Abstract
Objectives
To estimate the population burden of heart failure and the influence of modifiable risk factors.
Background
Heart failure is a common, costly, and fatal disorder, yet few studies have evaluated the population-level influence of modifiable risk factors.
Methods
From 14,709 Atherosclerosis Risk in Communities study participants we estimated incidence rate differences (IRD) for the association between five modifiable risk factors (cigarette smoking, diabetes, elevated low density lipoproteins, hypertension, and obesity) and heart failure. Potential impact fractions were used to measure expected changes in the heart failure incidence assuming achievement of a 5% proportional decrement in the prevalence of each risk factor.
Results
Over an average of 17.6 years of follow-up, 1 in 3 African American and 1 in 4 Caucasian participants were hospitalized with heart failure, defined as the first hospitalization with ICD-9 discharge codes of 428.x. Of the five modifiable risk factors, the largest IRD was observed for diabetes, which was associated with 1,058 (95% CI: 787–1,329) and 660 (95% CI: 514–805) incident hospitalizations of heart failure/100,000 person-years among African American and Caucasian participants, respectively. A 5% proportional reduction in the prevalence of diabetes would result in approximately 53 and 33 fewer incident heart failure hospitalizations per 100,000 person-years in African American and Caucasian ARIC participants, respectively. When applied to U.S. populations, this reduction may prevent approximately 30,000 incident cases of heart failure annually.
Conclusions
Modest decrements in the prevalence of modifiable heart failure risk factors such as diabetes may substantially decrease the incidence of this major disease.
Keywords: heart failure, epidemiology, diabetes
INTRODUCTION
Heart failure is a common, costly, disabling, and often fatal disorder that affects approximately 5.8 million Americans (1). Despite improvements in medical care and advances in therapy, hospital discharges for heart failure have increased 155% over the past two decades (2) and heart failure has become the most common cause for hospital admission (3) and readmission (4). Although the considerable morbidity and mortality attributed to heart failure can be reduced by treatment (5,6), approximately half of those with heart failure die within five-years of diagnosis (2). Thus, the aging U.S. population combined with heart failure treatment costs that are the most expensive of all Medicare diagnoses (7), make heart failure a major - and growing-public health burden.
Numerous studies have evaluated associations between heart failure and risk factors including previous coronary heart disease (CHD), elevated blood pressure, hypercholesterolemia, overweight/obesity, cigarette smoking, and arrhythmias (8,9). Yet, few have explicitly measured the population burden of heart failure attributable to modifiable risk factors (10–12). Population burden measures, for example the population impact fraction (13), are of direct relevance for primary prevention, resource allocation and research prioritization, as they estimate the number of events that may be preventable if the prevalence of a modifiable risk factor was reduced. Similarly useful for informing heart failure research agendas and primary prevention efforts are disability adjusted life years (DALYs), which inform on premature mortality and years lived with disability (14,15). Therefore, we evaluated the population burden of heart failure measured by the lifetime risk, incidence rate differences (IRD), DALYs, and potential impact fractions (PIF) in 14,709 Atherosclerosis Risk in Communities (ARIC) Study participants.
MATERIALS AND METHODS
Study Population
The ARIC study includes an ongoing population-based cohort of 15,792 Caucasian and African American males and females aged 45–64 years at baseline that was recruited in 1987–89 using probability sampling from four United States communities: Forsyth County, North Carolina, the northwest suburbs of Minneapolis, Minnesota, Washington County, Maryland, and Jackson, Mississippi (16). Standardized physical examinations and interviewer-administered questionnaires were conducted at baseline, and at three triennial follow-up examinations. Participant follow-up through annual telephone interviews, review of hospitalization records and vital status is ongoing. Institutional Review Boards at each participating institution approved the ARIC Study and all participants provided informed consent before each examination.
We excluded a total of 1,083 participants (6.8% of the total ARIC cohort) from the analysis: 755 participants with prevalent heart failure; 280 participants missing data on variables used to assess prevalent heart failure; and 48 participants who did not self-report a race of Caucasian or African American. After these exclusions, the total sample size was 14,709 participants.
