Abstract
The field of affective neuroscience has emerged from the efforts of Jaak Panksepp in the 1990s and reinforced by the work of, among others, Joseph LeDoux in the 2000s. It is based on the ideas that affective processes are supported by brain structures that appeared earlier in the phylogenetic scale (as the periaqueductal gray area), they run in parallel with cognitive processes, and can influence behaviour independently of cognitive judgements. This kind of approach contrasts with the hegemonic concept of conscious processing in cognitive neurosciences, which is based on the identification of brain circuits responsible for the processing of (cognitive) representations. Within cognitive neurosciences, the frontal lobes are assigned the role of coordinators in maintaining affective states and their emotional expressions under cognitive control. An intermediary view is the Damasio-Bechara Somatic Marker model, which puts cognition under partial somatic-affective control. We present here our efforts to make a synthesis of these views, by proposing the existence of two interacting brain circuits; the first one in charge of cognitive processes and the second mediating feelings about cognitive contents. The coupling of the two circuits promotes an endogenous feedback that supports conscious processes. Within this framework, we present the defence that detailed study of both affective and cognitive processes, their interactions, as well of their respective brain networks, is necessary for a science of consciousness.
Keywords: Affective neuroscience, Cognition, Consciousness, Emotions, Endogenous feedback, Feelings
Introduction
The cognitive revolution that occurred in the last decades was an interdisciplinary movement arguing – among other ideas - that emotions are mental states that fall outside the domain of cognitive explanation. For instance, Gardner (1987[31]) argued that one of the five main features of the cognitive revolution was the lack of emphasis on affective/emotional and nonconscious aspects of brain functions.
According to Damasio, the cognitive revolution neglected the role of emotions in human and animal behaviour, on the basis of the following assumptions: (i) emotions cannot be trusted (neither in real life, nor in the laboratory); (ii) emotions are too subjective; and (iii) emotions are elusive and vague (Damasio, 2000[22]). Neuroscientists and cognitive scientists for some time had therefore been much more interested in cognitive aspects of the mind than in affects, emotions, and nonconscious processes.
Within the cognitivist perspective, the shaping of a conceptual framework devoted to the study of emotions and nonconscious processes would have been unnecessary or inadequate (LeDoux, 1999[46], 2000[47]). Researchers prioritised the analysis of mental states that could be translated in terms of computational operations, e.g., perception, learning, and memory. This keen interest in our cognitive functions partially explains why cognitive scientists did not include the study of affects and emotions among the research interests of the cognitive neurosciences (LeDoux, 1996[44], 2000[47]).
The cognitive revolution began to be overcome from the remarkable work of Darwin (1872/1998[19]). The attempt to complement and overcome the limitations and misconceptions inherent in the cognitive revolution gave rise to the emergence of an “affective revolution”, associated with the understanding that affects and emotions indeed play a key role in intelligent life (Panksepp, 2004[55]; Panksepp, 2001[53]; Davidson and Suton, 1995[23]). The new approach to the matter of unconscious emotions highlighted the limits of the cognitivist conception (Berridge and Winkielman, 2003[12]; Dubois, 2010[26]). Human actions have since been conceived as being greatly influenced by implicit processes that may not reach conscious attention (Bechara, 2004[8]; Bechara, Damasio and Damasio, 2000[9]; Burns and Bechara, 2007[15]; Damasio, 1994[20]; Dubois, 2010[26]), but may be considered within the range of “affective consciousness” (Panksepp, 2005a[56]).
In this paper, we analyse the work of Jaak Panksepp and Joseph LeDoux and introduce our perspective on the role of affects and emotions in a theory of consciousness. This perspective is based on the Endogenous Feedback Model, which suggests that affective/emotional and cognitive processes are mediated by distinct and interacting brain networks, and that degrees of consciousness correspond to the level of resonance of the networks (Augustenborg, 2010[4]; Carrara-Augustenborg and Pereira Jr., 2012[17]). After a brief historical review, we summarise central concepts present in current approaches to affects and emotions, make a brief review of their neurobiological basis, and propose a framework to define their role in a theory of consciousness.
At the onset, it is relevant to clarify our understanding of the concepts of affect, feeling, and emotion. These concepts are used with slightly different meanings by authors. We assume the terminology proposed by Russell (2003[64]; 2009[65]), by which core affect defines a state grossly characterised by positive or negative valence (Baars, 2008[6]). Such state conveys the degree of agreeableness of specific stimuli and events with respect to the organism's contextual state and current intentions. Proposed as a constant flow (Barrett, 2005[7]), core affect relate to neural changes determined by detection and processing of internal and external stimulia. The subjective result of these processes is the feeling, a category that includes sensations as pleasure and pain, affective states as being happy or sad, and also cognitive intuitions as grasping the meaning of a word or a sentence.
While the ability to produce core affect is present at birth (Barrett, 2005[7]), automatic processes such as attention can influence the feelings that compound it. They can be modified by associative learning, and in turn they can affect our behaviour. Feelings can then be expressed into overt behaviours, in terms of emotions (anger, sadness, happiness, shame expressions), which William James (1884[40]) defined as the experience emerging from individual's self-perception of automatic processes (see also Prinz, 2005[62]).
We can therefore define emotions as expressed bouts of affect, which we are able to explicitly assess in terms of intensity, quality, and contextual appropriateness, and also to convey by means of symbolic language (e.g., facial mimic). Emotions, which Freud (1950[29]) considered to be always conscious, can therefore express feelings, but they do not necessarily reflect them (i.e. someone can mimic sadness, without necessarily experiencing it). Therefore, there should always be a degree of consciousness related to our feelings and emotions, but such a consciousness may occur without awareness (attention to represented content). For instance, a hungry and thirsty person in a restaurant remains conscious of her hunger while choosing a drink, although her focus of attention is directed to the items of the beverage menu. Therefore, in the framework presented here we classify this sensation of hunger as an example of a peripheral, non-attended, or unaware conscious state. Affects and feelings frequently occur in our conscious life in this modality.
The major premises of Panksepp's affective neuroscience
Affective neuroscience is an area of research that focusses on the neural bases of emotions, and assumes a role of great relevance for emotions and affects in the modulation of cognition and behaviour. The approach advocated by Panksepp (1998[52]) is an attempt to understand, among other things, (i) the genesis of affective consciousness; (ii) how basic emotions and feelings are organised in the brain; and (iii) how the basic emotional processing systems of the brain generate internally experienced feelings.
