Abstract
Obesity has been associated with altered cerebral functions including cognitive control. The stop signal task (SST) has been widely used to study cognitive control by producing high conflict stop trials among many low conflict go trials. Contrasting these stop trials with go trials provides a measure of saliency processing and response inhibition. By comparing functional magnetic resonance images of obese (BMI >30) and lean (BMI <22) females performing the SST, we observed differences in regional brain activations despite similar behavioral performance between groups. Specifically, lean females had greater activations in the insula, inferior parietal cortex, cuneus, and supplementary motor area than obese females during stop as compared to go trials. This difference was caused by diminished brain activations in obese females in stop as compared to go trials. Furthermore, the brain activations in these regions inversely correlated to BMI across subjects. These preliminary findings suggest altered neural processes of cognitive control in obesity.
INTRODUCTION
Obesity is a growing problem in industrialized countries, where approximately a third of the population is obese, and was declared a global epidemic by the World Health Organization in 2003. Obesity has been associated with a number of physical health problems and decreased life expectancy (1). There is growing evidence that it is also associated with impaired cognitive functions (2–5). Although many individuals have been exposed to high-fat foods, not everyone becomes obese, suggesting individual variability and the importance of cognitive functioning including self control in association with obesity.
For many people suffering from obesity, there is decreased ability to resist highly fattening foods that would suggest impaired impulse control, and indeed, self-reported impulsivity positively correlates with caloric intake and BMI score (6). Self control can be assessed with behavioral paradigms such as the stop signal task (SST) (7,8). This as well as other cognitive control tasks evaluate inhibitory control and error-related behavioral adjustment. For instance, the stop signal reaction time (SSRT) measures the time it takes to stop a prepotent motor response and short SSRT indicates greater capacity of inhibitory control. In obese children, Nederkoorn et al. (9) found that longer SSRTs correlated with higher weight as well as a resistance to losing weight. Additionally, longer SSRTs were found to correlate to greater food consumption in college-aged women of normal weight (6). Obese children and adolescents were also found to have greater “inattention” or more variable reaction times in a go/nogo task (10).
Noninvasive brain imaging provides an opportunity to examine the cerebral processes associated with obesity. Many studies have evaluated how obese individuals responded to and made choices between different food stimuli (2,11–13). For instance, Jastreboff et al. (13) found that stress increased ventral striatum activity in obese and overweight individuals as compared to normal weight that might contribute to non-homeostatic feeding. Additionally, a recent positron emission tomography study examining glucose metabolism in the brain found that many prefrontal cortical areas showed a negative correlation between BMI and metabolic activity (14).
To investigate the neural processes of cognitive control in relation to obesity, we used the SST in conjunction with functional magnetic resonance images to compare obese (BMI >30) and lean (BMI <22) females. Specifically, we examined stop trials as compared to go trials, which typically elicit activation of a group of brain regions involved in response inhibition and saliency processing (15). After comparing basic brain activations in stop as compared to go trials for lean as they differed from obese subjects, we used the activity in those regions to see how well they correlated to BMI for women across a range of BMIs (16–37).
METHODS AND PROCEDURES
Subjects
A total of 43 healthy women were used in this study. Eighteen women were classified as lean (BMI <22, age 26.2 ± 6.7 years), 12 of intermediate weight (22 < BMI < 30, age 33.2 ± 16.7 years), and 13 obese (BMI >30, age 34.8 ± 9.6 years). Age did not differ between the three groups (ANOVA, F = 2.67, P < 0.082). All participants denied history of medical, neurological, or psychiatric illnesses. All participants denied use of illicit substances and tested negative for stimulants, opioids, benzodiazepines, and marijuana in urine toxicology tests prior to functional magnetic resonance images. None of the lean and two of the obese participants were smokers (χ = 2.96, P < 0.085). Subjects were instructed to eat as they normally would and scans were collected at approximately the same time of day across subjects. Smokers were allowed to smoke until ~1 h prior to functional magnetic resonance images if they chose to.
