Abstract
The differences in function, location, and migratory pattern of conventional dendritic cells (cDC) and plasmacytoid DCs (pDC) not only point to specialized roles in immune responses but also signify additive and interdependent relationships required to clear pathogens. We studied the in vivo requirement of cross-talk between cDCs and pDCs for eliciting antitumor immunity against in situ released tumor antigens in the absence or presence of the Toll-like receptor (TLR) 9 agonist CpG. Previous data indicated that CpG boosted tumor-specific T-cell responses after in vivo tumor destruction and increased survival after tumor rechallenges. The present study shows that cDCs are indispensable for cross-presentation of ablation-released tumor antigens and for the induction of long-term antitumor immunity. Depletion of pDCs or applying this model in type I IFN receptor–deficient mice abrogated CpG-mediated responses. CD8α+ cDCs and the recently identified merocytic cDCs were dependent on pDCs for CpG-induced upregulation of CD80. Moreover, DC transfer studies revealed that merocytic cDCs and CD8α+ cDCs were most susceptible to pDC help and subsequently promoted tumor-free survival in a therapeutic setting. By transferring wild-type pDCs into TLR9-deficient mice, we finally showed that TLR9 expression in pDCs is sufficient to benefit from CpG as an adjuvant. These studies indicate that the efficacy of CpG in cancer immunotherapy is dependent on cross-talk between pDCs and specific subsets of cDCs.
Introduction
In the last decades, technological advances catalyzed a shift in the treatment of solid tumors from open surgical resection toward less-invasive (radiofrequency ablation, cryosurgery, and laser ablation) or noninvasive techniques (high-intensity focused ultrasound; ref. 1). These techniques not only induce direct cell death by protein denaturation and membrane disruption but also induce apoptotic cell death due to cell damage or vascular disruptions. In situ tumor destruction hence creates a depot of tumor antigens consisting of dead and dying cells that becomes instantly available for phagocytes such as dendritic cells (DC). DCs are well equipped to internalize dying cells and cellular debris and subsequently process tumor antigens for presentation to T cells. The main arm of the adaptive immune system to fight cancer is the activation of tumor-specific cytotoxic CD8 T lymphocytes (CTL) that recognize and kill tumor cells. In vivo generation of CTLs is dependent on the unique mechanism of cross-presentation by DCs; presentation of exogenous antigens on MHC class I. Exploiting the CTL-priming capacity of DCs is of major interest for cancer immunotherapy, in particular, to enhance antitumor immunity after applying in situ tumor destruction techniques.
We previously developed a murine model in which a normally lethal melanoma tumor is destructed in situ by cryoablation (2). The released tumor antigens preferentially end up in CD11c+ DCs in the draining lymph node (3, 4). Although cryoablated mice remain tumor free, only approximately 20% to 50% of the mice survive a rechallenge with melanoma cells, indicating that a minority of the subjects developed efficient immunologic memory against the tumor. These findings emphasized the need to boost antitumor immunity by combining tumor-destructive treatment with adjuvant immunotherapy. Indeed, administration of the Toll-like receptor (TLR) 9 agonist, CpG oligodeoxynucleotides, immediately after the ablation elevates the numbers of CTLs in the lymph node and promoted survival rate upon rechallenge up to 90% to 100% (4-6). We aimed to elucidate the significance of different DC subsets in the induction of CpG-mediated antitumor responses after in situ tumor ablation and the importance of TLR9 in the different DC subsets herein.
The unique ability to cross-present antigens has traditionally been attributed to the CD8α+ subset (7), but recent reports showed that CD8α−CD11b− conventional dendritic cells (cDC) also possess the capacity to present exogenous antigens on MHC class I (8, 9). These cells were named merocytic DCs after their acquisition of small particles form dying cells through a “nibbling” process instead of engulfment. In contrast to these cDCs, plasmacytoid DCs (pDC) show a poor functional ability to stimulate naive T cells in mouse (10) and man (11). The cross-priming capacity of pDCs is still under debate but seems relatively meager when compared with cDCs and may be restricted to specific circumstances (12, 13). Yet, pDCs are able to effectively stimulate preactivated or memory-type T cells and deliver differentiation (10, 11) and activation (14) signals (particularly type I IFN) for cDCs. The differences in function, location, and migratory pattern of cDCs and pDCs may thus not only point to specialized roles in the elicitation of T-cell responses but also may signify additive and interdependent relationships, resulting in synergistic antitumor immunity.
In the present study, we elucidated the specific capacities and interactions of pDCs and different subsets of cDCs in (CpG stimulated) immune responses against tumor antigens released by in situ tumor destruction that requires internalization, processing, and presentation by the DCs in vivo. Furthermore, we studied whether the exclusive expression of TLR9 in either cDCs or pDCs would be sufficient for the induction of tumor-specific CTLs after combined treatment of ablation with CpG administration.
Materials and Methods
Mice, cell lines, and peptides
C57Bl/6J, B6.C-H2.bm1/ByJ (Kbm1), and B6.SJL.Ptpcra (B6. CD45.1) were obtained from The Jackson Laboratory. TLR9−/− mice were obtained from S. Akira (Department of Host Defense, Osaka University, Osaka, Japan). Act-mOVA transgenic mice were a gift from Dr. M. Jenkins (University of Minnesota Medical School, Minneapolis, NM) and were bred onto a B6.C-H2bm1/ByJ background (ActmOVA Kbm1). OT-I mice were bred on a CD45.1 background and crossed with Kbm1 mice. IFNα/βR (Ifnar−/−) knockout mice were kindly provided by Dr J. Sprent (The Scripps Research Institute Vivarium, La Jolla, CA). Mice were maintained at the animal facility of the La Jolla Institute for Allergy and Immunology) or at the animal laboratory of the Nijmegen Centre for Molecular Life Sciences under specific pathogen-free conditions and were used at 6 to 12 weeks of age. All experiments were in accordance with the guidelines by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Ovalbumin (OVA)-transfected murine melanoma cell line B16F10 (B16OVA, clone MO5) was kindly provided by Dr. K. Rock (Department of Pathology, University of Massachusetts Medical Center, Worchester, MA; ref. 15). Cells were frozen and thawed according to American Type Culture Collection guidelines for B16F10 and cultured as described before (4). No full authentication was carried out, but the expression of OVA, TRP-2 antigens, TLRs, MHC class I, and molecules was tested before the experiments by reverse transcriptase PCR or flow cytometry. In addition, the growth characteristics in vitro and in vivo are closely monitored and compared with previous growth curves. Peptides for the MHC class I-restricted OVA257–264 (SIINFEKL) were obtained from A&A Laboratories. B16-FLT3-L cells were cultured in 5% Iscove’s Modified Dulbecco’s Medium supplemented with 20 U/mL penicillin and 10 μg/mL streptomycin, and 5 × 106 cells were injected to establish FLT3-L–producing tumors in C57BL/6 mice.
