., 2006[75]). Despite its demonstrated relevance in human behaviour, the sources of these event-related BOLD responses (and related perception) variability are only partially understood (Dehaene and Changeux, 2005[59]; Fox et al., 2006[75]). Authors have recently made the hypothesis that variability in perception of identical stimuli may be due to differences in prestimulus baseline brain activity, in areas related to stimulus perception (Boly et al., 2007[25]).
A first possible source of baseline brain activity fluctuations observed in studies could be fluctuations of the arousal level of our subjects throughout the experiment. The medial thalamus is known to be involved in vigilance and alertness (Kinomura et al., 1996[108]; Portas et al., 1998[171]). This area has also been involved in attentional effects varying with the level of arousal (Coull et al., 2004[45]). A second possible source of slow neural activity fluctuations would be spontaneous changes in selective attention. A number of studies show enhanced lateral fronto–parietal activity during increased visual attention (Buschman and Miller, 2007[29]; Weissman et al., 2006[234]). Finally, attention to pain has also been shown to increase the BOLD signal in the pain matrix encompassing the anterior cingulate cortex and insula (Koyama et al., 2005[116]).
Although the neural correlates of external awareness begin to be better understood, much less is known about self-awareness and its relationships with environmental perception. On the other hand, even in the absence of cognitive tasks or sensory stimulation, baseline brain activity in the areas involved in the genesis of conscious perception continuously fluctuates in the human brain (Aglioti and Candidi, 2011[1]). Moreover, research data suggest that self- and external awareness are not concurrent; they would rather be in competition during the process of sensory perception. Taken as a whole, these data suggest that baseline brain activity fluctuations are likely to shape the contents of our ongoing ‘stream of consciousness’ in a decisive manner. However, the origin and physiology of these fluctuations remain a mystery, and will be the subject of future studies (Boly et al., 2008[27]; Boly et al., 2011[26]).
4. Altered Brain States and Consciousness
4.1 Altered brain states and consciousness
Consciousness has two major components: Awareness (i.e., the content of consciousness) and arousal (i.e. the level of consciousness) (Laureys, 2005[128]). Arousal and awareness are usually positively correlated: When one's arousal decreases, so does one's awareness (rapid eye movement (REM) sleep being a notable exception). Awareness can also be divided into two components: Self-awareness and external awareness. Self- and external awareness usually behave in an anti-correlated manner. When you are engaged in self-related processes, you are less receptive to environmental demands, and vice versa (Boly et al., 2007[25]). The vegetative state (VS) is a classic example of a dissociated state of unconsciousness. VS patients are fully aroused, but are unaware of themselves and their environment. They can show automatic reactions like moving their eyes, head, and limbs in a meaningless manner, and may even grimace, cry, or smile (albeit never contingently upon specific external stimuli). Some patients might evolve towards full recovery or remain in the minimally conscious state (Laureys, Owen, and Schiff, 2004[127]), where some non-reflexive or non-meaningful behaviours are shown, but patients are still unable to communicate. In addition to their clinical and ethical importance, the study of vegetative and minimally conscious states offers a still widely unexploited means of studying human consciousness. In contrast to other unconscious states, such as, general anaesthesia and deep sleep, where impairment in arousal cannot be disentangled from impairment in awareness, these states represent a unique lesional approach, enabling us to identify the neural correlates of (un) awareness.
Voxel-based statistical analyses have sought to identify regions showing metabolic dysfunction in VS patients as compared to the conscious resting state in healthy controls. These studies have identified a systematic metabolic dysfunction, not in one brain region, but in a wide frontoparietal network encompassing the polymodal associative cortices in VS: lateral and medial frontal regions bilaterally, parietotemporal and posterior parietal areas bilaterally, posterior cingulated, and precuneal cortices, known to be the most active ‘by default’ in resting non-stimulated conditions (Gusnard and Raichle, 2001[90]).
In contrast, arousal structures (encompassing the pedunculopontine reticular formation, the hypothalamus and the basal forebrain) are relatively preserved in these patients (Salinas and Sejnowski, 2001[182]). The same frontoparietal functional impairment is found in various other states of unconsciousness, that is, in sleep (Maquet, 2001[137]), coma (Baars, Ramsoy, and Laureys, 2003[10]), general anaesthesia (Baars, 1988[11]), generalised seizures, or in other dissociated unconscious states like absence seizures, complex partial seizures, or somnambulism (Baars, 2002[13]).
In addition to activity in frontoparietal network, awareness seems also to relate to the functional connectivity within this network, and with the thalami. Functional disconnections in long-range corticocortical (between laterofrontal and midline-posterior areas) and corticothalamocortical (between nonspecific thalamic nuclei and lateral and medial frontal cortices) pathways have been identified in the vegetative state (White and Alkire, 2003[236]). In the same line, disruptions in thalamocortical and corticocortical connectivity have been reported during other unconscious states like sleep or anaesthesia. Moreover, recovery from VS is accompanied by a functional restoration of the frontoparietal network and some of its corticothalamocortical connections. These results are in line with Dehaene and Changeux's relatively recent computational model of the relationships between spontaneous brain activity and external stimuli awareness (Dehaene and Changeux, 2005[59]). This model emphasises the importance of both thalamocortical and corticocortical cerebral connections to create patterns of spontaneous brain activity, hypothesised to allow conscious perception.
Even if states of extremely low or high brain activity are often associated with unconsciousness, the precise link between global brain metabolism and awareness remains difficult to assert. On the contrary, regional brain activity in a widespread frontoparietal associative network has been shown to be systematically altered in all documented states of unconsciousness. In line with the studies on awake volunteers, these data emphasise the potential role of frontoparietal association cortices in the genesis of awareness. Recent functional MRI studies have identified coherent low-frequency fluctuations among well-documented neuroanatomical networks (Tononi, 2005[221]; Tononi, 2012[222]).
4.2 The link between anaesthesia and consciousness
A proposed mechanism of general anaesthesia must in some way describe how anaesthetic actions affect functional end-points, such as the loss of consciousness. Current theories that attempt to explain the general anaesthetic mechanism, have indeed, focussed on the functional processes related to the neural correlates of consciousness (Changeux, 2012[36]). The information processing theory (Flohr, 1995[74]), the unified narcosis theory (Alkire, Haier, and Fallon, 2000[3]), the cognitive unbinding theory (Mashour, 2004[140]) and the proposed anaesthetic cascade (John and Prichep, 2005[102]) have all, in some way, considered synthetic processes in the brain as the functional targets of general anaesthetics. In 1995, Flohr[74] proposed an ‘information processing’ theory of anaesthetic mechanism. The premise was based on the role of glutamatergic NMDA receptors in the function of Hebbian cell assemblies responsible for the cortical representation of conscious perceptions (Hebb, 1949[92]). Flohr postulates that general anaesthesia results because of the interruption of NMDA receptor-mediated, higher-order representation, as supported by the anaesthetic actions of known NMDA-receptor antagonists such as ketamine, nitrous oxide and xenon. The GABA-mediated actions of anaesthetics are posited to have an indirect and common final effect through NMDA pathways. This theory represents an important step in linking a molecular action of anaesthetics to a functional neural correlate of consciousness (Mashour, 2011[142]).
The anaesthetic cascade mechanism of general anaesthesia (John and Prichep, 2005[102]) endorsed the concept of cognitive unbinding, at the same time postulating a specific stepwise process by which anaesthetics suppress consciousness. The proposed ‘cascade’ is as follows:
Depression of the brainstem reduces the influences of the ascending reticular activating system on the thalamus and cortex
Depression of the mesolimbic-dorsolateral prefrontal cortex interactions leads to blockade of memory storage
Further depression of the ascending reticular activating system leads to hyperpolarisation of the GABAergic neurons in the nucleus reticularis of the thalamus, resulting in
Blockade of thalamocortical reverberations
Functional uncoupling of parietal-frontal cortical activity, interrupting cognition
Reduced awareness and increase of frontal and band activity
Interestingly, the cascade is organised by first describing the molecular actions of anaesthetics and then linking these actions to the neural correlates of consciousness, such as, thalamocortical resonance and oscillations. It is unclear how drugs such as ketamine or nitrous oxide, which can lead to electroencephalogram activation rather than depression (Laureys, 2005[128]), fit within the proposed anaesthetic cascade. The previously discussed frameworks of consciousness and anaesthesia are not dependent on the depressive actions of anaesthetics, but instead focus on the disorganisation of the processes that bind information together. Thus, while the anaesthetic cascade incorporates many aspects of the proposed mechanisms of general anaesthesia that are based on the neural correlates of consciousness, perhaps the early steps can be revised to accommodate both excitatory and depressive phenomena.