Behavioral task
We employed a simple reaction time task in this stop signal paradigm (7,36). There were two trial types: “go” and “stop,” randomly intermixed. A small dot appeared on the screen to engage attention at the beginning of a go trial. After a randomized time interval (fore-period) between 1 and 5 s, the dot turned into a circle (the “go” signal), which served as an imperative stimulus, prompting the subjects to quickly press a button. The circle vanished at a button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. Three quarters of all trials were go trials. The remaining one quarter were stop trials. In a stop trial, an additional “X,” the “stop” signal, appeared after and replaced the go signal. The subjects were told to withhold button press upon seeing the stop signal. Likewise, a trial terminated at button press or when 1 s had elapsed since the appearance of the stop signal. The stop signal delay (SSD)—the time interval between the go and stop signal—started at 200 ms and varied from one stop trial to the next according to a staircase procedure: if the subject succeeded in withholding the response, the SSD increased by 67 ms; conversely, if they failed, SSD decreased by 67 ms. There was an inter-trial interval of 2 s. Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. In the scanner each subject completed four 10-min runs of the task with the SSD updated manually across runs. Depending on the actual stimulus timing (trials varied in fore-period duration) and speed of response, the total number of trials varied slightly across subjects in an experiment. With the staircase procedure, we anticipated that the subjects would succeed in withholding their response in approximately half of the stop trials.
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio; Siemens, Malvern, PA). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC-PC line with repetition time = 300 ms, echo time = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4 mm and no gap. Functional, blood oxygenation level dependent signals were then acquired with a single-shot gradient echo echo-planar imaging sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with repetition time = 2,000 ms, echo time = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4 mm and no gap. Three hundred images were acquired in each run for a total of four runs.
Data analysis and statistics
Data were analyzed with Statistical Parametric Mapping (SPM8; Wellcome Department of Imaging Neuroscience, University College London, UK). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between radiofrequency pulsing and relaxation. Images of each individual subject were first corrected for slice timing and realigned (motion-corrected). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were normalized to an Montreal Neurological Institute echo-planar imaging template with affine registration followed by nonlinear transformation (37). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each subject. Finally, images were smoothed with a Gaussian kernel of 8 mm at full width at half maximum.
Four main types of trial outcome were first distinguished: go success (G), go error (F), stop success (SS), and stop error (SE) trial. An SS or SE, together stop (S), trial involves incongruent goals between the prepotency to respond and the motor intention to withhold the response, and thus is highly salient, compared to a G trial. A single statistical analytical design was constructed for each individual subject, using the general linear model with the onsets of go signal in each of these trial types convolved with a canonical hemodynamic response function and with the temporal derivative of the canonical hemodynamic response function and entered as regressors in the model (38). Realignment parameters in all six dimensions were also entered in the model. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts. Serial autocorrelation of the time series violated the general linear model assumption of the independence of the error term and was corrected by a first-degree autoregressive (1) model. The general linear model estimated the component of variance that could be explained by each of the regressors.
The con or contrast (difference in β) images of the first-level analysis were used for the second-level group statistics (random effect analysis; (39)). Brain regions were identified using an atlas (16). All templates are in Montreal Neurological Institute space and voxel activations are presented in Montreal Neurological Institute coordinates. We used MarsBaR to derive for each individual subject the effect size of activity change for regions of interest ((17); http://marsbar.sourceforge.net/). Regions of interest were defined from SS + SE > G (S > G) contrast comparing lean and obese women. Effect sizes were then extracted from these regions of interest for all 43 women, and correlated with their BMI.
RESULTS
Behavioral performance
Performance of lean (n = 18) and obese (n = 13) women was not statistically different on stop signal performance measures, including go trial RT, SSRT (36), and posterror slowing (36) (Table 1). The SSRT was calculated by subtracting the critical SSD (an estimated SSD that participants required to succeed in half of stop trials) from the median go trial RT (7,8). The effect size of posterror slowing is calculated by comparing the RT of poststop and postgo go trials (36).
Table 1.
Performance data (mean ± s.d.) for lean (n = 18), intermediate (n = 8), and obese (n = 14) groups
| BMI range | Average BMI | GoRT (ms) | SSRT (ms) | Posterror slowing (effect size) | Go success rate (%) | Stop success rate (%) | |
|---|---|---|---|---|---|---|---|
| Lean | 18.2–21.9 | 20.2 ± 1.0 | 584.5 ± 96.9 | 214.8 ± 40.9 | 1.90 ± 1.45 | 94.1 | 51.5 |
| Intermediate | 22.1–29.1 | 25.6 ± 2.0 | 633.3 ± 86.9 | 183.1 ± 45.1 | 1.84 ± 1.02 | 90.4 | 53.1 |
| Obese | 30.1–38.4 | 33.2 ± 2.6 | 584.4 ± 110.3 | 210.7 ± 37.1 | 1.59 ± 1.80 | 93.3 | 50.8 |
| ANOVA (F)a | 176.264 | 1.502 | 2.362 | 0.176 | 1.982 | 2.376 | |
| P value | 7.33E-21 | 0.235 | 0.107 | 0.839 | 0.151 | 0.106 |
ANOVA, analysis of variance; GoRT, reaction time of go trials; SSRT, stop signal reaction time.