In situ release of tumor antigens by cryoablation
Mice were subcutaneously injected with 5 × 105 B16OVA cells in PBS and Matrigel. After 9 to 11 days of injection, tumors (7–10 mm) were treated in 2 cycles with cryosurgery using a CRY-AC device armed with a 6 mm probe (Brymill Cryogenic Systems). To test long-term tumor protection, the mice were challenged with 25 × 103 to 50 × 103 B16OVA cells 40 days after the ablation of the primary tumor and monitored for tumor growth. CpG-ODN 1668 (5′-TCCATGACGTTCCTGATGCT-3′) with phosphorothioated backbone was obtained from IDT and injected peritumorally (50 μg per mouse in 2 × 15 μL) within 15 minutes after ablation. CpG-ODN 1668 is a type B CpG, similar to the clinical grade available CpG-ODNs used in clinical trials.
Functional ablation of cross-presenting DCs and pDCs
We used a method that was recently described to functionally ablate cross-presentation in vivo (16). Tumor-bearing mice were injected with horse cytochrome c (5 mg per mouse; Sigma) on 2 consecutive days before ablation. At this time point, the number of CD8α+ DCs was reduced 2- to 3-fold. Other DC subsets (CD11c+CD11b+CD172+ and CD11cintB220+) were not affected. pDCs were depleted using the 120G8 antibody administered 1 and 2 days prior to ablation (0.5 mg per mouse). Rat IgG was used as a control. We did not observe differences in the number of other lymph node cells. To analyze DCs from the tumor-draining inguinal lymph nodes, excised lymph nodes were torn apart using needles in medium containing collagenase D and DNase and incubated at room temperature for 15 minutes. Suspensions were dislodged by resuspending, and EDTA was added before filtration.
Tetramer and intracellular cytokine staining
Seven to 10 days after ablation, blood, spleen, and lymph node cells were stained with allophycocyanin- or phycoerythrin-labeled iTag OVA-Kb tetramers (Beckman Coulter); CD8α (clone 53-6.7) and CD44 (clone IM7; eBioscience or BD Pharmingen). Cytokine profiles were assessed after re-stimulation with 0.1 μg/mL SIINFEKL peptide. Subsequently, cells were stained with monoclonal antibodies specific for CD8 TCRβ, CD44, and CD62L, fixed and permeabilized according to Cytofix/Cytoperm kit instructions (BD Pharmingen), and intracellular cytokine staining was carried out for IFN-γ (clone XMG1.2) and/or TNF-α (clone MP6-XT22; all antibodies were purchased from either BD Pharmingen or eBioscience). Samples were collected on a LSRII flow cytometer with Diva software (BD Pharmingen), and data were analyzed with FlowJo software (Tree Star).
Adoptive transfer of OT-I cells and in vivo cytotoxicity
OT-I SJL splenocytes were enriched for CD8+ T cells by negative selection according to the manufacturer’s instructions (CD8 T cell isolation kit; Miltenyi Biotec). Purity of Vα2+ Vβ5+ was typically greater than 98%. Cells were labeled with 1 μmol/L carboxyfluorescein succinimidyl ester (CFSE), and 5 × 105 cells were intravenously transferred to mice that were cryoablated. As a control, the same number of CFSE-labeled splenocytes from wild-type (WT) C57Bl/6J mice was transferred to the same mice. Four days later, spleens were harvested and CFSE dilution was analyzed in CD8+CD45.1+ (OT-I) and CD8+CD45.2+ (controls) cells using a LSRII flow cytometer. To address the killing capacity of endogenously generated CTLs, splenocytes from CD45.1+ mice were loaded (1 hour incubation at 37°C) with 1 μg/mL SIINFEKL peptide or an irrelevant peptide (E1B192–200 peptide; VNIRNCCYI) and labeled with CFSE in a concentration of 0.1 μmol/L (SIIN-FEKL) or 2 μmol/L (E1B192–200). A total of 5 × 106 cells of each population was injected into mice of which the B16OVA tumor had been treated with cryoablation with or without CpG 8 days before. Eighteen hours later, spleens were harvested and the differently labeled CD45.1+ populations were analyzed using flow cytometry. The percentage killing was calculated using the formula: 100 − [(sample-CFSEOVA/sample-CFSEcontrol)/(naive-CFSEOVA/naive-CFSEcontrol) × 100].
Splenic DC isolation and transfer of antigen-loaded DCs
Spleens were isolated from C57Bl/6 mice 10 to 12 days after injection with 5 × 106 B16-FLT3-L cells. Spleens were injected with collagenase/DNase medium and left for 5 to 10 minutes before they were cut in small pieces. After occasional resuspending during 20 minutes at room temperature, the preparation was filtered and washed. The single-cell suspension was loaded onto a 2-fraction OptiPrep gradient (Sigma) and spun for 20 minutes at room temperature. The low-density cells were washed and generally contained more than 90% CD11cint/+ that contained 10% to 15% pDCs (CD11cintBST-2+). Total DC fractions were divided in 2 suspensions of which the first (cDCs with pDCs) was cocultured overnight with irradiated (1,500 rad) ActmOVA Kbm1 cells (DC:dying cells = 1:3). The second fraction was depleted of pDCs by magnetic bead sorting and incubated in a similar way (cDCs without pDCs). Cells were harvested and sorted on fluorescence-activated cell sorter on the basis of the expression of CD11c, B220, BST-2, CD24, CD8α, CD172, and CD11b. A total of 1 × 105 DCs were injected into each mouse, which is approximately 4.5 × 106 per kg. [This is a relatively low amount of cells compared with what is generally used in mouse studies, especially in a therapeutic setting. However, human DC vaccinations are generally carried out with multiple injections (n = 2–3) in average 15 × 106 cells, which is 0.20 × 105 to 0.25 × 105 per kg.]
