Abstract
Early detection of oral premalignant lesions (OPL) and oral cancers (OC) is critical for improved survival. We evaluated if the addition of autofluorescence visualization (AFV) to conventional white-light examination (WLE) improved the ability to detect OPLs/OCs. Sixty high-risk patients, with suspicious oral lesions or recently diagnosed untreated OPLs/OCs, underwent sequential surveillance with WLE and AFV. Biopsies were obtained from all suspicious areas identified on both examinations (n = 189) and one normal-looking control area per person (n = 60). Sensitivity, specificity, and predictive values were calculated for WLE, AFV, and WLE + AFV. Estimates were calculated separately for lesions classified by histopathologic grades as low-grade lesions, high-grade lesions (HGL), and OCs. Sequential surveillance with WLE + AFV provided a greater sensitivity than WLE in detecting low-grade lesions (75% versus 44%), HGLs (100% versus 71%), and OCs (100% versus 80%). The specificity in detecting OPLs/OCs decreased from 70% with WLE to 38% with WLE + AFV. Thirteen of the 76 additional biopsies (17%) obtained based on AFV findings were HGLs/OCs. Five patients (8%) were diagnosed with a HGL/OC only because of the addition of AFV to WLE. In seven patients, additional HGL/OC foci or wider OC margins were detected on AFV. Additionally, AFV aided in the detection of metachronous HGL/OC in 6 of 26 patients (23%) with a history of previously treated head and neck cancer. Overall, the addition of AFV to WLE improved the ability to detect HGLs/OCs. In spite of the lower specificity, AFV + WLE can be a highly sensitive first-line surveillance tool for detecting OPLs/OCs in high-risk patients.
Oral and oropharyngeal cancers (OC) account for more than 3% of all cancers diagnosed annually (1). Two thirds of these patients have advanced stage disease at the time of initial diagnosis. Despite advances in treatment strategies, patients with late-stage cancers have a poor 5-year survival rate and a significant risk of treatment related morbidity (2). Invasive squamous cell carcinoma (SCC) of the oral cavity is often preceded by various oral premalignant lesions (OPL; refs. \3, 4). The higher-grade OPLs (moderate dysplasia, severe dysplasia, and carcinoma in situ) have a greater likelihood of progression to invasive SCC (5, 6). OPLs detected in patients with a history of OC have an even greater risk of progression (7–9). As a result, almost 4% of OC patients develop a second primary within 1 year of their initial treatment (7). The quality of life and survival of such high-risk patients is dependent on our ability to detect these OPLs and early-stage OCs.
Conventional white-light examination (WLE) remains the most widely used modality for OC surveillance and case detection. A recent randomized trial has shown that periodic screening with conventional WLE can improve the long-term survival, especially in a high-risk patient population (10). This result underscores the need for oral health care providers to perform regular surveillance on high-risk patients to detect early symptoms of malignancy. However, early malignant changes are often subtle, with visible symptoms occurring relatively late in the malignant process. Even specialized health care professionals often find it difficult to clinically differentiate between benign and early malignant changes on WLE alone. Therefore, it is essential to develop efficient visual aids that can differentiate and detect OPLs and early OCs.
Recently, there has been considerable interest in evaluating autofluorescence visualization (AFV) as a potential screening tool for epithelial cancers arising at various anatomic sites (11–14). The mechanism behind tissue autofluorescence has been extensively discussed elsewhere (15–17). In brief, carcinogenesis of squamous epithelium involves complex and progressive morphologic and biochemical changes. Some of these morphologic alterations, such as thickening of the epithelium, hyperchromatism, increase in nuclear size and nuclear cytoplasmic ratio, and increase in microvascularity, can cause subtle and often subclinical alterations in the autofluorescence characteristics of tissues. Similar alterations in fluorescence profile can also result from carcinogenesis-induced biochemical changes, such as increases in NADH, decreases in flavin adenine dinucleotide concentration, and altered elastin and keratin compositions (16, 18). When excited by violet/blue light in particular, the premalignant and malignant tissues typically exhibit a characteristic loss of fluorescence, especially in the green wavelength range. This loss of autofluorescence can be used to differentiate between malignant and surrounding normal tissues (16, 17, 19).
Several studies have evaluated the ability of AFV to differentially detect and demarcate tumors of the oral cavity (20–29). Although the early results are promising, there is still limited evidence to definitively conclude that the addition of AFV to conventional WLE will be beneficial to the patient overall. At Roswell Park Cancer Institute, we undertook a prospective study to evaluate AFV as a surveillance and detection tool for OPLs and OCs. High-risk patients are being followed over time to determine if AFV findings and baseline biomarker profiles can prognosticate malignant progression in early OPLs. In this report, we present the preliminary results from our baseline examination of high-risk patients with the combination of WLE and AFV. In addition to the usual estimates of efficacy, our analysis also aimed at answering the following fundamental questions: (a) Did AFV improve the clinician’s ability to detect OPLs and OCs that were invisible on WLE alone? (b) Did more patients benefit from the surveillance with the combination of AFV and WLE than from WLE alone? Positive answers to these questions are critically important in confirming the utility of AFV as a surveillance tool in this high-risk patient population.
Materials and Methods
Eligibility and consent
Patients were enrolled from the Departments of Dentistry and Head and Neck Surgery at Roswell Park Cancer Institute, Buffalo, NY. Patients were eligible if they met at least one of the three inclusion criteria: (a) presence of clinically suspicious oral lesions (including leukoplakia, erythroplakia, lichen planus, or pemphigus vulgaris); (b) a history of previously treated OCs, with no evidence of cancer recurrence for at least 6 mo after cessation of treatment; (c) presence of recently diagnosed untreated OPLs or OCs. Participants were at least 21 years of age and not undergoing active treatment for any malignancy at enrollment. The participants provided an informed consent and completed a detailed questionnaire, which included information on demographics, current medications, smoking and alcohol use, family history, and general health and dental care history. This study was approved by the Roswell Park Cancer Institute Institutional Review Board.
