Abstract
Psychiatric polypharmacy refers to the prescription of two or more psychiatric medications concurrently to a patient. It can be categorised as same-class, multi-class, adjunctive, augmentation and total polypharmacy. Despite advances in psychopharmacology and a better understanding of the principles of therapeutics, its practice is increasing rapidly. The prevalence of polypharmacy in psychiatry varies between 13%-90%. There are various clinical and pharmaco-economic factors associated with it. Dealing with polypharmacy requires an understanding of its associated factors. Education, guidelines and algorithms for the appropriate management of various conditions are effective ways to avoid irrational polypharmacy.
Keywords: Drug combinations, Multiple medications, Polypharmacy, Psychopharmacology
Introduction
The field of clinical psychiatry is challenging and physicians have been exploring methods to deal with complex situations. Some of these conditions present with life-threatening concerns, while others present with patients becoming unresponsive and resistant to treatment. Polypharmacy has been one method which physicians have been using in difficult circumstances since a long time; however, its appropriateness has of–late come under scrutiny.
Polypharmacy is the use of multiple medications for the treatment of a patient's medical condition. The basis for this definition is solely quantitative and does not take into account the clinical pertinence of the use of these medications (for example, the presence of multiple diseases) or the adequacy of the proposed therapeutic regimen (Rollason and Vogt, 2003[41]). The term polypharmacy suggests that more medication is being used than is ‘clinically indicated.’ The number of medications that constitute polypharmacy however, has not been defined in the available literature (Hidalgo et al., 1997[24]; Jörgensen et al., 2001[17]; Linjakumpu et al., 2002[23]).
The commonly used definition of psychiatric polypharmacy is the use of two or more psychiatric medications in the same patient, (NASMHPD Technical Report, 2001[32]) or using two or more medications (of the same chemical class or same pharmacologic actions) to treat the same condition (Kingsbury et al., 2001[20]).
Types of Polypharmacy
Due to the increasing prevalence and complexity in psychiatric polypharmacy, it is categorised as follows by the National Association of State Mental Health Programme Directors (NASMHPD) (NASMHPD Technical Report, 2001[32]):
Same-Class Polypharmacy refers to the use of more than one medication from the same class (e.g. use of two selective serotonin reuptake inhibitors in a case of depression).
Multi-Class Polypharmacy is the use of full therapeutic doses of more than one medication from different classes for the same symptom cluster (e.g. use of valproate along with an atypical antipsychotic, such as olanzapine, for treatment of mania).
Use of one medication to treat the side effects of another medication from a different class, is described as Adjunctive Polypharmacy (e.g. using trazodone for insomnia caused by bupropion).
Augmentation Polypharmacy refers to the use of one medication at a lower than normal dose along with another medication from a different class in full therapeutic dose for the same symptom cluster (e.g. addition of low dose haloperidol in a patient responding partially to risperidone); or the addition of a medication that would not be used alone for the same symptom cluster (e.g. augmentation of antidepressants with lithium or thyroid hormone).
Total Polypharmacy is the total count of medications used in a patient, or total drug load.
Epidemiology
Polypharmacy has become a common clinical practice for many psychiatric conditions (Ghaemi, 2002[13]). Up to one-third patients visiting outpatient psychiatry department have been found to be on three or more psychotropic drugs (Mojtabai and Olfson, 2010[29]). Rittmannsberger (2002[40]) reviewed available literature on the number of psychotropic drugs administered during inpatient treatment and reported a significant decline in patients being treated with monotherapy and increase in those being treated with polypharmacy during the last few decades. Studies originating before 1980 reported monotherapy in 48% patients, studies between 1981-1990 in 31%, and studies between 1991-2000 in 20% patients (Rittmannsberger, 2002[40]). The reported overall prevalence rates of polypharmacy in psychiatry vary between 13%-90% with a continuing debate about its merits and demerits (David, 200 2[7]; De las Cuevas and Emilio, 2004[8]; Stahl, 2002a[48]). A study from NIMH shows that prescription of 3 or more medication at discharge increased from 5% in 1974 to 40% in 1995 (Presborn and Flockhart, 2006[36]). Even evaluation of baseline medication data of schizophrenia patients in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial revealed that schizophrenia patients were being given poly-pharmacotherapy. Around 6% patients were taking two antipsychotics, 38% antidepressants; 22% anxiolytics; 4% lithium; and 15% other mood stabilizers (Chakos et al., 2006[4]).
De las Cuevas andand Sanz ( 2004[8]) conducted a cross-sectional survey of patients (n = 2,647) with mental disorders receiving psychotropic medication. They found that psychiatric polypharmacy is more prevalent in adult men than in women, and those between 25 and 45 years of age. Psychiatric polypharmacy is not only widespread in adult population, but is also increasingly been seen in child and adolescent population and the geriatric age group. In their nationally representative sample of 3,466 children and adolescents, Comer et al., (2010[6]) reported the prevalence of multi-class psychotropic treatment to be 19% in this population. Antidepressants were the most common co-prescribed medication class in multi-class visits followed by ADHD medications, antipsychotics, mood stabilisers, and sedative-hypnotics. The percentage of pair-wise multi-class medication combinations ranged from ADHD medications and antidepressants (7.3% of psychotropic visits) to antipsychotics and sedative-hypnotics (0.6%). Loyola et al., (2008[25]) studied a cohort of elderly people in Bambuν city to evaluate the prevalence of polypharmacy and the influence of income on the association between medication use and cognitive impairment among elderly people. They found that within the geriatric age group (age > 60 years), 44.8% of the 1,554 elderly Bambuν cohort were consuming 2-4 medications and 25.5% were consuming five or more medications.
When one looks at polypharmacy in various psychiatric diagnoses, it is seen that polypharmacy is more common in patients who receive ICD-10 diagnosis of schizophrenia, schizotypal and delusional disorders (De las Cuevas and Sanz, 2004[5]). It is also worth noting that psychiatric patients with better neuro-cognitive functioning are usually less likely to be taking several concomitant psychotropic medications (Chakos et al., 2006[4]).
