Abstract
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder that manifests as a progressive loss of memory and deterioration of higher cognitive functions. AD is characterized by accumulation in the brain of the β-amyloid peptide (Aβ) generated by β- and γ-secretase processing of amyloid precursor protein (APP). Epidemiological studies have linked elevated plasma cholesterol and lipoprotein levels in mid-life with AD development. Cholesterol-fed animal models exhibit neuropathologic features of AD including accumulation of Aβ. Specific isoforms of the cholesterol transporter apolipoprotein (apo) E are associated with susceptibility to AD. Although multiple lines of evidence indicate a role for cholesterol in AD, the exact impact and mechanisms involved remain largely unknown. This review summarizes the current state of our knowledge of the influence of cholesterol and lipid pathways in AD pathogenesis in vitro and in vivo.
Keywords: Alzheimer’s disease, amyloidprecursor protein, β-amyloid peptide, oxysterol, apolipoprotein E, ABCA1, LXR
INTRODUCTION
Alzheimer’s disease (AD), the most common form of dementia, is a slowly progressive neurodegenerative disease clinically characterized by memory impairment and pathologically characterized by the formation of extracellular senile plaques containing amyloid beta (Aβ) and neurofibrillary tangles containing hyperphosphorylated tau protein. in the brain.1 Substantial synaptic and neuronal loss occurs in critical brain areas, especially in cholinergic neurons in the basal forebrain region, with cortical shrinkage predominant.2,3 Ultimately, the pathology spreads throughout the cerebral cortex. In addition to memory deficits, patients with AD also experience visuospatial dysfunction, degradation of language, and a decline in their ability to carry out activities of daily living. Changes in mood and affect often accompany or precede memory decline. AD is a major public health problem due to increasing prevalence, prolonged course, challenges to caregivers and high expenditures for care.4,5
Although multiple lines of evidence indicate a role for cholesterol in AD, the exact impact and mechanisms involved remain largely unknown6–8. This review summarizes the current state of our knowledge of the influence of cholesterol and lipid pathways in AD pathogenesis in animal, human and cell culture systems.
THE AMYLOID HYPOTHESIS
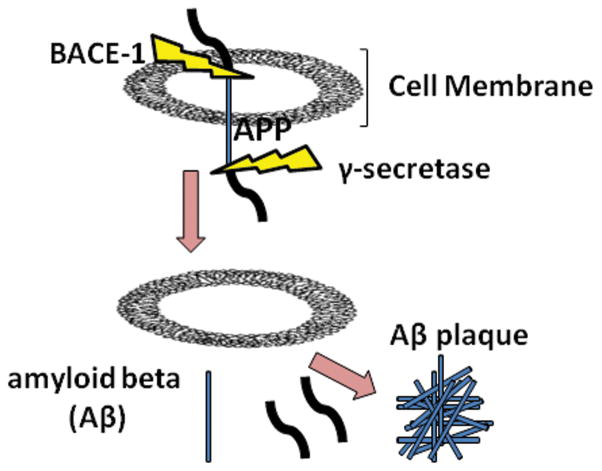
Aβ, the major peptide constituent of senile plaques, accumulates in the brain several decades before the disease is evident.9 The presence of Aβ plaques in the brain is a central neuropathologic feature of AD.10 Aβ is formed by the sequential cleavage of its parent protein, the amyloid precursor protein (APP), a type I transmembrane protein with a large extracellular domain and a short cytoplasmic region (Fig. 1). APP can undergo proteolytic processing via either of two different pathways, one of which is amyloidogenic while the other is not.11 In the amyloid-forming pathway, APP is acted upon first by the aspartyl protease β-site APP cleaving enzyme-1 (BACE-1).12 BACE-1 within the trans-Golgi network and early endosomes cuts APP 99 amino acids from the C-terminal to generate a soluble N-terminal fragment (APPsβ) and a membrane-bound 12-kDa C-terminal fragment. Then, the γ-secretase releases pathogenic Aβ from the lipid bilayer in a process known as regulated intramembrane proteolysis.13,14 Inhibition of either of these secretases can block Aβ generation.15 Depending upon the site of γ-secretase cleavage, two major forms of Aβ are generated, the 40 amino acid Aβ40 and the 42 amino acid Aβ42. Aβ42 is the predominant species of Aβ in senile plaques.16, 17 Once amyloid forms, a cascade is initiated resulting in additional intracellular aggregations of the tau protein, which then form tangles.18 The nonamyloidogenic processing pathway involves the cleavage of APP at the Lys16-Leu17 bond within the Aβ peptide sequence, and thus precludes Aβ formation.19
FIGURE 1. Aβ formation in the brain.