Fluorescence imaging system
Tissue autofluorescence was studied using a fluorescence imaging and point spectroscopy prototype designed by the Division of Biophysics & Bioimaging, Ontario Cancer Institute, University Health Network, Ontario, Canada (30). This system consists of a tissue illumination source (300-W xenon arc lamp) set to blue excitation centered at a wavelength of 405 nm with a spectral bandwidth of 80 nm. The excitation light was transmitted through a Storz liquid light guide and focused on the target area. The light remitted from the oral mucosa was then observed through a 10-mm rigid scope (Storz model 8711) via a fluorescence filter, which filters the blue excitation light and allows imaging in two fluorescence emission bands (green at 530–550 nm and red at 630–650 nm). This image was recorded and amplified by a three-chip charge-coupled device video camera (Sony DXC-C33). The illumination source could be switched between white light and autofluorescent blue light at different times during the exam, allowing examination under both lights on the same scope. Both autofluorescence and white-light imaging were done at 30 frames per second video rate. The spatial resolution of the fluorescence images acquired with this device was measured to be 0.12 mm (8.5 line pairs/mm). This was defined at a modulation transfer function of 50%, measured using a resolution pattern at a working distance of 2 cm from the laparoscope tip and a 12.7 × 8.2-mm field of view. The system was connected to a computer and equipped to record the videos of both the white-light and the autofluorescence examinations for future reference.
Baseline clinical examination and sample collection
The clinical examination included a general oral hygiene assessment followed by a detailed examination of the entire oral cavity (floor of the mouth, vestibule, gingiva, buccal mucosa, hard palate, and dorsal, ventral, and lateral oral tongue) and parts of the oropharynx (base of the tongue, soft palate, retromolar trigone, and tonsillar pillar). All clinical examinations were conducted by one clinician (M.S.) who specializes in dental oncology. First, all patients underwent a comprehensive WLE, and based on clinical evaluation, the findings were classified as (a) WLE grade 1: clinically unremarkable mucosa; (b) WLE grade 2: “abnormal but innocuous” (clinically explainable conditions like inflammation, scar, cheek bite, etc.); or (c) WLE grade 3: “suspicious/possibly premalignant or malignant.” The WLE was subsequently followed by AFV. The AFV images were viewed live as a video on the monitor and graded by the examining clinician. No image processing was done before the grading of the AFV images. The AFV findings were categorized based on a three-point grading system as (a) AFV grade 1: no loss of fluorescence/fluorescent green areas; (b) AFV grade 2: moderate loss of fluorescence/gray or brownish looking ill-defined areas; and (c) AFV grade 3: significant loss of fluorescence/dark gray or black areas with better-defined borders. The grading was based only on qualitative judgment. These categories were based on a similar grading system previously described by the autofluorescence screening studies in the lung (11). Due to the paucity of lesions in certain categories, the three grades were subsequently condensed into two categories as “suspicious” and “nonsuspicious.” The suspicious lesions were categorized as (a) WLE suspicious (WLE grade 3), (b) AFV suspicious (AFV grade 2/3), and (c) WLE + AFV suspicious (WLE grade 3 or AFV grade 2/3). Adequate biopsies were obtained from every suspicious lesion identified on either examination and sent for histopathologic evaluation. One control biopsy per patient was also obtained from a “WLE and AFV nonsuspicious” site, preferably on the contralateral side of the oral cavity. Whenever a contralateral biopsy was not possible, the control biopsy was obtained away from the lesion site on the same side. A photograph of each lesion was obtained, and a detailed description of each lesion, including size, clinical appearance, tenderness, and previous treatment history, was recorded. In addition, the borders of all identified lesions were recorded on an anatomic diagram of the oral cavity.
Premalignant lesions in certain anatomic subsites such as the floor of the mouth, ventral and lateral oral tongue, soft palate, and tonsillar pillar have been associated with a greater risk of cancer development (31–33). In this analysis, these subsites were classified as “high-risk sites” and the remaining subsites (including the dorsal tongue, vestibule, gingiva, buccal mucosa, and hard palate) were classified as “low-risk sites.”
Pathology review of tissue biopsy
All biopsies were fixed in 10% buffered formaldehyde, paraffin embedded, cut as 4-μm sections, and stained with H&E. For the purpose of this study, a centralized review of all samples was done by a pathologist with practice focus in head and neck oncologic pathology (M.M.). The pathologist was blinded to the clinical impression, patient history, lesion site, and WLE and AFV findings. Histopathology findings were graded as (1) normal, (2) simple hyperplasia, (3) parakeratosis, (4) parakeratosis with hyperplasia, (5) parakeratosis with cytologic atypia, (6) mild dysplasia, (7) moderate dysplasia, (8) severe dysplasia, (9) in situ carcinoma (CIS) and microinvasive SCC, and (10) invasive OC. For analysis purposes and clinical relevance, the histopathology findings were further consolidated based on the estimated risk of malignant transformation into four categories: (a) “benign”—no or minimal risk for malignant transformation (grades 1–4); (b) “low-grade lesions (LGL)” with mild architectural and/or cytologic atypia—low risk for malignant transformation (grades 5–6); (c) “high-grade lesions (HGL)”—high risk for malignant transformation (grades 7–9); and (d) “invasive OC” (grade 10). The pathology diagnoses and groupings were based on the current WHO classification system (34, 35).
In addition to benign histopathologic entities like hyperplasia and parakeratosis, we also recorded other benign parameters like hyperkeratosis, hyper-ortho/parakeratosis, chronic inflammation, fibrosis, edema, scar tissue, koilocytes, lichenoid lesions, proliferative leukoplakia, and salivary glands. These are the some of the morphologic parameters that can potentially alter the autofluorescence profile of the tissue.
Database and analysis
All the information regarding patient demographics, clinical findings, lesion description, and histopathologic diagnoses was logged into a structured database. The statistical software STATA (Stata-Corp LP, v. 10.0) was used for the current analysis. Absolute sensitivity, specificity, and predictive values (with 95% confidence intervals) were used to compare the efficacy of AFV to that of WLE, and the efficacy of the combination of WLE and AFV (WLE + AFV) to that of WLE alone. Absolute sensitivity was calculated using the formula [true positives/(true positives + false negatives)]. The sensitivity estimates were calculated separately for LGLs, HGLs, and OCs. The absolute specificity for OPLs and OCs was calculated using the formula [true negatives/(true negatives + false positives)]. A benign histopathology finding was considered as proof of absence of disease. Additionally, relative sensitivity estimates (the ratio of the sensitivity of WLE + AFV as compared with WLE alone) were calculated across different grade lesions. A relative sensitivity of greater than 1 would reflect a statistically significant improvement in the sensitivity of AFV or WLE + AFV compared with WLE alone.