Multi-Class Polypharmacy is the most prevalent type of polypharmacy found in 20.9% of patients. In Multi-Class Polypharmacy, the combination of SSRI with a benzodiazepine is the most common, followed by combination of a tricyclic antidepressant and a benzodiazepine. In Same-Class polypharmacy, treatment with several benzodiazepines is the most common (De las Cuevas and Sanz, 2004[8]).
Within the Indian context polypharmacy is common (Trivedi et al., 2010[54]). In a one year evaluation (January 2003 to December 2003) of discharge prescriptions of all patients of schizophrenia from St. Johns Medical College, Bangalore, Padmini et al., (2007)[35] reported polypharmacy in 9% of cases. While another 6-monthly prescription evaluation done a year later (2004) by Sawhney et al., (2004)[43] at another end of the country (Jammu) revealed a high percentage of cases (72.72%). Ramadas et al., (2010)[39] reviewed case charts of 656 psychiatric inpatients at a tertiary care centre in Kerala in order to audit prescription pattern of antipsychotic drugs in patients with schizophrenia and found that all patients who were on typical antipsychotics were receiving more than one antipsychotic, while those who were on atypical antipsychotic, only 3% were on combination of medications. However, the total number of patients with schizophrenia in their sample was small (around 29). Even a recent cross-sectional study on a cohort from a long-term outcome study by Shrivastava et al., (2012)[46], found that a large percentage of schizophrenia patients (30.1%) were taking more than one second-generation antipsychotic.
Factors in polypharmacy
The primary reason a person receives polypharmacy is because clinical staff determines that administration of a single medication is ineffective in adequately treating the individual's psychiatric symptoms (NASMHPD Technical Report, 2001[32]; Procyshyn et al., 2001[38]). Other reasons for prescribing more than one medication are to target specific symptoms, to treat two distinct but co-morbid illnesses in one patient, to address unremitting symptoms, and to treat extrapyramidal effects produced by a primary drug (Preskorn, 2007[37]). An increase in psychiatric outpatient polypharmacy over a ten-year period (Mojtabai and Olfson, 2010[29]), geographic differences in psychotropic prescribing (Baandrup et al., 2010[2]) and differences in prescribing practices between psychiatrists in the same setting (Owen et al., 2003[34]) all suggest a complex response to refractoriness or perceived insufficient responses to a single psychiatric medication. Using a survey study, Sernyak and Rosenheck (2004[44]) identified clinician's reasons for co-prescribing antipsychotics: 65% cited refractory illness, and 39% cited an incomplete switch attempt. Greater patient, parent, or physician emphasis on symptom reduction has also contributed to polypharmacy (Stahl, 2008[50]). In addition to this, an increasing number of psychiatrists are specialising in pharmacotherapy, to the exclusion of psychosocial treatments (Mojtabai andand Olfson, 2008[28]), most of which have limited availability placing heavy clinical demands on pharmacological dimensions of mental health care.
Ghaemi (2002[13]) described five factors associated with the rise of polypharmacy: scientific, clinical, economic, political, and cultural.
Scientific factor emphasised that research on biogenic amines conducted in depression and schizophrenia expanded on early clinical evidence on psychotropic medications, which contributed to polypharmacy.
According to the clinical factor, the advent of medications influenced the move to standardise diagnostic criteria in an “a-theoretical” way in DSM-III. This has resulted in many diagnoses with extensive overlap, excellent reliability, but limited validity. To some extent, poly-nosology and polypharmacy go together.
The economic factor was important as the pharmaceutical industry produced and marketed medications and influenced their demand significantly.
Political factor was evident by the US Food and Drug Administration (US FDA) imposing certain minimal guidelines for drug approval that became viewed as scientific facts, rather than political rules. One of these was that drugs should be indicated only for specific conditions designated by the US FDA.
The cultural factor specifies Americans having a large appetite for pharmacological treatments, dating back to the nineteenth century, which contributed to increased polypharmacy in psychiatry elsewhere.
Freudenreich et al., (2012)[12] described four aetiological factors that can lead to polypharmacy:
Disease factors or Biological factors include refractory disease, suboptimal treatment, side-effect management, misdiagnoses and missed diagnoses. Often some patients are started on medications whose doses are not optimised in time. This may lead to insufficient response, after which more medications may be added without a consideration for optimising their doses. Freudenreich et al., (2012)[12] also warn that taking a checklist approach to psychiatry biases the diagnostic process in favour of some diagnosis which may actually not be present. This leads to administration of medications to an individual who may not require them.
Patient factors (Psychological factor I) are insufficient compliance, personality type, consumer-choice paradigm, illness behaviour etc. Non-compliance and insufficient compliance by patients is a very important factor in polypharmacy. Often some patients also seem to self-medicate themselves.
Physician factors (Psychological factor II) include pharmacological hedonism (viewing drugs as a primary solution to life's problems) or Calvinism (viewing drugs as not so-important compared to other therapeutic methods), early or late adopter, symptom-based prescribing, self-image as powerful healer, fear of patient dissatisfaction. Many psychiatrists look at symptoms rather than a disorder. For instance, in case of depression with insomnia, one can use a sedating antidepressant, but some psychiatrists would use an antidepressant along with a benzodiazepine, resulting in two medications being prescribed.
The Systems (Sociological factor) include market-based system with consumer choice, fragmented health care system, and outside pressures (other stakeholders). Well-informed consumers often have greater demands and higher expectations regarding their treatments. This may put pressure on the treating psychiatrist who may then start the patient on multiple medications for quick improvement. In a country like India where the patient load is high, psychiatrists often have to compromise on the time spent on each patient. This reduces possibilities of providing non-pharmacological therapies which are more time-consuming. Consequently the treating psychiatrist often resorts to quick-fixes to the patient's complaints leading to increased reliance on polypharmacy.