Aβ is formed by the sequential cleavage of its parent protein, APP, by two enzymes, BACE-1 and γ-secretase in the membrane. β-cleavage of APP occurs at the N-terminus of Aβ. This cleavage releases a membrane-associated fragment of 99 amino acids which is then further processed at a second site within its transmembrane domain by γ-secretase to release the Aβ peptide which can be 38–42 amino acids in length. Newly generated molecules of Aβ then aggregate into plaques.
Approaches to AD treatment are under development. Strategies focus on preventing or reversing Aβ formation by influencing APP processing through manipulation of secretase expression/activity. Other disease-modifying therapies might inhibit Aβ aggregation or boost Aβ clearance.20
CHOLESTEROL
Cholesterol is an essential constituent of eukaryotic membranes that regulates lipid bilayer dynamics and structure and determines membrane biophysical properties. 21 Tight regulation of brain cholesterol homeostasis is necessary to maintain neurological function.22 Nearly all cholesterol present in the brain is synthesized within the central nervous system (CNS) and its metabolism is regulated independently of that in peripheral tissues.23,24 An intact blood brain barrier (BBB) prevents transport of cholesterol from peripheral circulation to the brain.25 Lipoproteins in human, mouse, and rat do not cross the BBB. The major sterol in the adult human brain is unesterified cholesterol, with small amounts of desmosterol and cholesteryl ester also detected.26 Brain cholesterol turnover is slow, with a half-life in humans of about a year.27
Neurons do not efficiently synthesize cholesterol and rely on astrocytes as an external source important for neuronal development and remodeling.28 It has been proposed that lipoproteins from glial cells are taken up by axons of neighboring neurons.29
Epidemiological observations have identified mid-life hypercholesterolemia, but not later life cholesterol elevation, as a major risk factor for AD.30, 31 A study that followed 9,844 members of the Kaiser Permanente Northern California Medical Group ages 40–45 who had blood work done between 1964 and 1973 found that even borderline elevations in cholesterol (greater than 220 mg/dl) increased the risk of developing AD three decades later. 32 In contrast, The Prospective Population Study of Women conducted in Sweden starting in 1968–1969 with 1,462 women without dementia aged 38–60 years, found that a higher cholesterol level in 1968 was not associated with an increased risk of AD in 2000–2001. 33
Some early epidemiological studies suggested that subjects who take statins, drugs that reduce plasma cholesterol by inhibiting 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis, exhibit reduced susceptibility to AD. 31,34,35 Statins are well characterized and tolerated and are the most prescribed lipid-lowering medications (Fig. 2).36 They are efficacious in decreasing cholesterol levels and also exert beneficial anti-inflammatory, anti-platelet aggregatory and antioxidant effects that are “pleiotropic” and unrelated to lipid-lowering.37 In addition to cholesterol, downstream