Results
We herein report the results from 60 patients who underwent baseline surveillance examination with WLE and AFV. A total of 189 lesions (mean, 3.2; range, 1–8 lesions/patient) were biopsied based on suspicious findings on either WLE or AFV. Of these 189 suspicious lesions, 26 were detected on WLE only, 76 were detected on AFV only, and 87 were suspicious on both WLE and AFV. Additionally, one control biopsy was taken per patient (60 biopsies), thus accounting for a total of 249 biopsy samples. The 249 biopsies were obtained from the following anatomic sites: dorsal oral tongue (19%), lateral oral tongue (26%), vestibule/gingiva (23%), floor of the mouth/ventral oral tongue (13%), hard palate/alveolar ridge (9%), and soft palate/tonsils (10%).
The demographic characteristics and medical history of these patients are listed in Table 1. Two thirds of the patients enrolled were males and the overall mean age was 60 years. A majority of the patients were smokers, and a majority also reported moderate alcohol intake. Thirty-four (57%) patients were evaluated for clinically suspicious oral lesions and 26 (43%) patients had a recently diagnosed untreated OPL or OC. Twenty-eight (47%) patients had a history of at least one previously treated head and neck cancer.
Table 1.
Demographic characteristics and medical history of 60 patients who participated in the surveillance study
| Characteristics | N = 60 patients |
|---|---|
| Age, y | |
| Mean (SD) | 59.8 (± 12.5) |
| Range | 34–84 |
| Gender, n (%) | |
| Male | 41 (68.3) |
| Female | 19 (31.7) |
| Race, n (%) | |
| Caucasian | 56 (93.3) |
| African American | 1 (1.7) |
| Other | 3 (5.0) |
| Smoking status, n (%) | |
| Never smoker | 21 (35.0) |
| Former smoker | 26 (43.3) |
| Current smoker | 13 (21.7) |
| Alcohol intake, n (%) | |
| Never/less than once a month | 14 (23.3) |
| Less than one drink per day | 20 (33.3) |
| 1–3 drinks per day | 12 (20.0) |
| More than 3 drinks per day | 6 (10.0) |
| Unknown | 8 (13.3) |
| Reason for exam, n (%) | |
| Suspicious oral lesion(s) | 34 (56.6) |
| Recently diagnosed OPL* | 13 (21.7) |
| Recently diagnosed cancer* | 13 (21.7) |
| H/O previously treated H&N cancer, n (%)† | |
| H/O one previous primary H&N cancer | 24 (40.0) |
| H/O more than one previous primary H&N cancer | 4 (6.7) |
| Frequency of oral/dental visit, n (%) | |
| More than once a year | 34 (56.7) |
| Once a year | 3 (5.0) |
| Only with dental problem | 11 (18.3) |
| Never | 3 (5.0) |
| Unknown | 9 (15.0) |
Abbreviations: H/O, history of; H&N, head and neck.
With at least one recently histopathologically diagnosed lesion, referred to rule out lesions in other parts of the oral cavity and for treatment.
Treated at least 6 mo before the current visit.
Efficacy in identifying individual lesions
The WLE and AFV findings and the corresponding histopathologic diagnoses from all of the 249 biopsy specimens are shown in Table 2. Of these samples, 34 (14%) were diagnosed as HGLs and 15 (6%) were diagnosed as invasive OCs. Thirteen of these biopsies [10 HGLs (29%) and 3 invasive OCs (20%)] were obtained from sites that were considered nonsuspicious on WLE alone (false negatives) but were suspicious on AFV. Conversely, two biopsies [one HGL (3%) and one invasive OC (6%)] were obtained from sites that were suspicious only on WLE, and not on AFV. On evaluation of the 60 control biopsies, 53% displayed benign unremarkable findings, 27% showed parakeratosis with cytologic atypia, and 20% showed mild dysplasia. None of the control biopsies were HGLs or OCs. Overall, 82% (91 of 111) of LGLs and all of HGLs and OCs were detected on either WLE or AFV.
Table 2.
Results from WLE and AFV with corresponding histopathologic diagnosis for 249 individual sites that were biopsied for this surveillance study
| Pathology diagnosis | WLE
|
AFV
|
WLE + AFV
|
Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Nonsuspicious | Suspicious | Nonsuspicious | Suspicious | P* | Nonsuspicious | Suspicious† | P‡ | ||
| Benign | |||||||||
| Normal | 19 | 4 | 11 | 12 | 9 | 14 | 23 | ||
| Simple hyperplasia | 1 | 3 | 3 | 1 | 1 | 3 | 4 | ||
| Parakeratosis | 22 | 8 | 17 | 13 | 15 | 15 | 30 | ||
| Parakeratosis with hyperplasia | 13 | 9 | 8 | 14 | 5 | 17 | 22 | ||
| Total | 55 | 24 | 39 | 40 | 0.02 | 30 | 49 | <0.001 | 79 |
| Low-grade lesions | |||||||||
| Parakeratosis with atypia | 33 | 23 | 23 | 33 | 17 | 39 | 56 | ||
| Mild dysplasia | 35 | 30 | 22 | 43 | 13 | 52 | 65 | ||
| Total | 68 | 53 | 45 | 76 | 0.004 | 30 | 91 | <0.001 | 121 |
| High-grade lesions | |||||||||
| Moderate dysplasia | 5 | 13 | 1 | 17 | — | 18 | 18 | ||
| Severe dysplasia | — | 6 | — | 6 | — | 6 | 6 | ||
| CIS OR microinvasive SCC | 5 | 5 | — | 10 | — | 10 | 10 | ||
| Total | 10 | 24 | 1 | 33 | 0.006 | 0 | 34 | 0.006 | 34 |
| Cancers | |||||||||
| Invasive SCC | 2 | 10 | 1 | 11 | — | 12 | 12 | ||
| Other carcinoma§ | 1 | 2 | — | 3 | — | 3 | 3 | ||
| Total | 3 | 12 | 1 | 14 | 0.59 | 0 | 15 | 0.22 | 15 |
| Total | 136 | 113 | 86 | 163 | 60 | 189 | 249 | ||
P value for WLE findings compared with AFV calculated by two-tailed Fisher’s exact test.