‘Internally experienced affective states’ is an expression used by Panksepp and others to refer to mental events that “reflect our neurobiological ability to subjectively experience certain states of the nervous system” (Panksepp, 1998, p9[52]). Based on this conception, the approach advocated by Panksepp aims at showing how neurobiological systems mediate basic emotions (such as fear and anger). It further attempts to show how these systems elicit, “the valenced affective feeling states that provide fundamental values for the guidance of behavior” (Panksepp, 1998, p. 122[52]).
These purposes are grounded in the idea that emotional processes are experienced feelings, which play a key role in “the causal chain of events that control the actions of both humans and animals” (Panksepp, 1998, p14[52]). “Internal feelings” (corresponding to our concept of core affect) arise from neurobiological events that mediate and modulate the instinctual nature of many human action patterns, and that provide efficient ways to ensure human behavioural adaptation to environment. The causal chain of internal feelings that controls the actions of both humans and nonhuman animals is largely crafted from non-conscious processes (Panksepp, 2001[53]). This is why one of the major premises of Panksepp's affective neuroscience is that feelings sustain some unconditioned behavioural tendencies, and play a key role in the unconscious constitution of new behaviours through providing mechanisms that allow organisms to categorise world events efficiently so as to control future behaviours (Panksepp, 1998, p14[52]).
‘Natural internal values’ is an expression used by Panksepp (1998, p14[52]) to refer to the set of types of values upon which we carry out complex behaviours, efficiently categorise events and control future behaviours. These values are triggered by the arousal of various subcortical circuits, located in evolutionarily ancient areas of the mammalian brain. One of the best ways to understand how the values are composed is to delineate a ‘natural taxonomy’ of these systems through an analysis of the brain circuits from which such tendencies arise. Based on these assumptions, Panksepp intends to demonstrate that the function of emotional systems is to integrate many types of behavioural responses and physiological processes in the brain and rest of the body. More so, Panksepp defends the view that emotional systems trigger subjectively experienced feeling states that provide efficient ways to guide and sustain behaviour patterns and certain types of learning (Panksepp, 1998, p15[52]). He believes that such a psychoneurological analysis of human and animal emotions makes possible an understanding of the basic underlying nature of the human mind. All mammals “come into the world with a variety of abilities that do not require previous learning, but which provide immediate opportunities for learning to occur” (Panksepp, 1998, p25[52]). These emotional abilities emerge from basic systems in the brain, and their development depends on the interactions with higher brain areas that allow the organisms to make effective behavioural choices.
The human brain contains seven “basic emotional systems” (Panksepp, 1998, P 51[52]), among them four primal circuits. These circuits - i.e. seeking, rage, fear and separation distress panic -- mature early in life to ensure the survival of individuals.
The seeking-system is the neural network that provides us efficient ways to elaborate “energetic search and goal-directed behaviours in behalf of any of a variety of distinct goals objects” (Panksepp, 1998, p52[52]). The rage-system is easily aroused by thwarting and frustrations, helping us to defend ourselves and prompting behaviour when we are irritated or restrained (Panksepp, 1998[52]). The fear-system tries to minimise the probability of bodily destruction. This specific circuit arose during animal evolution and it serves to reduce pain (Panksepp, 1998[52]). Finally, separation distress panic is a neural system that is very important in the constitution and elaboration of social emotional processes related to attachment: “to be a mammal is to be born socially dependent” (Panksepp, 1998, p54[52]). This system provides both safeguards to assure that parents (usually the mother) “take care of the offspring and that offspring have powerful emotional systems to indicate that they are in need of care” (Panksepp, 1998, p54[52]).
Panksepp (1998, p54[52]) adds to the above primal circuits, three socio-emotional systems, which are engaged at appropriate times in the lives of all mammals, namely: (i) lust, (ii) maternal care, and (iii) play. These systems depend on abilities acquired during mammalian evolution, being developed in the individual by the action of specific hormones and social interaction. In addressing lust, Panksepp (1998, p225[52]) says that there are specific brain circuits that promote both sexual instinct and maternal motivation (Panksepp, 1998, p54[52]). Regarding care, Panksepp (1998, p246[52]), illustrates the existence of intrinsic brain systems that promote nurturing behaviours of mothers and fathers. Finally, when Panksepp designates the rough-and-tumble play as an emotional system (Panksepp, 1998, p280[52]), he understands that playing may reveal some major secrets of the brain and yield important insights into certain childhood psychiatric problems, among them autism and attention deficit disorders. Panksepp (1998, p297[52]) postulates that many children diagnosed with ADHD may, in fact, be exhibiting heightened play tendencies. He writes:
Their hyperactivity, impulsiveness, and rapid shifting from one activity to another may be partly due to their unconstrained and unfocused playful tendencies. Indeed, the medications that are used to treat the disorder – psycho-stimulants such as methylphenidate (i.e. Ritalin) and amphetamines – are all very effective in reducing playfulness in animals. (Panksepp, 1998, p297[52]).
From the standpoint of clinical neuroscience and clinical psychiatry, Panksepp's affective neurosciences aims at showing that it is possible that many cognitive deficits could be ameliorated by tackling the underlying emotional feelings (Panksepp, 2001[53]).
This objective cannot be reached by using a single disciplinary approach. Indeed, neuro-scientific terminology is not sufficient by itself to embrace the full nature of brain processes related to both emergence and constitution of internal emotional states (Panksepp, 2001[53]). Behavioural and psychological perspectives are not sufficient either. The demarcation of the neurobiological nature of human and non-human animal feelings requires the synthesis and further integration of behavioural, psychological, and neurobiological perspectives. Many disciplines have contributed to the improvement of our knowledge of how emotions arise from ancient brain circuits, but Panksepp (1998[52]) remarks that there is a missing piece that can bring all these disciplines together. In his words, this is the “neurobiological understanding of the basic emotional operating systems of the mammalian brain, and the various conscious and non-conscious internal states they generate” (Panksepp, 1998, p5[52]).
Joseph LeDoux: Two pathways of affective-emotional processing
In The Emotional Brain (1996[44]), LeDoux established the notion of the Low and the High roads, in order to show, among other things, that emotional responses can occur without the involvement of cognitive processing systems of the brain. According to LeDoux (1996[44]):
When a certain region of the brain is damaged, animals or humans lose the capacity to appraise the emotional significance of certain stimuli without any loss in the capacity to perceive the same stimuli as objects. The emotional meaning of a stimulus can begin to be appraised by the brain before the perceptual systems have fully processed the stimulus. The brain mechanisms through which memories of the emotional significance of stimuli are registered, stored, and retrieved are different from the mechanisms through which cognitive memories of the same stimuli are processed. (LeDoux,1996, p69[44]).