F value of ANOVA.
Voxelwise imaging results and correlations with BMI
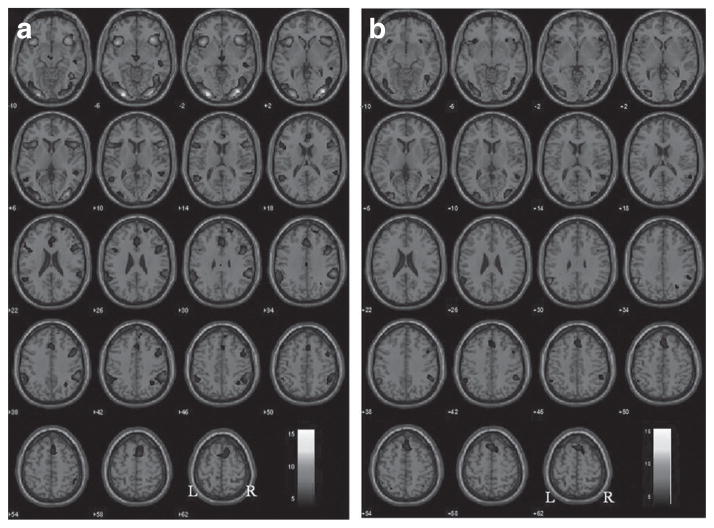
At P < 0.001, uncorrected, lean women showed activation of bilateral anterior insula extending on the right side to the inferior frontal gyrus, bilateral primary visual cortices, supplementary motor area, thalamus, and right inferior parietal cortex during stop as compared to go trials (Figure 1a; Table 2). Obese women show similar brain activations in the insula, visual cortices, and supplementary motor area though to a lesser extent (Figure 1b; Table 2).
Figure 1.
Brain regions showing activation in stop as compared to go trials for (a) lean and (b) obese subjects (P < 0.001, uncorrected). Blood oxygenation level dependent contrasts are superimposed on a T1 structural image in axial sections from z = −10 to z = 62. The adjacent sections are 4 mm apart. The color bar represents voxel T value. Neurological orientation: right = right.
Table 2.
Brain regions activated in stop as compared to go trials for lean and obese subjects
| Cluster size (voxels) | Voxel Z value | MNI coordinates (mm)
|
Side | Identified region | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Lean | ||||||
| 549 | 6.75 | −27 | −97 | −8 | L | Cuneus |
| 4.87 | −48 | −76 | −2 | L | Middle occipital G | |
| 3.35 | −39 | −64 | −2 | L | Middle occipital G | |
| 1311 | 6.73 | 36 | −85 | 1 | R | Middle occipital G |
| 6.18 | 27 | −97 | 1 | R | Middle occipital G | |
| 5.19 | 45 | −55 | −8 | R | Middle occipital G | |
| 1018 | 6.12 | 36 | 23 | −8 | R | Inferior frontal G |
| 5.7 | 36 | 14 | −14 | R | Inferior frontal G | |
| 5.4 | 60 | 20 | 10 | R | Inferior frontal G | |
| 669 | 6.09 | −39 | 17 | −5 | L | Inferior frontal G |
| 4.98 | −57 | 17 | 7 | L | Inferior frontal G | |
| 4.04 | −45 | 11 | 25 | L | Inferior frontal G | |
| 422 | 4.83 | −48 | −55 | 13 | L | Superior temporal G |
| 4.53 | −60 | −37 | 34 | L | Inferior parietal G | |
| 4.45 | −60 | −55 | 31 | L | Supramarginal G | |
| 656 | 4.41 | 0 | 26 | 34 | R | Cingulate G |
| 4.28 | 9 | 11 | 58 | R | Superior frontal G | |
| 4.24 | 15 | 23 | 61 | R | Superior frontal G | |
| 77 | 4.18 | −3 | −16 | −8 | L | Midbrain |
| 3.15 | 9 | −10 | 1 | R | Thalamus | |
| 24 | 3.77 | −51 | 41 | −5 | L | Middle frontal G |
| 3.6 | −45 | 50 | −2 | L | Middle frontal G | |
| 20 | 3.51 | 0 | −22 | 34 | R | Cingulate G |
| Obese | ||||||