Suspicious on either WLE or AFV.
P value for WLE findings compared with (WLE + AFV) calculated by two-tailed Fisher’s exact test.
Includes one adenocarcinoma (salivary gland carcinoma) and two verrucous carcinomas with SCC component.
In total, 76 additional biopsies were obtained from these 60 patients due to the addition of AFV to the conventional WLE (mean, 1.3 additional biopsies/person). Of these biopsies, 13 (17%) were diagnosed as either HGLs or invasive OCs. Eight of these 13 AFV suspicious biopsies (seven HGLs and one OC) were obtained from sites that showed no visible abnormality on WLE (WLE grade 1), and the other five (three HGLs and two OCs) were obtained from sites that looked like innocuous lesions on WLE (WLE grade 2). Figure 1 shows sites that showed no visible abnormality on WLE, but were biopsied based on the suspicious AFV findings and histopathologically confirmed to be an OPL/OC.
Fig. 1.
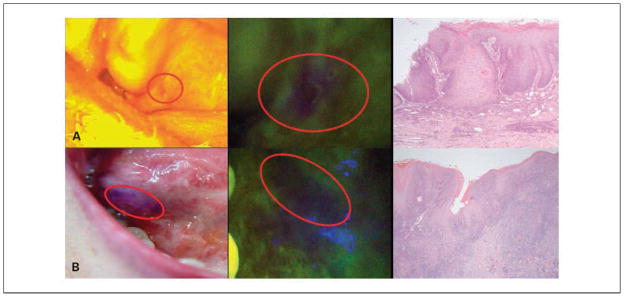
SCC and dysplasia identified based on loss of autofluorescence. A, moderate dysplasia on soft palate, identified as an innocuous irritation spot on WLE but biopsied because of the loss of autofluorescence. B, SCC at the base of the tongue, nonsuspicious on WLE but biopsied because of the loss of autofluorescence. The anterior and posterior extents of the area showing autofluorescence loss were marked in blue for biopsy purposes.
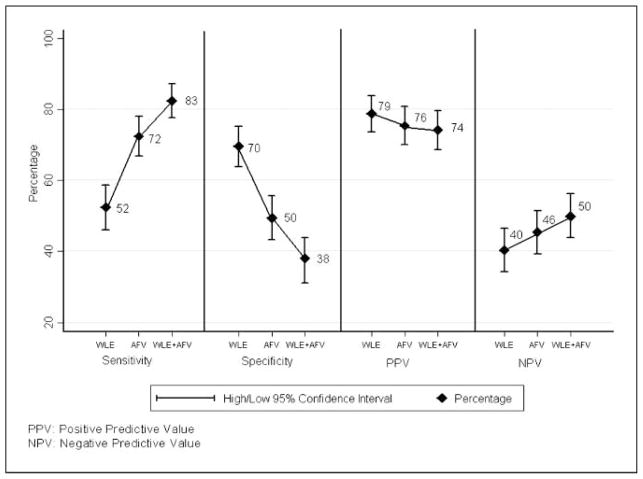
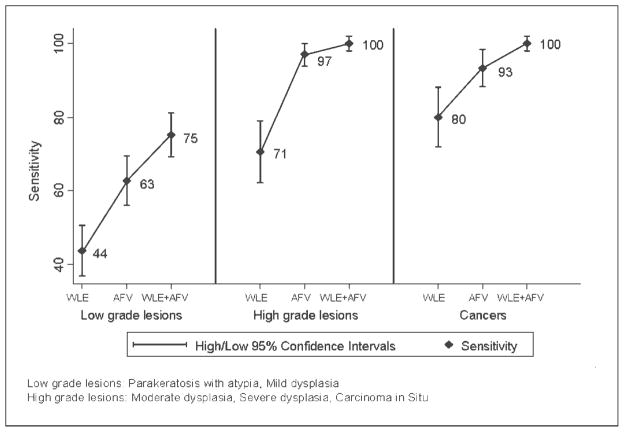
Figure 2 presents the overall absolute sensitivity, specificity, positive predictive value, and negative predictive value for all grades of premalignant lesions and cancers. The overall sensitivity for detecting all grades of premalignant lesions and cancers improved from 52% with WLE to 72% with AFV and to 83% with WLE + AFV. However, the specificity in detecting OPLs and OCs decreased from 70% with WLE to 50% with AFV and to 38% with WLE + AFV. We also evaluated the absolute sensitivity and negative predictive value of WLE, AFV, and WLE + AFV separately for different grades of premalignant lesions and cancers. Absolute sensitivity for detecting HGLs was 71% with WLE alone, and it improved to 97% with AFV and to 100% with WLE + AFV. Similarly, the absolute sensitivity for detecting OCs also improved from 80% with WLE alone to 100% with WLE + AFV (Fig. 3). The negative predictive value for ruling out OCs was 95% with WLE, and it improved to 100% with WLE + AFV.
Fig. 2.
Sensitivity, specificity, and predictive values of WLE, AFV, and WLE + AFV for all grades of premalignant lesions and cancers. PPV, positive predictive value; NPV, negative predictive value.
Fig. 3.
Sensitivity of WLE, AFV, and WLE + AFV across different grades of premalignant lesions and cancers. Low-grade lesions: parakeratosis with atypia, mild dysplasia. High-grade lesions: moderate dysplasia, severe dysplasia, carcinoma in situ.
The relative improvement in sensitivity (relative sensitivity estimates) due to the addition of AFV to the conventional WLE was estimated based on the grade of the lesion (data not shown). Compared with WLE, the relative sensitivity of AFV improved by 43% for LGLs, by 38% for HGLs, and by 17% for OCs. Similarly, when compared with WLE, the combination of AFV and WLE improved the relative sensitivity by 72% for LGLs, by 42% for HGLs, and by 25% for OCs.