One of the arguments made by LeDoux (1996[44]) is that emotional appraisal may have more rapid effect in the determination of behavioural responses than perceptual and cognitive processing:
The systems that perform emotional appraisals are directly connected with systems involved in the control of emotional responses. Once an appraisal is made by these systems, responses occur automatically. In contrast, systems involved in cognitive processing are not so tightly coupled with responses control systems. The linkage of appraisal mechanisms with response control systems means that when the appraisal mechanisms detect a significant event, the programming and often the execution of a set of appropriate responses will occur. (LeDoux,1996, 69-70[44]).
Prior to cognitive activity, the amygdala detects and modulates responses to natural dangers (like predators), and it binds contingencies between novel threats and the stimuli that predict their occurrence (Dalgleish, 2004[18]; LeDoux, 1998[45]). Based on the knowledge that external stimuli reach the amygdala by means of direct pathways from the thalamus, as well as through an indirect route that, from the thalamus, crosses the cortex before converging to the amygdala, LeDoux establishes the notion of the low and the high roads (LeDoux, 1996[44]; 1998[45]; 2000[47]). The low road is the short and more rapid path that modulates our ability to respond promptly to those stimuli, which we have learned to associate with aversive outcomes prior to their cognitive assessment. The high road is instead the connective pathway between the corpus geniculatum, cortical tissue, and amygdala. This pathway integrates the emotional response with the more detailed analysis of the distinctive features of stimuli, and it shapes our awareness of contingency between specific stimuli and responses.
The amygdala operates as a central threat detector in the brain, responding to relevant stimuli and triggering a cascade of events – both through the high and low roads – that mediate emotional and behavioural responses to them. It projects to a variety of brain stem areas, each of which controls particular responses (LeDoux, 1996[44]). In cases of lesions in the central gray matter, the freezing response to fear is impaired, without alteration in autonomic (changes in blood pressure) and endocrine responses (release of stress hormones). Lesions to the lateral hypothalamus determine instead the reduction of autonomic responses (blood pressure), but not the freezing or autonomic responses (LeDoux, 1996, p160[44]). Finally, startle reflex and emotion modulation are impaired after a right amygdala lesion (LeDoux, 1996, p160[44]).
Experimental research has shown that the amygdala plays a pivotal role especially in the modulation of fear responses (Bechara et al., 1995[9]; Davidson, 1995[23]; Kapp et al., 1992[41]; LeDoux, 1996[44]; 1998[45]; 2000[47]). Indeed, many neuroimaging studies have demonstrated that the human amygdala is a critical component of the neural substrates of emotional experience, and that this structure has a central role in the mediation of fear, anxiety, and negative affectivity.
A neuroscientific approach to the interactions of cognition and emotion
Although cognition and emotions are research topics originally rooted into different disciplines (i.e. neuroscience and clinical psychology), the evidence accumulated through years of empirical work unequivocally shows that their mechanisms interact closely one with another. We have already suggested in previous work (Augustenborg, 2010[4]; Pereira Jr. and Furlan, 2010[59]; Pereira Jr. and Almada, 2011[58]) the role of feelings and emotions in the mediation of stimuli significance and in the modulation of behaviour. In fact, the Endogenous Feedback Network (EFN) model (Augustenborg, 2010[4]) defends that they contribute to both stimuli-response integration, and to processional speed. Furthermore, the proposed framework plausibly accounts for the occurrence of emotions also in absence of a corresponding stimuli. In this section, we will consider in greater details how such functions are indeed supported by empirical research, and how interaction between cognition and emotions are endorsed by our growing knowledge of brain connectivity.
Cognitive and affective processes appear to interact (Panksepp, 2003[54]), although to a substantial degree they correspond to distinct neurobiological events, in terms of anatomical, neurochemical, and functional criteria. According to Panksepp, “the arousal of feeling states helps to channel activities of the cognitive apparatus and thereby facilitates behavioral choices” (1998, p39[52]). Gray et al. (2002[36]), among others, have elaborated on models that describe how emotions and cognition are integrated. They assume that the two systems are distinct, but at the same time interact to become functional and adaptive (Gray et al., 2002[36]; Gray, 2004[35]). Their interactions involve a large circuit including several limbic and cortical areas. For instance, studies using functional brain imaging have shown that induction of emotional states correlates with activity in lateral prefrontal cortex (Braver, Cohen and Barch, 2002[14]) and many others have shown the role of orbitofrontal cortex in emotion and emotion-related learning (O’Doherty et al., 2001[63]). The latter study indicates that both lateral and medial orbitofrontal cortical areas play a substantial role in the brain regions responsible for reward and punishment. This research corroborates the idea according to which the lateral area of the orbitofrontal cortex is associated with an aversive outcome, while the medial area of the orbitofrontal cortex is associated with reward. According to O’Doherty et al. (2001[63]), evidence from lesion studies suggests that the orbitofrontal cortex, but not other regions of prefrontal cortex, is essential for performance on an emotion-related reversal learning task. Beer et al. (2006[3]) also advocate the idea that orbitofrontal cortex has a significant role in emotion-cognition interactions.
Some emotions have selective effects on cognitive control, and may influence cognitive mechanisms that support action control and goal-directed behaviour (Gray, 2001[34]). Many aspects of human mental life, including empathy, beliefs, attitudes, the self, altruism, creativity, decision making, and moral reasoning, are hence believed to reflect a “true marriage of cognitive and affective abilities” (Gray, 2004, p48[35]). In fact, feelings and emotions play crucial functions both in the selection of stimuli and in our choice of eventual responses. Such roles indicate a close interaction between cortical and subcortical areas, respectively, involved in cognitive and affective (automatic) processes.
Recognised as one of the brain structures primarily linked to emotions, the amygdala appears to cover a significant role in the mediation of such interactions, as the above reviewed work of Joseph LeDoux has made evident. The amygdala is situated in the medial temporal lobe, anterior to the hippocampal structure, and it is connected to visual cortex, visual thalamus, dorsolateral prefrontal cortex as well as to subcortical structures. Damage to the amygdala determines patients’ inability to produce learned fear responses and deficits in long-term memory (Bechara et al., 1995[10]). In instructed fear paradigms, for example, the participants are informed about the negative valence and the contingency of a stimulus. While in healthy subjects awareness of the negative valence of a stimulus is sufficient to activate the amygdala in magnitude similar to the arousal that an unexpected, aversive stimulus would have determined; patients with amygdala lesions fail to show any physiological expression of fearb (Phelps, 2002[61]). However, as outlined by Bechara et al. (1995[10]), such patients preserve both awareness of stimulus contingency and the understanding of its valence, that is, they are able to verbally account for the potential danger of the stimulus. It has to be noted however that the amygdala is per se not responsible for the expression of fear, as it basically possesses no neurons able to process the meaning of stimuli (LeDoux, 1996[44]). Instead, information from cortical sensory areas directly affects the sensory thalamus, from where the input reaches the amygdala (Damasio, 1994[20]). Consequently, activation of the amygdala triggers physiological responses via connective pathways from the central nucleus of the amygdala toward the brainstem (where they activate the sympathetic nervous system), and toward the hypothalamus (which in turn affects the hormonal secretions of the pituitary gland), (Kapp and Cain, 2001[42]; Kapp et al. 1992[41]). Although this has not yet reached the stage of explicit naming and accessible recognition (so far no cognitive mechanism has been involved), the information is sufficient to shape a rudimentary assessment of the stimulus’ valence, which can then motivate toward a specific response.