These aetiological factors bear some resemblance to Ghaemi's five factors. However, the role played by physicians and patients, both individually and in tandem, has been emphasised in these factors as each can contribute significantly to polypharmacy.
Merits
In a number of situations, particularly in presence of psychiatric and physical comorbidities, use of polypharmacy and/ or co-prescribing, results in better perceived outcomes. Although, currently it is difficult and academically premature to pass a judgement about merits of polypharmacy, many patients who were previously unresponsive to a single medication often improve after taking multiple medications (Niculescu and Hulvershorn, 2010[33]). This seems to be a mixed blessing. The degree of risk and benefit associated with polypharmacy varies depending on the medications used and the characteristics of the patient [Table 1].
Table 1.
Merits and demerits of psychiatric polypharmacy
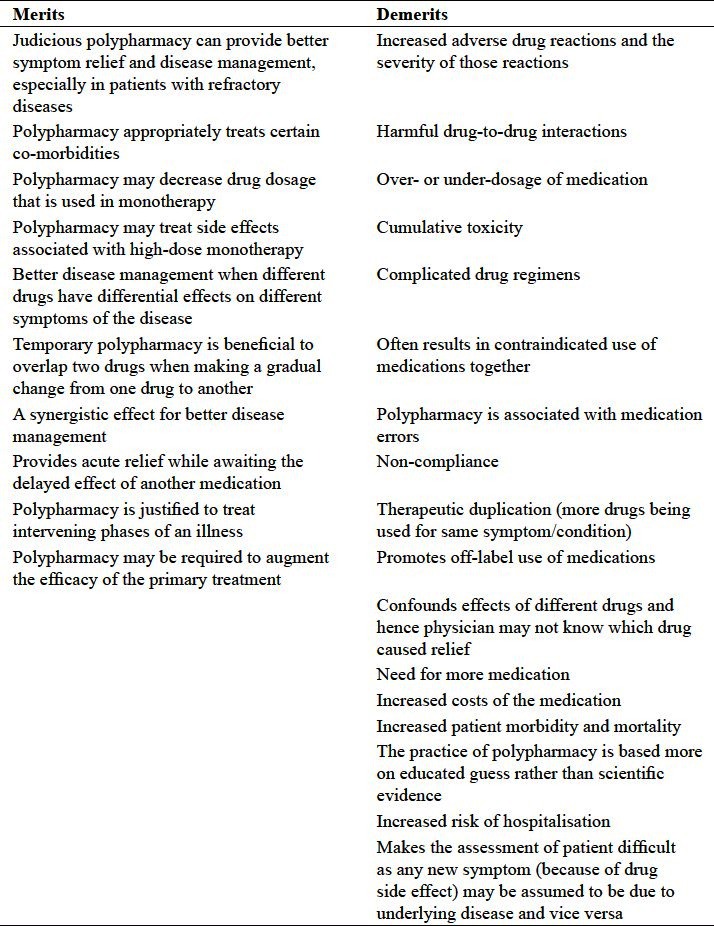
To define what constitutes an adequate psychotropic drug prescription is a complex task, as pharmacological, clinical, social and economic factors influence both the adequacy and rationality of prescriptions. There are clearly times when polypharmacy is necessary, particularly when there are co-morbidities requiring more than one class of medication, or when monotherapy provides insufficient improvement. It is not appropriate to always view polypharmacy as a poor prescribing pattern, because using more than one psychotropic drug can be effective in some patients. For instance, different antipsychotics have differential effects on different symptoms of psychosis (Taylor, 2002[42]). The Royal College of Psychiatrists’ consensus statement in the UK justifies some cases of temporary polypharmacy, including making a gradual change from one drug to another (Royal College of Psychiatrists, 1993[35]). However, some patients get caught in the “cross-over trap.” Eighty percent of patients switching from a typical to an atypical antipsychotic medication became “caught” in a study by Tapp et al., 2003[51]) . Although the intention was to completely switch the patient to the second medication, the patient's level of improvement while on both medications made the patient and/or physician unwilling to discontinue the first conventional medication (Tapp et al., 2003[51]).
The goal is not to simply avoid polypharmacy, but to practice rational instead of indiscriminate polypharmacy. For example, although evidence for the efficacy of combining antipsychotics is limited (Englisch and Zink, 2012[9]), sulpiride augmentation of clozapine has shown beneficial effects clinically (Shiloh et al., 1997[45]). Preskorn andand Lacey (2007)[37] described the conditions under which clinicians may justifiably use polypharmacy (‘rational polypharmacy’)-
To treat two patho-physiologically distinct but co-morbid illnesses in the same patient in contradistinction to treating the same condition or two ‘co-morbid’ syndromes in the same patient. For instance, in patients having epilepsy and psychosis, the patient would require an antiepileptic drug for control of seizure disorder and an antipsychotic medication for control of psychosis.
To treat an adverse effect produced by the primary drug. For example, for antipsychotic medication induced extrapyramidal reaction or akathisia, trihexyphenidyl or propranolol is added to the existing antipsychotic medications.
To provide acute amelioration while awaiting the delayed effect of another medication. Thus one may add hypnotics like zolpidem or lorazepam for immediate symptomatic relief of insomnia, while waiting for antidepressant drugs to act.
To treat intervening phases of an illness. A substantial number of patients of schizophrenia develop depressive symptoms at some point of time during the course of their illness (post-psychotic depression). One may have to add an antidepressant to the existing regimen of antipsychotic medication in such cases.
To boost or augment the efficacy of primary treatment. A number of agents such as lithium, thyroxin, modafinil, l-methyl folate etc. are used to augment anti-depressant therapy when patient does not show adequate response.
However, these are also the same factors where practice of polypharmacy gives rise to adverse effects. It is important to note that demerits of polypharmacy are not contained in ‘where’ the drugs are being used but in ‘how’ these drugs are being used. Therefore a rational and optimised usage of multiple medications together is likely to offer better outcome of pharmacotherapy.