Efficacy in identifying the highest histologic grade lesion in a patient
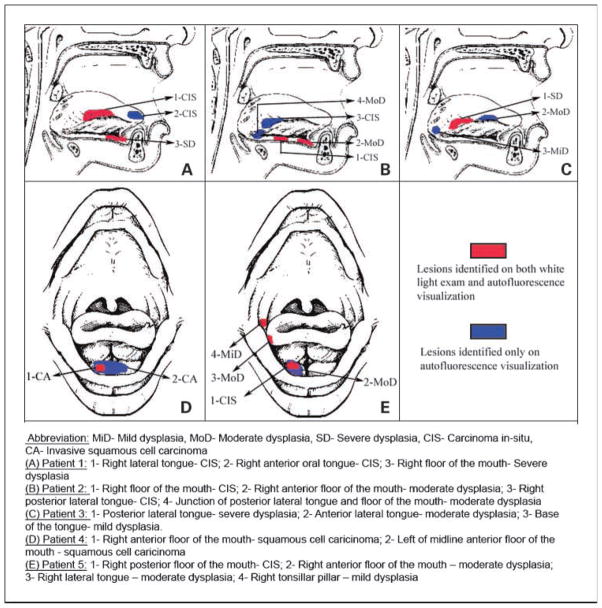
Of the 60 patients who participated in this study, 29 patients (48%) were diagnosed with at least one LGL and 27 patients (45%) were diagnosed with at least one HGL/OC (Table 3). In 75% of the patients, all of the highest-grade lesions were detected on AFV, compared with 40% of the patients on WLE. In two patients with multifocal HGLs, only one of the highest histologic lesion (CIS) was detected on WLE, whereas both the CIS lesions were detected on AFV (Fig. 4, patients 1 and 2). Overall, 8% (5 of 60) of our patients were diagnosed with a HGL or invasive OC solely because of the addition of AFV to the conventional WLE.
Table 3.
Ability of WLE and AFV in detecting the highest histologic grade lesion in 56 patients with at least one OPL or cancer
| Highest histologic grade lesion in the patient | WLE
|
AFV
|
|||||
|---|---|---|---|---|---|---|---|
| None of the highest-grade lesions diagnosed | Some of the highest-grade lesions diagnosed* | All of the highest-grade lesions diagnosed | None of the highest-grade lesions diagnosed | Some of the highest-grade lesions diagnosed* | All of the highest-grade lesions diagnosed | P† | |
| Low-grade lesions | |||||||
| Parakeratosis with atypia (n = 8) | 5 | 2 | 1 | 1 | 2 | 5 | |
| Mild dysplasia (n = 21) | 11 | 6 | 4 | 2 | 5 | 14 | |
| Total | 16 | 8 | 5 | 3 | 7 | 19 | 0.001 |
| High-grade lesions | |||||||
| Moderate dysplasia (n = 8) | 2 | — | 6 | — | — | 8 | |
| Severe dysplasia (n = 2) | — | — | 2 | — | — | 2 | |
| CIS or micro invasive SCC (n = 7) | 1 | 2 | 4 | — | — | 7 | |
| Total | 3 | 2 | 12 | 0 | 0 | 17 | 0.22 |
| Cancers | |||||||
| Invasive SCC (n = 8) | 1 | 1 | 6 | — | 1 | 7 | |
| Other carcinoma (n = 2)‡ | 1 | — | 1 | — | — | 2 | |
| Total | 2 | 1 | 7 | 0 | 1 | 9 | 0.47 |
| Total | 21 | 11 | 24 | 3 | 8 | 45 | <0.001 |
Some, but not all, of the highest-grade lesions diagnosed.
P value for WLE findings compared with AFV calculated by two-tailed Fisher’s exact test.
Includes one patient with adenocarcinoma (salivary gland carcinoma) and one with verrucous carcinomas with SCC component.
Fig. 4.
Diagram depicting the field changes identified by WLE and AFV on five high-risk patients who participated in the surveillance study. MiD, mild dysplasia; MoD, moderate dysplasia; SD, severe dysplasia; CA, invasive squamous cell carcinoma. A, patient 1: 1, right lateral tongue—CIS; 2, right anterior oral tongue—CIS; 3, right floor of the mouth—severe dysplasia. B, patient 2: 1, right floor of the mouth—CIS; 2, right anterior floor of the mouth—moderate dysplasia; 3, right posterior lateral tongue—CIS; 4, junction of posterior lateral tongue and floor of the mouth—moderate dysplasia. C, patient 3: 1, posterior lateral tongue—severe dysplasia; 2, anterior lateral tongue—moderate dysplasia; 3, base of the tongue—mild dysplasia. D, patient 4: 1, right anterior floor of the mouth—squamous cell carcinoma; 2, left of midline anterior floor of the mouth—squamous cell carcinoma. E, patient 5: 1, right posterior floor of the mouth—CIS; 2, right anterior floor of the mouth—moderate dysplasia; 3, right lateral tongue—moderate dysplasia; 4, right tonsillar pillar—mild dysplasia.
Ability of AFV in detecting synchronous and metachronous lesions
In five patients with multifocal HGL/OC, additional foci were detected only on AFV. In two patients, AFV aided in the detection of wider-contiguous field changes around the WLE detectable OC/CIS (Fig. 4, patients 4 and 5). Additionally, 6 of the 26 patients (23%) with a history of previously treated head and neck cancer had diffuse metachronous HGLs and OCs that were identified only on AFV. A total of seven HGLs/OCs (one invasive SCC, three CIS, and three moderate dysplasia) were identified in these six patients.
Efficacy based on the anatomic site of the lesion
We also evaluated the AFV findings based on the anatomic location of the lesion (data not shown). A greater percentage of HGLs were noted on the high-risk anatomic sites (65%) compared with the low-risk anatomic sites (35%). A significantly greater number of mild dysplasia (P = 0.05) and hyperplasia (P = 0.04) located on high-risk sites were “AFV suspicious” compared with the ones located on low-risk sites. However, the number of lesions per individual location was insufficient to permit detailed analysis.