This is by no means the first time that a relationship between the science of consciousness and anaesthesia has been proposed. This may, however, be the first time that the field of anaesthesiology is ready for such a proposal. There was recognition in the late 1980s and early 1990s that the study of consciousness and anaesthesiology shared a common interest (Kulli and Koch, 1991[118]). It is important to consider that this was a time in which a paradigm shift was occurring: Investigation of anaesthetics was shifting from lipids to proteins, and the diversity of molecular actions was becoming appreciated. Thus, integrative or unitary hypotheses were not the focus at that stage of investigation. In fact, it is only in recent years that discussions of anaesthesia and neural correlates of consciousness have appeared in the literature of anaesthesiology (Mashour, 2004[140]), rather than the literature of psychology or consciousness. It has been suggested that anaesthetics may not be a useful tool to study consciousness because of its diverse molecular actions (Koch, 2005[112]). It is arguable, however, that differing molecular actions can nonetheless result in a single common mechanism. General anaesthetics act on diverse substrates on the molecular level, yet may nonetheless result in a similar phenotype with a common functional outcome. The common feature that is currently proposed in multiple frameworks of anaesthetic mechanism is the disruption of integrated neural processes that are normally required for consciousness (Mashour, 2006[141]; Mashour, 2011[142]).
4.3 The split-brain syndrome and consciousness [Figure 9]
Figure 9.
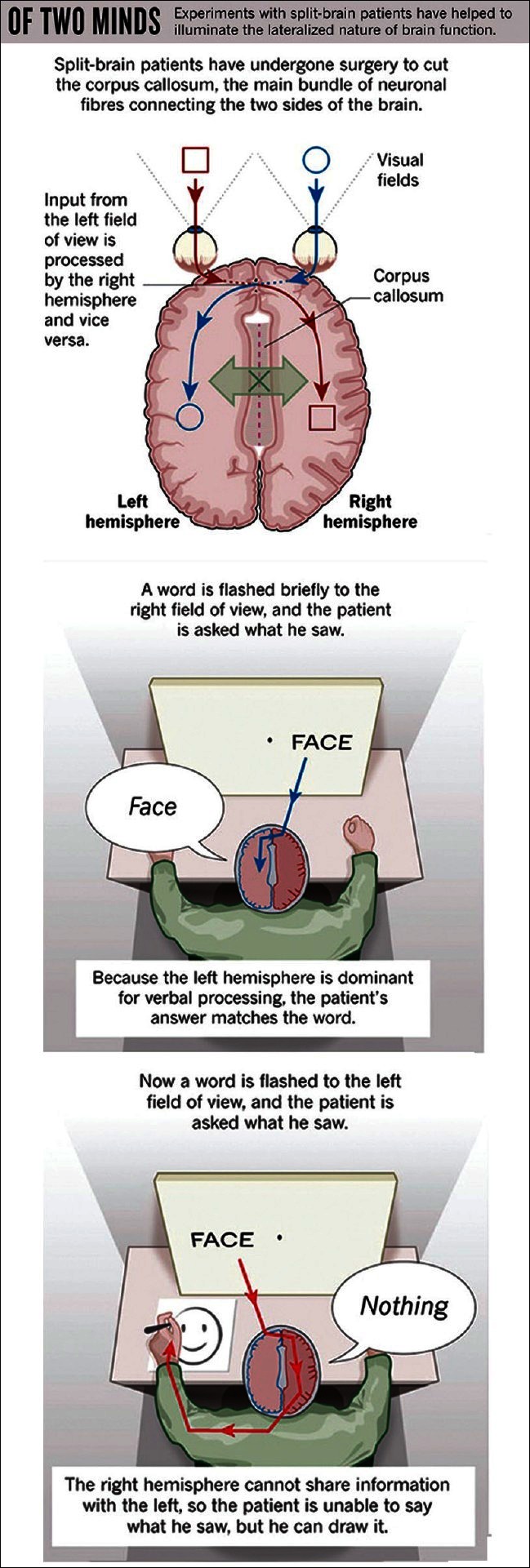
The split brain patient and consciousness
[Author's note: Taken from the website http://teachneuro.blogspot.in/2012/03/split-brain-left-brain-v s-right-brain.html. May be subject to copyright, although no copyright issues mentioned at website.]
First performed on humans in the late 1930s, the split-brain procedure involved severing the corpus callosum in order to prevent the spreading of epileptic seizures from one hemisphere to another (Springer and Deutsch, 1998[206]). The original version of the procedure, known as a commissurotomy, involved severing a number of interhemispheric tracts (such as the anterior commissure, the hippocampal commissure and the massa intermedia of the thalamus) in addition to the corpus callosum (Squire and Butters, 1984[207]). In the later versions of the procedure, known as a callosotomy, only the corpus callosum was sectioned. The differences between these two patient groups were not pronounced, and I would refer to both commissurotomy and callosotomy patients as split-brain patients (Nagel, 1971[154]).
Such studies have revealed two kinds of disunities in the split-brain, that is, behavioural disunities and representational disunities. Behavioural disunities are most striking. When asked to report what she sees, the patient in the key-ring experiment will typically say that she sees only the word ‘ring’; yet, with her left hand, the patient may select a picture of a key and ignore pictures of both a ring and a key-ring. Generally speaking, visual information projected to the right visual field (RVF) cannot be verbally reported, and visual information projected to the left visual field (LVF) is unavailable for behaviour involving the right hand. In the tactile modality, the patient cannot describe, or use her right hand to respond to objects palpated by her left hand, and objects palpated by the right hand cannot be reported via left-handed actions (Gazzaniga, 2000[78]; Gazzaniga, 2012[79]).
There is something to the claim that the continuity of consciousness requires physical continuity of some kind, although spelling out just what kind of continuity is required is not easy. Two important qualifications must be made. First, some forms of conscious content, such as affective content, involve subcortical systems that are not separated by the split-brain procedure. The mechanisms responsible for such states constitute a form of physical continuity that underlies inter-hemispheric switches. Second, we should not think of the cortical mechanisms responsible for the content of consciousness as mechanisms of consciousness per se. Arguably, cortical activity does not generate consciousness under its own steam, but contributes to the contents of consciousness only when integrated with subcortical processing. Again, these subcortical networks can provide any physical continuity that might be required for the continuity of consciousness (Laureys, 2005[128]; Vogeley and Fink, 2003[229]).
Although few contemporary theories would follow the seventeenth century anatomist Giovanni Lancisi in identifying the corpus callosum as the seat of the soul, it is widely assumed that splitting the corpus callosum also splits consciousness. Researchers have attempted to loosen the grip that disunity models of the split-brain have on us and to present the above-mentioned model as a live alternative to them. Not only does it do better in accounting for the behaviour of split-brain patients in both experimental and everyday contexts, it also avoids the philosophical baggage that accompanies the two-streams and partial unity models. I leave open the question of whether consciousness is necessarily unified. Even if consciousness in the split-brain syndrome remains unified, it is possible that the unity of consciousness breaks down in the context of other pathologies of consciousness; and of course, it is possible that the unity of consciousness may fail in non-human animals. All that can be said at this stage is that the case against the unity of consciousness remains unproven (Bayne, 2008[17]; Mehta, 2011[145]).
5. Cognitive Psychology and Consciousness
5.1 The relation between attention and consciousness
The debate is intensifying between those who believe that attention is necessary (but not sufficient) for consciousness (Mack and Rock, 1998[135]) and those who regard these two brain processes as independent (Koch and Tsuchiya, 2007[110]; Lamme, 2003[125], 2006[124]; Pollen, 2003[169]; Mograbi, 2011[147]). The debate is presently based on arguments of the latter protagonists, who assume that attention and consciousness are simple processes. However neither of the processes is likely to be simple. The complexity of attention is indicated by the subtle nature of the priming and masking effects, and by a variety of deficits in attention, such as, neglect and extinction, as well as the fact that there are both exogenous and endogenous varieties of attention, as also attention focussed on sensory input or motor response modes (Badgaiyan, 2012[14]). The complexity of consciousness arises from the wealth of different states of consciousness: In the normal waking state, under various drugs, in meditation (such as in the so-called pure consciousness), in dreaming, hypnosis, dissociation of identity disorder and so on (Mograbi, 2011[147]).
The general class of attention models is of an engineering control form, with many varieties, as is known from the range of motor control models proposed as existing in the brain (Cohen et al., 2012[42]). However, in particular, there is support given for the existence of the corollary discharge of attention movement signal (CODAM), especially from the motor theory of attention (Rizzolatti et al., 1987[178]). Further identification of this signal as being observed as part of the N2 signal is supported by experimental data (Taylor and Fragopanagos, 2007[210]). The CODAM model has been interpreted as possessing the ability to create both the conscious experience of content, as well as providing a neural underpinning for the phenomenological experience of ownership (the sense of the ‘inner self’).