In a study performed by Anderson and Pelphs (2002[3]), no differences in the magnitude and frequency of self-reported positive or negative affect were found between control subjects and patients with amygdala damage. This research was divided into two studies, and both concluded that the structure of affective states was not altered by amygdala damage. In this sense, Anderson and Pelphs (2002[3]) substantiated the notion according to which the amygdala is not necessary for the generation of the phenomenal affective states, even though the human amygdala may be recruited in the constitution of affective states in the intact brain. The core of Anderson and Pelphs’ idea is that human affective experiences depend on internal processes, even in the absence of stimuli and contexts related to threats and rewards.
This controversy about the centrality of the amygdala for affective/emotional processing can be bypassed by a model of brain affective/emotional information processing based on a broader circuit, as in the case of the integrative and modulating role attributed to the astroglial network by Pereira Jr and Furlan (2010[59]), and Pereira Jr., Furlan and Pereira (2011[60]). This framework can accommodate the findings and theoretical views of LeDoux, without commitment to a central position of the amygdala in emotional processing.
In accordance with the presence of two pathways connecting the amygdala, both directly to the thalamus and also to higher processing areas (LeDoux, 1996[44]), emotional responses do not require perceptual consciousness. Besides being conveyed towards the amygdala, information from the thalamus is simultaneously sent to cortical areas where the stimulus is thoroughly assessed (e.g., per category, meaning, contextual relevance etc.), leading eventually to the generation of an intentional response. It is only at this stage that conceptual knowledge will emerge and – via descending pathways toward the amygdala - determine whether the physiological arousal has to be maintained (that is, stimulus is recognised as a threat), or the organism may relax (that is, stimulus is in fact harmless).
In sum, pathways from subcortical structures toward cortical areas can trigger both behavioural responses (e.g., fleet) and cognitive mechanisms (e.g., attention), while pathways from cortical areas toward the brain inner structures can either inhibit or increment the emotional reactions. Therefore, such reciprocal interactions between our cognitive and emotional mechanisms highlight one of the functions of emotions: that is, to prepare the organism toward an adaptive response which, by not depending from the time-consuming constraints of a cognitive evaluation, can occur rapidly. Our ability to detect fear expressed by unconsciously (masked) perceived faces (Adolph, 2002[1]) supports an adaptive role of emotions as early detectors of stimuli bearing possible negative consequences for the organism.
Thus, emotions appear significantly involved in shaping the content of our cognition, since they can affect the chances that given stimuli and events are granted increased attentional resources compared to others. This function is clearly demonstrated by studies employing the attentional blink paradigm. These experiments are based on the knowledge that visual stimuli following too closely the presentation of a target has decreased chances of being detected than those which appear after a longer time interval. However, a number of studies (e.g., Schwabe et al., 2011[66]; Anderson, 2005[2]) have shown that stimuli bearing emotional significance are likely to be detected even if they are presented in the refractory window that closely follows the appearance of the preceding target. Similar experiments involving brain-damaged patients have shown that amygdala lesions determine the disappearance of such emotional advantage. Accordingly, studies carried on by Öhman et al., 2001[50] have shown that masked threatening stimuli attract attention more rapidly than neutral ones. This is also supported by evidence collected by Vuilleumier et al., 2004[69] showing that the amygdala - via connective pathways to visual areas - is involved in the enhanced activation of visual processing areas during the presentation of emotional stimulic. The role of emotions as attention-markers is further confirmed by evidence that patients suffering from specific neurological conditions (e.g., visual extinction, neglect, and blindsight) show amelioration of deficits when they are presented with emotionally significant stimuli (see Hamm et al., 2003[39]; Grandjean et al., 2008[33]; Grabowska et al., 2011[32]).
A shift of attention towards one stimulus above others is also implicit in decision-making processes. As Gray et al., 2005[37] have pointedly argued, the benefit of possessing a system able to process multiple options and to simultaneously assess various behavioural responses is counterbalanced by an increased risk of conflicts between different possible choices. The bias shaped by emotions toward a given option can in this context contribute to solve the so-called “control-dilemmas” that may emerge between our cognitive knowledge and our emotional preference for a specific choice. Thus summarising, while cognition may affect our emotional responses by means of inhibitory control, our emotions may affect cognition by means of tipping the scale towards specific decisions. Emotions can additionally affect our cognitive style as indicated by evidence showing that negative affect (moods) stimulate systematic processing, while positive affect appears to lead to more heuristic attitudes in decision-making (Bolte, Goschke, and Kuhl, 2003[13]).
As previously mentioned, damage to the amygdala determines also impairments in long-term memory. This structure, which itself is not responsible for the encoding and storing of memories, affects instead the quality of our memories, and it contributes to selection of stimuli and events most relevant to encoding. In situations of reduced attentional resources for example, the emotional valence of a specific stimulus increases its chances of being attended to, and therefore remembered. Consequently, the emotional tag placed by the amygdala on given stimuli also improves our faculty to learn from past events. In fact, McGaugh (2000[48]) has shown that the amygdala modulates our ability to remember by affecting the hippocampal complex which is responsible for the initial encoding of episodic and declarative memory. The stress hormones released during emotional arousal trigger a mechanism that improves the consolidation and storage of hippocampus-dependent memories. Such interaction has been demonstrated by studies in which the inhibition of stress hormones, by administration of beta-blockers, had determined the attenuation of emotional memories’ recollection (van Stegeren, 2008[68]).
By means of attentional modulation, our emotions can boost the likelihood of assigning increased processional resources toward some stimuli above others. Emotions can also facilitate both the encoding and retrieval of specific memories and representations, determine the triggering of behavioural responses, and also affect our decision-making processes. By the same token, attention, memory, accessible knowledge and our intentional behaviour can affect emotions both in terms of intensity and valence. In sum, the feelings of our experiences are shaped by the meanings we have learned to ascribe to specific stimuli and events: Such meaning is emotionally and cognitively encoded in a unitary data-clusterd bearing the subjective “what-it-is-likeness” of every experience.