Evaluation of the false-positive biopsies
We analyzed the benign parameters associated with the false-positive findings on autofluorescence. Our results show that whereas the benign parameters individually were not significantly associated with the loss of fluorescence, the presence of two or more coexisting parameters was associated with loss of fluorescence. For example, loss of fluorescence was noticed in 72% of the benign lesions that showed both parakeratosis and hyperplasia, compared with 19% of the benign lesions that showed only one of these changes (P = 0.003). Similarly, 77% of the benign lesions that showed both parakeratosis and inflammation exhibited loss of fluorescence, compared with 21% of the lesions that showed only one of these changes (P = 0.002). Also, 74% of all the false-positive biopsies showed at least two of the benign parameters. These results suggest that presence of multiple benign morphologic changes might influence the autofluorescence profile of the tissue. However, fewer lesions in each subcategory limited the possibility of a detailed analysis on these false-positive biopsies.
Discussion
Oral carcinogenesis often involves diffuse and/or multifocal premalignant changes with variable potential to progress to invasive cancers (4, 36, 37). The poorer prognostic implications associated with late-stage disease make it critical to efficiently detect these early lesions. However, the limited ability to detect the premalignant and early malignant changes on conventional oral exam poses a significant challenge to OC screening. Several innovative diagnostic aids have recently been designed to address this limitation (22–25, 38–46). Some of these diagnostic techniques such as toluidine blue staining, tissue reflectance, in vivo AFV, and spectroscopy have shown early promising results (22, 23, 39, 41–45). Direct comparison of these techniques has not yet been done. However, the ease of use, wide-area imaging capability, nonrequirement of exogenous agents, and the ability to detect diffuse lesions provide AFV a functional advantage as a surveillance tool.
In vivo autofluorescence screening for OCs was first proposed in the late 1980s (47). Early studies using UV light and porphyrin-based fluorescence reported inconsistent results, with the sensitivity for detecting OCs varying between 67% and 96% (20, 26, 28, 29). Later studies have evaluated near-UV to green range wavelengths for fluorescence visualization (21–25, 27). Paczona et al. and Kulapaditharom et al. evaluated the efficiency of autofluorescence endoscopy in detecting head and neck cancers (21, 25). They reported 93% to 100% sensitivity with AFV, which was a significant improvement over WLE. However, both these studies mainly examined laryngeal lesions and evaluated only a limited number of oral lesions. Svistun et al. evaluated freshly resected oral tissue with autofluorescent light of four different wavelengths and reported that the best contrast was achieved at the 400-nm illumination. They achieved improved sensitivity and specificity of 91% and 86%, respectively. In two other recent reports, Poh et al. used a handheld autofluorescence device and successfully demonstrated the ability to detect new lesions and extended tumor margins invisible on WLE (22, 23). Similar to other studies, our results also showed a significant improvement in detection of HGLs/OCs due to the addition of AFV to conventional WLE. Although all these studies evaluated the autofluorescence technique, critical comparison between studies is complicated because of a few reasons. First, the studies used different excitation and/or emission wavelength bands and evaluated dissimilar patient populations, making it hard to directly compare the results. Second, as with many other screening and case-detection tools, there is a learning curve during which the operator is less confident of the exam findings. This learning curve effect makes it difficult to accurately evaluate clinical studies with a small sample size. Third, the lack of consistent grading system for dysplasias poses challenges in evaluating the efficacy of AFV in detecting OPLs. A binary classification system, based on the WHO Working Group guidelines, has recently been proposed to grade OPLs as “low-risk” or “high-risk” lesions (34, 35). In our study, we used these suggested criteria to classify the lesions as LGLs or HGLs.
The sensitivity of detecting OCs improved to 93% with AFV alone and to 100% with WLE + AFV. Conversely, the specificity dropped to 38% with WLE + AFV. Although we reported absolute sensitivity and specificity for consistency purposes, one should be cautious while interpreting the results of autofluorescence screening studies. Ideally, to obtain an unbiased estimate of the sensitivity and specificity, we would need the accurate estimates of “true negatives” and “false negatives.” These can be obtained only by histopathologic diagnosis from all the normal-looking areas on WLE and AFV. Because biopsying the entire oral cavity is not a feasible option, we examined the control biopsies as a sub-sample of the nonsuspicious looking areas. None of these control biopsies showed any HGL/OC. We also confirmed the improvement in sensitivity by calculating the relative sensitivity of our surveillance tools. AFV and WLE + AFV consistently showed a relative improvement in sensitivity across all grades of lesions.
However, the clinical utility of AFV can be evaluated much better by summarizing the individual results at both the “per-lesion” and “per-patient” levels. About 17% of the additional biopsies obtained based on AFV findings alone were diagnosed as either a HGL or an OC. Sixty percent of these HGLs/OCs were diagnosed from sites that showed no visible abnormality on WLE. AFV also aided in the detection synchronous and metachronous OCs that were missed on WLE alone. Most important of all, one in every five patients with a HGL or an OC had their diagnosis made possible only due to the addition of AFV surveillance.
In spite of these encouraging preliminary findings, certain limitations need to be considered while interpreting these results. Our study was done in a tertiary care facility where the prevalence of HGL/OC was high (45%). Furthermore, most of our patients were at high risk for OC and would have warranted at least one biopsy on WLE. Therefore, the additional morbidity due to the AFV-guided biopsies was considered to be minimal. Hence, the benefits of diagnosis by AFV-guided biopsies outweighed the risk of additional biopsy-related morbidity. This may not be the case in the screening of the general population, where disease prevalence is lower. Thus, poor specificity is the major limitation for using AFV as a screening tool in a primary care setting.
To understand the reason for the poor specificity, one needs to be aware of the complexity involved in the mechanism of AFV. The AFV technique uses the changes in the optical characteristics of these altered tissues to enhance the visual contrast between benign and cancerous tissues (15–17). In essence, AFV uses optical changes as an indirect measurement of the cancerous changes, compared with histopathology, which involves direct visualization of the morphologic alterations. It is important to note that several benign morphologic changes and patient factors (such as pigmentation, dentures, and previous treatment) may also induce tissue optical changes, thereby affecting the AFV findings (48). These factors could potentially contribute to a greater number of false-positive findings. Due to these inherent limitations, refinements to the AFV technique are likely to improve the specificity only to a certain level. Some of these false positives can be clinically ruled out based on the appearance and/or patient history, but most need biopsy confirmation. Therefore, AFVat present is not a substitute to WLE or histopathology, but rather a complementary diagnostic aid for improved lesion detection.