Researchers have noted cases of subjects being unaware to change blindness, but yet sensing that it occurred (Fernandez-Duque and Thornton, 2000[70]). The CODAM model or variants of it have in general a boosting effect from the corollary discharge to speed up the access of stimulus activity from the lower level semantic maps onto their associated buffer sites, for report and use at a cognitive level. The corollary discharge also provides various levels of inhibition of distracters to prevent their access to the buffer sites. Overall the process of consciousness creation, according to CODAM, involves activation of two sites: Ownership on the corollary discharge buffer site and content on the sensory buffer. The second of these activations is expected to have a correlated lower level activity (brought about possibly through synchronisation or by amplitude correlation, such as by attention feedback). The ownership activity is not correlated with these low-level cortical sites, but may still involve a network of similar sites, providing a sense of the unity of self and also rapid access to material (Deco and Rolls, 2005[56]).
As an overall conclusion, we see that in all this discussion of the dynamical processes involved in various attention-based paradigms, there is a clear message, that is, attention is necessary for consciousness, whereby, attention is meant to be signal generated by the attention movement controller; without that there will be no efference copy, or any amplification of the lower level cortical activity to achieve access to the sensory buffer. However, on the other hand with the attention signal there will not necessarily be consciousness, unless the corollary discharge and the amplified lower level activity both attain their appropriate buffers. If various modules involved in the generation of the attention control signals are damaged, then it is to be expected that the sense of self will itself be compromised. This can occur, for example, in Alzheimer's disease or in schizophrenia, as well as in other diseases of a cognitive nature, such as neglect and frontotemporal lobar degeneration (Drubach et al., 2011[63]). At the same time varieties of consciousness – in dreams, under drugs – begin to be explicable in terms of the deconstruction of consciousness, which can be achieved through CODAM (Taylor, 2003[211]).
5.2 The global workspace model of consciousness
It has been proposed that the contents of consciousness can be similar to that of a global workspace, which can broadcast widely through the nervous system, to recruit the operation of numerous specialised subsystems to the task on hand (Baars, 1988[11]; Baars, 1997[12]). The global workspace provides a central information exchange, receiving its input processors that currently command attention, and broadcasts it widely from the brain. The two processing principles include competition through the global workspace, lower activation levels of global messages and raising it. The inputs selected for conscious awareness depend on the activity of the reticular activating system and thalamus, along with areas like the parietal and temporal cortex (Baars, 1988[11]).
The global workspace has flexibility of response, with relatively slow, voluntary, limited capacity serial processing (Zeman, 2001[243]). A similar model known as the neuronal workspace model (Dehaeene and Naccache, 2003[60]) suggests that a significant piece of neural machinery of what is called ‘access to consciousness’ is found in the workspace neurons, which have long excitatory axons for communication between parts of the brain, allowing cognitive performances that are otherwise unattainable. These include inhibition of habitual responses, planning, evaluation and monitoring of novel strategies and higher level semantics.
5.3 The cognitive hierarchy model of consciousness
This model (a) examines some of the characteristics of processes, which, in humans, are described either as conscious or unconscious, (b) links this to their biological embodiment and (c) speculates on their evolutionary history. To achieve this, it theorises the evolutionary origins of the processes: It shows where certain precursor processes, which are evident in a range of species, exhibit a similar distinction in performance to human non-conscious and conscious processes (Kunde et al., 2012[119]). That is, it will argue for evolutionary continuity. The model also looks at interactions between conscious and non-conscious processing, in the context of similar processes possessed by a range of species. As a framework, a model of behaviour and cognition (Toates, 2002[216], 2004[217]) will be extended to the study of consciousness. It is suggested that researchers may benefit from a greater appreciation of design solutions employed more widely across species.
The model investigates how a system of multiple layers of an interacting control can describe features of behaviour, cognition and consciousness. As a start, it assumes that the simplest and evolutionarily the oldest solutions to producing adaptive behaviour involve neither sophisticated cognition nor consciousness (Reber, 1992[175]). The model then suggests what the strengths and weaknesses of such relatively simple stimulus-based solutions are. It identifies the adaptive value of the evolutionary emergence of additional and higher-order processes. Such processes are commonly seen to involve representations of the environment, as is implied by such terms as cognitive map and expectation. There is a controversy on whether the term ‘representation’ is really needed to account for behaviour (Clark, 1999[39]; Van Gelder, 1998[224]). This, however, has presently been resolved with the acceptance of the term ‘representation’ (Aly and Yonelinas, 2012[4]; Pfaff, 2006[164])
The higher-order processes are defined by their flexibility and also by exclusion, as those that are evolutionarily more recent and which do not mediate behaviour in a direct stimulus-based manner. However, the fact that terms such as expectancy, error, model, disparity and cognitive map will be used in various places implies a belief in something resembling a traditional representation, even though the strength of the model does not rest or fall with the validity of this notion. Of course, the question of animal awareness (phenomenal consciousness) is not a new one and we do not know whether non-humans experience phenomenal consciousness. Others have speculated about animal awareness on the basis of behaviour, brain mechanisms and evolutionary considerations (Dawkins, 1990[54]). However, this model adopts a somewhat different perspective from these authors by pursuing a contrast between processes that appear to map onto the human conscious/unconscious divide. We know that many species exhibit higher-order cognition of a kind that would be open to conscious introspection in humans, for example, goal directedness, flexible use of memory, extrapolation to beyond sensory stimulation and resisting habitual behaviour (Dawkins, 2011[55]). We can also identify the brain regions underlying these cognitive capacities. It is argued that a study of the bases of such information processing in non-humans is relevant to how, in humans, features of consciousness are associated with similar cognition and underlying brain regions. These ideas represent a form of hierarchical control and this notion will provide a framework throughout. The hierarchical model conforms to evidence suggesting that new structures emerge in evolution by building on the existing structures, so-called tinkering (Jacob, 1977[98]). However, I could not find any further study in this direction after 1977.
5.4 The relation between intuitive decision-making and consciousness
The influential two-system framework, within decision-making and judgement research, dichotomises between processes that are characterised as heuristic, affective and intuitive, versus those that are seen as more deliberative, cognitive and rational. We refer to these supposedly separable systems, System 1 (S1) and System 2 (S2), respectively (Griffin and Kahneman, 2002[81]). Researchers declare that there is considerable agreement over the properties of these systems, which for S1 include being fast, automatic, effortless, associative and difficult to control or modify, and for S2 include being slower, serial, effortful, deliberately controlled and relatively flexible (Evans, 2008[68]). Recent advances in neural models have led researchers to study vertical and adaptive decision-making and they have developed an epistemological reflection on the neural basis of decision-making (Mograbi, 2011[148]).
Despite a tendency to associate S1 with non-conscious processing and S2 with conscious processing, it is suggested that within the representative formulations of this two-system framework, such as Kahneman's (2002)[104] summary of his Nobel Prize winning work and Epstein's (1994)[67] seminal article, there is considerable ambiguity over how to map the System 1/System 2 axis and the notion of intuitive processing onto the distinction between conscious and non-conscious processes. One must outline this ambiguity, to argue why clarification is important, to describe how the clarification can be made both theoretically and empirically and to suggest how consideration of the consciousness dimension can soften the two-system dichotomy (Kahneman, 2002[104]).
Kahneman (2002)[104] describes S1 as an intuitive mode of processing that leads to people having an intuition, defined as, “thoughts and preferences that come to mind quickly and without much reflection”. Is the label of intuition being used here to refer stipulatively to a family grouping of information processing characteristics that include lack of consciousness? Or is it referring to a genre of subjective experience that may be taken to imply an association with the conscious S2? Or, it depends? Or is there a sense in which intuitions are a bit of both? (Kahneman, 2002[104])
It is suggested that a more profitable way to categorise mental phenomena is to place them in a multidimensional space where three of the principle axes are:
The distinction between automatic and controlled processing styles: Typical comparative lists of S1 versus S2 properties (Epstein, 1994[67]; Kahneman, 2002[104]) read very much as lists of the distinguishing properties of the familiar cognitive division between automatic and controlled or attentional processes. It must therefore be noted that the automatic versus controlled distinction is a labelling convenience applied to a continuum of dissociable properties, rather than an inviolate black and white dichotomy (Price and Norman, 2008[173]; Reber, 1992[175]).
The distinction between non-conscious and conscious processing: Again note this is a gradation rather than a dichotomy. The degree of consciousness can be operationalised by batteries of behavioural measures, as exemplified by research on implicit learning.