Implications for a theory of consciousness: The endogenous feedback network model
The alliance between cognition and emotions bears crucial implications for any theory of consciousness that imposes limited capacities to our unconscious processes. Our sense organs are an open window toward our environment: at any one time, sounds, visual and perceptual stimuli, smells and flavours impinge our senses and, while we might be able to limit their occurrence (e.g., by closing our eyes, blocking our ears), the magnitude and variety of perceptions often exceed our contextual needs and the processional capacities of our brain. Nevertheless, it is crucial for us to be able to discern between relevant and irrelevant stimuli, to draw pertinent information from our environment, and to allow such knowledge to reflect on our responses and decisions. How can we carry on such selections, become aware of specific stimuli above others, and at the same time optimally administrate our resources?
It is unlikely that, in order to assess the value of all the information to which we have access, we must carry on an extensive cognitive evaluation of each given stimulus. Global Workspace theories of consciousness (e.g., Baars, 1988[5]; Dehaene and Naccache, 2001[25]) have proposed models in which stimuli gain processional resources on the bases of their contextual relevance. In the context of visual perception, it is therefore suggested that the object's physical features, its meaning and the subjective relevance attributed to it, play a crucial role in determining whether the stimulus is granted attentional resources or ignored. By means of an eventual allocation of attention, we may then become conscious of the specific stimulus above others, and we may consequently be able to better assess its value, to mnemonically store its meaning, and to strategically and intentionally retrieve such information in future circumstances. Although these models take into consideration the weight that subjective values and pre-existing information may have on the selection and interpretation of specific stimuli, they assume a role of minor relevance to the phenomenality of perceptions and to the capacities of our unconscious processes. It is in this context that affective neuroscience takes on the crucial role of a mediator by creating a bridge between our cognitive processes and the phenomenal elements that both accompany and affect them [Figure 1].
Figure 1.
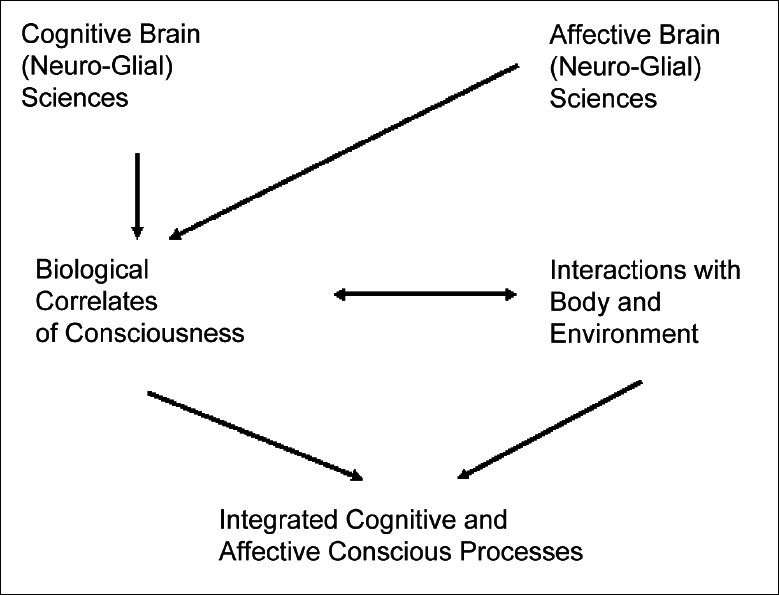
Epistemological interdisciplinary requirements for a science of consciousness
Affective and cognitive processes should be given equal emphasis when modelling the dynamics of conscious experience. The interaction with the rest of the body and with the environment should also be taken into account.
Engel and Singer (2001[27]) had suggested that temporal synchronisation of unconscious neuronal coalitions leads to dynamic binding, and therefore to the emergence into consciousness of specific representation above others. Global Workspace Theory (Baars, 1998[5]) has also proposed that consciousness emerges as the result of unconscious integration of data from a distributed neural network. What is common to both suggestions is their assumption that consciousness shapes knowledge, and it does so by binding distributed information into meaningful concepts. Nevertheless, the evidence we have discussed earlier in this section shows that emotions participate actively in the processing of information by modulating a broad variety of cognitive mechanisms (e.g., attention, meaning-making). We have furthermore considered the many instances in which empirical studies have demonstrated that emotions, although not consciously perceived, can determine cognitive styles, intentional behavioural responses, memory formation (by means of attention enhancement), as well as intentional mnemonic retrieval. In other words, the information borne by feelings (i.e. emotions deprived of cognitive evaluation) appears to contribute significantly to the content and the quality of our cognitive processes. It can therefore be argued that information can cluster into meaningful events even though these might not be precisely reportable. Such information may then be manipulated both by emotional and cognitive processes, it can be modified by associative learning, and be deployed in intentional behavioural responses. As accessible (attended) and inaccessible (non-attended) processes appear to significantly affect one another, it should appear clear that a convincing theory of consciousness must therefore offer an equally valid theory of degrees of consciousness and unconsciousness (see Carrara-Augustenborg and Pereira Jr., 2012[17]).
The Endogenous Feedback Network (EFN) theory shapes a framework able to account for both attended and unattended perceptual mechanisms, as well as for degrees of consciousness between the two. It acknowledges that a significant amount of information integration unfolds below our awareness’ thresholds and, importantly, that such information can be flexibly and strategically deployed within a broad range of cognitive processes. The EFN conceptualises consciousness as the global broadcast of the neural changes triggered by stimuli sensorial detection and processing. In such sense, consciousness reflects a constant flow of information that unfolds independently from attentional mechanisms, and that also includes the computations carried by the neural pathways modulating affective responses.
Within the frames of the EFN, the term consciousness is employed in adherence with its etymological root (lat. cum scientia: “with knowledge of”) , and it is taken to indicate the presence of knowledge within the organism. The eventual allocation of attention toward specific segments of such information flow may then allow specific contents of the broadcast to emerge into the accessible and reportable state that characterises awarenessse. At the functional level, the role of the conscious broadcast is to promptly mediate the stimulus’ significance in order to allow an appropriate response also prior to the occurrence of an eventual cognitive assessment. In this light, phenomenal consciousness reflects the feeling derived from the detection of a percept and – in the instance of a negative stimulus – it would correlate with the activation of the amygdala following the exposure to the emotionally significant input. It is however relevant to note that – as suggested by the EFN - behavioural and emotional responses may occur also in absence on external stimuli. Thoughts, mnemonic retrieval, and internal sensations (e.g., hunger, anxiety) may in fact trigger a chain of neural computations able to reverberate and consequently be broadcast along the endogenous feedback network.