Statistical analyses
Data were analyzed using Prism software (GraphPad Software, Inc.). Comparisons of one-variable data were conducted using a 2-tailed unpaired Student’s t test. Statistical analyses of the survival curves were carried out using a log-rank test. Bar graphs represent mean levels ± SEM. P < 0.05 was considered statistically significant.
Results
Conventional DCs cross-present tumor antigens that are released by in situ tumor ablation
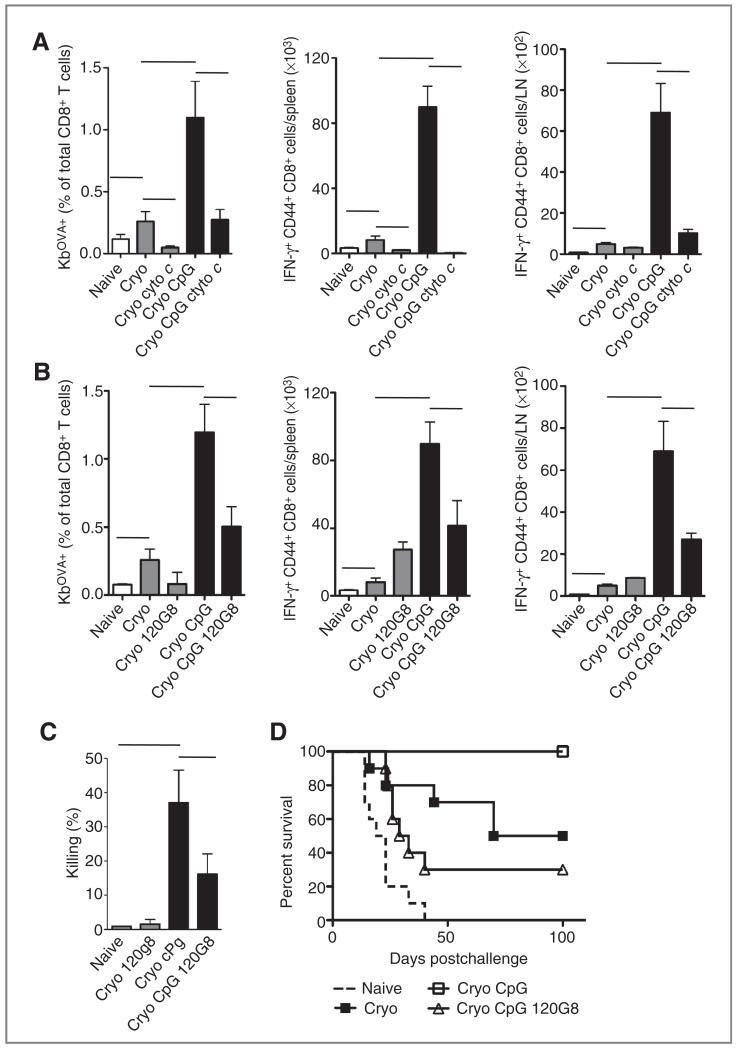
C57Bl/6 mice were inoculated subcutaneously with 5 × 105 OVA-transfected B16-melanoma cells. When tumors measured 7 to 10 mm in diameter (generally 9–11 days after injection), the tumor was destructed in situ by cryoablation (4-6). Mice were additionally treated with 2 injections of cytochrome c, an initiator of the mitochondrial apoptosis pathway in cells capable of endosome-to-cytosol transport such as cDCs (16). In agreement with Lin and colleagues (16), cytochrome c selectively reduced the numbers of splenic CD11c+CD11b− cDCs (generally 2- to 3-fold) whereas other cDC populations (CD11c+CD11b+) and pDCs (CD11cintB220+SiglecH+) were unaffected. After cryoablation alone, OVA-specific CD8+ T cells were present in blood (Fig. 1A), spleen, and draining lymph nodes. After in vitro restimulation with MHC class I–restricted SIINFEKL peptide, CD8+ T cells expressed the activation marker CD44+ and produced IFN-γ (Fig. 1A). Additional treatment with CpG significantly increased the numbers of CTLs in vivo. Selective suicide of cross-presenting cDCs by cytochrome c prevented the increment of CTLs in both the absence and the presence of CpG. This indicates that the process of DC cross-presentation is crucial for the induction of immune responses against in situ released tumor antigens.
Figure 1.
Both cDCs and pDCs are required to benefit from immune potentiating effects of CpG in vivo. A, C57Bl/6 mice were injected with 5 × 104 B16OVA cells. When the tumors measured 8 to 11 mm, tumor antigens were released by cryoablation (cryo) of the tumor in situ. Some groups received cytochrome c (cyto c; 5 mg per mouse intraperitoneally) 1 day before and on the day of the ablation and/or CpG (50 μg per mouse peritumorally) after the ablation. Eight to 10 days after ablation, blood cells were stained for OVA-Kb tetramers. Cells from spleen and draining lymph nodes (LN) were restimulated with SIINFEKL peptide and TCR-β+CD8α+ cells were analyzed for the expression of CD44+ and IFN-γ+. B, similar experiments were carried out to study the involvement of pDCs. pDCs were depleted using 120G8 monoclonal antibodies injected 1 and 2 days before the ablation. C, the killing capacity of the endogenously induced CTLs was assessed by an in vivo cytotoxicity assay; splenocytes from CD45.1+ mice were differentially CFSE labeled and coated with SIINFEKL peptide (CFSElow) or E1B192–200 (CFSEhigh) and injected into mice that had been treated with cryoablation 8 days before. Cytolytic capacity was analyzed 18 hours later by determining the presence of CFSE-labeled cells in the spleen. Mean levels ± SEM are shown. Each group consisted of 4 to 6 mice and these data are representative of 2 similar experiments. Naive mice and mice that were tumor free for 40 days after ablation were rechallenged with 50 × 103 B16OVA cells to determine long-term tumor protection. Tumors measuring 1,500 mm3 were killed according to the animal welfare guidelines. Groups consisted of 8 to 12 mice. D, the survival curves of naive, Cryo + CpG, and Cryo + CpG + 120G8 differed significantly (P < 0.05) from that of cryo only. 120G8 reduced the survival ratio in the cryo + CpG group.