However, it is worth noting that rational polypharmacy is not a blanket sanction for using multiple medications. This refers to the conditions (mentioned above) wherein use of multiple medications may not necessarily be an improper prescribing pattern.
Demerits
While evidence for the added benefit of psychiatric polypharmacy is limited, there is growing evidence regarding the increased adverse effects associated with such combinations. Concerns with polypharmacy include not only possibilities of cumulative toxicity and increased vulnerability to adverse events (Stahl, 2008[50]) but also adherence issues which emerge with increasing regimen complexity (Murray and Kroenke, 2001[30]). Combinations of drugs may lead to various pharmaco-dynamic and pharmaco-kinetic interactions. Presence of one drug alters the nature, magnitude, and/or duration of the effect of another drug. One drug may affect another drug's absorption, distribution within the body, or its metabolism or excretion thereby changing the blood levels of other drugs (Preskorn, 2007[37]). For example, combining valproate and carbamazepine (CBZ) may lead to neurotoxicity by CBZ since valproate increases CBZ levels. One example of cumulative toxicity is when mood-stabilising drugs - carbamazepine and valproate, are taken together; valproate blocks the hydrolysis of CBZ 10, 11-epoxide by inhibiting epoxide hydrolase, so that the ratio of carbamazepine to CBZ 10, 11-epoxide becomes 2:1. Higher concentrations of the epoxide metabolite contribute to neurotoxicity (Stahl, 2008[50]). Examples of adverse drug-drug events in polypharmacy include - venlafaxine and atenolol in treating a patient with depression and hypertension (the two drugs have opposing mechanism of action). Another example is the occurrence of orthostatic hypotension and syncope when risperidone (because of its action as an alpha-1 adrenergic blocker) is added to a diuretic. Similarly, typical neuroleptics may reduce mood stabilisation (Stahl, 2008[50]). This is of particular importance when using antipsychotics in the treatment of persons with bipolar disorder.
Other serious concerns with polypharmacy are lack of evidence-based strategies to guide this practice (NASMHPD Technical Report, 2001[32]), and drug costs for patients (Stahl, 2002b[49]). There have been only few randomised, controlled, scientific studies evaluating the effectiveness, risks and long term effects of using two psychiatric drugs in combination (Campos et al., 2010[3]; Muscatello et al., 2011[31]). Polypharmacy is strongly associated with excessive dosing and early death (Ito et al., 2005[16]). Use of thioridazine is a predictor of QTc prolongation, and even moderate doses of antipsychotics would increase the risk of sudden cardiac death (Wang et al., 2012[55]). Antipsychotic polypharmacy, compared with monotherapy, is found to be independently associated with an increased risk of having pre-metabolic syndrome, even after adjusting for patients’ lifestyle characteristics (Misawa et al., 2011[27]).
Polypharmacy often becomes a cycle of treating one condition, experiencing side effects, and treating the side effects, until the patient and the clinician cannot remember where the cycle began.
The most important is to understand merits verses demerits of polypharmacy, but unfortunately, this has not been scientifically studied. The core issue in relevance of polypharmacy, if any, is whether or not it affects the outcome of psychiatric illnesses in absolute or relative terms. Unequivocal evidence of its merits and demerits is urgently needed.
Dealing with polypharmacy
Dealing with polypharmacy is complex, and ‘art of prescribing’ plays the most important role. There is no single answer to this clinical challenge but combination of methods of prescribing can minimise polypharmacy. Specific measures are targeted at individual patients, their diagnosis and the therapeutic milieu. Some specific methods include dealing with compliance, a dialogue with pharmacists and psycho-education for the patient and family. Besides specific measures, there are some general measures which are quite helpful (Spinewine et al., 2007[47]; Anthierens et al., 2010[1]). Though little evidence exits, we believe that implementing advanced measures of psycho-education, psycho-rehabilitation programmes and ‘standard of care’, shall improve compliance and lead to better evidence based pharmacotherapy. Periodic review of pharmacotherapy, ‘patient engagement programmes’, and assessment of physical health for early identification of adverse effects are newer measures which are required to be scientifically evaluated (Ketter, 2010[19]).
Evidence-based treatment and continued physician's education go a long way in optimising usage of medications. Recently developed concept of ‘personalised medicine’ holds the key for prescribing patterns for the future. This model recommends customised health care with decisions and practices tailored to individual patients based upon genetic findings. Such methods might be used for assessing patient's risk factor and offer a preventive approach (Evers, 2009[10]).
Lee described SAIL protocol in the management of polypharmacy (Lee, 1998[22]) whereby physicians focus on Simple drug regimens, know various Adverse effects of drugs, use drugs with clear Indications, and keep a precise List of all medications with the patient to appropriately manage a patient's drug regimen. Simplicity involves keeping the drug regimen as simple as possible with once or twice daily dosing. Any drug not achieving its defined therapeutic goal is discontinued. One should treat multiple symptoms using a single drug with multiple effects, rather than treating each symptom individually. One should be aware of adverse effects of each drug including drug-drug interactions. Whenever practical, drugs with broad rather than narrow therapeutic indices should be prescribed. Each prescribed drug should have a clear Indication and a well-defined therapeutic goal both of which should be evidence-based. It is also wise to List names and dosages of each drug in the patient's chart, and he is also informed about the same. Use of computers in health service administration has been successfully used. Computer data entry and feedback procedures, which have been shown to decrease polypharmacy and drug-drug interactions, should be considered (Lapane et al., 2008[21]).
Werder and Preskorn (2007[56]) described the TIDE approach to be used with SAIL protocol to avoid polypharmacy's negative consequences. TIDE means allow Time to address medication issues. The understanding of Individual variability, pharmacokinetics, and pharmacodynamics should be applied while prescribing. Potentially dangerous Drug-drug interactions should be carefully avoided. Patients should be educated regarding potential adverse effects of each drug such as extra-pyramidal reactions, tardive dyskinesia, metabolic syndrome, weight gain etc.
