Abstract
In 2011, the FDA approved the first new therapy for melanoma in over a decade, ipilimumab (Yervoy). Ipilimumab is a novel antibody that blockscytotoxic T lymphocyte-associated antigen 4 (CTLA-4), a regulatory molecule expressed on activated T cells. Blockade of this important immune checkpoint can lead to durable tumor regression and Phase III studies showed an overall survival benefit for patients with advanced melanoma. During the clinical development of ipilimumab, several unique features of thisimmunotherapywere identified including the remarkable durability of responses and a distinct side-effects profile. Herein we review the preclinical and clinical development of CTLA-4 blocking antibodies, and describe current practices using ipilimumab for the treatment of advanced melanoma. Unique clinical issues related to ipilimumab will be summarized. Lastly, we will briefly previewcombination therapies that incorporate ipilimumab and new checkpoint targeting antibodies currently in clinical development.
Introduction
In the past year, the standard of care for the treatment of advanced melanoma has been transformed by the FDA approval of two new agents, ipilimumab and vemurafenib. Ipilimumab is a novel immunotherapy that works by blocking the engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), a regulatory molecule expressed on activated T cells. Blockade of this important immune checkpoint can potentiate a robust antitumor immune response and lead to durable tumor regression. Ipilimumab was the first agent to demonstrate a benefit in overall survival for patients with metastatic melanoma.1 During the clinical development of ipilimumab,several unique features of this ‘checkpoint blocking’ antibodywere identified including the remarkable durability of responses and a distinct side-effects profile. The success of ipilimumab offers a template for the development of the next generation of immunomodulatory antibodies. We shall review the preclinical and clinical development of CTLA-4 blocking antibodies, and describe current practices using ipilimumab for the treatment of advanced melanoma. Unique clinical issues related to ipilimumab will be summarized. Lastly, we will briefly previewcombination therapies that incorporate ipilimumab and new checkpoint targeting antibodies currently in clinical development.
Checkpoints that Regulate T cell Activation and Antitumor Immunity
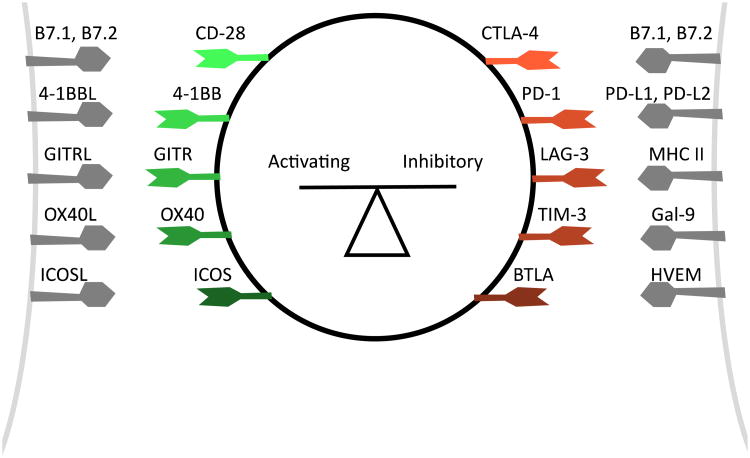
Research into the fundamental mechanisms that regulate T cell activation informed the clinical development of CLTA-4 blocking antibodies (Figure 1). In 1970, Bretscher and Cohn proposed the “two signal” model of T cell activation.2In this model, antigen-specific T cell activation requires both T cell receptor (TCR) engagement (signal 1) and a co-stimulatory signal (signal 2).2-5 In subsequent decades, this simple model was expanded to incorporate additional signals that fine-tune this process. A diversity of co-stimulatory and co-inhibitory molecules are required to both promote and regulate the complex orchestration of T cell activation (Figure 2).8-14 CTLA-4 plays a pivotalrole as an inhibitory receptor, or checkpoint, during T cell activation.CTLA-4 was cloned in 1987,and its similarity to the costimulatory molecule CD28 was recognized.15 Like CD28, CTLA-4 binds to B7-1 and B7-2, ligands expressed on antigen presenting cells,but with higher affinity.16 Unlike CD28, engagement of CTLA-4 inhibits T cell activation.17-19 CTLA-4 engagement on activated T cells inhibits cytokine synthesis and restricts cell proliferation.The characterization of CTLA-4 -/- knockout mice established the importance of CTLA-4-mediated regulation in vivo; these mice develop a lethal hyperproliferative lymphocyte expansion.23-25
Figure 1.
Pre-Clinical and Clinical Development of CTLA-4 blocking Antibodies.