Next, we tested whether pDCs are involved in the generation of tumor-specific CTLs. Both murine cDCs and pDCs express TLR9 mRNA and are responsive to CpG, but only cDCs are able to cross-present cell-associated antigens to CD8+ T cells in vitro (data not shown). pDCs were depleted in B16OVA-bearing mice using 120G8 monoclonal antibodies before cryoablation with or without CpG treatment. Compared with control Ig-treated animals, CpG-treated, but pDC-depleted, mice showed reduced numbers of OVA-specific CD8+CD44+ T cells in blood and IFN-γ+CD8+ (CD44+) T cells in both spleen and lymph nodes (Fig. 1B). In the absence of pDCs, OVA-specific T cells were still generated after cryosurgery alone (no CpG), suggesting that CTL priming in this model is a unique feature of cDCs but not pDCs. The injection of CpG also drastically increased the cytolytic capacity to kill SIINFEKL-pulsed CFSE-labeled CD45.1+ target cells, which was shown to be dependent on the presence of pDCs during priming (Fig. 1C). It was additionally tested whether the presence of pDCs during the priming phase of the immune response was required to induce CpG-mediated long-term immunity. Mice that remained tumor free for 40 days after cryosurgery received a rechallenge with a lethal dose of B16OVA tumor cells. As reported previously, only approximately 50% of the cryoablation-only mice survive the rechallenge whereas all animals treated with cryoablation and CpG survive (4-6). In contrast, pDC-depleted mice did not show the protective effect of CpG (Fig. 1D). These results indicate that CpG-mediated protective immune responses to in situ released tumor antigens are highly regulated by pDCs.
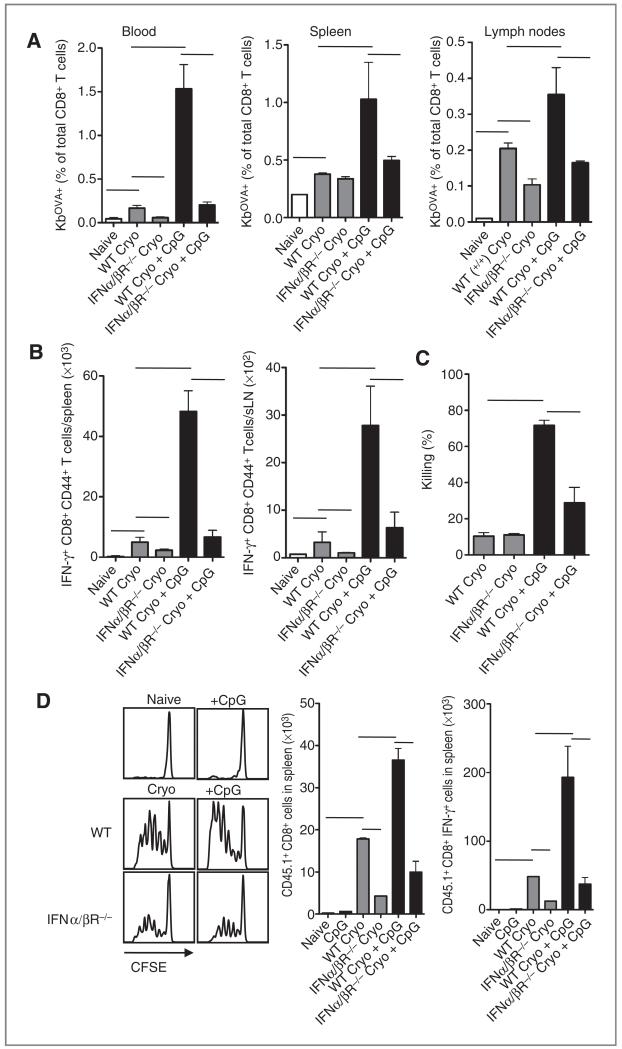
CTL priming after cryoablation is dependent on type I IFN receptor signaling
One of the hallmarks of pDCs is the production of massive amounts of type I IFN in response to CpG in vitro and in vivo. It was tested whether cryoablation with or without CpG treatment could induce antitumor responses in mice deficient for the type I IFN receptor (IFNα/βR−/−). As expected, the efficacy of CpG to increase OVA-specific (Fig. 2A) and IFN-γ–producing (Fig. 2B) CD8+ T cells after cryoablation was drastically diminished in IFNα/βR−/− mice. Coadministration of CpG increased the in vivo killing of SIINFEKL-loaded target cells in WT mice (Fig. 2C). Also, IFNα/βR−/− mice started to show increased killing of target cells, indicating that CpG might stimulate the immune response to some extent even in the absence of type I IFN signaling.
Figure 2.
Priming of endogenous CD8+ T cells and transferred OT-I T cells is abrogated in IFNα/βR−/− mice. A, B16OVA-bearing WT and IFNα/βR−/− mice were treated with cryoablation. After the ablation, indicated groups were additionally treated with CpG. Eight to 10 days after ablation, blood, spleen, and draining lymph nodes were isolated and stained with OVA-Kb tetramers. B, spleen and draining lymph nodes were restimulated with SIINFEKL peptide for 5 hours and analyzed for IFN-γ production. C, 6 to 8 days after ablation, mice received 5 × 106 SIINFEKL-coated CFSE-labeled (low dose) splenocytes from C57Bl/6 SJL (CD45.1) mice and a similar number of CFSE-labeled (high dose) splenocytes coated with irrelevant peptide E1B192–200. After 18 hours, spleens were harvested and analyzed for the presence of CD45.1 CFSE-labeled cells. D, tumor-bearing mice were subjected to ablation with or without CpG and injected with CFSE-labeled purified CD8α+ OT-I SJL cells or WT cells as control population. Three or 4 days after transfer, CFSE dilution was analyzed in splenocytes. Histograms show representative data of cells gated on CD8, Vα2, and Vβ5. Bar graphs show mean values ± SEM of 4 to 6 mice and are representative of 2 similar experiments.