Relative cognitive versus emotional content: The relationship of these types of feelings to the more emotional feelings discussed by other contributors (Pfister and Böhm, 2008[165]) is an important topic for future exploration. For example, do all intuitive feelings contain an emotional component? Or in what sense can emotions sometimes be thought of as intuitive emotional feelings? Mangan (2001)[136] certainly considers emotions to be an important part of everyday fringe consciousness. Moreover, our current analysis of intuitive feeling has a great overlap with the insightful fractionation of the concept of emotion by Pfister and Böhm[165] (2008). Their proposal that emotions are an integral part of most decision-making processes, rather than external influences acting on rational processes, is very much in line with the Jamesian notion of transient fringe consciousness pervading all our mental life. Also, at least some of the functional dimensions that Pfister and Böhm[165] use to characterise emotions are extremely close to the proposed functions of fringe consciousness.
5.5 Voluntary brain processing and consciousness
In traditional models of cognitive psychology, voluntary behaviour and automatic behaviour have been kept rather separate. To many theorists, automatic and unconscious actions appear to be fast, but inflexible, making them categorically different from the flexible and controlled nature of conscious, voluntary actions. Likewise, there has been a traditional division between the roles of the subcortical and cortical regions of the brain. The subcortex is often associated with unconscious behaviour, such as primitive reflexes and motor commands that have grown automatic and inflexible through practice. The cortex, on the other hand, is associated with consciousness, flexibility and control. Here, we review several lines of study that may challenge these traditional distinctions. We are led to conclude that first, automatic motor activity may arise ubiquitously, forming an intrinsic part of all behaviour, and second, that nonconscious processes are not as inflexible as has been previously assumed (Kanwisher, 2010[105]; Edelman et al., 2011[64]; Dehaene and Changeux, 2011[58])
Automatic and unconscious mechanisms have traditionally been regarded as inflexible and contrasted with flexible conscious behaviour. However, recent evidence has shown that nonconscious mechanisms can be modulated both by current task goals and by attention, and therefore, do not occur inflexibly, regardless of context. For example, masked prime stimuli do not generally cause priming unless they resemble the stimuli that are relevant for the task currently being performed (Enns and Oriet, 2008[66]; Naccache, Edlin and Dehaene, 2002[153]). This kind of evidence has led to the concept of conditional automaticity that given a certain internal and external context, the stimuli may trigger motor activation automatically, but in another context, they will not.
Studies on masked priming in healthy humans have also found that focussing attention in time or space can modulate the effectiveness of invisible stimuli (Lachter, Forster and Ruthruff, 2004[122]; Schlaghecken and Eimer, 2000[190]). One way that attention is thought to modulate the priming effects of invisible stimuli is by boosting the perceptual representation of weak stimuli towards the conscious threshold, enhancing their ‘perceptual strength’ (even if they still do not quite reach awareness). There is also some evidence that attention may act directly on the sensorimotor priming process, independently from the perceptual representations that support conscious awareness (Rose, 1998[179]).
Stimuli can automatically activate motor plans. When stimuli appear, they attract eye movements and partially activate the potential actions afforded by the object. Such automatic activations are likely to occur during most behaviours, and although they are hidden in most of us, they can be dramatically revealed following brain damage (e.g., in patients with alien limb syndrome or utilisation behaviour) (Kim, 2000[106]). Thus, a prerequisite for flexible behaviour is the ability to deal efficiently with the competition between different motor plans, including those automatically and partially activated by objects around us. Here, both medial frontal areas and the parietal cortex appear to play important roles. We have yet to understand these complex control mechanisms, but it appears that unconscious automatic inhibition may form a component part, blurring the distinction between voluntary and automatic control (Zelazo et al., 2007[242]). Evidence that unconscious processes are modulated by current goals and attention also challenges the traditional view that these processes are automatic and inflexible. Perhaps unconscious processes may not be categorically different from flexible and controlled voluntary actions after all (Andersen and Buneo, 2002[5]).
5.6 Brain learning and consciousness
The processes whereby our brains continue to learn about a changing world in a stable fashion throughout life are proposed to lead to conscious experiences. These processes include the learning of top-down expectations, the matching of these expectations against the bottom-up data, the focussing of attention upon the expected clusters of information and the development of resonant states between the bottom-up and top-down processes as they reach an attentive consensus between what is expected and what is there in the outside world. It is suggested that all conscious states in the brain are resonant states, and that these resonant states trigger learning of sensory and cognitive representations (Goodale and Milner, 1992[83]; Custer and Aarts, 2011[51]).
The models that summarise these concepts are therefore called Adaptive Resonance Theory models or ART models. Psychophysical and neurobiological data in support of ART are presented from early vision, visual object recognition, auditory streaming, variable-rate speech perception, somatosensory perception and cognitive-emotional interactions, among others. It is noted that ART mechanisms seem to be operative at all levels of the visual system, and how these mechanisms are realised by known laminar circuits of visual cortex is proposed. It is predicted that the same circuit realisation of ART mechanisms will be found in the laminar circuits of the entire sensory and cognitive neocortex. Concepts and data are summarised with regard to how some visual perceptions may be visibly or modally perceived, whereas, amodal perceptions may be consciously recognised, even though they are perceptually invisible (Lynch, McGaugh and Weinberger, 1984[134]; Zeki, 2003[241]). We await a more recent study in this field.
Conclusions
The theories reviewed herewith have looked at certain domains of consciousness. To start with we have looked at certain conceptual and methodological issues in consciousness. We have looked at how research paradigms define consciousness, conscious processes and neural correlates of consciousness. From there we have moved to the macro-structure of consciousness, covering various anatomical models of consciousness. It is worthwhile for the reader to note that all the topics covered in this article have been presented in a concise manner to fit this article and its purpose. All the theories and models mentioned are in fact worthy of single reviews by themselves [Figure 10].
Figure 10.
Flowchart of the article
The study of consciousness is not restricted to psychology and neurobiology alone. Various sub-specialties like cognitive psychology, cognitive neuroscience, neuroimaging, neurobiology, neurochemistry and cellular neuroscience have contributed in the developments of an integrated theory of consciousness. They deserve mention and a careful study for a better understanding of the complexities of consciousness.
Take home message
Consciousness involves processes that are at both micro and macro levels in neurobiology. Various models have been proposed and studied in this regard. This is intertwined with issues related to the definition and meaning of consciousness and a study of conscious processes at an abstract and cognitive level. The key to understanding the ultimate neurobiology of consciousness is a holistic encompassment of all these perspectives.
Questions that this Paper Raises
Is an integrated theory encompassing all fields on the neurobiology of consciousness possible? Is it necessary?
Is it possible to understand true biological substrates of consciousness or are we looking at pure phenomenology?
Does the study of altered states of consciousness, with an approach from pathology to normalcy, hold the key to the neurobiology of consciousness?
Should research groups from various arenas come together to form a more integrative research protocol to study consciousness?
Do we need further focussed neurobiological studies both at a micro and macro level to understand consciousness?
Do we need to go top down from cognitive psychology to neurobiology to understand consciousness or should the reverse be done?
Will we in the near future impinge upon an ultimate holistic theory that solves the riddle of the neurobiology of consciousness?
Will better neuroimaging studies and modalities in the future, together with advances in cellular neuroscience, help solve the puzzle of consciousness?
Will all stakeholders agree to a comprehensive definition of consciousness?
About the Author

Dr. Avinash De Sousa is a consultant psychiatrist and psychotherapist with a private practice in Mumbai. He is an avid reader and has over 130 publications in national and international journals. His main areas of research interest are alcohol dependence, child and adolescent psychiatry, transcranial magnetic stimulation and consciousness. He is also the academic director of the Institute of Psychotherapy Training and Management, Mumbai. He teaches psychiatry, child psychology and psychotherapy in over 25 institutions as a visiting faculty. He is one of the few psychiatrists who in addition to a psychiatry degree has an MBA in Human Resource Development, a Masters in Psychotherapy and Counselling and an MPhil in Psychology.
Acknowledgments
The author would like to thank Dr. Ajai Singh for his guidance and encouragement throughout the preparation of this article.
Footnotes
Conflict of interest: None declared.
Declaration
This is my original and unpublished study and has not been submitted for publication elsewhere.
CITATION: De Sousa A. Towards An Integrative Theory Of Consciousness: Part 1 (Neurobiological And Cognitive Models). Mens Sana Monogr 2013;11:100-50.
