Abstract
BACKGROUND
Primary percutaneous coronary intervention (PPCI) is considered as a choice of treatment in ST-elevation myocardial infarction (STEMI). PPCI has been performed in the Isfahan Province for several years. This study was performed to describe the situation, and determine in-hospital and early (30 days) clinical outcomes of the patients in order to provide sufficient evidence to evaluate and modify this treatment modality if necessary.
METHODS
All patients, who underwent PPCI for STEMI from July to December 2011 at Chamran and Saadi Hospitals (PPCI centers in the Isfahan Province), were included in this case series study. Premedication, angioplasty procedure, and post-procedural treatment were performed using standard protocols or techniques. All discharged patients were followed for 30 days by phone. Endpoints consisted of clinical success rate, and in-hospital and 30 day major adverse cardiac events (MACEs) (death, reinfarction, stroke, and target vessel revascularization).
RESULTS
93 patients (83 (89.2%) at Chamran Hospital and 10 (10.8%) patients at Saadi Hospital) had PPCI. Mean Age of the patients was 59.60 ± 11.10 and M/F ratio was 3.89. From the 181 involved vessels (involved vessels/patient ratio = 1.97 ± 0.70), the treatment of 105 lesions (lesions/patient ratio = 1.13 ± 0.368) was attempted. The clinical success rate was 72%. Pain-to-door and door-to-balloon times were, respectively, 255.1 ± 221.4 and 148.9 ± 168.5 min. The reason for failure was impaired flow (n = 17 (18.3%)), failure to cross with a guidewire (n = 2 (2.2%)), suboptimal angiographic results (n = 2 (2.2%)), and death in one patient. The in-hospital and 30 days MACE rates were, respectively, 8.6% and 3.2%.
CONCLUSION
Low success rate in our series could be due to prolonged pain-to-door and door-to-balloon times and lack of an established, definite protocol to regularly perform PPCI in a timely fashion. We should resolve these problems and improve our techniques in order to prevent and treat slow/no-reflow phenomenon.
Keywords: Acute Coronary Syndrome, Myocardial Infarction, Percutaneous Transluminal Coronary Angioplasty, Cardiogenic Shock, No-Reflow Phenomenon
Introduction
ST-elevation myocardial infarction (STEMI) is a dangerous manifestation of coronary artery disease (CAD) and continues to be a significant public health problem in industrialized and developing countries.1,2
The cornerstone of treatment of these patients is the rapid and effective restoration of blood flow with fibrinolytic therapy, and/or primary percutaneous coronary intervention (PPCI).3 PPCI has been shown to be the superior strategy resulting in a markedly lower occurrence of short-term major adverse cardiac events (MACEs).4-9
Impaired or ceased flow in the absence of anatomical obstruction may occur after PPCIN; this can influence the prognosis negatively.10,11 this event known as angiographic slow/no-reflow phenomenon is recognized angiographically in 5-20% of patients undergoing PPCI for acute myocardial infarction (AMI).10,12,13
A major disadvantage of PPCI is related to the availability of facility and an experienced team; PPCI is the treatment of choice for reperfusion therapy of STEMI whenever available and feasible.14,15 Its golden time is within 90 min of admission to the hospital (door-to-balloon time 90 min) especially when thrombolytic therapy has failed (known as rescue PCI).2,4,16
In the Isfahan Province, PPCI has been performed since 2006. It was performed in Chamran Hospital for the first time, and has recently been performed in Saadi Hospital. However, after 6 years of experience of PPCI we could not find any study describing the situation, problems, and clinical outcomes of PPCI in Isfahan. Therefore, the objective of this study is to describe the situation and determine in-hospital and early (after discharge until 30 days) clinical outcomes of the patients who underwent primary or rescue PCI in the Isfahan Province. This study was done in order to provide sufficient evidence to evaluate and modify our system if necessary.
Materials and Methods
All patients who underwent primary or rescue PCI for the STEMI from July to December 2011 in the Isfahan Province (at Chamran and Saadi Hospitals) were included in this case series study.