To dissect whether the disabilities observed in IFNα/βR−/− mice were intrinsic for antigen-presenting cells or for the CD8 T cells themselves, IFNα/βR−/− mice were injected with CFSE-labeled WT OT-I cells just after ablation and analyzed for proliferation by CFSE dilution. In all circumstances where antigen was released after ablation, OT-I cells were able to divide (Fig. 2D). However, the absolute numbers of dividing OT-I cells showed that less cells accumulated in the IFNα/βR−/− mice than in WT mice, indicating that the type I IFN feedback loop in antigen-presenting cells is important in the onset of the response. Interestingly, IFNα/βR−/− mice also showed lower numbers of CTL priming after cryoablation alone, suggesting that type I IFN signaling is involved in the stimulation of immune responses against in situ released antigens from dead cells. Because pDC-depleted mice were able to mount effective CTL responses after ablation only, these data suggest that other cells may be the source of type I IFN. Indeed, we previously showed that merocytic cDCs produce type I IFN upon the encounter with dying cells (9, 17).
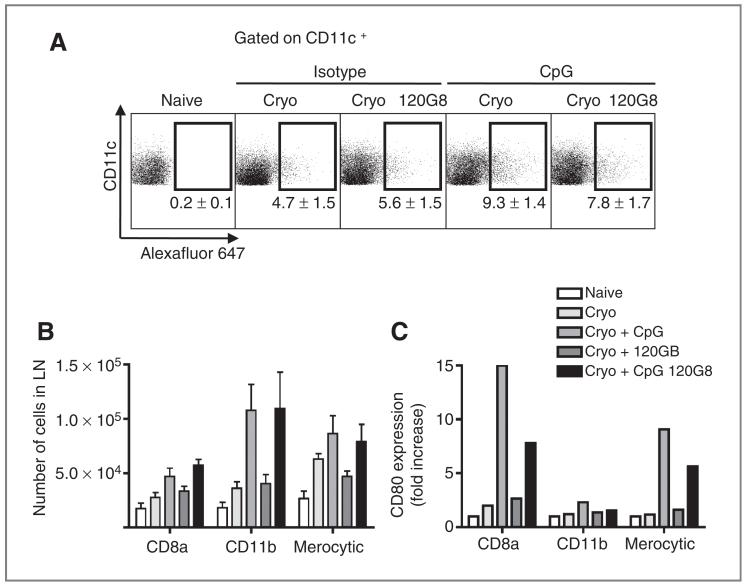
pDCs determine cDC maturation and antigen loading in CpG-modulated responses
To get more insight in cDC subsets and their susceptibility to pDC-mediated factors, we addressed the antigen uptake capacity and maturation status of the cDCs directly ex vivo. Tumor-draining lymph nodes were isolated from 120G8- or isotype-treated mice 1 to 2 days after cryoablation with or without CpG treatment. Just prior to the ablation, OVA-Alexa Fluor 647 was injected in the tumor to determine the ability of DCs to internalize soluble antigens after ablation with or without CpG. CpG increased the internalization of OVA-Alexa Fluor 647 by CD11c+B220− cDCs after ablation whereas 120G8 did not seem to have an effect on antigen uptake after ablation alone (Fig. 3A). Generally, lower uptake of OVA-Alexa Fluor 647 was observed in DCs from 120G8-treated mice injected with CpG. In all circumstances, approximately 80% of the CD11c+ cells that had internalized OVA-Alexa Fluor 647 expressed CD11b+. These CD11b-expressing DCs were present in the draining lymph nodes after CpG treatment, irrespective of 120G8 treatment (Fig. 3B). Also, the numbers of antigen-loaded CD8α+ DCs and merocytic DCs increased after CpG treatment, although to a lesser extent. In addition, the expression of costimulatory markers on cDCs was very much increased following CpG treatment, in particular, in CD8α+ DCs and merocytic DCs (Fig. 3C). In pDC-depleted mice, this CpG-mediated upregulation of CD80 expression was much less pronounced, suggesting that differences in CTL priming may be due to impairment of activation of cross-presenting cDCs.
Figure 3.
Plasmacytoid DCs determine the function of cDCs. A, OVA-Alexa Fluor 647 (20 μg/20 μL) was injected into the tumor just prior to ablation with or without CpG. Two days after ablation, lymph node cells were analyzed for the uptake of Alexa Fluor 647 in CD11c+ cells. B, CD11c+ cells that were present in the draining lymph node were subfractionated into CD8α+ DCs (CD11c+B220−-CD24+CD172loCD11b−), CD11b+ DCs (CD11c+B220−CD24lo-CD8α−), and merocytic DCs (CD11c+B220−CD172loCD8α−-CD11blo). Bar graphs show mean values ± SEM of 4 to 6 mice. C, the relative increase in CD80 expression was determined in each subset.