Figure 2. Co-stimulatory and Co-inhibitory Molecules Regulate T cell Activation.
A diversity of activating and inhibitory signals are integrated to modulate the process of T cell activation. CTLA-4 is one of many inhibitory checkpoint molecules that regulate T cell activation.
Based upon the observation that CTLA-4 attenuates T cell activation, it was hypothesized that blockade of CTLA-4 could enhance antitumor immune responses.26 This concept was initially validated using transplantable murine tumor lines of fibrosarcoma and colon carcinoma.27 This finding has now been expanded to transplantable tumors of many types including prostate carcinoma, breast carcinoma, melanoma, ovarian carcinoma, lymphoma, and others.27-31 For some poorly immunogenic tumors, such as the B16 melanoma, CTLA-4 monotherapy isinsufficient, but combinations of CTLA-4 blockade with vaccines are active.32-34Mice treated successfully with CLTA-4 blockade are protected from subsequent tumor challenge, consistent with the generation of protective antitumor immunity.
The Development of Human Reagents Ipilimumab and Tremelimumab
Based on the preclinical activity seen in mouse models, antibodies that blockCTLA-4 were subsequently developed forclinical use. Both ipilimumab (Yervoy™, Bristol Meyers Squibb, Princeton, NJ) and tremelimumab (formerly CP-675, 206 or ticilimumab, Pfizer, New York, NY) are fully human antagonist antibodiesrecognizing human CTLA-4.35-37 Ipilimumab is an IgG1 antibody with a half-life of 12-14 days, whereas tremelimumab is an IgG2 antibody with a half-life of approximately 22 days. Both of these agents have been widely tested in patients with metastatic melanoma, where durable clinical responses have been well documented for both antibodies. Based on an overall survival benefit in phase III studies, the US Food and Drug Administration (FDA) approved ipilimumab for the treatment of patients with unresectable or metastatic melanoma in 2011;1however, aphase III study of tremelimumab was halted after an interim analysis failed to demonstrate an overall survival benefit compared to standard chemotherapy, though a follow up analysis did show a trend favoring tremelimumab.At present, it is unclear if differences in the dosing, schedule, clinical trial design/execution, or clinical activity explain the apparent shortcomings of tremelimumab in this study.
Ipilimumab in Clinical Trials
In 2002, the results of a pilot study of 17 patients with unresectable melanoma treated with a single dose of ipilimumab(3 mg/kg) were reported. There were 2 objective durable partial responses (PR), and no serious toxicities were reported.40Subsequent early phase studies introduced a schedule of repeated dosing every 3 weeks.These studies demonstrated tolerability and clinical activity, and the dosing regimen of 3 mg/kg every 3 weeks for 4 doses was adopted in several subsequent studies.Unique toxicities were seen in these early studies with reports of colitis, dermatitis, hepatitis, hypophysitis, thyroiditis, and uveitis.This spectrum of toxicities was felt to be related to immune activation, latercategorized asimmune-related adverse events (irAEs). A dose-response relationship for ipilimumab was defined in a double-blind phase II study comparing doses of 0.3, 3, and 10 mg/kg every 3 weeks for 4 doses, followed by maintenance doses administered every 12 weeks. The highest dose cohort, 10 mg/kg, had the greatest response rate (11%), followed by 3 mg/kg (4.2%), and 0.3 mg/kg (0%). The irAEs followed a similar pattern.46
A randomized, double-blinded, phase III study examining 676 patients with advancedmelanoma demonstrated an improved median overall survival for patients receiving ipilimumab (10.1 vs. 6.4 months, P=0.003). 47 This three-armed study compared patients treated with ipilimumab at a dose of 3 mg/kg every 3 weeks for 4 doses versusthe gp100 peptide vaccine alone or gp 100 peptide vaccine plus ipilimumab. The survival rates for patients treated with ipilimumab alone were 45.6% at 1 year and 23.5% at the 2-years. Patients who initially achieved a confirmed partial or complete response or at least stable disease ≥24 weeks were eligible for re-induction within their original treatment arm ifthey subsequentlydeveloped disease progression.
A second randomized, placebo-controlled, phase III clinical trial compared dacarbazine plus ipilimumab versus dacarbazine plus placeboand accrued 502treatment naïve patients with metastatic melanoma. Patients received ipilimumab at a dose of 10 mg/kg every 3 weeks for 4 doses, followed by maintenance doses of ipilimumab given every 3 months. Again, a benefit in OS (11.2 vs. 9.1 months) was reported.48 Survival rates for patients who received dacarbazine with ipilimumab were higher than patients who receiveddacarbazine alone at 1 year (47.3% vs. 36.3%), 2 years (28.5% vs. 17.9%), and 3 years (20.8% vs. 12.2%).