products of the mevalonate pathway include key isoprenoid intermediates. By inhibiting L-mevalonic acid synthesis, statins also block the production of these isoprenoids. Some of the cholesterol-independent effects of statins may be due to decreased prenylation of proteins involved in signal transduction in cells, particularly Rho and Rab family GTPases.38 In cultured neurons, statin inhibition of protein isoprenylation decreases Aβ secretion.39 Statins differ in their biochemical properties and particularly in blood brain barrier permeability. Some statins such as simvastatin and lovastatin are lipophilic and are able to cross the blood-brain barrier even if it is intact. Rosuvastatin and pravastatin are more hydrophilic. Thus, statins are not considered to be therapeutically interchangeable. Since cholesterol is vital to normal brain function, there has been concern that more lipophilic statins may decrease cognitive function by lowering cholesterol in neuronal membranes.40
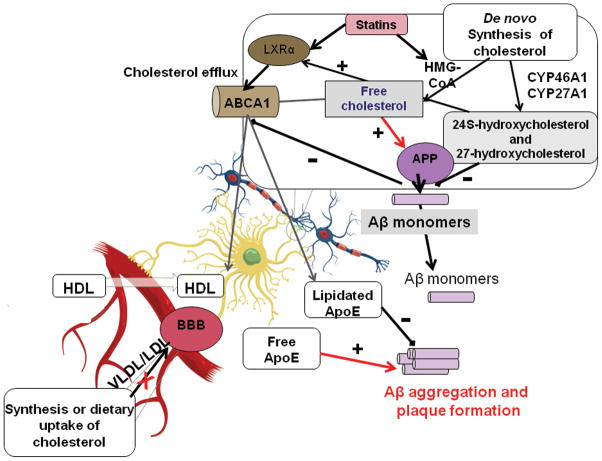
FIGURE 2. The role of cholesterol metabolism in AD pathogenesis.
The cholesterol pool in the brain contains cholesterol synthesized de novo and small amounts delivered from the periphery by HDLs that cross the BBB. Cholesterol binds to both APP and Aβ, and elevated levels of cholesterol increase amyloid plaque formation. HMG-CoA reductase regulates de novo cholesterol biosynthesis, while CYP46A1 and CYP27A1 mediate formation of 24S- and 27-hydroxycholesterol, respectively. Accumulation of these oxysterols inhibits aggregation of Aβ and activates LXRs. Aβ aggregation has an inhibitory effect on the expression of ABCA1, while LXR ligation upregulates ABCA1. ApoE-containing HDL-like particles inhibit the aggregation of Aβ, whereas free apoE has been shown to promote Aβ aggregation.
Based on the relationship between cholesterol and AD and inflammation and AD, statins were thought to hold promise, but conflicting data continues to accumulate with a number of studies failing to find a positive impact.41–43 Recently, in the Rotterdam Study, a prospective evaluation of AD incidence in almost 7,000 persons, use of statins, but not of non-statin cholesterol-lowering drugs, was associated with a lower risk of AD.44
Cholesterol homeostasis is closely linked to multiple aspects of Aβ biology. Studies have shown that the cholesterol level influences the production of the pathogenic Aβ peptide. In 1998, Simons and colleagues 45 showed in rat hippocampal neurons infected with recombinant Semliki Forest virus carrying APP that reducing cellular cholesterol content decreased formation of Aβ. Cells were depleted of about 70% of their cholesterol content using a combination of the HMG CoA reductase inhibitor lovastatin with the cholesterol sequestering agent methyl-β-cyclodextrin.
Using lovastatin, Frears et al. 46 lowered the cholesterol levels in human 293 cells transfected with a gene coding for APP under the control of a cytomegalovirus promoter. The lovastatin treatment markedly inhibited β-secretase cleavage. Supplementing the APP-transfected cells with cholesterol increased secretion of both Aβ40 and Aβ42.