All patients received orally 325 mg of chewable aspirin, and 600 mg of Plavix in the emergency room. After coronary angiography if the anatomy was eligible for PCI additional heparin (100 units/kg) was administered intravenously, and angioplasty procedure was performed using standard techniques.2,16 However, strategic planning of the procedure and device selection were dependent on the operator’s discretion.
After the angioplasty, patients received 325 mg of aspirin daily, beta-blockers, and angiotensin-converting enzyme inhibitors if not contraindicated. All patients (DES or BMS) received 75 mg of Plavix daily for the first month, and were suggested to continue using it for 12 months under the supervision of their physician.
Lesion types were noted according to the American College of Cardiology/American Heart Association’s (ACC/AHA) lesion characteristics classification.16
All Patients who were discharged alive from hospitals were eligible to be followed by a phone survey for 30 days.
Definitions: Myocardial infarction (MI) was defined as Ischemic symptoms accompanied by at least one of the following criteria: positive cardiac enzymes, electrocardiographic changes (pathologic Q wave or new ST changes), and new cardiac motion abnormality on echocardiographic or radionuclide imaging.
Coronary blood flow after PPCI is graded on a scale of 0 through 3 depending on flow characteristics. Thrombolysis In myocardial infarction (TIMI) 0 is defined as no contrast flow beyond the site of occlusion (no perfusion), TIMI 1 as contrast flow beyond the site of occlusion but failing to opacify the entire artery (penetration with minimal perfusion), TIMI 2 is defined as contrast flow beyond the site of occlusion and opacification of the entire artery but at a rate slower than normal (partial reperfusion), and TIMI 3, known as normal flow, as opacification of the entire artery at a normal rate. No-reflow is traditionally defined as TIMI grade 0 or 1, and slow flow is defined as TIMI grade 2 in this scheme.13
Angiographic success was defined as post-procedure TIMI flow grade 3 and a residual stenosis of less than 20%.2 The procedure was considered as successful if it was angiographically successful in all attempted lesions.
Clinical success was defined as a successful procedure in the absence of in-hospital major adverse cardiac events (MACEs: death, reinfarction, stroke and target vessel revascularization (TVR)) during hospitalization.2,16 Reinfarction after PCI was defined as recurrent symptoms of ischemia with new electrocardiographic changes, and/or a rise in cardiac troponin more than twice the normal limits. Early MACEs were defined as the occurrence of mentioned events during the first 30 days after STEMI. TVR was defined as ischemia-driven repeat percutaneous intervention, or bypass surgery of the target vessel. Target lesion revascularization (TLR) was defined as ischemia-driven repeat percutaneous intervention, or bypass surgery for the target lesion. Other adverse events in this study included arrhythmia, congestive cardiac failure, allergy, access site complications, and bleeding.
The left ventricular ejection fraction was determined using either echocardiography, or contrast ventriculography during the procedure.
Data Collection and Management: The data were collected by specific data collection forms. Data entry was done using the forms designed in EPI Info™ 3.3.2 (Center for Disease Control and Prevention; Atlanta, GA). Moreover, data were analyzed using the Statistical Package for Social Sciences (SPSS) for Windows 15.0 (SPSS Inc., Chicago, IL, USA).
All the continuous data were expressed as mean ± SD or range (min-max) and categorical data were expressed as number, and percentages. After descriptive analyses, categorical variables were compared using the chi-square test (or Fisher’s exact test if required), and continuous variables by using student’s t-test or Mann-Whitney test. P values of less than 0.05 were considered as statistically significant.
Results
From July to December 2011, 83 (89.2%) patients at Chamran Hospital and 10 (10.8%) patients at Saadi Hospital (93 patients in total) underwent PPCI. Table 1 describes baseline characteristics of the patients at the time of reaching the hospital. Mean age of the patients was 59.60 ± 11.10, and M/F ratio was 3.89.
Table 1.