Exposure and covariate definitions
We examined five potentially modifiable cardiovascular risk factors, which were assessed at study baseline and three triennial examinations: current smoking, diabetes, elevated low density lipoprotein concentration (LDL), hypertension, and obesity. Criteria for inclusion in this study included the availability of measures at all four ARIC visits, previously documented associations with the incidence of heart failure, and amenability to change. Current smoking status was ascertained by interviewer-administered questionnaire. Diabetes was defined as fasting plasma glucose ≥ 126 mg/dl, non-fasting glucose ≥ 200 mg/dl, self-reported use of diabetes medications, or self-reported physician diagnosis of diabetes. Elevated LDL was defined as LDL ≥ 160 mg/dl or self-reported use of lipid-lowering medications. Participants with systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or who reported the use of medications for blood pressure control were classified as hypertensive. Body mass index dichotomized at ≥ 30 kg/m2 was used to define obesity. Serum creatinine was measured and analyzed as previously described (17). We calculated estimated glomerular filtration rate using the CKD-EPI equation (18). Medication use was ascertained by medication inventory using Therapeutic Classification System codes.
Ascertainment of incident heart failure events
Heart failure events were ascertained through active surveillance of local hospital discharge lists to identify hospitalizations with cardiovascular disease diagnoses. Yearly interviews of cohort members during which all hospitalizations were reported were also conducted to identify hospitalizations that occurred outside the ARIC catchment areas. Incident heart failure hospitalizations were defined as the first recorded hospitalization with International Classification of Diseases, Ninth Revision discharge codes of ‘428.x’ in any position. Validation of heart failure hospitalizations indicated that the positive predictive value of ‘428.x’ was 93% for acute decompensated heart failure and 97% for chronic heart failure (19). Heart failure hospitalization data were available from study baseline through December 31, 2008.
Ascertainment of incident coronary heart disease and stroke events
To facilitate comparisons with lifetime risk estimates of heart failure, we also examined incident CHD and stroke events. Incident CHD was defined as a validated definite or probable hospitalized MI, a definite CHD death, or an unrecognized MI defined by ARIC electrocardiogram readings. The criteria for definite or probable hospitalized MI were based on combinations of chest pain symptoms, ECG changes, and cardiac enzyme levels (20). An incident stroke occurrence was defined as a first definite or probable hospitalized stroke occurring in a participant free of a history of physician-diagnosed stroke at the baseline interview. Adjudicated events were available from baseline through December 31, 2008.
Statistical analysis
Race-specific lifetime risk estimates conditional on survival free of each disease to age 45 and accounting for the competing risk of death were calculated as:
where A represents age, j indexes ordered failure times among N participants, h is the hazard or conditional probability estimate of developing the disease at time tj given survival beyond time tj−1, and Ûis the estimated survival probability (21). The timescale of interest was age; participants contributed to the risk set beginning at their age of entry into the cohort. Accounting for competing risks was necessary because traditional survival analytic techniques (e.g. the Kaplan-Meier method (22)) assume that heart failure is still possible beyond the time of censoring (21); since heart failure, stroke, and CHD cannot occur after death, death was defined as a competing risk. The variance of the cumulative incidence was estimated using a Taylor series linear expansion (23). Confidence intervals for incidence rates were estimated using a method described by Haenszel (24). Age-adjusted rates were directly standardized to the entire population. Poisson regression was used to estimate race-specific IRDs for the five modifiable risk factors adjusting for age (in 10-year categories) and sex. Event time was defined as the time until the first heart failure hospitalization.
Using the PIF, we estimated the expected proportional change in the average heart failure incidence assuming a reduction in the prevalence of a categorical outcome with n discrete levels:
where RR(i) represents the relative risk at exposure level i, P(i) is the population prevalence, and P’(x) is the counterfactual population prevalence (13). Proportional reductions in the prevalence of a given risk factor imply a shift below the risk factor threshold of the corresponding proportion of the population (i.e. into the unexposed population group). Race-, sex-, and age-specific (10-year categories) RRis were recombined by race using a case-load weighted sum method (10).
DALYs were used to quantify disability, as direct measures of disability and functional limitations were unavailable. DALYs are a commonly used health metric that was introduced in the mid-1990s by the World Health Organization Global Burden of Disease project so that nonfatal outcomes spanning cancers, cardiovascular diseases, and infectious diseases could be considered on a common scale for the evaluation of health resource use and research prioritization (15,25). Specifically, DALYs combine data on mortality (years of life lost, YLL) and non-fatal health outcomes (years of life lived with disability, YLD) into a single measure that estimates the difference between current and ideal health states (26–28). DALYs were developed DALYs were calculated as the sum of YLL and YLD:
where:
and:
Disability following heart failure hospitalizations was not directly measured. Instead, a disability weight was used to indicate the severity of disease on a scale from 0 to 1. A disability weight of ‘0’ indicates perfect health (i.e. no disability) and a disability weight of ‘1’ indicates death. The disability weight assigned to heart failure by the Global Burden of Disease panel of reviewers was 0.201 (29).