Cholesterol binds to both APP and Aβ. 47, 48 APP binds to cholesterol specifically through its C-terminal transmembrane domain. Cholesterol has also been shown to directly modulate the processing of APP in neuronal cell cultures. Ehehalt et al. 49 found that BACE-1 activity in mouse neuroblastoma N2a cells depends upon intact lipid rafts, cholesterol- and sphingolipid-enriched microdomains within cellular membranes. Lipid rafts may thus act as surface catalysts, fostering the aggregation of Aβ. On the other hand, α-secretase is believed to reside primarily in the non-raft phase of the plasma membrane. The Ehehalt group hypothesized that cholesterol depletion shifts the partitioning of APP from lipid rafts to the lipid bilayer, leading to increased non-amyloidogenic α-cleavage.
In multiple animal models, elevated dietary cholesterol has been shown to increase amyloid plaque formation (Fig. 2). In an Aβ-depositing double transgenic (APP-presenilin) mouse model, consumption of a high cholesterol diet for seven weeks elevated Aβ levels in the CNS.50 Cholesterol co-localizes with fibrillar Aβ in the amyloid plaques of transgenic mice. 51 In transgenic mice, a typical Western diet containing 1% cholesterol consumed between ages 6 and 18 months increased Aβ accumulation and plaque burden particularly in the dentate gyrus of the hippocampus.52 Fitz et al. 53 fed a high cholesterol diet to APP23 mice that overexpress human APP751 (familial Swedish AD mutant) for a 4 month period. This resulted in significantly worse memory deficits than in the same mouse model fed a normal diet.
Studies using New Zealand white rabbits have demonstrated that diet-induced hypercholesterolemia causes a twofold increase in brain Aβ concentration in the hippocampal cortex.54 The Aβ accumulation seen in cholesterol-fed rabbits can include senile plaque-like structures in the hippocampus and temporal lobe. In addition to increasing Aβ, cholesterol-enriched diets increase tau phosphorylation and oxidative stress in rabbit brains.55–57
A study in guinea pigs showed that lowering whole body cholesterol with statins decreased cerebral Aβ formation. 58
APOE
ApoE is a 35-kDa, 299 amino acid glycoprotein and a major apolipoprotein in the CNS. It is responsible for transport of cholesterol between cells in the brain and the best known bona fide genetic risk factor in sporadic AD.59 The ApoE gene is localized on chromosome 19. Humans express three naturally occurring variants of ApoE, differing by a single amino acid at either position 112 or 158: E2 (Cys112/Cys158), E3 (Cys112/Arg158) and E4 (Arg112/Arg158).60 ApoE3 is the most common allele. The ApoE2 allele reduces the risk of AD while the apoE4 allele dose dependently increases AD risk. Individuals homozygous for the E4 allele have a 50% to 90% chance of developing AD by age 85 years, and heterozygotes with 1 copy of E4 have an approximately 45% chance. Carriers of ApoE4 manifest dementia symptoms earlier than non-carriers with the disease and convert from mild cognitive impairment to AD at an accelerated rate.61 Murine studies have shown that, astrocytes and microglia are the primary ApoE secreting cells in the brain, but neurons can express ApoE under conditions of excitotoxic injury. 62
The mechanisms by which ApoE isoforms affect risk of AD are uncertain. ApoE is found in the amyloid plaques of AD in humans.63, 64 ApoE promotes the formation of Aβ fibrils in vitro and in transgenic mice with mutated APP (Fig. 2), more fibrillar Aβ deposits form in mice that express apoE4 compared with apoE3.65, 66 In transgenic AD mouse models, a striking reduction in amyloid deposits is observed when the mice are apoE-null.67 It has been postulated that ApoE functions as a chaperone for Aβ and that it regulates its conversion from a mixed random coil/α-helix to a β-sheet amyloid conformation.68 It has also been suggested that in AD patients with apoE4, a decreased ability to clear Aβ contributes to increased Aβ accumulation and amyloid plaque formation.69