| 179 | 4.89 | −57 | −55 | 31 | L | Supramarginal G |
| 3.77 | −51 | −52 | 52 | L | Inferior parietal G | |
| 3.28 | −33 | −61 | 55 | L | Inferior parietal G | |
| 295 | 4.72 | −33 | −88 | −8 | L | Inferior occipital G |
| 4.61 | −30 | −91 | 7 | L | Middle occipital G | |
| 4.03 | −45 | −76 | −5 | L | Middle occipital G | |
| 356 | 4.67 | 39 | −85 | 4 | R | Middle occipital G |
| 4.28 | 42 | −52 | −14 | R | Middle occipital G | |
| 4 | 48 | −73 | 4 | R | Middle occipital G | |
| 71 | 4.41 | 51 | −46 | 40 | R | Inferior parietal G |
| 292 | 4.25 | 3 | 26 | 61 | R | Superior frontal G |
| 3.88 | 0 | 29 | 52 | R | Superior frontal G | |
| 3.8 | 3 | 38 | 52 | R | Superior frontal G | |
| 44 | 3.93 | 54 | −49 | 10 | R | Superior temporal G |
| 68 | 3.65 | −33 | 23 | −8 | L | Inferior frontal G |
| 3.49 | −54 | 23 | 4 | L | Inferior frontal G | |
| 3.43 | −42 | 20 | −2 | L | Inferior frontal G | |
| 18 | 3.63 | 30 | 23 | −8 | R | Inferior frontal G |
| 18 | 3.43 | 48 | 23 | −8 | R | Inferior frontal G |
| 25 | 3.41 | 45 | 11 | 37 | R | Inferior frontal G |
Within individual clusters, all peak activations 8 mm apart are identified. Statistical threshold: P < 0.001, uncorrected; extent, 10 voxels.
G, gyrus; L, left; R, right; S, sulcus.
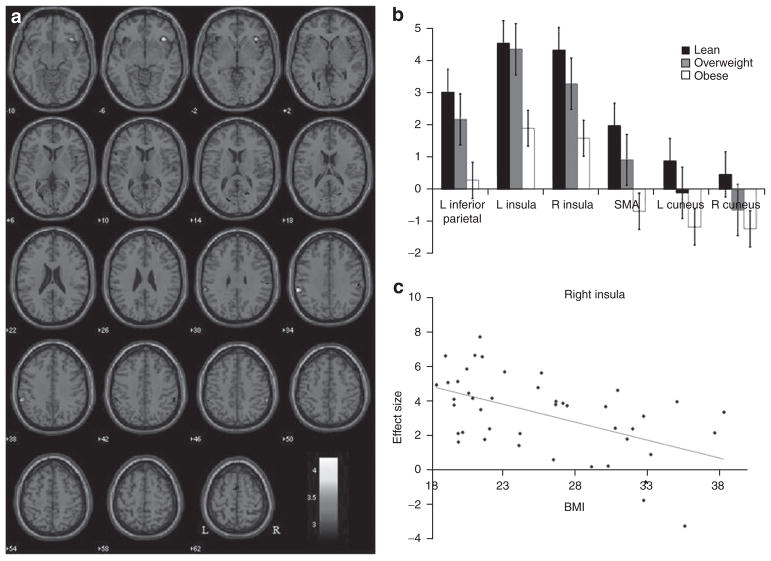
In a two sample t-test to compare lean to obese women for stop as compared to go trials, lean women showed greater brain activations overall than obese women at a threshold of P < 0.005, uncorrected with ten voxels in the extent of activation (Figure 2a). In particular, lean subjects show greater activations in bilateral insula, supplementary motor area, bilateral cuneus, and bilateral inferior parietal cortices (Table 3). We extracted the effect sizes of these regional brain activities. Figure 2b shows the mean effect sizes of lean and obese participants as well as those who are overweight. In linear regressions, we found that activity in all regions active in the stop as compared to go contrast correlated significantly to BMI across lean, overweight, and obese women (Table 4). In particular, activation of the right anterior insula during saliency processing showed the strongest correlation with BMI (r = −0.5127, P < 0.0004; Figure 2c). In an additional regression, we included age as a covariate. The results showed that activation of the right anterior insula was still significantly correlated with BMI (adjusted r = −0.5307, P < 0.003).