Baseline characteristics of patients
| Age | ||
| Mean(years) | 59.60 ± 11.10 | |
| Range (years) | 33-86 | |
| Age≥ 65 years | 34 (36.6) | |
| Gender, M/F ratio | 74/19 | |
| MI location | ||
| Anterior | 58 (63) | |
| Inferior | 32 (34.8) | |
| Other | 2 (2.2) | |
| Killip class | ||
| 1 | 72 (77.4) | |
| 2 | 12 (12.9) | |
| 3 | 4 (4.3) | |
| Ischemic time (pain-to-door Time) | ||
| Mean(minutes) | 255.1 ± 221.4 | |
| Range (minutes) | 16-720 | |
| < 2hr | 29 (31.2) | |
| ≤ 2 hr < 4 hr | 21 (22.6) | |
| ≤ 4hr lt; 6 hr | 6 (6.5) | |
| ≤ 6hr < 12 hr | 30 (32.3) | |
| Missed | 7 (7.5) | |
| Unconsciousness at admission | 4 (4.3) | |
| Cardiogenicshock at admission | 9 (9.7) | |
| Renal insufficiency(Cr > 1.5) | 12 (12.9) | |
| Smoker * | 28 (30.1) | |
| Diabetes mellitus | 19 (20.4) | |
| Hypertension† | 24 (25.8) | |
| Hyperlipidemia‡ | 18 (19.4) | |
| Previous stroke | 1 (1.1) | |
| Previous CAD | 25 (26.9) | |
| EF | ||
| Mean(%) | 36.02 ± 11.58 | |
| Range (%) | 15-60 | |
| LowEF (< 40%) | 40 (43.0) | |
Categorical variables are expressed as n (%) and continuous variables are expressed as Mean ± SD or range (Min-Max).
M/F: Male/Female; MI: Myocardial infarction; SBP: Systolic blood pressure; CAD: Coronary artery disease; EF: Ejection fraction
Smoker: a person who has smoked at least 1 cigarette (or cigar, pipe) in the last month.
Hypertension: Systolic blood pressure > 140 mmHg; diastolic blood pressure > 90 mmHg; or taking hypertensive drugs
Hyperlipidemia: LDL cholesterol ≥ 130 mg/dl; triglycerides ≥150 mg/dl; and HDL ≤ 40 mg/dl; or on treatment of hyperlipidemia
Table 2 reveals angiographic success, lesion characteristics, and treatment strategies of the patients. The interventionalists attempted to treat 105 lesions (lesions/patient ratio = 1.13 ± 0.368) of the 181 involved vessels (involved vessels/patient ratio = 1.97 ± 0.70) in our patients. In total 116 stents (62 BMS and 54 DES) were deployed in 98 lesions, and 4 lesions were treated only by balloon angioplasty. 3 lesions remained inaccessible during the PPCI.
Table 2.
Basic angiographic success, lesion characteristics, and treatment strategies*
| Attemptedlesions | 105 (100) | |
| Left main | 1 (1.0) | |
| LAD | 58 (55.2) | |
| D1 | 5 (4.8) | |
| LCX | 9 (8.6) | |
| OM1 | 4 (3.8) | |
| RCA | 25 (23.8) | |
| PDA | 2 (1.9) | |
| Ramus | 1 (1.0) | |
| Lesioncharacteristics | ||
| Mean preprocedural stenosis,% | 96.19 ±7.44 | |
| Total occlusion | 61 (59.9) | |
| Proximal location | 44 (41.9) | |
| Small vessels (RVD < 3mm) | 23 (21.9) | |
| Long ( >10, < 20 mm) | 52 (49.5) | |
| Diffuse ( ≥ 20 mm) | 49 (46.7) | |
| Treatmentstrategy | ||
| Predilation balloon | 74 (70.5) | |
| Stenting | 98 (93.3) | |
| Postdilation balloon | 11 (10.5) | |
| Thrombectomy | 25 (23.8) | |
| TIMI gradeafter procedure | ||
| 0–1 | 9 (8.6) | |
| 2 | 10 (9.5) | |
| 3 | 84 (80.0) | |
| Angiographicsuccess | 83 (79.0) | |
Categorical variables are expressed as n (%) and continuous variables are expressed as mean ± SD.