The burden of disease due to mortality (i.e. YLLs) was calculated as the race-, sex-, and age-specific (five-year age groups) one-year mortality rate multiplied by the age-specific life expectancy based on Year 2004 U.S. life tables. For YLD estimation, an incidence-based approach was adopted that measured years with disability as incidence × duration, defined as the incidence of heart failure multiplied by the mean duration of disability, in race, sex, and five year age intervals (14). The mean duration of disability was estimated by the median survival time following the first heart failure hospitalization. For the calculation of DALYs attributable to heart failure risk factors, DALYs were multiplied by the population attributable fraction (30) for each risk factor and the specified proportional reduction in the exposure (5% assumed), assuming a constant disease progression for each risk factor. This resulting metric is interpreted as the absolute reduction in DALYs (measured in years) associated with a population-level reduction in a given exposure. All statistical analyses were performed using SAS 9.1 (Cary, North Carolina).
RESULTS
ARIC participants contributed an average of 17.6 years of person-time through 2008, during which 2,102 heart failure cases were identified (Table 1). The one-year age-adjusted incidence rate of heart failure was higher in African Americans (1,126/100,000 person-years) compared to Caucasians (676/100,000 person-years) (Table 2), as was the prevalence of hypertension, diabetes, and chronic kidney disease. Median survival among Caucasian participants with heart failure following the index hospitalization was 8.6 years, approximately four years longer than the median survival among African American participants (4.9 years). An estimated 196 African Americans (27.4%) and 349 Caucasians (25.2%) died within one year of their incident heart failure hospitalization.
TABLE 1.
Baseline characteristics of ARIC Study participants (N=14,709) by race, 1987–1989.
| Characteristics | African Americans (n=3,902) |
Caucasians (n=10,807) |
|---|---|---|
| Mean age in years, (SD) | 53 (5.8) | 54 (5.7) |
| Female, N (%) | 2,374 (60.8) | 5,140 (47.6) |
| Mean BMI in kg/m2 (SD) | 29.4(6.1) | 26.9 (4.8) |
| Prevalent CHD, N (%) | 117(3.0) | 483 (4.5) |
| Current smoker, N (%) | 1,169(30.0) | 2,645 (24.5) |
| Diabetes, N (%) | 706 (18.6) | 918(8.5) |
| ≥ High school education, N (%) | 2,314(59.4) | 8,971 (83.1) |
| Hypertension, N (%) | 2,080 (53.3) | 2,741 (25.4) |
| eGFR in ml/min/1.73 m2, (SD) | 104.0 (19.6) | 95.7(14.9) |
| eGFR < 60 ml/min/1.73 m2, N (%) | 84 (2.2) | 159(1.5) |
| Mean LDL in mg/dl, (SD) | 137.4 (42.7) | 137.7 (37.9) |
| ACE inhibitor use, N (%) | 305 (2.8) | 161 (4.3) |
| Diuretics use, N (%) | 846(22.1) | 1,226(11.4) |
| Beta blocker use, N (%) | 367 (9.4) | 1,083 (10.1) |
ACE, angiotensin-converting enzyme; ARIC, Atherosclerosis Risk in Communities Study; BMI, body mass index; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; LDL, low density lipoprotein concentration; N, number; SD, standard deviation.
TABLE 2.
Race-specific incidence rates of heart failure per 100,000 person-years, the ARIC cohort study, 1987–2008.