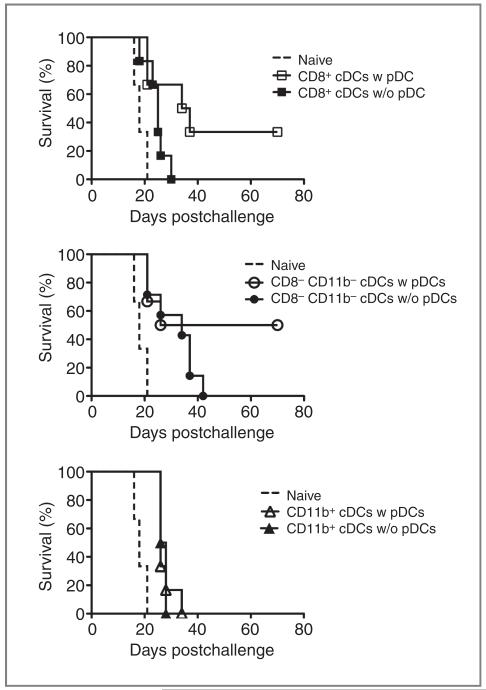
pDCs stimulate the intrinsic cross-presenting ability of cDCs
In Fig. 3, we showed that the in vivo maturation of especially CD8α+ cDCs and CD8α−CD11b− merocytic DCs after combinational therapy of ablation and CpG is dependent on pDCs. We next studied whether the cross-priming function of resting cDC subsets was differentially regulated by pDC help. A total of 1 × 105 sorted DC subsets, loaded with dying ActmOVA Kbm1 cells (to prevent direct presentation), were transferred into donor mice bearing an established B16OVA tumor (~4 × 4 mm2). Therapeutic vaccinations with CD8α+ cDCs were not successful. However, when CD8α+ cDCs were allowed to interact with pDCs during incubation with irradiated cells, almost 40% of the mice were able to clear the established B16OVA tumor after cDC transfers (Fig. 4). In line with our previous observations (17), vaccinations with CD8α−CD11b− alone already extended the period of tumor-free survival. These CD8α−CD11b− cDCs were also highly responsive to “pDC help” as approximately 50% of the mice survived the outgrowth of a lethal melanoma tumor. DC vaccination with CD11b+ cDCs cultured in either the absence or presence of pDCs failed to increase survival. This study suggests that CD8α+ cDCs and CD8α−CD11b− cDCs develop a better intrinsic capacity for immune stimulation when they are permitted to interact with pDCs.
Figure 4.
pDCs stimulate the intrinsic cross-priming ability of cDCs against cell-associated antigens. Spleens of B16-FLT3-L–bearing C57Bl/6 mice were harvested. Dendritic cells were enriched using a density gradient and divided in 2 volumes. One volume was depleted of pDCs using magnetic beads and both volumes were cocultured with irradiated ActmOVA Kbm1 splenocytes (DC:dying cells = 1:3). After 18 hours of coculture and maturation with CpG (0.1 μg/mL), the cells were sorted and 1 × 105 cells were injected into tumor-bearing recipients (tumor size: 4 mm in diameter). Graphs show survival curves of groups consisting of 6 to 8 mice.
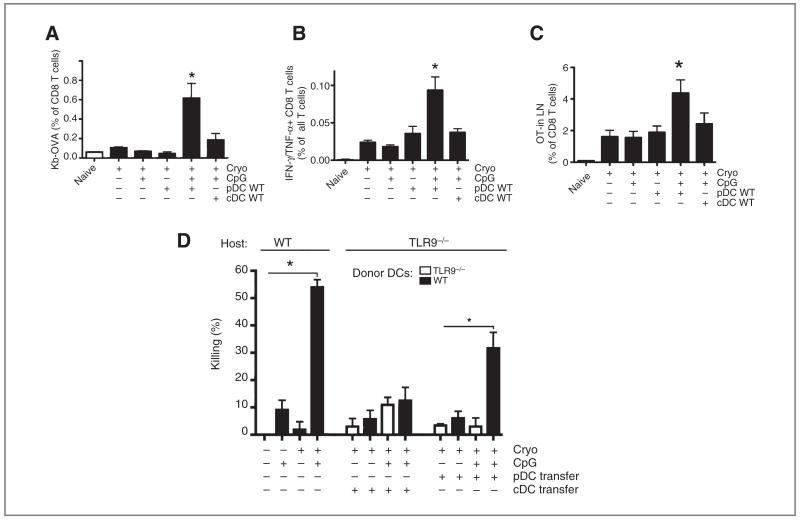
Exclusive TLR9 expression in pDCs suffices to benefit from CpG
The expression of TLR9 may be differently regulated in mice and man with the main discrepancy being the low expression or absence in human cDCs. To study the significance of TLR9 expression in cDCs versus pDCs, B16OVA melanomas in TLR9-deficient mice were cryoablated in the absence or presence of CpG. Cryoablation in TLR9−/− mice resulted in the generation of endogenous OVA-Kb+ CD8 T cells in blood (Fig. 5A) and IFN-γ/TNF-α–producing CD8 T cells (Fig. 5B) in lymph nodes. Additional treatment with CpG failed to modulate these responses. Interestingly, the efficacy of CpG was restored when TLR9-deficient mice received a transfer with WT pDCs. Transfer of pDCs, but not cDCs, after combined treatment of cryoablation and CpG increased the percentages of IFN-γ/TNF-α–producing CD8 T cells in the lymph node and OVA-specific T cells in blood when compared with cryoablation alone. To exclude possible effects of CpG directly on the T cells, we also transferred CFSE-labeled OT-I T cells (similar as Fig. 2D) and confirmed that exclusive expression of TLR9 in pDCs stimulates antigen-specific T-cell proliferation (Fig. 5C). In addition, pDC transfer, but not cDC transfer, enhanced the in vivo capacity of endogenous T cells to kill SIINFEKL-labeled target cells (Fig. 5D). In all, these data suggest that the restricted expression of TLR9 in pDCs, but not in cDCs, suffices for CpG to further stimulate antitumor immune responses.
Figure 5.
Selective expression of TLR9 in pDCs suffices to benefit from CpG as an adjuvant. B16OVA tumors (7–9 mm in diameter) in TLR9-deficient mice were subjected to cryoablation with or without CpG. Indicated groups subcutaneously received WT, FLT3-L–grown bone marrow–derived pDCs just after ablation. The percentages of Kb-OVA–expressing CD8 T cells in blood and IFN-γ/TNF-α–producing CD8 T cells in the draining lymph node are shown in (A) and (B), respectively. C, alternatively, CD8+ OT-I cells were transferred intravenously after ablation and the accumulation was analyzed in the draining lymph node of the TLR9-deficient mice 3 to 4 days later. D, at day 10 after ablation, mice received control and SIINFEKL-pulsed and CFSE-labeled target cells and the killing capacity was determined 18 hours after injection. Bar graphs show mean values ± SEM of 4 to 6 mice. A–C, *, significantly different from all other groups, P < 0.05.