Immune-related Adverse Events
The potent ability of CTLA-4 blockade to activate the immune system can result in tissue specific inflammation characterized as immune-related adverse events (irAEs). Tissues that are most often involvedinclude the skin (dermatitis), gastrointestinal tract (enterocolitis), liver (hepatitis), and endocrine organs (hypophysitis, thyroiditis).In general, irAEs are transient and reversible; depending upon the severity of symptoms, interventions may include interruption of ipilimumab dosing, treatment with a course of steroids, or stronger immunosuppressants.
In particular, cases of enterocolitis can have serious consequences if appropriate treatment is not initiated promptly. In the phase III study reported by Hodi et al.,29% of patients treated with ipilimumab at a dose of 3 mg/kg developed gastrointestinal irAEs of any grade, and grade 3/4 colitis symptoms were reported in 8%.1 Colitis typically resolves when treated with steroids. In cases refractory to steroids, treatment with tumor necrosis factor (TNF)-blocking antibodies such as infliximab may be helpful.45 An algorithm for the management of colitis symptoms has been developed and adequate patient education and vigilance on the part of patient and physician are paramount. IrAEs involving the skin are common, but rarely serious and typically present as pruritus and/or a mild rash. Upon pathologic evaluation, findings of epidermal spongiosis and perivascular lymphocytic infiltrate with a predominance of eosinophils and CD4+ T cells have been described.Topical emollients, antihistamines, or topical steroids are often helpful to minimize dermatologic symptoms. Systemic steroids are rarely required. Other irAEs such as hepatitis or hypophysitis are seen infrequently (typically <5%). Depending upon the extent of damage to the endocrine organ, supplemental hormones may be required in patients who develop hypophysitis, thyroiditis, or adrenal insufficiency. Additional irAEs including pancreatitis, uveitis, myopathy, neuropathy, arthritis, cytopenias, or pneumonitishave been described but are quite rare (1-2% or less).
Kinetics and Durability of Responses to Ipilimumab—Immune-related Response Criteria
While radiographic responses to ipilimumab are relatively infrequent, the durability of these responses can be measured in years rather than months. The remarkable stability of disease control was well represented in the phase III studies of ipilimumab, where a clear plateau in the 1-, 2- and 3-year survival rates were observed. Taking a closer look at the question of long-term responses to ipilimumab, 177 patients were treated on some of the earliest clinical trials. In the study population,15 patients achieved long-term durable complete responses (CR) and 14 of these are ongoing with the longest lasting 99+ months (median 83 months). Surprisingly, patients who achieved partial responses (PR) can also achieve long-term disease control. Nine patients who achieved PRs are alive many years after ipilimumab treatment, 3 without further treatment.
In addition to the remarkable durability of responses to ipilimumab, unusual patterns of radiographic responses were seen. Whereas responses to chemotherapy are usually seen within the first weeks or months of therapy, responses to ipilimumab can be quite delayed.63 Furthermore, some ipilimumab treated patients will initially appear to have progressive disease with the development of new lesions but will ultimately go on to achieve a response. The distinct response patterns associated with ipilimumab were evaluated in a larger group of patients through a retrospective analysis of 487 patients treated across three multicenter phase II clinical trials.64Following this analysis, the immune-related response criteria (irRC) were proposed, to better characterize the response pattern. The irRC are based upon principles of the traditional modified World Health Organization (mWHO) criteria, but they differ in several important ways. According to the irRC, new lesions are included in the determination of the overall tumor burden and do not automatically indicate progressive disease. Additionally, evidence of disease progression requires confirmation with a subsequent radiographic assessment at least 4 weeks later. The irRC are being prospectively validated in ongoing studies of immunotherapeutic agents.
Immune-monitoring, the search for biomarkers
Despite the OS improvement demonstrated in phase III trials for patients who receive ipilimumab, unfortunately only ∼30% of patients appear to derive benefit.With the delayed response kinetics, identifying patients who will fall into this favorable category can be especially challenging. Ongoing efforts continue to evaluate biomarkers which may help guide clinical decisions and/or better inform our understanding about the mechanism(s) of activity for ipilumumab in vivo. Thus far, no clear predictive biomarker for clinical response has been identified in peripheral blood samples. Several biomarkers that appear reflect immune activation by ipilimumab during treatment and correlate with responses to ipilimumabhave been identified in retrospective studies and includeabsolute lymphocyte count (ALC) 65,67, sustained upregulation of the T cell activation marker inducible co-stimulator (ICOS), 68-72the development of a polyfunctional T cell response to the tumor antigen NY-ESO-1.73Prospective validation in larger studies will be necessary to determine the significance of these findings.