LAD: Left anterior descending; D1: Diagonal1; LCX: Left circumflex artery; OM1: Obtuse marginal; RCA: Right coronary artery; PDA: Posterior descending artery;BMS: Bare metal stents; DES: Drug-eluting stents; BMS+DES: Combined DES and BMS stenting in a lesion, TIMI: Thrombolysis in myocardial infarction
Lesion-based Analysis
83 of 105 lesions were treated successfully (angiographic success rate = 79.0%).
Procedural details are described in table 3. Pain-to-door (time from onset of symptoms to hospital admission and door-to-balloon time were 255.1 ± 221.4 and 148.9 ± 168.5 min, respectively. Their medians were 255.1 and 148.8 min, respectively.
Table 3.
Procedural details* and complications of primary percutaneous coronary interventions
| SVD | 24 (25.8) | |
| Multivessel PCI|| | 11 (11.8) | |
| Primary | 69 (74.2) | |
| Rescue | 24 (25.8) | |
| Door-to-balloontime | ||
| Mean (min) | 148.9 ± 168.5 | |
| Range (min) | 24-900 | |
| IABP | 6 (6.5) | |
| Arrhythmia | 27 (29.0) | |
| Procedure | ||
| Plain old balloon angioplasty (POBA) | 3 (4.3) | |
| Guide wire crossfailure | 2 (2.2) | |
| Cardiogenic shock, only IABP / discontinue procedure for CPR | 1 (1.1) | |
| Use of stent | 87 (93.5) | |
| Only BMS | 42 (48.3) | |
| Only DES | 34 (39.1) | |
| BMS+DES | 11 (12.6) | |
| Stent/patientratio | 1.31 ± 0.64 | |
| Procedural acuteadverse events | ||
| Impaired flow | 17 (18.3) | |
| Access sitecomplications | 7 (7.5) | |
| Congestivecardiac failure | 13 (14) | |
| Bleeding | 1 (1.1) | |
| Proceduralsuccess rate | 71 (76.3) | |
Categorical variables are expressed as n (%) and continuous variables are expressed as mean ± SD.
SVD: Single vessel disease; PCI: Percutaneous coronary intervention; IABP: Intra-aortic balloon pump;
PVC: Premature ventricular contraction; VF: Ventricular fibrillation; VT: Ventricular Tachycardia; CPR: Cardiopulmonary resuscitation; LAD: Left anterior descending; LCX: Left circumflex artery; RCA: Right coronary artery
Patient Based Analysis
Multivessel PCI: PCI on more than one lesion in one stage
Pain-to-door time was significantly different in primary and rescue PCI (207.9 ± 203.9 min vs. 396.3 ± 217.3 min, P < 0.001), but the door-to-balloon time was not (137.1 ± 150.9 min vs. 184.7 ± 217.3 min, P = 0.359).
Thrombectomy was used in 23 (24.7%) patients, and stents were deployed in lesions of 87 (93.5%) patients.
The procedure failed due to impaired flow (n = 17 (18.3%)), failure to cross with a guide wire (n = 2 (2.2%)), suboptimal angiographic results (n = 2 (2.2%)), and death during procedure in one patient (procedural success rate = 76.3%). As mentioned above, impaired flow was the most frequent cause of failure. Slow flow (TIMI less than 3) was detected in 8 (47.1%) and no-reflow in 9 (52.9%) cases all of whom had been treated by stenting (BMS 9 (52.9%), DES 6 (35.3%), and combined stents 2 (11.8%)). This phenomenon was treated by intracoronary (IC) Integrilin in 12 cases (70.6%), IC epinephrine in 8 cases (47.1%), IC adenosine in 6 cases (35.3%), IABP in 3 cases (17.6%), and Nitrate in 3 cases (17.6%) in this series.