Niculescu and Hulvershorn (2010[33]) suggested a tri-dimensional approach towards early, personalised and rational psychiatric polypharmacy. Personalised tri-dimensional treatment approach involves concurrent treatment of anxiety, mood, and cognitive abnormalities plus modulation of environmental factors (e.g., stress). One medication per dimension (each acting primarily on anxiety, mood, or cognition, respectively) is desirable although not always achievable. Depending on the major pathology, one of these medications is used at a higher dose and the others at lower doses. For example, for major mood abnormalities such as bipolar disorder, a mood stabilizer at a higher dose would be the primary approach and an anxiolytic and antipsychotic secondary at lower doses. If more than one medication is used for optimal effects in one dimension, complementary medications, rather than redundant similar ones, should be used. For example in treating the cognitive dimension in schizophrenia, lower doses of both a strong dopaminergic-blocking antipsychotic (with primarily extrapyramidal side effects, for example, haloperidol) and a broader-spectrum antipsychotic (with primarily metabolic side effects, for example olanzapine) could be used to maximize benefits and minimize adverse effects.
Polypharmacy in psychiatry can be safely reduced with proper clinical titration, aided by guidelines and protocols (Goh et al., 2011[14]). Chong et al., (2006[5]) implemented a treatment algorithm for patients accepted into an Early Psychosis Intervention Programme (EPIP- 483 subjects) and compared their prescription patterns with a comparator group (pre-EPIP- 68 subjects) before the use of the algorithm. They found a significant reduction in the rate of antipsychotic polypharmacy, prolonged use of benzodiazepines and anticholinergic medication in EPIP patients. Thus, the implementation of a treatment algorithm coupled with audit can reduce polypharmacy in psychiatric practice.
Education, guidelines and algorithms are thus effective ways to avoid irrational polypharmacy (Thompson et al., 2008[53]). A rational prescribing strategy can thus lead to a decrease in adverse drug reactions and improve patient outcomes (McCue et al., 2003[26]).
Certain psychiatric drug combinations are considered irrational. These include combined use of drugs from the same class to treat the same symptoms such as: Typical antipsychotics (Kingsbury et al., 2001[20]). Other irrational practices include use of more than two antipsychotics, typical or atypical; change in medication dose before serum level has reached a steady state and sufficient time has lapsed for a therapeutic response; and failure to adequately evaluate and monitor patients prescribed a polypharmacy regimen (NASMHPD Technical Report, 2001[32]). Irrational polypharmacy practice has led to the recognition of ‘rational polypharmacy’ and the formulation of principles to regulate its practice (Freudenreich and Goff, 2002[11]; Kennedy and Procyshyn, 2000[18]). This concern has arisen from the growing body of experience which indicates that polypharmacy may be beneficial for a subset of patients who respond poorly to antipsychotic monotherapy.
The theoretical rationale for using combination of psychotropic drugs include boosting the effectiveness of monotherapy, optimising dopamine-2 receptor occupancy in refractory patients, targeting a diverse range of receptors and treating patients with partial, inadequate or no response (Freudenreich and, Goff, 2002[11]; Kennedy and Procyshyn, 2000[18]; Williams and Garner,2002[57]). Polypharmacy in such instances may lead to better symptom relief with minimal side effects. However, much of the evidence supporting psychotropic polypharmacy appears to come from clinical experience, small clinical trials and case reports. There is therefore a need for more systematic research and the drawing up of guidelines for polypharmacy practice (David, 2002[7]). Many have thus cautioned against a culture of ‘perception-based’ clinical practice as against that which is ‘evidence-based’ (David, 2002[7]; Isaacs and Fitzgerald, 1999[15]; NASMHPD Technical Report, 2011[32]).
Concluding Remarks [Figure 1: Flowchart of paper]
Figure 1.

Flow chart of paper
The literature highlights three important issues (1) polypharmacy is widely practiced, (2) its merits and demerits are undetermined (3) there are serious drug-drug interactions and sometimes life-threatening complications. There have been reasonable advances in understanding of polypharmacy as a clinical complexity and a number of criteria have been proposed to define polypharmacy; however, the commonly used definitions of polypharmacy focus on quantitative aspects while ignoring other important aspects. Areas such as biological underpinnings and outcome correlates still remain a challenging research area. Other important factors which lead to its undermined position are lack of clarity in phenomenology and biology of mental disorders. Usage of multiple psychotropic medications remains complex with a potential of clinical threat in presence of unknown individual vulnerability, pharmaco-dynamic variability in trans-cultural and ethnic subgroups. In order to address the issue of polypharmacy in psychiatry, the treating psychiatrists need to keep certain issues in mind including potential adverse effects and drug-drug interactions, and the specific indications for drugs. Sticking to such guidelines, polypharmacy may actually give a better symptom relief and disease management with minimal or no side-effects.
Take Home message
Physicians prescribing psychiatric drugs must be aware of the existence and high prevalence of polypharmacy.
The term polypharmacy suggests that two or more psychiatric medications are being used in the same patient or two or more medications (of the same chemical class or same pharmacologic actions) are being used to treat the same condition.
Polypharmacy may be necessary and justified particularly when there are co-morbidities requiring more than one class of medication, when monotherapy provides insufficient improvement and while making a gradual change from one drug to another.
-
One can deal with polypharmacy with SAIL and TIDE approaches:
SAIL: Keep drug regimen Simple, know drug Adverse effects, prescribed drug should have a clear Indication, keep List of drug name and dosage in patient's chart. TIDE: Allow Time to address medication issues, understand Individual variability, avoid potential dangerous Drug-drug interactions, and Educate patients regarding treatment.
Education, proper clinical titration aided by guidelines and protocols are effective ways to avoid irrational polypharmacy.