The tumor microenvironment may be a more relevant area to look for immunologic markers. ICOS upregulation on T cells after neoadjuvant ipilumumab treatment was first identified in the tumor microenvironment for bladder and prostate tumors.68-72More recently, the results of a prospective, double-blind phase II study exploring candidate biomarkers from the melanoma tumor microenvironment have ben reported.74Evaluation of tumor biopsies revealed significant associations between clinical benefitand high baseline expression of FoxP3 (P=0.014) and indoleamine 2,3-dioxygenase (P=0.012). Clinical activity also correlated with an increase in tumor-infiltrating lymphocytes (TILs) between baseline and after the second dose of ipilimumab (P=0.005). In a second study evaluating biopsy samples, Ji et al. reported on gene expression patterns in the tumor microenviroment. The investigatorsobserved that expression of inflammatory response genes at baseline predicts clinic benefit after ipilimumab treatment (P<0.01). 75 Several other small case series have described intratumoral changes after treatment with ipilimumab consistent with induction of a productive antitumor immune response.76-78
Ipilimumab in Clinical Use Today
Dosing and Schedule
The FDA has approved ipilimumab at a dose of 3 mg/kg to be administered once every three weeks for four doses, the schedule utilized by Hodi et al.1 It is not clear, however,that this regimen reflects the optimalclinical activity for ipilimumab and several important questions are unanswered. First, what is the most effective dose of ipilimumab? Phase I studies did not identify a maximum tolerated dose. In a randomized, double-blinded phase II study comparing ipilimumab at three dose levels, 0.3, 3, and 10 mg/kg,dose-dependent antitumor activity was observed with response rates of 0%, 4.2% and 11.1% respectively. This must be balanced against an increased rate of Grade 3/4 irAEs (0%, 7 %, 25%). The activity of ipilimumab at the 10 mg/kg dose will be formally compared to the FDA-approved 3 mg/kg dose in an upcoming randomized, double- blind phase III study (NCT01515189). A second outstanding question relates to the appropriate duration of ipilimumab treatment. Some clinical trials have permitted additional, so-called “maintenance”, doses of ipilimumab administered every three months after completion of the first 4 doses. Alternatively, some trials have permitted repeat dosing or “reinduction” therapy using the original four-dose induction schedule. The Hodi study provides some limited evidence that reinduction with ipilimumab may help some patients. In this study, 31 patients who initially benefitted from ipilimumab treatment and subsequently developed progressive disease were offered reinduction. After reinduction therapy, 1 patient achieved a CR, 5 patients achieved PRs, and 15 patients achieved SD.
BRAF mutant melanoma
For the approximately 50-60% of patients with advanced melanoma that harbor the BRAFV600E mutation, vemurafenib, a targeted inhibitor of mutated BRAF, has been approved by the FDA based upon an overall survival benefit.79 Within this population, vemurafenib has a superior response rate (∼50%) and faster kinetics of response when compared to ipilimumab.;however, unlike ipilimumab, responses to vemurafenib are rarely durable and progressionusually occurs within 6-8 months.80The presence or absence of a BRAF mutation does not appear to have any impact on the likelihood of response to ipilimumab.81 The sequencing of these two agents in patients with BRAF mutant melanoma has not been clearly established. At present, our practice has been to treat patients with symptomatic BRAF mutant melanoma with vemurafenib upfront in the hope of achieving rapid palliative reduction in disease burden, given the slower kinetics of responses to ipilimumab. Otherwise, both vemurafenib and ipilimumab are reasonable in the first-line setting and the merits and liabilities of each should be balanced for the individual patient. A phase I study combining ipilimumab and vemurafenib has recently opened (NCT01400451).
Patients with CNS metastases
For patients with advanced melanoma who develop CNS metastases, prognosis is especially poor. While the data are limited, it appears thatipilimumab may provide some benefit for this population.82 Initial observations supporting this notion came from two case reports of patients treated with ipilimumab who had either regression or stabilization of CNS disease.In a retrospective analysis of patients treated on a phase II study of ipilimumab, 12 patients with stable brain metastases before starting treatment were identified.85 In this group, 2/12 achieved a partial response and 3/12 had stable disease, with one patient developing grade 3 cerebral edema responsive to treatment with steroids. Lastly, a study prospectively evaluating the activity of ipilimumab in patients with brain metastases was reported in abstract form at ASCO in 2010.86In 51 patients with brain metastasis not requiring steroids whowere treated with ipilimumab, 4/51 achieved a systemic PR and 5/51 achieved SD for an overall disease control rate of 9/51 (18%). In evaluating CNS disease alone, 5 patients had a PR and 6 had SD at 12 weeks. Thus, ipilimumab appears to have similar activity for brain metastases as for non-CNS disease. The activity of ipilimumab in the setting of CNS metastases requiring steroids has not been reported. Aphase II study (NIBIT-M1)evaluating the combination of fotemustine with ipilimumab in patients with metastatic melanoma with or without asymptomatic brain metastases is underway.