References
- 1.Aglioti SM, Candidi M. Out of place bodies, out of body selves. Neuron. 2011;70:173–5. doi: 10.1016/j.neuron.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Aleksander I. Mapping the Mind Series. London: Weidenfeld and Nicolson; 2000. How to build a Mind. [Google Scholar]
- 3.Alkire MT, Haier RJ, Fallon JH. Toward a unified theory of narcosis: Brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic induced unconsciousness. Conscious Cogn. 2000;9:370–86. doi: 10.1006/ccog.1999.0423. [DOI] [PubMed] [Google Scholar]
- 4.Aly M, Yonelinas AP. Bridging consciousness and cognition in memory and perception: Evidence for both state and strength processes. PLoS One. 2012;7:e30231. doi: 10.1371/journal.pone.0030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- 6.Arnsten AF, Li BM. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–84. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Aru J, Bachmann T, Singer W, Melloni L. Distilling the neural correlates of consciousness. Neurosci Biobehav Rev. 2012;36:737–46. doi: 10.1016/j.neubiorev.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–26. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- 9.Baars BJ, Banks WP, Newman J. Essential Sources in the Scientific Study of Consciousness. Cambridge: MIT Press; 2003. [Google Scholar]
- 10.Baars BJ, Ramsoy TZ, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26:671–5. doi: 10.1016/j.tins.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Baars BJ. A Cognitive Theory of Consciousness. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- 12.Baars BJ. In the Theater of Consciousness. Oxford: Oxford University Press; 1997. [Google Scholar]
- 13.Baars BJ. The conscious access hypothesis: Origins and recent evidence. Trends Cogn Sci. 2002;6:47–52. doi: 10.1016/s1364-6613(00)01819-2. [DOI] [PubMed] [Google Scholar]
- 14.Badgaiyan RD. Nonconscious perception, conscious awareness and attention. Conscious Cogn. 2012;21:584–6. doi: 10.1016/j.concog.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balduzzi D, Tononi G. Integrated information in discrete dynamical systems: Motivation and theoretical framework. PLoS Comput Biol. 2008;4:e1000091. doi: 10.1371/journal.pcbi.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balleine BW, Killcross S. Parallel incentive processing: An integrated view of amygdala function. Trends Neurosci. 2006;29:272–9. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Bayne T. The unity of consciousness and the split brain syndrome. J Philos. 2008;105:277–300. [Google Scholar]
- 18.Beck DM, Rees G, Frith CD, Lavie N. Neural correlates of change detection and change blindness. Nat Neurosci. 2001;4:645–50. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- 19.Bernat JL. Chronic disorders of consciousness. Lancet. 2006;367:1181–92. doi: 10.1016/S0140-6736(06)68508-5. [DOI] [PubMed] [Google Scholar]
- 20.Blanquet PR. Advances in interdisciplinary researches to construct a theory of consciousness. J Behav Brain Sci. 2011;1:242–61. [Google Scholar]
- 21.Block N. On confusion about a function of consciousness. Behav Brain Sci. 1995;18:227–87. [Google Scholar]
- 22.Block N. Perceptual consciousness overflows cognitive access. Trends Cogn Sci. 2011;15:567–75. doi: 10.1016/j.tics.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Block N. The Nature of Consciousness: Philosophical Debates. Cambridge: MIT Press; 1997. [Google Scholar]
- 24.Bogen JE. On the neurophysiology of consciousness: An overview. Conscious Cogn. 1995;4:52–62. doi: 10.1006/ccog.1995.1003. [DOI] [PubMed] [Google Scholar]
- 25.Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA. 2007;104:12187–92. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, et al. Preserved feedforward but impaired top down processes in the vegetative state. Science. 2011;332:858–62. doi: 10.1126/science.1202043. [DOI] [PubMed] [Google Scholar]
- 27.Boly M, Phillips C, Balteau E, Schnakers C, Degueldre C, Moonen G, et al. Consciousness and cerebral baseline activity fluctuations. Hum Brain Mapp. 2008;29:868–74. doi: 10.1002/hbm.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruno MA, Fernández Espejo D, Lehembre R, Tshibanda L, Vanhaudenhuyse A, Gosseries O, et al. Multimodal neuroimaging in patients with disorders of consciousness showing “functional hemispherectomy”. Prog Brain Res. 2011;193:323–33. doi: 10.1016/B978-0-444-53839-0.00021-1. [DOI] [PubMed] [Google Scholar]
- 29.Buschman TJ, Miller EK. Top down versus bottom up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–2. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 30.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 31.Cavanna AE, Shah S, Eddy CM, Williams A, Rickards H. Consciousness: A neurological perspective. Behav Neurol. 2011;24:107–16. doi: 10.3233/BEN-2011-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 33.Cavanna AE. The precuneus and consciousness. CNS Spectr. 2007;12:545–52. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- 34.Chalmers DJ. Facing up to the problem of consciousness. J Conscious Studies. 1995;2:200–19. [Google Scholar]
- 35.Chalmers DJ. The Conscious Mind. Oxford: Oxford University Press; 1996. [Google Scholar]
- 36.Changeux JP. Conscious processing: Implications for general anesthesia. Curr Opin Anaesthesiol. 2012;25:397–404. doi: 10.1097/ACO.0b013e32835561de. [DOI] [PubMed] [Google Scholar]
- 37.Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology. 1993;43:1465–7. doi: 10.1212/wnl.43.8.1465. [DOI] [PubMed] [Google Scholar]
- 38.Christoff K, Owen AM. Improving reverse neuroimaging inference: Cognitive domain versus cognitive complexity. Trends Cogn Sci. 2006;10:352–3. doi: 10.1016/j.tics.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Clark A. Visual awareness and visuomotor action. J Conscious Studies. 1999;6:1–18. [Google Scholar]
- 40.Cleermans A. Computational correlates of consciousness. Prog Brain Res. 2005;150:81–98. doi: 10.1016/S0079-6123(05)50007-4. [DOI] [PubMed] [Google Scholar]
- 41.Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus and cerebellum. J Neurosci. 2001;21:6283–91. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen MA, Cavanagh P, Chun MM, Nakayama K. The attentional requirements of consciousness. Trends Cogn Sci. 2012;16:411–7. doi: 10.1016/j.tics.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Cohen MA, Dennett DC. Consciousness cannot be separated from function. Trends Cogn Sci. 2011;15:358–64. doi: 10.1016/j.tics.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Coleman MR, Davis MH, Rodd JM, Robson T, Ali A, Owen AM, et al. Towards a routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain. 2009;132:2541–52. doi: 10.1093/brain/awp183. [DOI] [PubMed] [Google Scholar]
- 45.Coull JT, Jones ME, Egan TD, Frith CD, Maze M. Attentional effects of noradrenaline vary with arousal level: Selective activation of the thalamic pulvinar in humans. Neuroimage. 2004;22:315–22. doi: 10.1016/j.neuroimage.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 46.Crick F, Koch C. Are we aware of the neural activity in primary visual cortex. Nature. 1995;375:121–3. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- 47.Crick F, Koch C. Consciousness and neuroscience. Cereb Cortex. 1998;8:97–107. doi: 10.1093/cercor/8.2.97. [DOI] [PubMed] [Google Scholar]
- 48.Crick F, Koch C. Towards a neurobiological theory of consciousness. Sem Neurosci. 1990;2:263–75. [Google Scholar]
- 49.Crick F. Function of the thalamic reticular complex: The searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–90. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–23. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 51.Custers R, Aarts H. Learning of predictive relations between events depends on attention, not on awareness. Conscious Cogn. 2011;20:368–78. doi: 10.1016/j.concog.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 52.da Silva FH. Contribution to a neurophysiology of consciousness. Suppl Clin Neurophysiol. 2004;57:645–56. doi: 10.1016/s1567-424x(09)70404-0. [DOI] [PubMed] [Google Scholar]
- 53.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 54.Dawkins MS. From an animal's point of view: Motivation, fitness and animal welfare. Behav Brain Sci. 1990;13:1–61. [Google Scholar]
- 55.Dawkins R. The magic of reality: How we know whats really true. New York: John Wiley and Sons; 2011. [Google Scholar]
- 56.Deco G, Rolls ET. Attention, short term memory, and action selection: A unifying theory. Prog Neurobiol. 2005;76:236–56. doi: 10.1016/j.pneurobio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious and subliminal processing: A testable taxonomy. Trends Cogn Sci. 2006;10:204–11. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 58.Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–27. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 59.Dehaene S, Changeux JP. Ongoing spontaneous activity controls access to consciousness: A neuronal model for inattentional blindness. PLoS Biol. 2005;3:e141. doi: 10.1371/journal.pbio.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4:752–8. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- 61.Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 62.Dehaene S, Sergent C, Changeux JP. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci USA. 2003;100:8520–5. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drubach DA, Rabinstein AA, Molano J. Free will, freedom of choice and frontotemporal lobar degeneration. Mens Sana Monogr. 2011;9:238–50. doi: 10.4103/0973-1229.77440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edelman GM, Gally JA, Baars BJ. Biology of consciousness. Front Psychol. 2011;2:4. doi: 10.3389/fpsyg.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edelman GM. The remembered present: A biological theory of consciousness. New York: Basic Books; 1989. [Google Scholar]