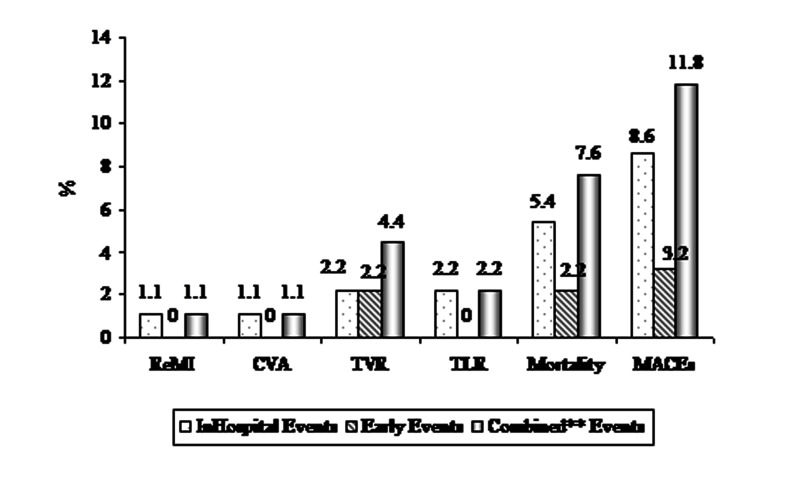
8 (8.6%) patients had MACEs during hospitalization which included 5 (5.4%) cases of in-hospital death (Figure 1). Of the five patients who died, 3 (60.0%) had cardiogenic shock, 3 (60%) had impaired flow. In-hospital mortality was significantly higher in the shock group (33.3% vs. 2.5%, P < 0.001), and in the older patients (over 65 years of age: 11.8% vs. 1.7%, P < 0.05). Successful PCI decreased in-hospital mortality significantly (33.3% vs 3.4%, P < 0.05) in our series.
Figure 1.
In-hospital and early* clinical outcome of the patients *Early clinical outcome: after discharge until 30 days; **Combined: In-hospital and early clinical outcome MACE: Major adverse cardiac events; ReMI: Repeated myocardial infarction; CVA: Cerebrovascular accident; TVR: Target vessel revascularization; TLR: Target lesion revascularization
PPCI was clinically successful in 67 (72.0%) patients. The response rate in the follow-up was 100%, and 3 other patients developed MACEs in this period (Figure 1). In total 11 (11.8%) patients had MACEs (combined MACEs) in our study. The rate of MACEs was significantly higher in the patients with impaired flow (29.4% vs. 7.0%, P = 0.009).
All of the PPCI failures and MACEs occurred in Chamran Hospital, but due to the small sample size at Saadi Hospital we could not compare clinical outcomes of the patients in these hospitals.
Discussion
PPCI is considered to be a superior strategy in treatment of STEMI.14,15 This procedure has been carried out for our patients since 2006, but, to our knowledge it has not yet been evaluated in any research project.
Our study revealed that procedural success rate was 76.3%, in-hospital MACEs was 8.6%, and combined MACEs was 11.8%. Alidoosti et al. described their experience of 83 primary angioplasty in STEMI based on their single center registry at Tehran Heart Center during a period of 2 years (2003-2005).17 Their reported procedural success rate was 95%, both in-hospital MACEs and mortality were 8.4%, and MACEs after 9 months was 12%.17 The results of our study are in accordance with that of the study by Alidoosti et al. regarding in-hospital MACEs, but are different in terms of success rates and in-hospital mortality. In comparison with other studies, although we reported lower success rates our patients’ early clinical outcomes were in accordance with international data.10,11,17 For instance, the in-hospital mortality, which was 5 (5.4%) cases, in our series of patients is comparable to international data, which showed in-hospital mortality of 5.2% in the second national registry of Myocardial infarction (NRMI2).18
In our study 9 patients had cardiogenic shock; 3 (33.3%) of them died, which is again in agreement with international data, which showed higher mortality in patients with cardiogenic shock (i.e. 32% in NRMI 2, 46.4% in shock registry, and 59.1% in American College of Cardiology-National Cardiovascular Data Registry (ACCNCDR).18-20
Poor angiographic and procedural results in our series were related to the most frequent cause of failure, which was impaired flow (18.3%). Although, the mechanisms of slow-flow and no-reflow phenomenon have been debated extensively, it has been proposed that obstruction of the myocardial microcirculation is a result of distal embolization or vasospasm.12 Moreover, it was revealed that the degree of impaired flow is associated with the duration of the preceding myocardial ischemia, infarct size, procedural variables, and patient characteristics.10
Our study revealed that pain-to-door and door-to-balloon times had an extremely wide range of almost 12, and 14.5 hours, respectively. These wide ranges, which were observed both in primary and rescue PCI, demonstrated that PPCI was not performed in a timely fashion.