Ipilimumab in the Adjuvant Setting
At present, the indications for ipilimumab restrict its use to patients with either stage IV or unresectable stage III melanoma, as the benefit of ipilimumab for earlier stage disease has not been established. Ipilimumab in the adjuvant setting is being evaluated in two ongoing phase III trials (NCT00636168 and NCT01274338). In NCT00636168, ipilimumab is being compared to placebo after resection of high risk stage III melanoma with recurrence-free survival as the primary endpoint. Accrual has been completed and results are anticipated. Ipilimumab is also being compared to high-dose recombinant interferon-alpha-2b (NCT01274338).
Beyond Ipilimumab Monotherapy
Combination Therapy
Combining ipilimumab with traditional or experimental therapies may improve upon response rates and expand the durable benefits of ipilimumab. Preclinical evidence from mouse models offers support for combinations with conventional cancer therapies including surgery 87, radiation, chemotherapy 90 cryoablation91, and radiofrequency ablation.92 CTLA-4 has also been combined successfully with a diversity of immunotherapies including tumor vaccines and immunomodulatory antibodies in the preclinical setting.Lastly, limited evidence supports the combination of CTLA-4 blockade with molecularly targeted agents, an area likely to enjoy increased attention.103
A number of combination strategies have been explored in clinical trials to date. Combinations of ipilimumab with tumor vaccines have been the most common, including peptide vaccines, cellular vaccines105, and DNA/RNA vaccines106. The combination of ipilimumab with a peptide vaccine against gp100 was tested in a randomized phase III study but failed to show superior activity to ipilimumab alone.1 Alternative vaccination strategies may be more successful in combination with ipilimumab but have not yet been tested in larger, randomized studies. A regimen combining ipilimumab and IL-2 was tested in a single arm phase I/II study.43 The combination proved tolerable and responses were seen in 22% of patients, but it is unclear if this regimen is superior to monotherapy.
The utility of combining ipilimumab with chemotherapy in advanced melanoma is unclear, perhaps reflecting the limited activity of standard chemotherapies like dacarbazine in this disease. In an open label, randomized phase II study, Hersh et al. reported a non-significant trend favoring ipilimumab combined with dacarbazine compared to ipilimumab alone, with disease control rates of 37.1% vs. 21.6%, respectively. In a phase III study, Robert et al. evaluated a similar combination of ipilimumab and dacarbazine and observed a response rate of 15% with over 40% of patients experiencing grade 3/4 toxicity. While there was no comparator arm of ipilimumab alone, it seems unlikely that this combination is superior given the historical response rates for ipilimumab. Lastly, in a case report, radiation therapy has been identified as an attractive partner for combination with ipilimumab and formal studies of this combination are underway (NCT01449279, NCT01497808). 107
On the horizon, combinations of ipilimumab with novel immunotherapies or molecularly targeted therapies are likely to be promising based upon preclinical studies. At present, ipilimumab is being tested in combination with MDX-1106, a programmed death-1 (PD-1) blocking antibody, in the phase I setting (NCT01024231). And, a first-in-human trial combining ipilimumab with vemurafenibhas recently opened (NCT01400451).
Expanding the Repertoire of Checkpoint Blocking Antibodies
Ipilimumab has clearly expanded and re-established the important role for immunotherapy in the treatment of melanoma and has demonstrated the robust clinical activity of a checkpoint blocking antibody. CTLA-4 is the first on a growing list of immunological checkpoints that now includes PD-1, LAG-3 (Lymphocyte-activation gene 3), TIM-3 (T cell immunoglobulin mucin-3), BTLA (B- and T-lymphocyte attenuator), and others (Figure 2). The development and clinical testing of antibodies for these newer checkpoint molecules are in various stages of pre-clinical or clinical development.108-111 Several antibodies targeting PD-1 or its ligand PD-L1 have been developed for clinical use including BMS-936558/MDX-1106, BMS-936559/MDX-1105 (both from Bristol-Myers Squibb), MK-3475 (Merck), MPDL3280A/RG7446 (Genentech), and CT-011 (Cure Tech); an anti-PD-1 fusion protein, AMP-224 (Amplimmune) is in development as well. BMS-936558 is a fully human IgG4 antibody, which has a serum half-life of 20 days at the highest doses tested.114 A first-in-human, Phase I, single-dose dose-escalation study of BMS-936558 showed activity and was followed by a second Phase I study investigating a schedule of bi-weekly dosing.On a biweekly schedule, BMS-936558 had an objective response rate of 37.5% (6/16) including 5 PRs (melanoma, renal-cell carcinoma, non-small cell lung cancer) and one CR (renal-cell carcinoma).