Questions that the Paper Raises
Is it wise to keep adding a new drug for every new symptom that the patient complains of, or for every symptom that fails to respond completely with a single drug?
Should a clinician treat the ‘symptom,’ the ‘disorder’ or the ‘patient’?
Should one wait for results from more systematic reviews and meta-analyses or rely on one's gut-feeling or clinical experience in psychopharmacological treatment?
In a bid to give the patient quick relief, are we as psychiatrists becoming pharmaco-psychiatrists and losing out on psychotherapeutic-psychiatry?
About the Author

Sanjay Kukreja M.B.B.S., F.C.L.R. is a Research Associate at Lokmanya Tilak Municipal Medical College and Sion General Hospital, Mumbai, India. His clinical and lab research has focussed on neuronal regeneration, cell therapy and biology of neurospinal and psychiatric disorders. His work has been published in international journals. He is experienced in research methodology and has designed and analysed many clinical trials. His areas of research interest include quality improvement, translational research and understanding the pathophysiology to find novel strategies of cure for incurable diseases. He has been the editorial coordinator and has chapter contributions in many books.
About the Author

Having completed his M.D. in psychiatry from Maharashtra University of Health Sciences (MUHS), Gurvinder Kalra, M.D., D.P.M. is now Assistant Professor of Psychiatry at M.G.M University of Health Sciences in New Mumbai, India. His main areas of interest are mood disorders, psychosexual disorders, alternate sexualities, and cinemeducation. With a keen interest in teaching, he makes use of cinema extensively in his teaching modules. He has presented and published extensively on the use of cinema in teaching. Some of his much-appreciated work in cinemeducation is on psychiatry movie clubs and portrayal of hijras in Bollywood cinema. He was awarded the Asian Young Psychiatrist Award (AYPA) at the 3rd World Congress of Asian Psychiatry at Melbourne in 2011 as an emerging early career psychiatrist from India
About the Author

Nilesh Shah, M.D., D.N.B., D.P.M is currently Professor and Head of department of Psychiatry at Lokmanya Tilak Medical College and Sion General Hospital in Mumbai, India. He has more than 25 years of experience in the field as a clinician and a teacher. He has more than 90 publications in various national/international journals and has presented at more than 150 conferences, seminars and symposiums. His areas of interest include pharmacotherapy, electroconvulsive therapy, consultation-liaison therapy and dementia. He also sits on the editorial board of Indian Journal of Psychiatry
About the Author

Amresh Shrivastava, MD, MRCPsych, FRCPC, trained at KEM hospital in Mumbai, India. Currently he is the Physician Lead, Early Intervention Programme, Regional Mental Health Care, St. Thomas; Associate Scientist, Lawson Health Research Institute, and Associate Professor of Psychiatry, The University of Western Ontario; London, Ontario, Canada. He works in the field of International mental health. He is Secretary of the psychoendocrinology section of the World Psychiatric Association. Dr. Shrivastava's teaching and research interest are related to suicide behaviour, schizophrenia, particularly early psychosis, outcome measures and risk assessment. He is also editor of recently published handbook ‘Suicide from Global Perspective’
Footnotes
Conflict of interest: None declared.
Declaration
This is our original unpublished work, not submitted for publication elsewhere.
CITATION: Kukreja S, Kalra G, Shah N, Shrivastava A. Polypharmacy In Psychiatry: A Review. Mens Sana Monogr 2013;11:82-99.
Peer Reviewers for this paper: J.K. Trivedi MD
Authors’ Contributions
1 and 2- Review of literature, preparation of manuscript, revising paper and responding to reviewers’ comments, checking and confirming final version for submission.
3 and 4- Conceptualisation of the theme of paper, developing outline, revising, checking and confirming the final version for submission.
References
- 1.Anthierens S, Tansens A, Petrovic M, Christiaens T. Qualitative insights into general practitioners views on polypharmacy. BMC Fam Pract. 2010;11:65. doi: 10.1186/1471-2296-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baandrup L, Allerup P, Nordentoft M, Lublin H, Glenthoj BY. Exploring regional variation in antipsychotic coprescribing practice: A Danish questionnaire survey. J Clin Psychiatry. 2010;71:1457–64. doi: 10.4088/JCP.09m05270yel. [DOI] [PubMed] [Google Scholar]
- 3.Campos RN, Costa LF, Bio DS, de Souza MG, Garcia CR, Demétrio FN, et al. LICAVAL: Combination therapy in acute and maintenance treatment of bipolar disorder. Trials. 2010;11:72. doi: 10.1186/1745-6215-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakos MH, Glick ID, Miller AL, Hamner MB, Miller DD, Patel JK, et al. Baseline use of concomitant psychotropic medications to treat schizophrenia in the CATIE trial. Psychiatr Serv. 2006;57:1094–101. doi: 10.1176/ps.2006.57.8.1094. [DOI] [PubMed] [Google Scholar]
- 5.Chong SA, Ravichandran N, Poon LY, Soo KL, Verma S. Reducing polypharmacy through the introduction of a treatment algorithm: Use of a treatment algorithm on the impact on polypharmacy. Ann Acad Med Singapore. 2006;35:457–60. [PubMed] [Google Scholar]