In Tehran, 88% of the patients arrived at the hospital in the first 6 hours.17 46% of our patients arrived during this time. This shows that our patients request medical help later. We think that this is a multifactorial issue (cultural, socioeconomic, political, and educational), which could be improved by intersectoral and cross-sectoral collaboration, and the contribution of all authorities of the province.
Door-to-balloon time is exclusively related to health management, and it is an important determinant of the quality of care. The door-to-balloon time recommended by the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines is 90 minutes.2 However, achieving this time is only possible in an ideal world scenario. In developing countries financial constraints, insurance coverage problems, and delay in decision making due to lack of knowledge are the major obstacles in following door-to-balloon time recommendations. In Pakistan the median door-to-balloon time was reported to be 115 minutes with 40% of patients having PCI performed at or over 90 minutes.3 In China the median door-to-balloon time was 132 min, and only 22% of patients had PCI performed in less than 90 minutes.21 In Germany this time, from admission to start of PPCI, was 86 ± 42.22 In Tehran door-to-balloon time was not reported.17 Solving the Insurance problems, facilitating the process of admittance, discharge, and transfer, providing well-established protocols and an expert team, and informing the community could improve this Index.
Conclusion
The low success rate in our series could be due to prolonged pain-to-door time; community education is necessary to decrease this type of delay.
Long door-to-balloon time could be due to lack of a definite protocol to regularly perform PPCI in a timely fashion. We should define the duty and role of different components of the process of patient admission, transfer, and treatment to reduce door-to-balloon time.
Finally, we should improve our technique, especially to prevent and treat slow/no-reflow phenomena, in order to reach a better outcome after PPCI.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104(3):365–72. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 2.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation. 2004;110(5):588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 3.Shaikh AH, Siddiqui MS, Hanif B, Malik F, Hasan K, Adhi F. Outcomes of primary percutaneous coronary intervention (PCI) in a tertiary care cardiac centre. J Pak Med Assoc. 2009;59(7):426–9. [PubMed] [Google Scholar]
- 4.Raff GL, O'Neill WW. Interventional therapy of the acute coronary syndromes. Prog Cardiovasc Dis. 2002;44(6):455–68. doi: 10.1053/pcad.2002.124414. [DOI] [PubMed] [Google Scholar]
- 5.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 6.Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328(10):680–4. doi: 10.1056/NEJM199303113281002. [DOI] [PubMed] [Google Scholar]
- 7.Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O'Keefe J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1993;328(10):673–9. doi: 10.1056/NEJM199303113281001. [DOI] [PubMed] [Google Scholar]
- 8.Stone GW, Grines CL, Rothbaum D, Browne KF, O'Keefe J, Overlie PA, et al. Analysis of the relative costs and effectiveness of primary angioplasty versus tissue-type plasminogen activator: the Primary Angioplasty in Myocardial Infarction (PAMI) trial. The PAMI Trial Investigators. J Am Coll Cardiol. 1997;29(5):901–7. doi: 10.1016/s0735-1097(97)00041-7. [DOI] [PubMed] [Google Scholar]