OXYSTEROLS
In order to maintain cholesterol homeostasis in the brain, a mechanism is required for efflux through the BBB into the circulation. This is accomplished through conversion of cholesterol to the relatively polar 24S-hydroxycholesterol, also called cerebrosterol, in a reaction catalyzed by the microsomal cytochrome P450 enzyme cholesterol 24-hydroxylase (CYP46A1).70 Cholesterol 24-hydroxylase is almost exclusively located in the brain and the brain is the source of over 90% of plasma 24-hydroxycholesterol.71 CYP46A1 is expressed in neurons of the brain but not the spinal cord, and not in myelin-producing cell types (oligodendrocytes) or in support cell types such as astrocytes.72 Approximately 6–7 mg of cholesterol crosses the BBB and leaves the human brain each day after conversion to 24S-hydroxycholesterol.73 About 60% of cholesterol clearance from the brain occurs in the form of 24S-hydroxycholesterol.74 To a lesser extent, cholesterol is oxidized to 27-hydroxycholesterol in the brain by cholesterol 27-hydroxylase (CYP27A1), an enzyme found in brain, and also in many other organs and tissues.75–77 In healthy male volunteers, Heverin and colleagues found that the 27-hydroxycholesterol concentration was higher in brachial artery blood than in blood taken from the internal jugular vein, indicating a significant net uptake of 27-hydroxycholesterol from the circulation into the brain, corresponding to 4–5 mg/day.78 Under conditions of elevated serum cholesterol, flux of 27-hydroxycholesterol into the brain is increased. 27-Hydroxycholesterol is taken up from the blood, acting as an important link between extra-cerebral and intra-cerebral pools of cholesterol and may contribute to negative effects of hypercholesterolemia in the brain.78–80
A number of studies indicate that oxysterols affect the amyloid cascade. In rat primary cortical neurons, both 24S-hydroxycholesterol and 27-hydroxycholesterol are potent inhibitors of Aβ secretion (Fig. 2), but 24S-hydroxycholesterol is about 1000 times more potent.81 In primary human neurons isolated from brain tissue, treatment with 10μM 27-hydroxycholesterol for 24h reduced the level of Aβ peptides detected by Western blot in the culture supernatants by 65%.82
Other studies have indicated that oxysterols may cause changes that favor AD development. In human neuroblastoma SH-SY5Y cells treatment with 5, 10 and 25 μM 24-hydroxycholesterol for 24h did not significantly alter secreted Aβ42 levels while treatment with 5, 10 or 25 μM 27-hydroxycholesterol substantially increased Aβ42 levels, possibly by elevating APP and BACE-1. In this study, 24-hydroxycholesterol favored APP processing via the non-amyloidogenic pathway that precludes generation of Aβ42. None of the treatments affected Aβ40 production.83
Halford and Russell cross-bred CYP46A1 knockout mice with an AD mouse model (APP-presenilin transgenic mice) and found that 24-hydroxylase deficiency did not impact formation of amyloid plaque in the brain, although it was associated with increased longevity in both male and female AD mice.84 As seen previously, the rate of cholesterol turnover was diminished in the brains of mice lacking the 24-hydroxylase and, in order to maintain a steady-state, cholesterol synthesis was reduced as a compensatory mechanism.85 Cholesterol synthesis in liver was unaffected. When oxysterols exit the brain, they are metabolized in the liver to form bile acids or other water-soluble metabolites.