| African Americans |
Caucasians |
|||
|---|---|---|---|---|
| Age group | N. events | Incidence rate/100,000 |
N. events | Incidence rate/100,000 |
| 45–54 years | 34 | 260 | 40 | 132 |
| 55–64 years | 238 | 818 | 308 | 379 |
| 65–74 years | 334 | 1,581 | 662 | 946 |
| >75 years | 110 | 2,410 | 376 | 2,039 |
| Total | 716 | 1,126* | 1,386 | 676* |
ARIC, Atherosclerosis Risk in Communities Study; HF, heart failure;
HF incidence rate is age-adjusted
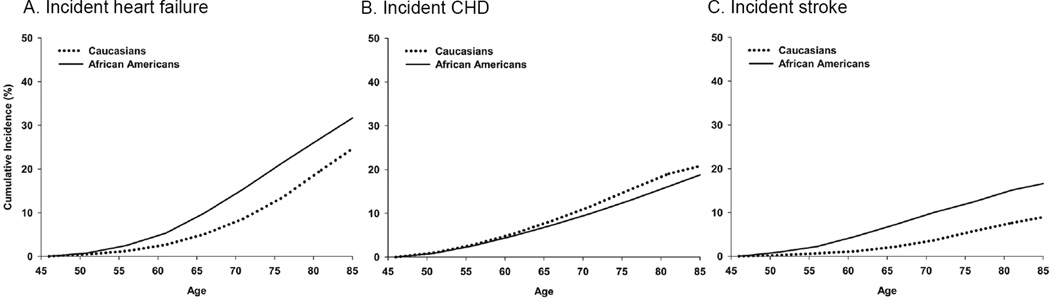
The lifetime risk of heart failure adjusted for competing risk of death for adults over 45 years old was 32.8% (95% CI: 27.4, 36.1) or 1 in 3 for African Americans and 25.9% (95% CI: 22.7, 28.0) or 1 in 4 for Caucasian participants (Figure 1). For both Caucasian and African American participants, the lifetime risk of hospitalized heart failure was higher than the lifetime risk of incident CHD and incident stroke.
FIGURE 1. Lifetime risk estimates of heart failure, CHD, and stroke.
Race-specific lifetime risk of incident hospitalized heart failure (panel A), incident CHD (panel B), and incident stroke (panel C), the Atherosclerosis Risk in Communities study, 1987–2008. Lifetime risks are estimated conditional on survival to age 45 and adjusted for a competing risk of death.
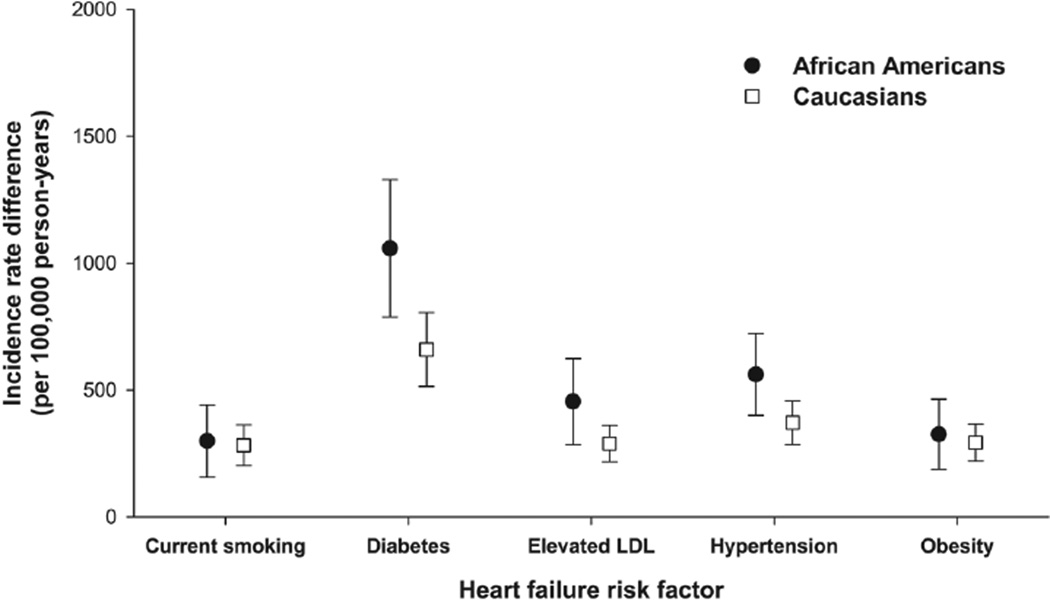
Of the five modifiable risk factors we examined (current smoking, diabetes, elevated LDL, hypertension, and obesity), diabetes was associated with the largest incidence rate difference (Figure 2). Specifically, diabetes accounted for 1,058 (95% CI: 787, 1,329) and 660 (95% CI: 514, 805) incident cases of heart failure per 100,000 person-years among African American and Caucasian participants, respectively. IRD estimates for current smoking, elevated LDL, hypertension and obesity were generally consistent across African American and Caucasian populations, although IRD estimates for elevated LDL and hypertension were slightly elevated among African American ARIC participants. As a sensitivity analysis, we examined how robust IRD estimates were to adjustment for all risk factors together and beta blockers, ACE inhibitors and diuretic use. Inferences were robust to adjustment for both (results not shown).