As discussed earlier in this section, our experience of a given emotion is an act of recollection which involves both cortical and subcortical structures. Exposure to a stimulus can activate the pertinent emotional and cognitive data-cluster which can then prepare the organism toward the response associated with the specific stimulus. In sum, feelings may reflect mental “short-cuts” which rapidly bind the stimulus assessment to specific responses and/or meanings independently from a complete (and time-consuming) cognitive appraisal. This suggestion is supported at the anatomical level by the short connective route between thalamus and amygdala. Such direct pathway may determine in fact a rapid response, which only later may be inhibited or supported by the outcomes of the cognitive processing mediated by the longer pathway between thalamus and amygdala via sensory and associative cortical areas.
The distinction between feelings (core affect lacking cognitive appraisal) and emotions (feelings we have learned to explicitly recognise and describe) correlates with our proposed distinction between consciousness (information that elicits a feeling, but lacking cognitive appraisal) and awareness (conscious segments which we can explicitly access). If we accept that feelings can affect a significant range of cognitive mechanisms, and that the information they bear can shape coherent percepts even prior to their formalisation into conceptsf (i.e., emotions), we might then also accept that broadcast knowledge may be coherently deployed without necessarily having been translated into accessible concepts (i.e., awareness).
If we then seem able to make significant use of both feelings and consciousness independently from the explicit acknowledgement of either one, what are the respective functions of emotions and awareness? As other theories have also previously suggested, our awareness of specific stimuli above others allows us the possibility to isolate the former from a potentially crowded background of perceptions. By means of directed attention, we are able to appraise the specific representation, devoting to its assessment our complete cognitive toolbox. Additionally, the ability to overtly recognise specific emotions might have great adaptive value since it would allow us to communicate such states to others, and conversely to experience empathy by grasping how others might perceive (and react to) specific stimuli and eventsg. In other words, emotions may represent the organism's feedback with regard to both own and others subjective experience of specific behaviours and decisions.
As previously mentioned, the role of consciousness is instead to broadcast the occurrence of neural activity within and between specialised areas prior to the emergence of awareness. This constant flow of computations allows rapid endogenous updates of the status of the organism since its informational content is communicated also prior to the emergence of the cognitive and conceptual appraisal of the percept.
Concluding Remarks [Figure 2: Flowchart of Paper]
Figure 2.
Flowchart of paper
We propose a comprehensive model of consciousness within which affects, feelings, and emotions play a crucial role in stimuli-response integration. We have suggested that the neural changes produced by sensorial detection and processing of stimuli and events merge into a constant informational broadcast. By mediating the integration of data in real-time, such global network shapes a flow of distributed information processing across the brain able to facilitate behavioural responses, and to optimise the allocation of mental resources (e.g., by eventually affecting attentional mechanismsh). Within such framework, feelings represent non-cognitive markers able to link the neural changes that accompany specific representations to their mnemonically stored data-clusters. The formalisation of such feelings into broadly nuanced emotions determines finally our faculty to explicitly recognise significant contingencies and experiences, and it boosts our ability to remember and to learn on the basis of previous experiences.
In conclusion, the EFN model appears to accommodate both the views proposed by Panksepp and LeDoux, and the approach suggested by Damasio and Bechara. While in the classical approach to the relation of emotion and cognition (Stuss and Benson, 1986[67]) the frontal lobes are assigned the role of coordinators - in maintaining affective states and their emotional expressions under cognitive control - the EFN model suggests a more distributed processing network, allowing relatively independent broadcastings of feeling and cognitively processed information, instantiating different degrees of consciousness in the presence and absence of external stimulation. In this sense, the EFN framework offers angles compatible with the model proposed by Pereira Jr. and Furlan (2010[59]), Pereira Jr., Furlan and Pereira, (2011[60]), which accounts for the variable degrees of interaction between cognitive and affective states.
Take home message
The conceptual revolution brought about by affective neuroscience led to an emphasis on experimental studies of affective states and processes which were not accessible to conscious attention. These states and processes were then called “unconscious emotion”. However, many of these states/processes elicit conscious feelings. In the perspective of our proposed theory of consciousness, the expression “unconscious emotion” refers to conscious feelings, which are components of episodes of “affective consciousness” (according to the proposal of Panksepp, 2005a[56]). Feelings are events of the endogenous feedback network, when the information content of a stimulus elicits a reactive process (e.g., an evoked electric potential), which feeds back on the network's processing units, leading to the formation of a conscious episode that may be (or not be) expressed in terms of emotions and respective covert/overt behaviours.
Questions that this Paper Raises
What does Affective Neuroscience mean for a Theory of Consciousness?
What are the differences between core affects, feelings, and emotions?
What are the brain circuits responsible for affective and cognitive processing? How do they run in parallel and at the same time reciprocally interact?
What are the main contributions of Panksepp and LeDoux in the emerging field of Affective Neuroscience?
How does the flux of consciousness extend beyond the focus of attention?
About the Author

Leonardo Ferreira Almada is Adjunct Professor at the Institute of Philosophy of the Federal University of Uberlandia. He studied Philosophy at the Federal University of Rio de Janeiro, and obtained his PhD in 2009 at the same university. Between 2009 and 2011, he did postdoctoral research at the State University of São Paulo (UNESP) in the field of Affective Neuroscience. This research was conducted under the supervision of Alfredo Pereira Jr. His current research seeks to outline the neural circuitry that underlies decision-making and moral judgments, and involves the analysis of unconscious component that support human behaviour
About the Author

Alfredo Pereira Júnior – Adjunct Professor of Philosophy of Science at the São Paulo State University (UNESP) – Undergraduate Courses on Philosophy and Administration – Master (1986) and PHD (1994) in Philosophy of Science – Post-Doctoral Fellow at the Dept. of Brain and Cognitive Sciences, Massachusetts Institute of Technology (1996 - 1998) – Researcher granted by the Brazilian National Research Council (CNPQ) since 2001, Project on the Science of Human Consciousness.
About the Author

Claudia Carrara-Augustenborg has a Master in Clinical and Neuropsychology, and she is currently completing her Ph.D. in Cognitive Neuroscience at the Dept. of Psychology of the University of Copenhagen (Denmark). Her research interests lie on the nature and mechanisms of consciousness, and on the functional and neural distinctions between conscious and unconscious processes.
Acknowledgments
CNPQ and FAPESP for supporting grants (A. P. Jr.) and Dr. Ajai Singh for inviting this work.