The remaining checkpoint molecules are in earlier stages of pre-clinical or clinical development. The relative contribution of each checkpoint in fostering a protected tumor environment are beginning to be unraveled and will likely be unique for eachtumor. Ultimately, assays that determine the most relevant checkpoints to target in an individual tumor may guide clinical decisions. Building upon the success of ipilimumab, the cannon of clinically available checkpoint blocking antibodies will likely expand over the next decade.
Conclusion
The CTLA-4 blocking antibody, ipilimumab, is the first in new class of checkpoint blocking antibodies. With a demonstrated survival benefit in two randomized phase III studies, ipilimumab has been recently approved by the FDA for the treatment of advanced melanoma. Unique clinical features of ipilimumab were identified during its clinical development including delayed response kinetics and a distinct profile of side effects. As ipilimumab is incorporated into the standard of care of advanced melanoma, patient and physician education is paramount to the successful and safe use of this promising new therapy. The success of ipilimumab as a monotherapy, opens the door for the development of new checkpoint blocking antibodies and new combinations of ipilimumab with standard and experimental therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169(950):1042–9. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 3.Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat Rev Immunol. 2002;2(6):439–46. doi: 10.1038/nri823. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165(2):302–19. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53(1):27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 6.Bernard A, Lamy, Alberti I. The two-signal model of T-cell activation after 30 years. Transplantation. 2002;73(1 Suppl):S31–5. doi: 10.1097/00007890-200201151-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16(3):321–7. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhu C, Anderson AC, Kuchroo VK. TIM-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol. 2011;350:1–15. doi: 10.1007/82_2010_84. [DOI] [PubMed] [Google Scholar]
- 9.Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24(12):619–22. doi: 10.1016/j.it.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–92. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 12.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229(1):5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS) Curr Opin Immunol. 2010;22(3):326–32. doi: 10.1016/j.coi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328(6127):267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 16.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–50. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 19.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 20.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183(6):2541–50. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162(10):5813–20. [PubMed] [Google Scholar]
- 22.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14(2):145–55. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 23.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 24.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 25.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7(6):885–95. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 26.Allison JP, Hurwitz AA, Leach DR. Manipulation of costimulatory signals to enhance antitumor T-cell responses. Curr Opin Immunol. 1995;7(5):682–6. doi: 10.1016/0952-7915(95)80077-8. [DOI] [PubMed] [Google Scholar]
- 27.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 28.Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94(15):8099–103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57(18):4036–41. [PubMed] [Google Scholar]
- 30.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11(4):483–93. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 31.Sotomayor EM, Borrello I, Tubb E, Allison JP, Levitsky HI. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A. 1999;96(20):11476–81. doi: 10.1073/pnas.96.20.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63(12):3281–8. [PubMed] [Google Scholar]
- 34.Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patino JA, Perales MA, et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22(13-14):1700–8. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 35.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23(35):8968–77. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 37.Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5950–6. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 38.Ribas A, Hauschild A, Kefford R, Punt CJ, Haanen JB, Marmol M, et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide or dacar- bazine) in patients with advanced melanoma. J Clin Oncol. 2008;26(2009) abstract LBA9011. [Google Scholar]
- 39.Marshall MA, Ribas A, Huang B. Evaluation of baseline serum C-reactive protein (CRP) and benefit from tremelimumab compared to chemotherapy in first-line melanoma. J Clin Oncol. 2010;28:15s. (suppl; abstr 2609) 2010. [Google Scholar]
- 40.Tchekmedyian S, Glasby J, Korman A, Keler T, Deo Y, Davis T. MDX-010 (human anti-CTLA4): a phase I trial in malignant melanoma. Proc Am Soc Clin Oncol. 2002;21 abstr 56. [Google Scholar]