Figure 2.
Lean women show greater activations than obese women during stop as compared to go trials. (a) Brain regions showing more activation in stop as compared to go trials for lean as compared to obese subjects (P < 0.005, uncorrected). Blood oxygenation level dependent contrasts are superimposed on a T1 structural image in axial sections from z = −10 to z = 62. The adjacent sections are 4 mm apart. The color bar represents voxel T value. Neurological orientation: right = right. (b) Histograms showing the effect sizes of activations in regions of interests for lean, overweight, and obese subjects. (c) The effect sizes for right insula activation during saliency processing showed the most significant linear correlation to BMI across all subjects (r = −0.5127, P < 0.0004).
Table 3.
Brain regions activated in stop as compared to go trials for lean as compared to obese subjects
| Cluster size (voxels) | Voxel Z value | MNI coordinates (mm)
|
Side | Identified region | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 63 | 3.7 | −60 | −37 | 34 | L | Supramarginal G |
| 71 | 3.54 | 39 | 26 | −5 | R | Insula |
| 26 | 3.4 | 9 | −94 | −2 | R | Striate area |
| 2.96 | 12 | −94 | 10 | R | Striate area | |
| 20 | 3.27 | −57 | 17 | 7 | L | Inferior frontal G |
| 16 | 3.24 | 60 | −40 | 46 | R | Supramarginal G |
| 14 | 3.19 | 21 | −64 | 16 | R | Cuneus |
| 70 | 3.17 | −18 | −70 | 4 | L | Cuneus |
| 2.87 | −15 | −76 | 16 | L | Cuneus | |
| 20 | 3.13 | 21 | 56 | 25 | R | Superior frontal G |
| 27 | 2.97 | −6 | −1 | 67 | C | Superior frontal G |
| 2.76 | 3 | 5 | 61 | C | Superior frontal G | |
| 16 | 2.95 | 48 | 8 | 1 | R | Insula |
| 35 | 2.94 | −39 | 20 | −11 | L | Insula |
| 2.74 | −36 | 29 | −5 | L | Insula | |
| 10 | 2.75 | −15 | 53 | −5 | L | Middle temporal G |
Within individual clusters, all peak activations 8 mm apart are identified. Statistical threshold: P < 0.005, uncorrected; extent, 10 voxels.
G, gyrus; L, left; R, right; S, sulcus.
Table 4.
Results of Pearson regressions for BMI with all regions of interest activated during stop as compared to go trials with P values
| Region | Correlation coefficient | P value |
|---|---|---|
| Left inferior parietal | −0.4476 | 0.0026 |
| Left insula | −0.4415 | 0.0030 |
| Right insula | −0.5127 | 0.0004 |
| Supplementary motor area | −0.4063 | 0.0068 |
| Left cuneus | −0.4086 | 0.0065 |
| Right cuneus | −0.4184 | 0.0052 |
DISCUSSION
Obesity and regional brain activations during saliency processing
Despite indistinguishable performance on the SST, lean women showed greater brain activations to stop as compared to go trials than obese women. Saliency processing in regions including the left inferior parietal cortex, supplementary motor area, bilateral insula, and bilateral cuneus inversely correlated with BMI across lean, overweight, and obese women, with the right anterior insula showing the most significant association.
As part of the ventral attention system, the left inferior parietal cortex responds to infrequent but behaviorally relevant stimuli (18). The cuneus is a visual area which shows greater activation with attention to visual stimuli (7). Medial frontal cortex including the supplementary motor area responds to conflict, such as the stop trials, as compared to nonconflict go trials (15). Similarly, the right anterior insula increased activation during stop trials (19,20). Taken together, a network of brain regions responded to saliency processing and differentiated between obese and lean individuals.
There is growing evidence that dopamine is a major effector of saliency processing. Dopamine neurons spike at the appearance of salient, nonreward stimuli (21,22) and mediate decision making based on the salience of visual cues (23). Patients with Parkinson’s disease, a disorder of decreased nigrostriatal dopamine, have less memory for angry faces, which are thought to be more salient (24).