Discussion
Accumulating evidence shows that adaptive immunity is shaped by cooperation between functionally distinct DC subsets. Cross-talk may include the transfer of antigenic material between migratory and lymph node–resident DCs (18) or cDCs and pDCs (19) that may also synergize to reach optimal activation (20) and physically interact to stimulate CTL-mediated eradication of viruses (14). This is the first study to show the in vivo importance of cDC–pDC cross-talk in generating productive CTL responses to in situ released tumor antigens. In cancer therapies where a tumor is instantly destructed in situ (e.g., radiofrequency ablation, cryosurgery, and irradiation), tumor antigens become readily available for phagocytic antigen-presenting cells and thus for presentation to T cells. We have previously shown that CpG synergizes with tumor ablation to induce long-term protection against tumor rechallenges through activation of tumor-specific CTLs. The same conclusions were drawn when using B16F10 parental cells instead of OVA-transfected cells (4-6), indicating that responses to classical tumor antigens follow similar principles. Here, we conclude that the clinical efficacy of CpG in mice is critically dependent on the presence of both cDCs and pDCs and the cross-talk between TLR9-expressing pDCs and specific subsets of cDCs.
The present data suggest that cDCs are required for the induction of tumor-specific immunity after ablation. Cytochrome c did not affect MHC class II presentation (16) but selectively decreased the number of cDCs without affecting the numbers of pDCs. This finding supports the notion that the ability of pDCs to phagocytose dead cells and cross-present exogenous antigens in vivo appears less efficient than that of cDCs (discussed in ref. 21). Moreover, tumor-specific CTLs were still present in pDC-depleted animals after ablation, emphasizing the leading role of cDCs and not pDCs for antigen presentation and cross-priming in this model. CpG-licensed pDCs were reported to cross-prime OT-I cells after isolation from OVA protein–challenged mice (9 mg per mouse; ref. 22). Compared with these levels, the amount of antigens released after ablation may be too low to be able to detect cross-presentation by pDCs. Functional pDCs were however required to optimally benefit from CpG-mediated immunotherapy evidenced by significantly lower numbers of tumor-specific CTLs and lower survival rates upon secondary challenge in pDC-depleted mice. In the ablation setting in vivo, the generation of both endogenous and transferred IFNα/βR+/+ OT-I CD8 T cells was dependent on type I IFN signaling, indicating that type I IFN signaling was important at the level of antigen presentation but was not an intrinsic defect of the IFNα/βR−/− CTLs.
The promoter elements of CD80-encoding genes include several IFN regulatory factor 2 sites, which suggest that the upregulation of CD80 may be a direct result of type I IFN. CD8α+ DCs and merocytic DCs failed to upregulate the costimulatory molecule CD80 in response to CpG in vivo when pDCs were depleted before treatment, suggesting that the lack of costimulatory signals may have prevented efficient CTL priming. Although our data strongly implicate a role for type I IFN production by pDCs, administration of recombinant IFN-α after cryoablation only slightly increased the influx of DCs in the draining lymph nodes and the expression of CD80 on cDCs (not shown). It is well possible that pDCs influence cDCs through other mechanisms as was previously reported in responses to Listeria monocytogenes. Kuwajima and colleagues (23) showed that CpG-induced protection from Listeria was dependent on CD40–CD40L interactions between cDCs and pDCs and subsequent interleukin 12 production by cDCs. Although adaptive immune responses against antigens from a destructed tumor develop following different mechanisms than those at work in an acute infection model, these data suggest that the cross-talk between pDCs and cDCs after cryoablation with CpG may also be costimulation dependent. It was previously shown in vitro that cross-talk between pDCs and cDCs requires physical interactions (24). The use of peptides in an in vitro coculture system as used in the study of Lou and colleagues (24) does however overcome the antigen uptake and processing machinery of DCs and may therefore not be directly translated to our preclinical model where antigens are derived from dead and dying cells in situ.
Type I IFN signaling was shown to be essential to generate antitumor responses after ablation both in the presence and absence of CpGs. In the latter situation, the data suggest that non-pDCs are the source of type I IFN, as pDC-depleted mice showed a trend toward increased antitumor responses. Indeed, we previously showed that merocytic DCs are a source of type I IFN upon an encounter with dying cells (9, 17). The activation pathways through which immunity is established against in situ released tumor antigens in such sterile inflammatory conditions are subject of intensive research. Previous studies indicated that CTL priming against antigens derived from dying cells injected into the host was independent of MyD88 but dependent on the type I IFN receptor (9). Similar results were found in the cryoablation model. In addition, type I IFN was found essential in local radiotherapy-mediated tumor control (25). The way in which these tumor cells die in vivo after ablation can thus be considered slightly immunogenic, but the independency of MyD88/TRIF suggests that the type I IFN pathway might be an alternative immune stimulatory pathway in addition to those previously proposed (26).
In situ tumor destruction techniques are increasingly applied for the treatment of solid tumors. The ad hoc availability of tumor antigens for immune cells makes these treatments particular good candidates for combinations with immunotherapy. The use of CpG in clinical trials so far resulted in variable success (27-30). A common argument on the efficacy of CpG in immunotherapy is the differential expression of TLR9 between mouse and man DCs. Others challenged this argument by showing TLR9 expression in both human pDCs and cDCs (31) and responsiveness to CpG by monocyte-derived DCs (32, 33) and FLT3-L–induced blood DCs (34). An alternative explanation could be suboptimal timing (4) and routing (6) conditions of CpG administration in patients. We here conclude that the exclusive expression of TLR9 in murine pDCs suffices to benefit from the clinical efficacy of CpG in this murine model. The transfer of WT pDCs, but not cDCs, into TLR9-deficient animals restored the ability to cross-prime antigen-specific and functional CTLs. These data show that although murine cDCs are able to respond to CpG, the exclusive reactivity of pDCs on CpG suffices to induce protective immunity. It will be interesting to determine CpG efficacy in clinical trials that take into consideration the optimal timing (4) and routing (6) prerequisites of CpG administration.