- 9.Reeder GS, Bailey KR, Gersh BJ, Holmes DR, Christianson J, Gibbons RJ. Cost comparison of immediate angioplasty versus thrombolysis followed by conservative therapy for acute myocardial infarction: a randomized prospective trial. Mayo Coronary Care Unit and Catheterization Laboratory Groups. Mayo Clin Proc. 1994;69(1):5–12. doi: 10.1016/s0025-6196(12)61604-8. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117(24):3152–6. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 11.Hillegass WB, Dean NA, Liao L, Rhinehart RG, Myers PR. Treatment of no-reflow and impaired flow with the nitric oxide donor nitroprusside following percutaneous coronary interventions: initial human clinical experience. J Am Coll Cardiol. 2001;37(5):1335–43. doi: 10.1016/s0735-1097(01)01138-x. [DOI] [PubMed] [Google Scholar]
- 12.Yip HK, Chen MC, Chang HW, Hang CL, Hsieh YK, Fang CY, et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest. 2002;122(4):1322–32. doi: 10.1378/chest.122.4.1322. [DOI] [PubMed] [Google Scholar]
- 13.Prasad S, Meredith IT. Current Approach to Slow Flow and No-Reflow. Cardiac Interventions Today. 2008:43–51. [Google Scholar]
- 14.Simes RJ, Topol EJ, Holmes DR, White HD, Rutsch WR, Vahanian A, et al. Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion. Importance of early and complete infarct artery reperfusion. GUSTO-I Investigators. Circulation. 1995;91(7):1923–8. doi: 10.1161/01.cir.91.7.1923. [DOI] [PubMed] [Google Scholar]
- 15.Ross AM, Coyne KS, Moreyra E, Reiner JS, Greenhouse SW, Walker PL, et al. Extended mortality benefit of early postinfarction reperfusion. GUSTO-I Angiographic Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries Trial. Circulation. 1998;97(16):1549–56. doi: 10.1161/01.cir.97.16.1549. [DOI] [PubMed] [Google Scholar]
- 16.Smith SC, Feldman TE, Hirshfeld JW, Jacobs AK, Kern MJ, King SB, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention-Summary Article: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47(1):216–35. doi: 10.1016/j.jacc.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Alidoosti M, Salarifar M, Hajizeinali A, Kassaian SE, Kasemisaleh D, Goodarzynejad H. Outcomes of primary percutaneous coronary intervention in acute myocardial infarction at Tehran Heart Center. Med Princ Pract. 2007;16(5):333–8. doi: 10.1159/000104804. [DOI] [PubMed] [Google Scholar]
- 18.Tiefenbrunn AJ, Chandra NC, French WJ, Gore JM, Rogers WJ. Clinical experience with primary percutaneous transluminal coronary angioplasty compared with alteplase (recombinant tissue-type plasminogen activator) in patients with acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2). J Am Coll Cardiol. 1998;31(6):1240–5. doi: 10.1016/s0735-1097(98)00094-1. [DOI] [PubMed] [Google Scholar]
- 19.Webb JG, Sanborn TA, Sleeper LA, Carere RG, Buller CE, Slater JN, et al. Percutaneous coronary intervention for cardiogenic shock in the SHOCK Trial Registry. Am Heart J. 2001;141(6):964–70. doi: 10.1067/mhj.2001.115294. [DOI] [PubMed] [Google Scholar]
- 20.Klein LW, Shaw RE, Krone RJ, Brindis RG, Anderson HV, Block PC, et al. Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol. 2005;96(1):35–41. doi: 10.1016/j.amjcard.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SY, Hu DY, Sun YH, Yang JG. Current management of patients with ST elevation myocardial infarction in Metropolitan Beijing, China. Clin Invest Med. 2008;31(4):E189–E197. doi: 10.25011/cim.v31i4.4779. [DOI] [PubMed] [Google Scholar]
- 22.Zeymer U, Schroder R, Machnig T, Neuhaus KL. Primary percutaneous transluminal coronary angioplasty accelerates early myocardial reperfusion compared to thrombolytic therapy in patients with acute myocardial infarction. Am Heart J. 2003;146(4):686–91. doi: 10.1016/S0002-8703(03)00326-0. [DOI] [PubMed] [Google Scholar]