Indeed, studies have linked obesity with altered dopaminergic neurotransmission. Severely obese individuals (BMI >40) were shown to have lower dopamine D2 receptor levels and these levels correlated to their BMI (25). Furthermore, striatal dopamine D2 receptor levels positively correlated with brain metabolism in the prefrontal cortices, and were lower in obese subjects (26). Similar to rats showing drug addictions, obese rats that have been exposed to a “cafeteria diet” of cakes, bacon, frosting, etc. have been found to have decreased dopamine D2 receptor and increased reward thresholds (27).
Deficits in saliency processing have also been reported in individuals with drug addictions or other mental conditions such as attention deficit hyperactivity disorder, in which altered dopaminergic neurotransmissions are implicated. For instance, cocaine dependent individuals show hypoactivity in presupplementary motor area, insula, and cingulate cortex during nogo as compared to go trials (28). Individuals with attention deficit hyperactivity disorder showed decreased prefrontal cortical responses to nogo trials in go/nogo tasks (29,30), paralleling our findings with obese subjects in the SST. Taken together, evidence is accumulating to suggest shared neural processes underlying obesity, drug addictions, and attention deficit hyperactivity disorder, which all appear to implicate the dopaminergic systems (31).
Implications and limitations of the current findings
There is some evidence that altered cortical functions as observed in obese individuals may be reversible after weight loss. For instance, after gastric bypass surgery, there was a reduction in brain activity to high-calorie foods as subjects lost weight (32). Gunstad and colleagues showed improvement cognitive performance in obese people after bariatric surgery and weight loss (33). In an earlier study, Kretsch et al. (34) found that after obese women dieted and lost weight, they showed marked improvement in word recall performance.
These findings also indicate a need to measure and control for BMI in brain imaging studies of mental illnesses that frequently coexist with obesity. Obesity has been associated with a major lifetime risk of both axis I and axis II disorders (35). The need for consideration of BMI was reflected by a recent study examining brain metabolite levels in alcohol-dependent patients, which discovered that many of these brain metabolites are altered as a function of BMI, independent of alcohol dependence (40).
There are some limitations to this study. First, we determined whether an individual is obese, overweight, or lean by BMI, which does not take into account muscle composition. Therefore, the current study focused on females, who are less likely to have muscle mass as a confound. Future studies should use either a skinfold test, where a piece of skin is measured by calipers at a standardized location to determine subcutaneous fat, or waist circumference in relation to height measurements to allow for the estimation of obesity in both men and women. Second, obesity is known to be associated with medical conditions. Although our participants reported no conditions worthy of medical attention, we did not perform a thorough medical examination on our subjects. On the other hand, in a study of 68 patients seeking surgical treatment for obesity, Boeka and Lokken (3) showed that cognitive dysfunction in obese individuals was independent of other common comorbidities including diabetes, sleep apnea, and hypertension. Third, the current results were not statistically significant when evaluated at a threshold corrected for multiple comparisons. These results thus must be deemed preliminary and replicated in the future. Finally, we also acknowledge that a contrast between stop and go trials in the SST could potentially involve processes related to attentional monitoring and response inhibition. For instance, the presupplementary motor area is implicated in response inhibition (7). Thus, future studies of a larger number of participants are needed to disambiguate how these different psychological processes are compromised in obesity (7,15,36).
Conclusion
To summarize, we demonstrated that obesity is associated with altered saliency processing, broadly consistent with other findings of cognitive impairment and altered catecholaminergic function in obese individuals.