Footnotes
Conflict of interest: None declared.
Declaration
This is our original, unpublished work, not under consideration for publication elsewhere.
CITATION: Almada LF, Pereira Jr A, Carrara-Augustenborg C. What Affective Neuroscience Means For Science Of Consciousness. Mens Sana Monogr 2013;11:253-73.
Peer reviewers for this paper: William Hirstein PhD.; Anon
aDamasio's model (1994[20]) grants a role of primary relevance to core affect and to feelings in the generation of conscious experience. In Panksepp's terminology, “basic emotional systems” (Panksepp, 1998, p.51[52]) would correspond to systems that generate core affects, and “core emotional feelings” (Panksepp, 2005b[57]) to core affects.
bEvidence suggests that inhibition of physiological responses correlates with damage in the left amygdala, but not in the right region of this structure (Funayama et al., 2001[30]).
cStudies have confirmed for example that the speed of processing of emotional facial expressions is faster than other features of the stimulus (Halgren et al., 2000[38]; Kawasaki et al., 2001[43]).
dDamasio's model of time-locked multiregional retroactivation (1989[21]) can well represent the way in which different processes can ally to produce integrated representations.
eSee Carrara-Augustenborg (2012[16]) for an in depth discussion regarding the distinction between consciousness, unconsciousness and awareness.
fBy concept is intended an information-cluster which we have learned to explicitly recognise, categorise and eventually describe.
gThe role of emotions is the development of Theory of Mind is also supported by evidence that individuals affected by autism present impairments in experiencing fear-learned startle responses (Wilbarger et al. 2009[66]).
hIn anatomical terms, this interaction is mediated by signals from ventral to dorsal region of the central nucleus of the amygdala which in turn modulates attention (De Gelder, 2005[24]).
References
- 1.Adolph R. Recognizing emotions from facial expressions: Psychological and neurological mechanisms. Behav Cog Neurosci Rev. 2002;1:21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- 2.Anderson AK. Affective influences on the attentional dynamics supporting awareness. J Exp Psychol Gen. 2005;134:258–81. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- 3.Anderson AK, Phelps EA. Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J Cogn Neurosci. 2002;14:709–20. doi: 10.1162/08989290260138618. [DOI] [PubMed] [Google Scholar]
- 4.Augustenborg CC. The endogenous feedback network: A new approach to the comprehensive study of consciousness. Conscious Cogn. 2010;19:547–79. doi: 10.1016/j.concog.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Baars B. A Cognitive Theory of Consciousness. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- 6.Baars B. Lecture 10. Consciousness and Emotion, Consciousness: the Webcourse: 2008. [Last accessed on 2011 Jan 10]. Available from: http://bernardbaars.pbworks.com/f/Lect10_Emotions+.pdf .
- 7.Barrett LF. Feeling is perceiving: Core affect and conceptualization in the experience of emotion. In: Barrett L, Niedenthal P, Winkielman P, editors. Emotion and Consciousness. New York: The Guilford Press; 2005. pp. 255–84. [Google Scholar]
- 8.Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Bechara A, Damasio H, Damasio AR. Emotion, decision-making, and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 10.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–8. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 11.Beer JS, John OP, Scabini D, Knight RD. Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006;18:871–9. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- 12.Berridge KC, Winkielman P. What is unconscious emotion.(The case for unconscious ‘Liking’) Cogn Emot. 2003;17:181–211. doi: 10.1080/02699930302289. [DOI] [PubMed] [Google Scholar]
- 13.Bolte A, Goschke T, Kuhl J. Emotion and intuition: Effects of positive and negative mood on implicit judgments of semantic coherence. Psychol Sci. 2003;14:416–21. doi: 10.1111/1467-9280.01456. [DOI] [PubMed] [Google Scholar]
- 14.Braver TS, Cohen JD, Barch DM. The role of the prefrontal cortex in normal and disordered cognitive control: A cognitive neuroscience perspective. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford UK: Oxford University Press; 2002. pp. 428–48. [Google Scholar]
- 15.Burns K, Bechara A. Decision making and free will: A neuroscience perspective. Behav Sci Law. 2007;25:263–80. doi: 10.1002/bsl.751. [DOI] [PubMed] [Google Scholar]
- 16.Carrara-Augustenborg C. Consciousness extended: Bridging informational broadcast and perceptual awareness within a comprehensive conceptualization of consciousness. In: Cavanna AE, Nani A, editors. Consciousness: States, Mechanisms and Disorders. Hauppauge, N.Y: Nova Science; 2012. [Google Scholar]
- 17.Carrara-Augustenborg C, Pereira A., Jr . Brain endogenous feedback and degrees of consciousness. In: Cavanna AE, Nani A, editors. Consciousness: States, Mechanisms and Disorders. Hauppauge, N.Y: Nova Science; 2012. [Google Scholar]
- 18.Dalgleish T. The emotional brain. Nat Rev Neurosci. 2004;5:582–5. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- 19.Darwin C. The Expression of Emotions in Man and Animals. New York: Oxford University Press; 1872/1998. [Google Scholar]
- 20.Damasio A. Descartes’ Error: Emotion, Reason, and the Human Brain. New York: Putnam's Sons; 1994. [Google Scholar]
- 21.Damasio A. Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- 22.Damasio AR. A second chance for emotions. In: Lane R, Nadel L, editors. Cognitive Neuroscience for Emotion. New York: Oxford Books; 2000. pp. 12–23. [Google Scholar]
- 23.Davidson RJ, Sutton SK. Affective neuroscience: The emergence of a discipline. Curr Opin Neurobiol. 1995;5:217–24. doi: 10.1016/0959-4388(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 24.De Gelder B. Nonconscious emotions: New findings and perspectives on nonconscious facial expression recognition and its voice and whole-body contexts. In: Barrett L, Niedenthal P, Winkielman P, editors. Emotion and Consciousness. New York: The Guilford Press; 2005. pp. 123–49. [Google Scholar]
- 25.Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 26.Dubois DM. Breakthrough in the human decision making based on an unconscious origin of free will. Acta Syst. 2010;10:13–8. [Google Scholar]
- 27.Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- 28.Flykt A, Esteves F, Ohman A. Skin conductance responses to masked conditioned stimuli: Phylogenetic/ontogenetic factors versus direction of threat? Biol Psychol. 2007;74:328–36. doi: 10.1016/j.biopsycho.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Freud S. Collected papers. London: Hogarth Press and The Institute of Psychoanalysis; 1950. [Google Scholar]