FIGURE 2. Incidence rate differences for five modifiable heart failure risk factors.
Race-specific estimated heart failure incidence rate differences for current smoking, diabetes, elevated LDL, hypertension and obesity, the Atherosclerosis Risk in Communities study, 1987–2008. Incidence rate difference estimates are adjusted for age and sex and presented per 100,000 person years.
Heart failure was also associated with disability and early mortality. In the ARIC cohort, heart failure was associated with 9,527 YLLs. Approximately 12,473 years of life were lived following the incident heart failure hospitalization; multiplying this estimate by the heart failure disability weight of 0.201 yielded 2,507 YLD. Together, the YLD and YLL estimates indicated that, on average, ARIC participant with heart failure experienced 5.7 DALYs, the majority representing early mortality.
Finally, we evaluated how population-level reductions in the prevalence of modifiable heart failure risk factors may influence estimates of the incidence of disease, disability, and early mortality. As shown in Table 3, modest decrements in the population prevalence of modifiable risk factors would decrease the heart failure incidence. For example, a 5% proportional reduction in the prevalence of diabetes in ARIC African American participants (i.e. reducing the diabetes prevalence from 31.0% to 29.5%) would result in approximately 53 fewer heart failure cases per 100,000 person-years (Table 3). A 5% proportional reduction in the prevalence of diabetes in ARIC would also decrease the estimated number of heart failure DALYs by 183 years overall, largely reflecting a reduction in the number of YLL.
TABLE 3.
Race-specific estimates of the preventable number of heart failure cases, years of life lost, and years of life lived with disability that would result from a 5% proportional reduction in the prevalence of five cardiovascular disease risk factors, the ARIC cohort study, 1987–2008.
| African Americans | Caucasians | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure | Period prevalence, 1987–1998* |
Preventable number of heart failure cases† |
Disability adjusted life vears |
Period prevalence, 1987–1998* |
Preventable number of heart failure cases† |
Disability adjusted life vears |
||
| Number of Years of Life Lost‡ |
Number of Years of Life with Disability‡ |
Number Years of Life Lost‡ |
Number of Years of Life with Disability‡ |
|||||
| Current smoking | 32.4 | 15 | 28 | 6 | 26.8 | 10 | 63 | 19 |
| Diabetes | 31.0 | 53 | 65 | 13 | 17.1 | 33 | 81 | 24 |
| Elevated LDL | 65.4 | 23 | 60 | 12 | 60.5 | 11 | 120 | 36 |
| Hypertension | 71.1 | 28 | 68 | 14 | 45.3 | 19 | 102 | 31 |
| Obesity | 50.2 | 16 | 39 | 8 | 33.9 | 15 | 69 | 21 |
Assessed at baseline and three triennial visits;
Per 100,000 person years;
Per year for all participants with heart failure. ARIC, Atherosclerosis Risk in Communities study; DALYs, disability adjusted life years; LDL, low-density lipoprotein;
DISCUSSION
In this biracial cohort of middle-aged males and females, we estimated a lifetime risk of heart failure of 1 in 3 for African Americans and 1 in 4 for Caucasians. Heart failure was also associated with early mortality and disability. However, we estimated that 5% reduction in modifiable heart failure risk factors would markedly decrease the incidence of disease as well as heart failure-related early mortality and disability in this population.
Few studies have reported estimates of the population burden of heart failure (10–12). Lifetime risk estimates, for example, are useful as they may be more easily understood by clinicians and patients than relative effect measures and can be compared across diseases to understand competing causes of morbidity and mortality (31). Several studies have reported lifetime risk estimates of heart failure in populations of European descent (11,32,33), which were comparable in magnitude to lifetime risks of heart failure we estimated among Caucasians. However, to our knowledge, we are the first to present such estimates in an African American population and show that the lifetime risk of heart failure is 1 in 3, which is higher than that of stroke or CHD in the same population.