In a recent study, Poh et al. evaluated histopathologically benign OC margins and reported a significant association between loss of autofluorescence and loss of heterozygosity, a molecular marker of cancer risk (22). Similarly, prospective AFV studies in the lung have also reported a higher rate of cancer development in histologically benign mucosae that exhibit loss of autofluorescence (49, 50). These results suggest a possibility that some of the AFV detectable changes might precede histologic changes, the current gold standard for diagnosis. In that case, some of the false-positive findings may, in retrospect, be classified as true positives. However, this can only be confirmed by large prospective studies evaluating the genetic changes and progression rates associated with “AFV suspicious” sites. Such studies also have the potential to provide complementary genetic markers that can improve the specificity of the AFV technique.
In summary, our study results underscore the utility of AFV technique as a potential complementary diagnostic aid in the surveillance of the high-risk patient population. However, further refinement in autofluorescence technology and the development of adjunct genetic and molecular markers may be needed to improve the specificity of this technique. Multi-institutional prospective clinical trials using standardized devices and end points would be needed to further evaluate and improve accuracy before being recommended as a screening tool in the general population.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L, Eveson J, Reichart P, Sidransky D, editors. World Health Organization classification of tumors. Lyon: IARC Press; 2005. [Google Scholar]
- 4.Greer RO. Pathology of malignant and premalignant oral epithelial lesions. Otolaryngol Clin North Am. 2006;39:249–75. v. doi: 10.1016/j.otc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Rosin MP, Poh CF, Guillard M, Williams PM, Zhang L, MacaUlay C. Visualization and other emerging technologies as change makers for oral cancer prevention. Ann N Y Acad Sci. 2007;1098:167–83. doi: 10.1196/annals.1384.039. [DOI] [PubMed] [Google Scholar]
- 6.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 7.Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer. 1992;70:14–9. doi: 10.1002/1097-0142(19920701)70:1<14::aid-cncr2820700103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz LH, Ozsahin M, Zhang GN, et al. Synchronous and metachronous head and neck carcinomas. Cancer. 1994;74:1933–8. doi: 10.1002/1097-0142(19941001)74:7<1933::aid-cncr2820740718>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Tabor MP, Brakenhoff RH, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523–32. [PubMed] [Google Scholar]
- 10.Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365:1927–33. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 11.Lam S, Kennedy T, Unger M, et al. Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest. 1998;113:696–702. doi: 10.1378/chest.113.3.696. [DOI] [PubMed] [Google Scholar]
- 12.Huh WK, Cestero RM, Garcia FA, et al. Optical detection of high-grade cervical intraepithelial neoplasia in vivo: results of a 604-patient study. Am J Obstet Gynecol. 2004;190:1249–57. doi: 10.1016/j.ajog.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Curvers WL, Singh R, Song LM, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57:167–72. doi: 10.1136/gut.2007.134213. [DOI] [PubMed] [Google Scholar]
- 14.Kelloff GJ, Sullivan DC, Baker H, et al. Workshop on imaging science development for cancer prevention and preemption. Cancer Biomark. 2007;3:1–33. doi: 10.3233/cbm-2007-3101. [DOI] [PubMed] [Google Scholar]
- 15.Ramanujam N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia. 2000;2:89–117. doi: 10.1038/sj.neo.7900077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Veld DC, Witjes MJ, Sterenborg HJ, Roodenburg JL. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005;41:117–31. doi: 10.1016/j.oraloncology.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Pavlova I, Williams M, El-Naggar A, Richards-Kortum R, Gillenwater A. Understanding the biological basis of autofluorescence imaging for oral cancer detection: high-resolution fluorescence microscopy in viable tissue. Clin Cancer Res. 2008;14:2396–404. doi: 10.1158/1078-0432.CCR-07-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlova I, Sokolov K, Drezek R, Malpica A, Follen M, Richards-Kortum R. Microanatomical and biochemical origins of normal and precancerous cervical autofluorescence using laser-scanning fluorescence confocal microscopy. Photochem Photobiol. 2003;77:550–5. doi: 10.1562/0031-8655(2003)077<0550:maboon>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Westra WH, Sidransky D. Fluorescence visualization in oral neoplasia: shedding light on an old problem. Clin Cancer Res. 2006;12:6594–7. doi: 10.1158/1078-0432.CCR-06-2253. [DOI] [PubMed] [Google Scholar]
- 20.Betz CS, Mehlmann M, Rick K, et al. Autofluorescence imaging and spectroscopy of normal and malignant mucosa in patients with head and neck cancer. Lasers Surg Med. 1999;25:323–34. doi: 10.1002/(sici)1096-9101(1999)25:4<323::aid-lsm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Kulapaditharom B, Boonkitticharoen V. Performance characteristics of fluorescence endoscope in detection of head and neck cancers. Ann Otol Rhinol Laryngol. 2001;110:45–52. doi: 10.1177/000348940111000109. [DOI] [PubMed] [Google Scholar]
- 22.Poh CF, Zhang L, Anderson DW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12:6716–22. doi: 10.1158/1078-0432.CCR-06-1317. [DOI] [PubMed] [Google Scholar]
- 23.Poh CF, Ng SP, Williams PM, et al. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head Neck. 2007;29:71–6. doi: 10.1002/hed.20468. [DOI] [PubMed] [Google Scholar]
- 24.Lane PM, Gilhuly T, Whitehead P, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt. 2006;11:024006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]