- 30.Funayama ES, Grillon CG, Davis M, Phelps EA. A Double dissociation in the affective modulation of startle in humans: Effects of unilateral temporal lobectomy. J Cogn Neurosci. 2001;13:721–9. doi: 10.1162/08989290152541395. [DOI] [PubMed] [Google Scholar]
- 31.Gardner H. The Mind's New Science: A History of the Cognitive Revolution. New York: Basic Books; 1987. [Google Scholar]
- 32.Grabowska A, Marchewka A, Seniów J, Polanowska K, Jednoróg K, Królicki L, et al. Emotionally negative stimuli can overcome attentional deficits in patients with visuo-spatial hemineglect. Neuropsychologia. 2011;49:3327–37. doi: 10.1016/j.neuropsychologia.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Grandjean D, Sander D, Lucas N, Scherer KR, Vuilleumier P. Effects of emotional prosody on auditory extinction for voices in patients with spatial neglect. Neuropsychologia. 2008;46:487–96. doi: 10.1016/j.neuropsychologia.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Gray JR. Emotional modulation of cognitive control: Approach-withdrawal states double-dissociate spatial from verbal two-back task performance. J Exp Psychol Gen. 2001;130:436–52. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- 35.Gray JR. Integration of Emotion and Cognitive Control. Curr Dir Psychol Sci. 2004;13:46–8. [Google Scholar]
- 36.Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. P Natl Acad Sci USA. 2002;99:4115–20. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray JR, Schaefer A, Braver TS, Most SB. Affect and the resolution of cognitive control dilemmas. In: Barrett L, Niedenthal P, Winkielman P, editors. Emotion and Consciousness. New York: The Guilford Press; 2005. pp. 67–94. [Google Scholar]
- 38.Halgren E, Raij T, Marinkovic K, Jousmäki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cereb Cortex. 2000;10:69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- 39.Hamm AO, Weike AI, Schupp HT, Treig T, Dressel A, Kessler C. Affective blindsight: Intact fear conditioning to a visual cue in a cortically blind patient. Brain. 2003;126:267–75. doi: 10.1093/brain/awg037. [DOI] [PubMed] [Google Scholar]
- 40.James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- 41.Kapp BS, Whalen PJ, Supple W, Pascoe JP. Amygdaloid contributions to conditioned arousal and sensory information processing.In: , editor. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley; 1992. pp. 229–54. [Google Scholar]
- 42.Kapp BS, Cain ME. The neural basis of arousal. In: Smelser NJ, Battes PB, editors. International encyclopedia of the social and behavioral sciences. New York: Elsevier; 2001. pp. 754–8. [Google Scholar]
- 43.Kawasaki H, Kaufman O, Damasio H, Damasio AR, Granner M, Bakken H, et al. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nat Neurosci. 2001;4:15–6. doi: 10.1038/82850. [DOI] [PubMed] [Google Scholar]
- 44.LeDoux J.E. The Emotional Brain. New York: Simon and Schuster Paperbacks; 1996. [Google Scholar]
- 45.LeDoux JE. Fear and the brain: Where have we been, and where are we going? Biol Psychiatr. 1998;44:1229–38. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- 46.LeDoux JE. Psychoanalytic theory: Clues from the Brain. Neuro-Psychoanalysis. 1999;1:44–9. [Google Scholar]
- 47.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 48.McGaugh JL. Memory: A century of consolidation. Science. 2000;287:248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 49.Ohman A, Soares JJ. “Unconscious Anxiety”: Phobic Responses to Masked Stimuli. J Abnorm Psychol. 1994;103:231–40. doi: 10.1037//0021-843x.103.2.231. [DOI] [PubMed] [Google Scholar]
- 50.Ohman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. J Exp Psychol Gen. 2001;130:466–78. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- 51.Panksepp J. Toward a general psychobiological theory of emotions. Behav Brain Sci. 1982;5:407–67. [Google Scholar]
- 52.Panksepp J. Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- 53.Panksepp J. The neuro-evolutionary cusp between emotions and cognitions: Implications for understanding consciousness and the emergence of a unified mind science. Evol Cogn. 2001;7:141–63. [Google Scholar]
- 54.Panksepp J. At the interface of affective, behavioral and cognitive neurosciences: Decoding the emotional feelings of the brain. Brain Cogn. 2003;52:4–14. doi: 10.1016/s0278-2626(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 55.Panksepp J. Affective consciousness and the origins of human mind: A critical role of brain research on animal emotions. Impuls. 2004;3:47–60. [Google Scholar]
- 56.Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Conscious Cogn. 2005a;14:30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Panksepp J. On the embodied neural nature of core emotional affects. J Conscious Stud. 2005b;12:158–84. [Google Scholar]
- 58.Pereira A, Jr, Almada LF. Conceptual spaces and consciousness: Integrating cognitive and affective processes. Int J Mac Cons. 2011;3:127–43. [Google Scholar]
- 59.Pereira A, Jr, Furlan FA. Astrocytes and human cognition: Modeling information integration and modulation of neuronal activity. Prog Neurobiol. 2010;92:405–20. doi: 10.1016/j.pneurobio.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Pereira A, Jr, Furlan FA, Pereira MAO. Recent Advances in Brain Physiology and Cognitive Processing. Mens Sana Monogr. 2011;9:183–92. doi: 10.4103/0973-1229.77434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phelps EA. Emotions. In: Gazzaniga M.S., Ivry R.B., Mangun G.R., editors. Cognitive Neuroscience: The biology of the mind. New York: Norton; 2002. pp. 537–76. [Google Scholar]
- 62.Prinz JJ. Emotions, embodiment and awareness. In: Barrett L, Niedenthal P, Winkielman P, editors. Emotion and Consciousness. New York: The Guilford Press; 2005. pp. 363–83. [Google Scholar]
- 63.O’Doherty ML, Kringelbach ET, Rolls J, Hornak, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neuros. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 64.Russell JA. Core affect and the psychological construction of emotion. Psychol Rev. 2003;110:145–72. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- 65.Russell JA. Emotion, core affect, and psychological construction. Cogn Emot. 2009;23:1259–83. [Google Scholar]
- 66.Schwabe L, Christian J, Merz CJ, Bertram Walter B, Dieter Vaitl D, Oliver T, et al. Emotional modulation of the attentional blink: The neural structures involved in capturing and holding attention. Neuropsychologia. 2011;49:416–25. doi: 10.1016/j.neuropsychologia.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 67.Stuss D, Benson F. The Frontal Lobes. New York: Raven Press; 1986. [Google Scholar]
- 68.Van Stegeren AH. The role of the noradrenergic system in emotional memory. Acta Psychol. 2008;127:532–41. doi: 10.1016/j.actpsy.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–8. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]