Evaluating disability is especially important for conditions such as heart failure, which occurs primarily in the elderly and is characterized by a heavy comorbidity burden. Yet, few studies have quantified the heart failure disability burden and compared it to other major diseases. One exception is a Dutch study from the mid-1990s, which reported that heart failure early mortality and disability were comparable in magnitude to dementia and colon and rectal cancer (34). It is difficult to extrapolate these results to present-day populations, given demographic shifts and differences in risk factor burdens among countries. Future studies of heart failure should therefore leverage validated outcome measurements from contemporary studies.
Analyses examining five modifiable heart failure risk factors suggested that in the ARIC study, diabetes was associated with the greatest population burden of heart failure when compared to current smoking, elevated LDL, hypertension and obesity. Diabetes is a known risk factor, being associated with stage A heart failure (35), although hypertension and CHD are traditionally viewed as the strongest heart failure risk factors. Although IRD estimates presented herein may seem of small magnitude, extrapolations to U.S. African American and Caucasian population aged 45 years or greater suggest that approximately 30,000 annual incident cases of heart failure may be prevented by modest decrements in this modifiable risk factor. Of note, this estimate does not consider the effect of reducing incident HF cases on subsequent rehospitalizations for these prevented incident events.
Although population attributable risk estimates are more commonly reported in studies examining the population burden of heart failure (12,36), we chose to present potential impact fractions, as these estimates do not presume complete elimination of a given risk factor. Here, we considered a 5% proportional reduction in five modifiable risk factors that have been consistently associated with heart failure. A 5% proportional reduction in the prevalence of diabetes is less ambitious than Healthy People 2010 goals, which aim to reduce the age-adjusted prevalence of clinically diagnosed diabetes from a baseline of 40 cases/1,000 people (in 1997) to the Healthy People 2010 target of 25 cases/1,000 people, a 37.5% reduction. However, age-adjusted rates for diabetes, obesity and cigarette smoking increased over the past decade (37), underscoring the need for more aggressive evidence-based prevention efforts. It also highlights the importance of focusing on interventions that are practical, as the estimates presented herein are meaningless unless interventions are available and feasible to implement.
The strengths of this study include the standardized and repeated assessment of heart failure risk factors in a biracial, population-based cohort and an extended follow-up of participants with high retention (>90%). One limitation is that we did not include outpatient events, although Roger (2004) suggested that the majority of heart failure cases identified in outpatient settings were hospitalized within 1.7 years (2). It is also unclear whether our results are generalizable to the present day U.S., as only four communities were represented in the ARIC study and we included heart failure events that occurred in the late 1980s and 1990s. However risk factor profiles estimated in ARIC participants are similar to those reported in other population-based U.S. studies (1). We also report PIF estimates that consider each potentially modifiable risk factor separately, although these five risk factors often cluster together and interventions to reduce the prevalence of one would likely affect the prevalence of the others. Likewise, we did not evaluate the influence of population shifts in physical activity and diet on the incidence of heart failure, although such interventions would likely be necessary to achieve lasting population-level changes in cardiometabolic risk factors. Future research examining the interrelationship between the modifiable heart failure risk factors examined herein and the utility of shifting physical activity and diet patterns is clearly needed.
Finally, we focused on clinically defined cutpoints for potentially modifiable risk factors, including diabetes, elevated LDL, adiposity, and elevated blood pressure, as opposed to interval scale exposure values. One advantage of predefined cutpoints was that we were able to avoid strong assumptions about the relation between the exposures and risk of disease. The clinical cutpoints are also well-understood and provide estimates of the potential impact clinicians could achieve by adhering to current practice guidelines. However, small reductions in heart failure risk factors, such as a one or two mg/dl shifts in the population distribution of fasting plasma glucose, may be more easily achievable on a population level and should be examined further (38,39). Examining the burden of modifiable risk factors in populations at high risk for heart failure, including those with atrial fibrillation, coronary heart disease, or chronic kidney disease, is also warranted.