Acknowledgments
Grant Support
This research was funded by a fellowship (S. Nierkens) and a grant (G.J. Adema) of the Dutch Cancer Society (KWF Kankerbestrijding) and by the Netherlands Organization for Scientific Research (918.66.615 to G.J. Adema).
Footnotes
Note: G.J. Adema and E.M. Janssen contributed equally to this work.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kennedy JE. Innovation: high-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–7. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 2.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–9. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 3.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95:896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nierkens S, den Brok MH, Sutmuller RP, Grauer OM, Bennink E, Morgan ME, et al. In vivo colocalization of antigen and CpG within dendritic cells is associated with the efficacy of cancer immunotherapy. Cancer Res. 2008;68:5390–6. doi: 10.1158/0008-5472.CAN-07-6023. [DOI] [PubMed] [Google Scholar]
- 5.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Toonen LW, Figdor CG, et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66:7285–92. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 6.Nierkens S, den Brok MH, Roelofsen T, Wagenaars JA, Figdor CG, Ruers TJ, et al. Route of administration of the TLR9 agonist CpG critically determines the efficacy of cancer immunotherapy in mice. PLoS One. 2009;4:e8368. doi: 10.1371/journal.pone.0008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedoui S, Prato S, Mintern J, Gebhardt T, Zhan Y, Lew AM, et al. Characterization of an immediate splenic precursor of CD8 dendritic cells capable of inducing antiviral T cell responses. J Immunol. 2009;182:4200–7. doi: 10.4049/jimmunol.0802286. [DOI] [PubMed] [Google Scholar]
- 9.Janssen E, Tabeta K, Barnes MJ, Rutschmann S, McBride S, Bahjat KS, et al. Efficient T cell activation via a Toll-Interleukin 1 receptor-independent pathway. Immunity. 2006;24:787–99. doi: 10.1016/j.immuni.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, et al. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naranjo-Gomez M, Fernandez MA, Bofill M, Singh R, Navarrete CV, Pujol-Borrell R, et al. Primary alloproliferative TH1 response induced by immature plasmacytoid dendritic cells in collaboration with myeloid DCs. Am J Transplant. 2005;5:2838–48. doi: 10.1111/j.1600-6143.2005.01097.x. [DOI] [PubMed] [Google Scholar]
- 12.Kasturi SP, Pulendran B. Cross-presentation: avoiding trafficking chaos? Nat Immunol. 2008;9:461–3. doi: 10.1038/ni0508-461. [DOI] [PubMed] [Google Scholar]
- 13.Colonna M, Cella M. Cross-presentation: plasmacytoid dendritic cells are in the business. Immunity. 2007;27:419–21. doi: 10.1016/j.immuni.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J Exp Med. 2005;202:425–35. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falo LD, Jr, Kovacsovics-Bankowski M, Thompson K, Rock KL. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat Med. 1995;1:649–53. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
- 16.Lin ML, Zhan Y, Proietto AI, Prato S, Wu L, Heath WR, et al. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc Natl Acad Sci U S A. 2008;105:3029–34. doi: 10.1073/pnas.0712394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reboulet RA, Hennies CM, Garcia Z, Nierkens S, Janssen EM. Prolonged antigen storage endows merocytic dendritic cells with enhanced capacity to prime anti-tumor responses in tumor-bearing mice. J Immunol. 2010;185:3337–47. doi: 10.4049/jimmunol.1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Megjugorac NJ, Jacobs ES, Izaguirre AG, George TC, Gupta G, Fitzgerald-Bocarsly P. Image-based study of interferongenic interactions between plasmacytoid dendritic cells and HSV-infected monocyte-derived dendritic cells. Immunol Invest. 2007;36:739–61. doi: 10.1080/08820130701715845. [DOI] [PubMed] [Google Scholar]
- 20.Piccioli D, Sammicheli C, Tavarini S, Nuti S, Frigimelica E, Manetti AG, et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood. 2009;113:4232–9. doi: 10.1182/blood-2008-10-186890. [DOI] [PubMed] [Google Scholar]
- 21.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–61. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Mouries J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood. 2008;112:3713–22. doi: 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwajima S, Sato T, Ishida K, Tada H, Tezuka H, Ohteki T. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat Immunol. 2006;7:740–6. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 24.Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol. 2007;178:1534–41. doi: 10.4049/jimmunol.178.3.1534. [DOI] [PubMed] [Google Scholar]
- 25.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–96. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 27.Manegold C, Gravenor D, Woytowitz D, Mezger J, Hirsh V, Albert G, et al. Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3979–86. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 28.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 29.Leonard JP, Link BK, Emmanouilides C, Gregory SA, Weisdorf D, Andrey J, et al. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin’s lymphoma. Clin Cancer Res. 2007;13:6168–74. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
- 30.Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29:558–68. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 31.Hoene V, Peiser M, Wanner R. Human monocyte-derived dendritic cells express TLR9 and react directly to the CpG-A oligonucleotide D19. J Leukoc Biol. 2006;80:1328–36. doi: 10.1189/jlb.0106011. [DOI] [PubMed] [Google Scholar]
- 32.Hellman P, Eriksson H. Early activation markers of human peripheral dendritic cells. Hum Immunol. 2007;68:324–33. doi: 10.1016/j.humimm.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Mao TK, Lian ZX, Selmi C, Ichiki Y, Ashwood P, Ansari AA, et al. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42:802–8. doi: 10.1002/hep.20859. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Chan AS, Dawson AJ, Liang X, Blazar BR, Miller JS. FLT3 ligand administration after hematopoietic cell transplantation increases circulating dendritic cell precursors that can be activated by CpG oligodeoxynucleotides to enhance T-cell and natural killer cell function. Biol Blood Marrow Transplant. 2005;11:23–34. doi: 10.1016/j.bbmt.2004.08.004. [DOI] [PubMed] [Google Scholar]