- 41.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hersh EM, O'Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. 2010 doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 43.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12(12):1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Giacomo AM, Biagioli M, Maio M. The Emerging Toxicity Profiles of Anti-CTLA-4 Antibodies Across Clinical Indications. Semin Oncol. 2010;37(5):499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 47.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med. 2011 doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 49.Jaber SH, Cowen EW, Haworth LR, Booher SL, Berman DM, Rosenberg SA, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Archives of dermatology. 2006;142(2):166–72. doi: 10.1001/archderm.142.2.166. [DOI] [PubMed] [Google Scholar]
- 50.Bashey A, Medina B, Corringham S, Pasek M, Carrier E, Vrooman L, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113(7):1581–8. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361(2):211–2. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 52.Hunter G, Voll C, Robinson CA. Autoimmune inflammatory myopathy after treatment with ipilimumab. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2009;36(4):518–20. doi: 10.1017/s0317167100007939. [DOI] [PubMed] [Google Scholar]
- 53.Maur M, Tomasello C, Frassoldati A, Dieci MV, Barbieri E, Conte P. Posterior Reversible Encephalopathy Syndrome During Ipilimumab Therapy for Malignant Melanoma. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.38.7886. [DOI] [PubMed] [Google Scholar]
- 54.Bompaire F, Mateus C, Taillia H, De Greslan T, Lahutte M, Sallansonnet-Froment M, et al. Severe meningo-radiculo-nevritis associated with ipilimumab. Invest New Drugs. 2012 doi: 10.1007/s10637-011-9787-1. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia S, Huber BR, Upton MP, Thompson JA. Inflammatory enteric neuropathy with severe constipation after ipilimumab treatment for melanoma: a case report. J Immunother. 2009;32(2):203–5. doi: 10.1097/CJI.0b013e318193a206. [DOI] [PubMed] [Google Scholar]
- 56.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–30. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon IO, Wade T, Chin K, Dickstein J, Gajewski TF. Immune-mediated red cell aplasia after anti-CTLA-4 immunotherapy for metastatic melanoma. Cancer Immunol Immunother. 2009;58(8):1351–3. doi: 10.1007/s00262-008-0627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmad S, Lewis M, Corrie P, Iddawela M. Ipilimumab-induced thrombocytopenia in a patient with metastatic melanoma. Journal of oncology pharmacy practice: official publication of the International Society of Oncology Pharmacy Practitioners. 2011 doi: 10.1177/1078155211411001. [DOI] [PubMed] [Google Scholar]
- 59.Akhtari M, Waller EK, Jaye DL, Lawson DH, Ibrahim R, Papadopoulos NE, et al. Neutropenia in a patient treated with ipilimumab (anti-CTLA-4 antibody) J Immunother. 2009;32(3):322–4. doi: 10.1097/CJI.0b013e31819aa40b. [DOI] [PubMed] [Google Scholar]
- 60.Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 61.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 Blockade with Ipilimumab: Long-Term Follow-up of 177 Patients with Metastatic Melanoma. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Postow MA, Callahan MK, Wolchok JD. The Antitumor Immunity of Ipilimumab: (T cell) Memories to Last a Lifetime? Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-0409. [DOI] [PubMed] [Google Scholar]
- 63.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- 64.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 65.Berman Dea. Association of peripheral blood absolute lymphocyte count (ALC) and clinical activity in patients (pts) with advanced melanoma treated with ipilimumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(Abstract):3020. [Google Scholar]
- 66.Maker AV, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29(4):455–63. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–6. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 69.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, et al. ICOS controls the pool size of effector-memory and regulatory T cells. Journal of immunology. 2008;180(2):774–82. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 70.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proceedings of the National Academy of Sciences of the United States of America; 2008; pp. 14987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proceedings of the National Academy of Sciences of the United States of America; 2009; pp. 2729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16(10):2861–71. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9(1):204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105(8):3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klein O, Ebert LM, Nicholaou T, Browning J, Russell SE, Zuber M, et al. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res. 2009;15(7):2507–13. doi: 10.1158/1078-0432.CCR-08-2424. [DOI] [PubMed] [Google Scholar]
- 78.Del Vecchio M, Mortarini R, Tragni G, Di Guardo L, Bersani I, Di Tolla G, et al. T-cell activation and maturation at tumor site associated with objective response to ipilimumab in metastatic melanoma. J Clin Oncol. 2011;29(32):e783–8. doi: 10.1200/JCO.2011.36.5957. [DOI] [PubMed] [Google Scholar]