Randomized clinical trials have supported the utility of pharmacological interventions to improve survival in populations with heart failure (5,6), yet prognosis remains bleak. Given the aging of the population, improved post-MI survival and the increasing prevalence of modifiable heart failure risk factors, primordial prevention of heart failure likely provides the greatest opportunity for reducing the incidence of this major disease. Although additional studies are needed to verify the estimated magnitude of effect between the modifiable risk factors we examined and heart failure, our results suggest that modest improvements in the population prevalence of modifiable heart failure risk factors may decrease both the incidence of disease and the number of years lost or lived with disability.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
CLA was supported by grant R00-HL-098458 from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS
- ARIC
Atherosclerosis Risk in Communities study
- CHD
coronary heart disease
- DALYs
disability adjusted life years
- IRD
incidence rate difference
- LDL
low density lipoprotein
- PIF
potential impact fractions
- YLD
years of life lived with disability
- YLL
years of life lost
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no relationships with industry to disclose.
REFERENCES
- 1.American Heart Association. 2010 Heart and stroke statistical update. Dallas, TX: American Heart Association; 2010. [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Kozak LJ, DeFrances CJ, Hall MJ. National hospital discharge survey: 2004 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2006;13:1–209. [PubMed] [Google Scholar]
- 4.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 5.SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions, congestive heart failure The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 6.CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 7.Titler MG, Jensen GA, Dochterman JM, et al. Cost of hospital care for older adults with heart failure: medical, pharmaceutical, and nursing costs. Health Serv Res. 2008;43:635–655. doi: 10.1111/j.1475-6773.2007.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 9.Kenchaiah S, Narula J, Vasan RS. Risk factors for heart failure. Med Clin North Am. 2004;88:1145–1172. doi: 10.1016/j.mcna.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Loehr LR, Rosamond WD, Poole C, et al. The potentially modifiable burden of incident heart failure due to obesity: the atherosclerosis risk in communities study. Am J Epidemiol. 2010;172:781–789. doi: 10.1093/aje/kwq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgenstern H, Bursic ES. A method for using epidemiologic data to estimate the potential impact of an intervention on the health status of a target population. J Community Health. 1982;7:292–309. doi: 10.1007/BF01318961. [DOI] [PubMed] [Google Scholar]
- 14.Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429–445. [PMC free article] [PubMed] [Google Scholar]
- 15.Murray CJ, Acharya AK. Understanding DALYs (disability-adjusted life years) J Health Econ. 1997;16:703–730. doi: 10.1016/s0167-6296(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 16.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design objectives The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NHLBI. ARIC Coordinating Center. Chapel Hill, NC: University of North Carolina; 1987. Atherosclerosis Risk in Communities (ARIC) Study Operations Manual No. 3: Surveillance Component Procedures. Version 1.0. [Google Scholar]
- 21.Beiser A, D'Agostino RB, Sr., Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer's disease in the Framingham Study The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–1522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 23.Gaynor JJ, Feuer EJ, Tan C, et al. On the use of cause-specific failure and conditional failure probabilities: examples for clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 24.Haenszel W, Loveland DB, Sirken MG. Lung-cancer mortality as related to residence, smoking histories. I White males. J Natl Cancer Inst. 1962;28:947–1001. [PubMed] [Google Scholar]
- 25.Michaud CM, Murray CJ, Bloom BR. Burden of disease--implications for future research. JAMA. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 26.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 27.Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124:314–323. doi: 10.1161/CIRCULATIONAHA.111.018820. [DOI] [PubMed] [Google Scholar]
- 28.Stevens G, Dias RH, Thomas KJ, et al. Characterizing the epidemiological transition in Mexico: national and subnational burden of diseases, injuries, and risk factors. PLoS Med. 2008;5:e125. doi: 10.1371/journal.pmed.0050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. The global burden of diseases: 2004 update. Geneva: WHO; 2004. Disability weights for diseases and conditions. [Google Scholar]
- 30.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 32.Bleumink GS, Knetsch AM, Sturkenboom MC, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. European heart journal. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 34.Melse JM, Essink-Bot ML, Kramers PG, Hoeymans N. A national burden of disease calculation: Dutch disability-adjusted life-years. Dutch Burden of Disease Group. Am J Public Health. 2000;90:1241–1247. doi: 10.2105/ajph.90.8.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 36.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 37.Sondik EJ, Huang DT, Klein RJ, Satcher D. Progress toward the healthy people 2010 goals and objectives. Annu Rev Public Health. 2010;31:271–281. doi: 10.1146/annurev.publhealth.012809.103613. [DOI] [PubMed] [Google Scholar]
- 38.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 39.Held C, Gerstein HC, Yusuf S, et al. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation. 2007;115:1371–1375. doi: 10.1161/CIRCULATIONAHA.106.661405. [DOI] [PubMed] [Google Scholar]