ABCA1 AND LXRs
ATP-binding cassette (ABC) transporters constitute a group of evolutionary highly conserved cellular transmembrane proteins that transport a variety of macromolecules across biological membranes. Many ABC transporters, particularly in the A and G subfamilies, transport lipids, such as sterols, phospholipids and bile acids.86
ATP-binding cassette transporter A1 (ABCA1) is a membrane-associated protein which transports cholesterol to high-density lipoproteins (HDLs) and mediates cellular cholesterol efflux.87, 88 It is reported to be involved in apoE metabolism and Aβ production in central nervous system (Fig. 2).89–92 In particular, ABCA1 has been shown to modulate amyloid plaque formation and ApoE levels in the brain of Alzheimer’s transgenic mice (PDAPP murine model).5, 90, 91 Canepa et al.5 demonstrate strong correlation between Aβ and ABCA1 levels. First, they observed that AD transgenic mice have reduced expression of ABCA1 in the brain. These finding were confirmed by in vitro experiments demonstrating that Aβ have an inhibitory effect on the expression of the cholesterol transporter ABCA1 in cultured astrocytes. Recent studies have shown that AD transgenic mice lacking ABCA1 develop increased Aβ levels and plaque pathology in the absence of changes in APP processing. Thus, Wahrle and colleagues,91 using PDAPP/ABCA−/− mice found that deletion of ABCA1 results in increased parenchymal Aβ levels, co-deposition of poorly lipidated apoE with insoluble Aβ, and facilitation of amyloidogenesis. The PDAPP/ABC1 double transgenic mouse generated by Holtzman’s group overexpresses ABCA1. The ABCA1 level in these mice significantly modulated Aβ deposition.92 The striking phenotype that these mice possess is a complete absence of Thio-S positive amyloid plaques, once again leading to the conclusion that increasing ABCA1 function may have a therapeutic effect on AD.
Liver X receptors (LXRs) are ligand-activated transcription factors that induce the expression of ApoE, ABCA1, and other genes of lipid metabolism. LXRs act physiologically as cellular cholesterol sensors and are activated by oxysterols. The LXR-ABCA1-ApoE regulatory pathway is now considered a promising therapeutic target in AD.93–95 The therapeutic approach includes transcriptional regulation of ABCA1 levels by LXR agonists controlling lipidation, Aβ aggregation and amyloid clearance. However, the identification of proper LXR agonists in this regard is still in development in many research groups.95–97
Another ABC transporter, ABCA7, has been found to stimulate cellular cholesterol efflux to apoE-containing particles in the same way as ABCA1.98 ABCA7 reduces Aβ production in transfected cells, not by altering the enzyme activities of APP secretase enzymes, but by retaining APP in a perinuclear location, thereby reducing the availability of APP for Aβ production.
ACAT
Acyl-CoA:cholesterol acyltransferase (ACAT) catalyzes the formation of cholesteryl esters from cholesterol and long-chain fatty-acyl-coenzyme A in nearly all mammalian cells. 99 ACAT is a membrane-associated enzyme that is primarily localized in the endoplasmic reticulum. ACAT serves as a regulator of intracellular cholesterol homeostasis by modulating intracellular free cholesterol concentration. In view of the critical role of ACAT in lipid metabolism, Huttunen et al100 used various cell- and animal based models to show that inhibition of ACAT strongly reduces generation of Aβ and protects from amyloid pathology. In APP-overexpressing transgenic mice, they showed that two months of treatment with the ACAT inhibitor CP-113,818 decreased maturation of APP, attenuated Aβ formation and reduced amyloid pathology. 100, 101
CONCLUSIONS
Multiple threads of evidence connect cholesterol and AD. Epidemiological studies suggest a positive correlation between hypercholesterolemia and increased risk of AD. High dietary intake of cholesterol results in hypercholesterolemia and enhances Aβ deposition in rabbit, mouse and guinea pig brain. 50, 54, 58 Statins inhibit cholesterol synthesis and decrease Aβ levels and AD pathology in several animal models of AD. However, human studies of statins show variable outcomes. More research is needed with particular attention to stage of AD at initiation of statin therapy, hydrophilicity of chosen drug and pleiotropic effects. 102
The lipid composition of neurons impacts trafficking and/or activity of membrane-associated proteins that determine Aβ levels, including APP, BACE1 and presenilins. A major risk factor for AD is the E4 allelic variant of ApoE, a protein that regulates the redistribution and homeostasis of cholesterol within the brain. It has recently been reported that apoE facilitates intracellular Aβ degradation by microglia via reduction of intracellular cholesterol levels. 103
Effective therapy for AD is a major unmet medical need. Although there is no cure at present, some therapeutic drugs inhibiting acetylcholinesterase have been used to alleviate the disease symptoms and improve the quality of life for patients with AD.104, 105 Currently, no disease modifying or anti-amyloid therapies are available. There is burgeoning interest in the development of therapeutics for prodromal AD. This is driven by the hypothesis that disease-modifying therapy is most likely to be efficacious in the earliest stages of the pathologic cascade of events leading to neuronal dysfunction and death. Current marketed therapies for AD offer palliative cognitive benefits with little to no impact on the underlying pathology, or on long-term disease progression. Effective treatments for AD that address the underlying disease may include antiamyloid antibodies to reduce Aβ in brain, neurotrophic factor drugs that enhance the neuroregenerative capacity of the brain. 106, 107 A new development linking a BACE-1 inhibitor to a cholesterol molecule enriches the expression of the inhibitor in lipid rafts, increasing its potency.108, 109 It is hoped that this type of targeted therapy, now in its infancy, may have substantial impact on this debilitating illness.