- 6.Comer JS, Olfson M, Mojtabai R. National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996-2007. J Am Acad Child Adolesc Psychiatry. 2010;49:1001–10. doi: 10.1016/j.jaac.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David T. Antipsychotic prescribing - time to review practice. Psychiatric Bulletin. 2002;26:401–2. [Google Scholar]
- 8.De las Cuevas C, Sanz EJ. Polypharmacy in psychiatric practice in the Canary Islands. BMC Psychiatry. 2004;4:18. doi: 10.1186/1471-244X-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englisch S, Zink M. Treatment-resistant Schizophrenia: Evidence-based Strategies. Mens Sana Monogr. 2012;10:20–32. doi: 10.4103/0973-1229.91588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evers K. Personalized medicine in psychiatry: Ethical challenges and opportunities. Dialogues Clin Neurosci. 2009;11:427–34. doi: 10.31887/DCNS.2009.11.4/kevers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freudenreich O, Goff DC. Antipsychotic combination therapy in schizophrenia. A review of efficacy and risks of current combinations. Acta Psychiatr Scand. 2002;106:323–30. doi: 10.1034/j.1600-0447.2002.01331.x. [DOI] [PubMed] [Google Scholar]
- 12.Freudenreich O, Kontos N, Querques J. Psychiatric polypharmacy: A clinical approach based on etiology and differential diagnosis. Harv Rev Psychiatry. 2012;20:79–85. doi: 10.3109/10673229.2012.677358. [DOI] [PubMed] [Google Scholar]
- 13.Ghaemi SN. Polypharmacy in psychiatry. New York: Marcel Dekker; 2002. [Google Scholar]
- 14.Goh YL, Seng KH, Chuan AS, Chua HC. Reducing antipsychotic polypharmacy among psychogeriatric and adult patients with chronic schizophrenia. Perm J. 2011 Spring;15:52–6. doi: 10.7812/tpp/11-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaacs D, Fitzgerald D. Seven alternatives to evidence based medicine. BMJ. 1999;319:1618. doi: 10.1136/bmj.319.7225.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito H, Koyama A, Higuchi T. Polypharmacy and excessive dosing: Psychiatrists’ perceptions of antipsychotic drug prescription. Br J Psychiatry. 2005;187:243–7. doi: 10.1192/bjp.187.3.243. [DOI] [PubMed] [Google Scholar]
- 17.Jörgensen T, Johansson S, Kennerfalk A, Wallander MA, Svärdsudd K. Prescription drug use, diagnoses, and healthcare utilization among the elderly. Ann Pharmacother. 2001;35:1004–9. doi: 10.1345/aph.10351. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy NB, Procyshyn RM. Rational antipsychotic polypharmacy. Can J Clin Pharmacol. 2000 Autumn;7:155–9. [PubMed] [Google Scholar]
- 19.Ketter TA. Strategies for monitoring outcomes in patients with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2010;12:10–6. doi: 10.4088/PCC.9064su1c.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kingsbury SJ, Yi D, Simpson GM. Psychopharmacology: rational and irrational polypharmacy. Psychiatr Serv. 2001;52:1033–6. doi: 10.1176/appi.ps.52.8.1033. [DOI] [PubMed] [Google Scholar]
- 21.Lapane KL, Waring ME, Schneider KL, Dubé C, Quilliam BJ. A mixed method study of the merits of e-prescribing drug alerts in primary care. J Gen Intern Med. 2008;23:442–6. doi: 10.1007/s11606-008-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee RD. Polypharmacy: A case report and new protocol for management. J Am Board Fam Pract. 1998;11:140–4. doi: 10.3122/15572625-11-2-140. [DOI] [PubMed] [Google Scholar]
- 23.Linjakumpu T, Hartikainen S, Klaukka T, Veijola J, Kivelä SL, Isoaho R. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol. 2002;55:809–17. doi: 10.1016/s0895-4356(02)00411-0. [DOI] [PubMed] [Google Scholar]
- 24.López-Torres Hidalgo J, Cerdá Díaz R, Fernández Olano C, Requena Gallego M, Fernández Casalderrey C, Otero Puime A. Factors associated with chronic drug consumption in the elderly. Med Clin (Barc) 1997;108:572–6. [PubMed] [Google Scholar]
- 25.Loyola Filho AI, Uchoa E, Firmo JO, Lima-Costa MF. Influence of income on the association between cognitive impairment and polypharmacy: Bambuí Project. Rev Saude Publica. 2008;42:89–99. doi: 10.1590/s0034-89102008000100012. [DOI] [PubMed] [Google Scholar]
- 26.McCue RE, Waheed R, Urcuyo L. Polypharmacy in patients with schizophrenia. J Clin Psychiatry. 2003;64:984–9. doi: 10.4088/jcp.v64n0902. [DOI] [PubMed] [Google Scholar]
- 27.Misawa F, Shimizu K, Fujii Y, Miyata R, Koshiishi F, Kobayashi M, et al. Is antipsychotic polypharmacy associated with metabolic syndrome even after adjustment for lifestyle effects.: A cross-sectional study? BMC Psychiatry. 2011;11:118. doi: 10.1186/1471-244X-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mojtabai R, Olfson M. National trends in psychotherapy by office-based psychiatrists. Arch Gen Psychiatry. 2008;65:962–70. doi: 10.1001/archpsyc.65.8.962. [DOI] [PubMed] [Google Scholar]
- 29.Mojtabai R, Olfson M. National trends in psychotropic medication polypharmacy in office-based psychiatry. Arch Gen Psychiatry. 2010;67:26–36. doi: 10.1001/archgenpsychiatry.2009.175. [DOI] [PubMed] [Google Scholar]
- 30.Murray MD, Kroenke K. Polypharmacy and medication adherence: small steps on a long road. J Gen Intern Med. 2001;16:137–9. doi: 10.1111/j.1525-1497.2001.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muscatello MR, Bruno A, Pandolfo G, Micò U, Scimeca G, Di Nardo F, et al. Effect of aripiprazole augmentation of clozapine in schizophrenia: A double-blind, placebo-controlled study. Schizophr Res. 2011;127:93–9. doi: 10.1016/j.schres.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Medical Directors Council and State Medicaid Directors. Alexandria, Virginia: 2001. National Association of State Mental Health Program Directors: Technical Report on Psychiatric Polypharmacy. [Google Scholar]