- 66.Enns JT, Oriet C. Visual similarity in masking and priming: The critical role of task relevance. Adv Cogn Psychol. 2008;3:211–26. doi: 10.2478/v10053-008-0026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Epstein S. Integration of the cognitive and the psychodynamic unconscious. Am Psychol. 1994;49:709–24. doi: 10.1037//0003-066x.49.8.709. [DOI] [PubMed] [Google Scholar]
- 68.Evans JS. Dual processing accounts of reasoning, judgment and social cognition. Annu Rev Psychol. 2008;59:255–78. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- 69.Farthing GW. The Psychology of Consciousness. In: Cliff Englewoods., editor. New Jersey: Prentice Hall; 1992. [Google Scholar]
- 70.Fernandez Duque D, Thornton IM. Change detection without awareness: Do explicit reports underestimate the representation of change in the visual system? Visual Cogn. 2000;7:323–44. [Google Scholar]
- 71.Fins JJ, Illes J, Bernat JL, Hirsch J, Laureys S, Murphy E. Neuroimaging and disorders of consciousness: Envisioning an ethical research agenda. Am J Bioeth. 2008;8:3–12. doi: 10.1080/15265160802318113. [DOI] [PubMed] [Google Scholar]
- 72.Fins JJ. Constructing an ethical stereotaxy for severe brain injury: Balancing risks, benefits and access. Nat Rev Neurosci. 2003;4:323–7. doi: 10.1038/nrn1079. [DOI] [PubMed] [Google Scholar]
- 73.Flohr H, Glade U, Motzko D. The role of the NMDA synapse in general anesthesia. Toxicol Lett. 1998;100-101:23–9. doi: 10.1016/s0378-4274(98)00161-1. [DOI] [PubMed] [Google Scholar]
- 74.Flohr H. An information processing theory of anaesthesia. Neuropsychologia. 1995;33:1169–80. doi: 10.1016/0028-3932(95)00056-9. [DOI] [PubMed] [Google Scholar]
- 75.Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial to trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–5. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 76.Fuster JM. The Prefrontal Cortex. 2nd ed. New York: Raven Press; 1989. [Google Scholar]
- 77.Gambrill AC, Storey GP, Barria A. Dynamic regulation of NMDA receptor transmission. J Neurophysiol. 2011;105:162–71. doi: 10.1152/jn.00457.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gazzaniga MS. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123:1293–326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 79.Gazzaniga MS. Who's in charge?: Free will and the science of the brain. New York: Harper Collins; 2012. [Google Scholar]
- 80.Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 81.Gilovich T, Griffin D, Kahneman D. Heuristics and Biases. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 82.Gold L, Lauritzen M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci U S A. 2002;99:7699–704. doi: 10.1073/pnas.112012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–5. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 84.Gray JA. Consciousness: Creeping up on the hard problem. Oxford: Oxford University Press; 2006. [Google Scholar]
- 85.Greenfield SA, Collins TF. A neuroscientific approach to consciousness. Prog Brain Res. 2005;150:11–23. doi: 10.1016/S0079-6123(05)50002-5. [DOI] [PubMed] [Google Scholar]
- 86.Greenfield SA. The Human Brain: A guided tour. London: Weidenfeld and Nicolson; 1997. [Google Scholar]
- 87.Greenfield SA. The private life of the brain. London: Penguin; 2000. [Google Scholar]
- 88.Greicius MD, Srivastava G, Reiss AL, Menon V. Default mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grossberg S. The link between brain learning, attention, and consciousness. Conscious Cogn. 1999;8:1–44. doi: 10.1006/ccog.1998.0372. [DOI] [PubMed] [Google Scholar]
- 90.Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 91.Haines DE, Dietrichs E. The cerebellum structure and connections. Handb Clin Neurol. 2012;103:3–36. doi: 10.1016/B978-0-444-51892-7.00001-2. [DOI] [PubMed] [Google Scholar]
- 92.Hebb DO. The organization of behavior. New York: John Wiley; 1949. [Google Scholar]
- 93.Hill CS. Consciousness. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 94.Hirstein W. The contribution of prefrontal executive processes to creating a sense of self. Mens Sana Monogr. 2011;9:150–8. doi: 10.4103/0973-1229.77432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holland O. Machine consciousness, Special issue of the journal of consciousness studies. Exeter: Imprint Academic; 2003. [Google Scholar]
- 96.Hurley SL. Consciousness in action. Cambridge, Massachusetts: Harvard University Press; 1998. [Google Scholar]
- 97.Husain M, Kennard C. Distractor dependent frontal neglect. Neuropsychologia. 1997;35:829–41. doi: 10.1016/s0028-3932(97)00034-1. [DOI] [PubMed] [Google Scholar]
- 98.Jacob F. Evolution and tinkering. Science. 1977;196:1161–6. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- 99.Jasper HH, Descarries L, Castelucci VF, Rossignol S. Consciousness at the Frontiers of Neuroscience. Philadelphia: Lippincott Raven; 1998. [Google Scholar]
- 100.Jennett B. The Vegetative State: Medical facts, Ethical and Legal Dilemmas. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 101.Jensen M, Overgaard M. Neural plasticity and consciousness. Front Psychol. 2011;2:1–4. doi: 10.3389/fpsyg.2011.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.John ER, Prichep LS. The anesthetic cascade: A theory of how anesthetics suppress consciousness. Anesthesiology. 2005;102:445–71. doi: 10.1097/00000542-200502000-00030. [DOI] [PubMed] [Google Scholar]
- 103.Jones EG. The Thalamus. 1 and 2. UK: Cambridge University Press; 2007. [Google Scholar]
- 104.Kahneman D. Maps of bounded rationality: A perspective on intuitive judgment and choice, Nobel Prize Lecture, December 8th 2002. [Last accessed on 2012 Sept 18]. Available from: http: www.nobelprize.org/nobel_prizes/economics/laureates/2002/kahnemann lecture.pdf .
- 105.Kanwisher N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci USA. 2010;107:11163–70. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim J. Mind in a Physical World: An Essay on the Mind-Body Problem and Mental Causation. Cambridge: MIT Press; 2000. [Google Scholar]
- 107.Kim SJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. Structural and functional connections of the amygdala: From normal emotion to pathological anxiety. Brain Behav Res. 2011;223:403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kinomura S, Larrson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–5. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 109.Kjaer TW, Nowak M, Lou HC. Reflective self awareness and conscious states: PET evidence for a midline parietofrontal core. Neuroimage. 2002;17:1080–6. [PubMed] [Google Scholar]
- 110.Koch C, Tsuchiya N. Attention and concentration: Two distinct brain processes. Trends Cogn Sci. 2007;11:16–22. doi: 10.1016/j.tics.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 111.Koch C. Consciousness: Confessions of a Romantic Reductionist. Cambridge: MIT Press; 2012. [Google Scholar]
- 112.Koch C. The Quest for Consciousness. Englewood, Colorado: Roberts and Company; 2005. [Google Scholar]
- 113.Kolmac C, Mitrofanis J. Organization of the basal forebrain projection to the thalamus in rats. Neurosci Lett. 1999;272:151–4. doi: 10.1016/s0304-3940(99)00614-x. [DOI] [PubMed] [Google Scholar]
- 114.Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders. J Psychiatry Neurosci. 2006;30:178–86. [PMC free article] [PubMed] [Google Scholar]
- 115.Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann NY Acad Sci. 1999;877:445–60. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- 116.Koyama T, McHaffie JG, Luarienti PJ, Coghill RC. The subjective experience of pain: Where expectations become reality. Proc Natl Acad Sci USA. 2005;102:12950–5. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2002;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- 118.Kulli J, Koch C. Does anesthesia cause loss of consciousness? Trends Neurosci. 1991;14:6–10. doi: 10.1016/0166-2236(91)90172-q. [DOI] [PubMed] [Google Scholar]
- 119.Kunde W, Reuss H, Kiesel A. Consciousness and cognitive control. Adv Cogn Psychol. 2012;8:9–18. doi: 10.2478/v10053-008-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 121.LaBerge D. Apical dendrite activity in cognition and consciousness. Conscious Cogn. 2006;15:235–57. doi: 10.1016/j.concog.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Lachter J, Forster KI, Ruthruff E. Forty five years after Broadbent (1958): Still no identification without attention. Psychol Rev. 2004;111:880–913. doi: 10.1037/0033-295X.111.4.880. [DOI] [PubMed] [Google Scholar]
- 123.Lamme VAF. How neuroscience will change our view on consciousness. Cogn Neurosci. 2010;1:204–40. doi: 10.1080/17588921003731586. [DOI] [PubMed] [Google Scholar]