Acknowledgments
The current study was supported by a National Institutes of Health (NIH) grant K02DA026990. The NIH had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 2.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeka AG, Lokken KL. Neuropsychological performance of a clinical sample of extremely obese individuals. Arch Clin Neuropsychol. 2008;23:467–474. doi: 10.1016/j.acn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Gunstad J, Paul RH, Cohen RA, et al. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Lokken KL, Boeka AG, Austin HM, Gunstad J, Harmon CM. Evidence of executive dysfunction in extremely obese adolescents: a pilot study. Surg Obes Relat Dis. 2009;5:547–552. doi: 10.1016/j.soard.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Guerrieri R, Nederkoorn C, Stankiewicz K, et al. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007;49:66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logan GD. Spatial attention and the apprehension of spatial relations. J Exp Psychol Hum Percept Perform. 1994;20:1015–1036. doi: 10.1037//0096-1523.20.5.1015. [DOI] [PubMed] [Google Scholar]
- 9.Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behav Res Ther. 2007;45:1071–1075. doi: 10.1016/j.brat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Pauli-Pott U, Albayrak O, Hebebrand J, Pott W. Association between inhibitory control capacity and body weight in overweight and obese children and adolescents: dependence on age and inhibitory control component. Child Neuropsychol. 2010;16:592–603. doi: 10.1080/09297049.2010.485980. [DOI] [PubMed] [Google Scholar]
- 11.Bruce AS, Holsen LM, Chambers RJ, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond) 2010;34:1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davids S, Lauffer H, Thoms K, et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes (Lond) 2010;34:94–104. doi: 10.1038/ijo.2009.193. [DOI] [PubMed] [Google Scholar]
- 13.Jastreboff AM, Potenza MN, Lacadie C, et al. Body mass index, metabolic factors, and striatal activation during stressful and neutral-relaxing states: an FMRI study. Neuropsychopharmacology. 2011;36:627–637. doi: 10.1038/npp.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkow ND, Wang GJ, Telang F, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 2009;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li CS, Yan P, Chao HH, et al. Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience. 2008;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duvernoy HM. The Human Brain: Surface, Blood Supply, and Three-Dimensional Sectional Anatomy. 2. Springer Verlag; New York, NY: 1999. [Google Scholar]
- 17.Brett M, Anton J-L, Valabregue R, Poline J-P. Region of Interest Analysis Using an SPM Toolbox. Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2–6 June 2002. [Google Scholar]
- 18.Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond, B, Biol Sci. 1998;353:1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramautar JR, Slagter HA, Kok A, Ridderinkhof KR. Probability effects in the stop-signal paradigm: the insula and the significance of failed inhibition. Brain Res. 2006;1105:143–154. doi: 10.1016/j.brainres.2006.02.091. [DOI] [PubMed] [Google Scholar]
- 21.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 22.Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Guo JZ, Peng Y, Xi W, Guo A. Dopamine-mushroom body circuit regulates saliency-based decision-making in Drosophila. Science. 2007;316:1901–1904. doi: 10.1126/science.1137357. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian L, Hindle JV, Jackson MC, Linden DE. Dopamine boosts memory for angry faces in Parkinson’s disease. Mov Disord. 2010;25:2792–2799. doi: 10.1002/mds.23420. [DOI] [PubMed] [Google Scholar]
- 25.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 30.Fallgatter AJ, Ehlis AC, Seifert J, et al. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clin Neurophysiol. 2004;115:973–981. doi: 10.1016/j.clinph.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 31.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond, B, Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochner CN, Kwok Y, Conceição E, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunstad J, Strain G, Devlin MJ, et al. Improved memory function 12 weeks after bariatric surgery. Surg Obes Relat Dis. 2010 doi: 10.1016/j.soard.2010.09.015. e-pub ahead of print 30 October 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretsch MJ, Green MW, Fong AK, Elliman NA, Johnson HL. Cognitive effects of a long-term weight reducing diet. Int J Obes Relat Metab Disord. 1997;21:14–21. doi: 10.1038/sj.ijo.0800353. [DOI] [PubMed] [Google Scholar]
- 35.Carpiniello B, Pinna F, Pillai G, et al. Psychiatric comorbidity and quality of life in obese patients. Results from a case-control study. Int J Psychiatry Med. 2009;39:63–78. doi: 10.2190/PM.39.1.e. [DOI] [PubMed] [Google Scholar]
- 36.Li CS, Huang C, Yan P, et al. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 38.Friston KJ, Josephs O, Zarahn E, et al. To smooth or not to smooth? Bias and efficiency in fMRI time-series analysis. Neuroimage. 2000;12:196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- 39.Penny W, Holmes AP. Random-effects analysis. In: Frackowiak SJ, Ashburner JT, Penny WD, et al., editors. Human Brain Function. Elsevier; San Diego: 2004. pp. 843–850. [Google Scholar]
- 40.Gazdzinski S, Durazzo TC, Mon A, Meyerhoff DJ. Body mass index is associated with brain metabolite levels in alcohol dependence– a multimodal magnetic resonance study. Alcohol Clin Exp Res. 2010;34:2089–2096. doi: 10.1111/j.1530-0277.2010.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]