- 33.Niculescu AB, Hulvershorn L. Toward Early, Personalized, Rational Polypharmacy In Psychiatry: A Tri-Dimensional Approach. Psychopharm Review. 2010;45:9–16. [Google Scholar]
- 34.Owen RR, Fischer EP, Kirchner JE, Thrush CR, Williams DK, Cuffel BJ, et al. Clinical practice variations in prescribing antipsychotics for patients with schizophrenia. Am J Med Qual. 2003;18:140–6. doi: 10.1177/106286060301800402. [DOI] [PubMed] [Google Scholar]
- 35.Padmini DD, Amarjeeth R, Sushma M, Guido S. Prescription patterns of psychotropic drugs in hospitalized schizophrenic patients in a tertiary care hospital. Calicut Med J. 2007;5:e3. [Google Scholar]
- 36.Presborn SH, Flockhart D. Guide to Psychiatric Drug Interactions. Primary Psychiatry. 2006;13:35–64. [Google Scholar]
- 37.Preskorn SH, Lacey RL. Polypharmacy: when is it rational? J Psychiatr Pract. 2007;13:97–105. doi: 10.1097/01.pra.0000265766.25495.3b. [DOI] [PubMed] [Google Scholar]
- 38.Procyshyn RM, Kennedy NB, Tse G, Thompson B. Antipsychotic polypharmacy: A survey of discharge prescriptions from a tertiary care psychiatric institution. Can J Psychiatry. 2001;46:334–9. doi: 10.1177/070674370104600404. [DOI] [PubMed] [Google Scholar]
- 39.Ramadas S, Kuttichira P, Sumesh TP, Ummer SA. A study of an antipsychotic prescription pattern of patients with schizophrenia in a developing country. Indian J Psychol Med. 2010;32:13–6. doi: 10.4103/0253-7176.70520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rittmannsberger H. The use of drug monotherapy in psychiatric inpatient treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:547–51. doi: 10.1016/s0278-5846(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 41.Rollason V, Vogt N. Reduction of polypharmacy in the elderly: A systematic review of the role of the pharmacist. Drugs Aging. 2003;20:817–32. doi: 10.2165/00002512-200320110-00003. [DOI] [PubMed] [Google Scholar]
- 42.Council Report CR26. London: Royal College of Psychiatrists; 1993. Royal College of Psychiatrists Consensus Statement on the Use of High Dose Antipsychotic Medication. [Google Scholar]
- 43.Sawhney V, Chopra V, Kapoor B, Thappa JR, Tandon VR. Prescription trends in schizophrenia and manic depressive psychosis. J K Sci. 2005;7:156–8. [Google Scholar]
- 44.Sernyak MJ, Rosenheck R. Clinicians’ reasons for antipsychotic coprescribing. J Clin Psychiatry. 2004;65:1597–600. doi: 10.4088/jcp.v65n1203. [DOI] [PubMed] [Google Scholar]
- 45.Shiloh R, Zemishlany Z, Aizenberg D, Radwan M, Schwartz B, Dorfman-Etrog P, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry. 1997;171:569–73. doi: 10.1192/bjp.171.6.569. [DOI] [PubMed] [Google Scholar]
- 46.Shrivastava A, Johnston M, Terpstra K, Stitt L, Shah N. Atypical antipsychotics usage in long-term follow-up of first episode schizophrenia. Indian J Psychiatry. 2012;54:248–52. doi: 10.4103/0019-5545.102425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370:173–84. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 48.Stahl SM. Antipsychotic polypharmacy: Evidence based or eminence based? Acta Psychiatr Scand. 2002a;106:321–2. doi: 10.1034/j.1600-0447.2002.2e011.x. [DOI] [PubMed] [Google Scholar]
- 49.Stahl SM. Antipsychotic polypharmacy: Squandering precious resources? J Clin Psychiatry. 2002b;63:93–4. doi: 10.4088/jcp.v63n0201. [DOI] [PubMed] [Google Scholar]
- 50.Stahl SM. Stahl's essential pschopharmacology: Neuroscientific basis and practical applications. New York: Cambridge University Press; 2008. [Google Scholar]
- 51.Tapp A, Wood AE, Secrest L, Erdmann J, Cubberley L, Kilzieh N. Combination antipsychotic therapy in clinical practice. Psychiatr Serv. 2003;54:55–9. doi: 10.1176/appi.ps.54.1.55. [DOI] [PubMed] [Google Scholar]
- 52.Taylor DM, Young C, Paton C. Prior antipsychotic prescribing in patients currently receiving clozapine: A case note review. J Clin Psychiatry. 2003;64:30–4. doi: 10.4088/jcp.v64n0107. [DOI] [PubMed] [Google Scholar]
- 53.Thompson A, Sullivan SA, Barley M, Strange SO, Moore L, Rogers P, et al. The DEBIT trial: An intervention to reduce antipsychotic polypharmacy prescribing in adult psychiatry wards - a cluster randomized controlled trial. Psychol Med. 2008;38:705–15. doi: 10.1017/S003329170700147X. [DOI] [PubMed] [Google Scholar]
- 54.Trivedi JK, Dhyani M, Yadav VS, Rai SB. Anti-psychotic drug prescription pattern for schizophrenia: Observation from a general hospital psychiatry unit. Indian J Psychiatry. 2010;52:279. doi: 10.4103/0019-5545.70996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang YC, Chen CY, Kuo TB, Lai CJ, Yang CC. Influence of antipsychotic agents on heart rate variability in male WKY rats: Implications for cardiovascular safety. Neuropsychobiology. 2012;65:216–26. doi: 10.1159/000337459. [DOI] [PubMed] [Google Scholar]
- 56.Werder SF, Preskorn SH. Managing polypharmacy: walking the fine line between help and harm. Curr Psychiatry. 2003;2:24–36. [Google Scholar]
- 57.Williams DD, Garner J. The case against “the evidence”: A different perspective on evidence-based medicine. Br J Psychiatry. 2002;180:8–12. doi: 10.1192/bjp.180.1.8. [DOI] [PubMed] [Google Scholar]