- 124.Lamme VAF. Towards a neural stance on consciousness. Trends Cogn Sci. 2006;10:494–501. doi: 10.1016/j.tics.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 125.Lamme VAF. Why visual attention and awareness are different. Trends Cogn Sci. 2003;7:12–8. doi: 10.1016/s1364-6613(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 126.Lau HC, Passingham RE. Unconscious activation of the cognitive control system in the human prefrontal cortex. J Neurosci. 2007;27:5805–11. doi: 10.1523/JNEUROSCI.4335-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laureys S, Owen AM, Schiff ND. Brain in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–46. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 128.Laureys S. The neural correlate of unawareness: Lessons from the vegetative state. Trends Cogn Sci. 2005;9:556–9. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 129.Lewis CS. Studies in Words. Cambridge: Cambridge University Press; 1960. [Google Scholar]
- 130.Libet B. The neural time factor in conscious and unconscious events. Ciba Foundn Symp. 1993;174:123–37. doi: 10.1002/9780470514412.ch7. [DOI] [PubMed] [Google Scholar]
- 131.Llinas R, Ribury U, Contreras D, Pedroarena C. The neuronal basis of consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–9. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Llinas RR, Pare D. Of dreaming and wakefulness. Neurosci. 1991;44:521–35. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 133.Logothetis NK. Single units and conscious vision. Philos Trans R Soc Lond B Biol Sci. 1998;353:1801–18. doi: 10.1098/rstb.1998.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lynch G, McGaugh JL, Weinberger NM. Neurobiology of Learning and Memory. New York: Guilford Press; 1984. [Google Scholar]
- 135.Mack MA, Rock SA. Inattentional Blindness. Cambridge: MIT Press; 1988. [Google Scholar]
- 136.Mangan B. Sensations ghost: The non sensory “fringe” of consciousness. Psyche. 2001;7:7–18. [Google Scholar]
- 137.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res. 2001;9:207–31. doi: 10.1046/j.1365-2869.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 138.Maren S. What the amygdala does and doesn’t do in aversive learning. Learn Mem. 2003;10:306–8. doi: 10.1101/lm.68403. [DOI] [PubMed] [Google Scholar]
- 139.Marois R, Yi DJ, Chun MM. The neural fate of consciously perceived and missed events in the attentional blink. Neuron. 2004;41:465–72. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- 140.Mashour GA. Consciousness unbound: Towards a paradigm of general anesthesia. Anesthesiology. 2004;100:428–33. doi: 10.1097/00000542-200402000-00035. [DOI] [PubMed] [Google Scholar]
- 141.Mashour GA. Integrating the science of consciousness and anesthesia. Anesth Analg. 2006;103:975–82. doi: 10.1213/01.ane.0000232442.69757.4a. [DOI] [PubMed] [Google Scholar]
- 142.Mashour GA. Sleep, anaesthesia and consciousness. Sleep. 2011;34:247–8. doi: 10.1093/sleep/34.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 144.McFadden J. Quantum Evolution. London: Harper Collins; 2000. [Google Scholar]
- 145.Mehta N. Mind Body Dualism: A critique from a health perspective. Mens Sana Monogr. 2011;9:202–9. doi: 10.4103/0973-1229.77436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Milner AD, Goodale MA. The Visual Brain in Action. Oxford: Oxford University Press; 1995. [Google Scholar]
- 147.Mograbi GJ. Meditation and the brain: Attention, control and emotion. Mens Sana Monogr. 2011;9:76–83. doi: 10.4103/0973-1229.77444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mograbi GJ. Neural basis of decision making and assessment: Issues on testability and philosophical relevance. Mens Sana Monogr. 2011;9:251–9. doi: 10.4103/0973-1229.77441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Monaco F, Mula M, Cavanna AE. Consciousness, epilepsy and emotional qualia. Epilepsy Behav. 2005;7:150–60. doi: 10.1016/j.yebeh.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 150.Moody T. Consciousness and complexity. Prog Complex Info Design. 2003;2:1–6. [Google Scholar]
- 151.Moruzzi G, Magoun HW. Brain stem reticular formation and the activation of EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 152.Mudrik L, Breska A, Lamy D, Deouell LY. Integration without awareness: Expanding the limits of unconscious processing. Psychol Sci. 2011;22:764–70. doi: 10.1177/0956797611408736. [DOI] [PubMed] [Google Scholar]
- 153.Naccache L, Blandin E, Dehaene S. Unconscious masked priming depends upon temporal attention. Psychol Sci. 2002;13:416–24. doi: 10.1111/1467-9280.00474. [DOI] [PubMed] [Google Scholar]
- 154.Nagel T. Brain bisection and the unity of consciousness. Synthese. 1971;22:396–413. [Google Scholar]
- 155.Newman J, Baars BJ. A neural attentional model of access to consciousness: A global workspace perspective. Cogn Neurosci. 1993;4:255–90. [Google Scholar]
- 156.Northoff G, Qin P, Feinberg TE. Brain imaging of the self conceptual, anatomical and methodological issues. Conscious Cogn. 2011;20:52–63. doi: 10.1016/j.concog.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 157.Overgaard M, Mogensen J. A framework for the study of multiple realizations: The importance of levels of analysis. Front Psychol. 2011;2:79. doi: 10.3389/fphys.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Owen AM, Coleman MR. Functional MRI in disorders of consciousness: advantages and limitations. Curr Opin Neurol. 2007;20:632–7. doi: 10.1097/WCO.0b013e3282f15669. [DOI] [PubMed] [Google Scholar]
- 159.Pandya SK. Understanding brain, mind and soul: Contributions from neurology and neurosurgery. Mens Sana Monogr. 2011;9:129–49. doi: 10.4103/0973-1229.77431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parsuraman R. The Attentive Brain. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 161.Parvizi J, Damasio A. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–36. doi: 10.1093/brain/awg166. [DOI] [PubMed] [Google Scholar]
- 162.Passingham RE. The Frontal Lobes and Voluntary Action. Vol. 21. New York: Oxford University Press; 1993. [Google Scholar]
- 163.Perruchet P, Vinter A. The self organizing consciousness. Behav Brain Sci. 2003;25:297–300. doi: 10.1017/s0140525x02000067. [DOI] [PubMed] [Google Scholar]
- 164.Pfaff D. Brain arousal and information theory. Cambridge: Harvard University Press; 2006. [Google Scholar]
- 165.Pfister HR, Bohm J. The multiplicity of emotions: A framework of emotional functions in decision making. Judgm Decis Mak. 2008;3:5–17. [Google Scholar]
- 166.Phelps EA. Emotion and cognition: Insight from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 167.Pockett S. The Nature of Consciousness: A hypothesis. Lincoln NE: Writers Club Press; 2000. [Google Scholar]
- 168.Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 169.Pollen DA. Explicit neural representations, recursive neural networks and conscious visual perception. Cereb Cortex. 2003;13:807–14. doi: 10.1093/cercor/13.8.807. [DOI] [PubMed] [Google Scholar]
- 170.Pollen DA. On the neural correlates of visual perception. Cereb Cortex. 1999;9:4–19. doi: 10.1093/cercor/9.1.4. [DOI] [PubMed] [Google Scholar]
- 171.Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18:8979–89. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Praharaj SK, Goyal N, De Sarkar P, Arora M. Neurobiology of consciousness: What do we know yet? Indian J Soc Psychiatry. 2009;25:3–10. [Google Scholar]
- 173.Price MC, Norman E. Intuitive decisions on the fringes of consciousness: Are they conscious and does it matter? Judgm Decis Mak. 2008;3:28–41. [Google Scholar]
- 174.Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion. J Cogn Neurosci. 1999;11:110–25. doi: 10.1162/089892999563175. [DOI] [PubMed] [Google Scholar]
- 175.Reber AS. The cognitive unconscious: An evolutionary perspective. Conscious Cogn. 1992;1:93–133. [Google Scholar]
- 176.Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–70. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- 177.Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–85. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- 178.Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- 179.Rose S. Essays on the new sciences of the mind. Princeton (NJ): Princeton University Press; 1998. From brains to consciousness? [Google Scholar]
- 180.Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71:209–23. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: Anatomy and physiology. Physiol Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 182.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–50. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sara SJ. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 184.Sass LA, Parnas J. Schizophrenia, consciousness and the self. Schizophr Bull. 2003;29:427–44. doi: 10.1093/oxfordjournals.schbul.a007017. [DOI] [PubMed] [Google Scholar]
- 185.Schaefer A, Gray JR. A role for the human amygdala in higher cognition. Rev Neurosci. 2007;18:355–63. doi: 10.1515/revneuro.2007.18.5.355. [DOI] [PubMed] [Google Scholar]
- 186.Scharnowski F, Rüter J, Jolij J, Hermens F, Kammer T, Herzog MH. Transcranial magnetic stimulation (TMS) of early visual cortex reveals a window of integration of substantial duration. J Vis. 2007;7:1016. [Google Scholar]