- 79.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shahabi V, Whitney G, Hamid O, Schmidt H, Chasalow SD, Alaparthy S, et al. Assessment of association between BRAF-V600E mutation status in melanomas and clinical response to ipilimumab. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Margolin KA, Di Giacomo AM, Maio M. Brain metastasis in melanoma: clinical activity of CTLA-4 antibody therapy. Semin Oncol. 2010;37(5):468–72. doi: 10.1053/j.seminoncol.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Hodi FS, Oble DA, Drappatz J, Velazquez EF, Ramaiya N, Ramakrishna N, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol. 2008;5(9):557–61. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 84.Schartz NE, Farges C, Madelaine I, Bruzzoni H, Calvo F, Hoos A, et al. Complete regression of a previously untreated melanoma brain metastasis with ipilimumab. Melanoma Res. 2010;20(3):247–50. doi: 10.1097/CMR.0b013e3283364a37. [DOI] [PubMed] [Google Scholar]
- 85.Weber JS, Amin A, Minor D, Siegel J, Berman D, O'Day SJ. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011;21(6):530–4. doi: 10.1097/CMR.0b013e32834d3d88. [DOI] [PubMed] [Google Scholar]
- 86.Lawrence D, Hamid O, McDermott D, Puzanov I, Sznol M, Clark J, et al. Phase II trial of ipilimumab monotherapy in melanoma patients with brain metastases. J Clin Oncol. 2010;28:15s. (suppl; abstr 8523). 2010. [Google Scholar]
- 87.Kwon ED, Foster BA, Hurwitz AA, Madias C, Allison JP, Greenberg NM, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci U S A. 1999;96(26):15074–9. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–34. [PubMed] [Google Scholar]
- 90.Mokyr MB, Kalinichenko T, Gorelik L, Bluestone JA. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998;58(23):5301–4. [PubMed] [Google Scholar]
- 91.Waitz R, Solomon SB, Petre EN, Trumble AE, Fasso M, Norton L, et al. Potent Induction of Tumor Immunity by Combining Tumor Cryoablation with Anti-CTLA-4 Therapy. Cancer Res. 2012;72(2):430–9. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95(7):896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95(17):10067–71. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194(4):481–9. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–8. [PubMed] [Google Scholar]
- 96.Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Totterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 2010;33(3):225–35. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- 97.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69(19):7747–55. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Met O, Wang M, Pedersen AE, Nissen MH, Buus S, Claesson MH. The effect of a therapeutic dendritic cell-based cancer vaccination depends on the blockage of CTLA-4 signaling. Cancer Lett. 2006;231(2):247–56. doi: 10.1016/j.canlet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 99.Daftarian P, Song GY, Ali S, Faynsod M, Longmate J, Diamond DJ, et al. Two distinct pathways of immuno-modulation improve potency of p53 immunization in rejecting established tumors. Cancer Res. 2004;64(15):5407–14. doi: 10.1158/0008-5472.CAN-04-0169. [DOI] [PubMed] [Google Scholar]
- 100.Gao Y, Whitaker-Dowling P, Griffin JA, Barmada MA, Bergman I. Recombinant vesicular stomatitis virus targeted to Her2/neu combined with anti-CTLA4 antibody eliminates implanted mammary tumors. Cancer Gene Ther. 2009;16(1):44–52. doi: 10.1038/cgt.2008.55. [DOI] [PubMed] [Google Scholar]
- 101.Youlin K, Li Z, Xiaodong W, Xiuheng L, Hengchen Z. Combination Immunotherapy with 4-1BBL and CTLA-4 Blockade for the Treatment of Prostate Cancer. Clin Dev Immunol. 2012;2012:439235. doi: 10.1155/2012/439235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6(4):e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17(9):1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22 Pt 1):6681–8. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 106.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Postow M, Callahan M, Barker C, Yamada Y, J Y, S K, et al. Immunologic Correlates of an Abscopal Effect in a Patient with Melanoma. N Engl J Med. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–78. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 110.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paulos CM, June CH. Putting the brakes on BTLA in T cell-mediated cancer immunotherapy. J Clin Invest. 2010;120(1):76–80. doi: 10.1172/JCI41811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19(10):1223–34. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 113.Brahmer JR, Topalian SL, Powderly J, Wollner I, Picus J, Drake CG, et al. Phase II experience with MDX-1106 (Ono-4538), an anti-PD-1 monoclonal antibody, in patients with selected refractory or relapsed malignancies. J Clin Oncol. 27:15s. (suppl; abstr 3018). 2009. [Google Scholar]
- 114.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sznol M, Powderly JD, Smith DC, Brahmer JR, Drake CG, McDermott DF, et al. Safety and antitumor activity of biweekly MDX-1106 (Anti-PD-1, BMS-936558/ONO-4538) in patients with advanced refractory malignancies. J Clin Oncol. 2010;28:15s. suppl; abstr 2506. [Google Scholar]