- 25.Paczona R, Temam S, Janot F, Marandas P, Luboinski B. Autofluorescence videoendoscopy for photodiagnosis of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2003;260:544–8. doi: 10.1007/s00405-003-0635-6. [DOI] [PubMed] [Google Scholar]
- 26.Onizawa K, Saginoya H, Furuya Y, Yoshida H, Fukuda H. Usefulness of fluorescence photography for diagnosis of oral cancer. Int J Oral Maxillofac Surg. 1999;28:206–10. [PubMed] [Google Scholar]
- 27.Svistun E, Alizadeh-Naderi R, El-Naggar A, Jacob R, Gillenwater A, Richards-Kortum R. Vision enhancement system for detection of oral cavity neoplasia based on autofluorescence. Head Neck. 2004;26:205–15. doi: 10.1002/hed.10381. [DOI] [PubMed] [Google Scholar]
- 28.Onizawa K, Saginoya H, Furuya Y, Yoshida H. Fluorescence photography as a diagnostic method for oral cancer. Cancer Lett. 1996;108:61–6. doi: 10.1016/s0304-3835(96)04388-1. [DOI] [PubMed] [Google Scholar]
- 29.Fryen A, Glanz H, Lohmann W, Dreyer T, Bohle RM. Significance of autofluorescence for the optical demarcation of field cancerisation in the upper aerodigestive tract. Acta Otolaryngol. 1997;117:316–9. doi: 10.3109/00016489709117795. [DOI] [PubMed] [Google Scholar]
- 30.Moriyama E, Kim A, Bogaards A, Lilge L, Wilson B. A ratiometric fluorescence imaging system for surgical guidance. Adv Opt Technol. 2008:article ID 532368, 10. [Google Scholar]
- 31.Mashberg A, Meyers H. Anatomical site and size of 222 early asymptomatic oral squamous cell carcinomas: a continuing prospective study of oral cancer. II Cancer. 1976;37:2149–57. doi: 10.1002/1097-0142(197605)37:5<2149::aid-cncr2820370502>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Cheung KJ, Jr, Lam WL, et al. Increased genetic damage in oral leukoplakia from high risk sites: potential impact on staging and clinical management. Cancer. 2001;91:2148–55. doi: 10.1002/1097-0142(20010601)91:11<2148::aid-cncr1243>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 33.Boffetta P, Mashberg A, Winkelmann R, Garfinkel L. Carcinogenic effect of tobacco smoking and alcohol drinking on anatomic sites of the oral cavity and oropharynx. Int J Cancer. 1992;52:530–3. doi: 10.1002/ijc.2910520405. [DOI] [PubMed] [Google Scholar]
- 34.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42:987–93. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Warnakulasuriya S, Reibel J, Bouquot J, Dabelsteen E. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008;37:127–33. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 36.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 37.Ogden GR, Cowpe JG, Green MW. Evidence of field change in oral cancer. Br J Oral Maxillofac Surg. 1990;28:390–2. doi: 10.1016/0266-4356(90)90036-k. [DOI] [PubMed] [Google Scholar]
- 38.Sciubba JJ U.S. Collaborative OralCDx Study Group. Improving detection of precancerous and cancerous oral lesions. Computer-assisted analysis of the oral brush biopsy. J Am Dent Assoc (1939) 1999;130:1445–57. doi: 10.14219/jada.archive.1999.0055. [DOI] [PubMed] [Google Scholar]
- 39.Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97–103. doi: 10.1111/j.1600-0714.1996.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 40.Barrellier P, Babin E, Louis MY, Meunier-Guttin A. The use of toluidine blue in the diagnosis of neoplastic lesions of the oral cavity. Rev Stomatol Chir Maxillofac. 1993;94:51–4. [PubMed] [Google Scholar]
- 41.Huber MA, Bsoul SA, Terezhalmy GT. Acetic acid wash and chemiluminescent illumination as an adjunct to conventional oral soft tissue examination for the detection of dysplasia: a pilot study. Quintessence Int. 2004;35:378–84. [PubMed] [Google Scholar]
- 42.Ram S, Siar CH. Chemiluminescence as a diagnostic aid in the detection of oral cancer and potentially malignant epithelial lesions. Int J Oral Maxillofac Surg. 2005;34:521–7. doi: 10.1016/j.ijom.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Epstein JB, Gorsky M, Lonky S, Silverman S, Jr, Epstein JD, Bride M. The efficacy of oral lumenoscopy (ViziLite) in visualizing oral mucosal lesions. Spec Care Dentist. 2006;26:171–4. doi: 10.1111/j.1754-4505.2006.tb01720.x. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz RA, Gao W, Redden Weber C, et al. Non-invasive evaluation of oral lesions using depth-sensitive optical spectroscopy. Cancer. 2009;115:1669–79. doi: 10.1002/cncr.24177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heintzelman DL, Utzinger U, Fuchs H, et al. Optimal excitation wavelengths for in vivo detection of oral neoplasia using fluorescence spectroscopy. Photochem Photobiol. 2000;72:103–13. doi: 10.1562/0031-8655(2000)072<0103:OEWFIV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Muller MG, Valdez TA, Georgakoudi I, et al. Spectroscopic detection and evaluation of morphologic and biochemical changes in early human oral carcinoma. Cancer. 2003;97:1681–92. doi: 10.1002/cncr.11255. [DOI] [PubMed] [Google Scholar]
- 47.Harris DM, Werkhaven J. Endogenous porphyrin fluorescence in tumors. Lasers Surg Med. 1987;7:467–72. doi: 10.1002/lsm.1900070605. [DOI] [PubMed] [Google Scholar]
- 48.de Veld DC, Sterenborg HJ, Roodenburg JL, Witjes MJ. Effects of individual characteristics on healthy oral mucosa autofluorescence spectra. Oral Oncol. 2004;40:815–23. doi: 10.1016/j.oraloncology.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Sutedja TG, Venmans BJ, Smit EF, Postmus PE. Fluorescence bronchoscopy for early detection of lung cancer: a clinical perspective. Lung Cancer. 2001;34:157–68. doi: 10.1016/s0169-5002(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 50.Pasic A, Vonk-Noordegraaf A, Risse EK, Postmus PE, Sutedja TG. Multiple suspicious lesions detected by autofluorescence bronchoscopy predict malignant development in the bronchial mucosa in high risk patients. Lung Cancer. 2003;41:295–301. doi: 10.1016/s0169-5002(03)00191-0. [DOI] [PubMed] [Google Scholar]