- 187.Schiff ND, Plum F. The role of arousal and “gating” systems in the neurology of impaired consciousness. J Clin Neurophysiol. 2000;17:438–52. doi: 10.1097/00004691-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 188.Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–18. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 189.Schiff ND. Multimodal neuroimaging approaches to disorders of consciousness. J Head Trauma Rehabil. 2006;21:388–97. doi: 10.1097/00001199-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 190.Schlaghecken F, Eimer M. A central peripheral asymmetry in masked priming. Percept Psychophys. 2000;62:1367–82. doi: 10.3758/bf03212139. [DOI] [PubMed] [Google Scholar]
- 191.Schmahmann JD, Pandya DN. Course of the fibre pathways to pons from parasensory association areas in the rhesus monkey. J Comp Neurol. 1992;326:159–79. doi: 10.1002/cne.903260202. [DOI] [PubMed] [Google Scholar]
- 192.Schmahmann JD, Pandya DN. Prefrontal cortex projections to the basilar pons in rhesus monkey: Implications for the cerebellar contribution to higher function. Neurosci Lett. 1995;199:175–8. doi: 10.1016/0304-3940(95)12056-a. [DOI] [PubMed] [Google Scholar]
- 193.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum insights form the clinic. Cerebellum. 2007;6:254–67. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 194.Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 195.Schmahmann JD. The cerebellum and cognition. San Diego CA: Academic Press; 1997. pp. 613–34. [Google Scholar]
- 196.Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci. 2005;8:1391–400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- 197.Seth AK, Baars BJ, Edelman DB. Criteria for consciousness in humans and other mammals. Conscious Cogn. 2005;14:119–39. doi: 10.1016/j.concog.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 198.Seth AK. The grand challenge of consciousness. Front Psychol. 2010;1:5. doi: 10.3389/fpsyg.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sheinberg DL, Logothetis NK. The role of temporal cortical areas in perceptual organization. Proc Natl Acad Sci U S A. 1997;94:3408–13. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Simpson J, Weiner E. Oxford English Dictionary. Oxford (UK): Oxford University Press; 2009. [Google Scholar]
- 201.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–86. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 202.Singh AR, Godwin R, Mograbi GJ, Balasubramanian R, Garyali V. A discussion in the mind brain consciousness group 2010 2011: Let's study the structure that is the very raison de etre of our existence. Mens Sana Monogr. 2011;9:284–93. doi: 10.4103/0973-1229.77445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Singh AR, Singh SA. Brain mind dyad, human experience, the consciousness tetrad and lattice of mental operations: And further, the need to integrate knowledge from diverse disciplines. Mens Sana Monogr. 2011;9:6–41. doi: 10.4103/0973-1229.77412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Singh AR, Singh SA. Notes on a few issues in the philosophy of psychiatry. Mens Sana Monogr. 2009;7:128–83. doi: 10.4103/0973-1229.40731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Singh J, Hallmayer J, Illes J. Interacting and paradoxical forces in neuroscience and society. Nat Rev Neurosci. 2007;8:153–60. doi: 10.1038/nrn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Springer SP, Deutsch G. 5th ed. New York: WH Freeman and Co; 1998. Left brain, right brain: Prespectives from cognitive neuroscience. [Google Scholar]
- 207.Squire LR, Butters N. Neuropsychology of Memory. New York: Guilford Press; 1984. [Google Scholar]
- 208.Strata P, Scelfo B, Sacchetti B. Involvement of cerebellum in emotional behavior. Physiol Res. 2011;60:S39–S48. doi: 10.33549/physiolres.932169. [DOI] [PubMed] [Google Scholar]
- 209.Sturm T. Consciousness regained? Philosophical arguments for and against reductive physicalism. Dialogues Clin Neurosci. 2012;14:55–63. doi: 10.31887/DCNS.2012.14.1/tsturm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Taylor JG, Fragopanagos N. Resolving some confusions over attention and consciousness. Neural Netw. 2007;20:993–1003. doi: 10.1016/j.neunet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 211.Taylor JG. Paying attention to consciousness. Prog Neurobiol. 2003;71:305–35. doi: 10.1016/j.pneurobio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 212.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 213.Tegnér J, Compte A, Wang XJ. The dynamical stability of reverberatory neural circuits. Biol Cybern. 2002;87:471–81. doi: 10.1007/s00422-002-0363-9. [DOI] [PubMed] [Google Scholar]
- 214.Thiel U. The early modern subject: Self consciousness and personal identity from Descartes to Hume. Oxford UK: Oxford University Press; 2011. [Google Scholar]
- 215.Timmann D, Dimitrova A, Hein Kropp C, Wilhelm H, Dörfler A. Cerebellar ageneis: Clinical, neuropsychological and MR findings. Neurocase. 2003;9:402–13. doi: 10.1076/neur.9.5.402.16555. [DOI] [PubMed] [Google Scholar]
- 216.Toates F. Application of multilevel model of behavioural control to understand emotion. Behav Processes. 2002;60:99–114. doi: 10.1016/s0376-6357(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 217.Toates F. Cognition, motivation, emotion and action: A dynamic and vulnerable interdependence. Appl Anim Behav Sci. 2004;86:173–204. [Google Scholar]
- 218.Tong F, Nakayama K, Vaughan JT, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron. 1998;21:753–9. doi: 10.1016/s0896-6273(00)80592-9. [DOI] [PubMed] [Google Scholar]
- 219.Tong F. Primary visual cortex and visual awareness. Nat Rev Neurosci. 2003;4:219–29. doi: 10.1038/nrn1055. [DOI] [PubMed] [Google Scholar]
- 220.Tononi G, Koch C. The neural correlates of consciousness: An update. Ann N Y Acad Sci. 2008;1124:239–61. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- 221.Tononi G. Consciousness, information integration and the brain. Prog Brain Res. 2005;150:109–26. doi: 10.1016/S0079-6123(05)50009-8. [DOI] [PubMed] [Google Scholar]
- 222.Tononi G. Phi: A voyage from the brain to the soul. New York: Pantheon Books; 2012. [Google Scholar]
- 223.Trimble M. Body image and the parietal lobes. CNS Spectr. 2007;12:540–4. doi: 10.1017/s1092852900021283. [DOI] [PubMed] [Google Scholar]
- 224.van Gelder T. The dynamical hypothesis in cognitive science. Behav Brain Sci. 1998;21:615–28. doi: 10.1017/s0140525x98001733. [DOI] [PubMed] [Google Scholar]
- 225.Velmans M, Schneider S. The Blackwell companion to consciousness. Malden MA: Blackwell; 2007. [Google Scholar]
- 226.Velmans M. Consciousness from a first person perspective. Behav Brain Sci. 1991;14:702–19. [Google Scholar]
- 227.Velmans M. Understanding Consciousness. 2nd ed. London: Routledge; 2000. [Google Scholar]
- 228.Velmans M. Understanding consciousness. London: Routledge; 2000. [Google Scholar]
- 229.Vogeley K, Fink GR. Neural correlates of the first person perspective. Trends Cogn Sci. 2003;7:38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 230.von der Malsburg C, Schneider W. A neural cocktail party processor. Biol Cybern. 1986;54:29–40. doi: 10.1007/BF00337113. [DOI] [PubMed] [Google Scholar]
- 231.Wagner AD. Working memory contributions to human learning and remembering. Neuron. 1999;22:19–22. doi: 10.1016/s0896-6273(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 232.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–63. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- 234.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–8. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 235.Whalen PJ, Phelps EA. The human amygdala. New York: John Wiley and Sons; 2009. [Google Scholar]
- 236.White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general anesthetic induced unconsciousness. Neuroimage. 2003;19:402–11. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 237.Xu D, Liu T, Ashe J, Bushara KO. Role of the olivo cerebellar system in timing. J Neurosci. 2006;26:5990–5. doi: 10.1523/JNEUROSCI.0038-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–94. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Young GB, Pigott SE. Neurobiological basis of consciousness. Arch Neurol. 1999;56:153–7. doi: 10.1001/archneur.56.2.153. [DOI] [PubMed] [Google Scholar]
- 240.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 241.Zeki S. The disunity of consciousness. Trends Cogn Sci. 2003;7:214–8. doi: 10.1016/s1364-6613(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 242.Zelazo PD, Moscovitch M, Thompson E. Cambridge Handbook of Consciousness. New York: Cambridge University Press; 2007. [Google Scholar]
- 243.Zeman A. Consciousness. Brain. 2001;124:1263–89. doi: 10.1093/brain/124.7.1263. [DOI] [PubMed] [Google Scholar]
- 244.Zeman A. Persistent vegetative state: A review. Lancet. 1997;350:795–9. doi: 10.1016/S0140-6736(97)06447-7. [DOI] [PubMed] [Google Scholar]
- 245.Zhao J, Peng Y, Xu Z, Chen RQ, Gu QH, Chen Z, Lu W. Synaptic metaplasticity through NMDA receptor lateral diffusion. J Neurosci. 2008;28:3060–70. doi: 10.1523/JNEUROSCI.5450-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