In summary, although the exact function of cholesterol in the control of Aβ generation, aggregation, and toxicity remains unclear, there is substantial evidence that elevated cholesterol levels increase AD risk. Cholesterol-lowering strategies that decrease production (statins) or increase efflux (LXR agonists) are being explored as a means to reduce or remove Aβ with the ultimate goal of slowing or preventing AD.
Acknowledgments
This work was supported by a grant from the Neuroscience Education and Research Foundation and an Innovative Research Grant from the Arthritis Foundation.
References
- 1.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Auld DS, Kornecook TJ, Bastianetto S, et al. Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Canepa E, Borghi R, Vina J, et al. Cholesterol and amyloid-β: evidence for a cross-talk between astrocytes and neuronal cells. J Alzheimer’s Dis. 2011;25(4):645–653. doi: 10.3233/JAD-2011-110053. [DOI] [PubMed] [Google Scholar]
- 6.Rebeck GW, Reiter JS, Strickland DK, et al. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 7.Burns M, Duff K. Cholesterol in Alzheimer’s disease and tauopathy. Ann NY Acad Sci. 2002;977:367–375. doi: 10.1111/j.1749-6632.2002.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 8.Poirier J. Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer’s disease. Neurobiol Aging. 2005;26:355–361. doi: 10.1016/j.neurobiolaging.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 10.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]; Jellinger KA. The neuropathological diagnosis of Alzheimer disease. J Neural Transm Suppl. 1998;53:97–118. doi: 10.1007/978-3-7091-6467-9_9. [DOI] [PubMed] [Google Scholar]
- 11.Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts myloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 12.Yan R, Bienkowski MJ, Shuck ME, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 13.Edbauer D, Winkler E, Regula JT, et al. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 14.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh AK, Gemma S, Tang J. Beta-Secretase as a therapeutic target for Alzheimer’s disease. Neurotherapeutics. 2008;5:399–408. doi: 10.1016/j.nurt.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwatsubo T, Odaka A, Suzuki N, et al. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 17.Verdile G, Fuller S, Atwood CS, et al. The role of beta amyloid in Alzheimer’s disease: still a cause of everything or the only one who got caught? Pharmacol Res. 2004;50:397–409. doi: 10.1016/j.phrs.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenthaler SF. Alpha-secretase in Alzheimer’s disease: molecular identity, regulation and therapeutic potential. J Neurochem. 2011;116(1):10–21. doi: 10.1111/j.1471-4159.2010.07081.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin LF, Luo HM. Screening of treatment targets for Alzheimer’s disease from the molecular mechanisms of impairment by β-amyloid aggregation and tau hyperphosphorylation. Neurosci Bull. 2011;27(1):53–60. doi: 10.1007/s12264-011-1040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouritsen OG, Zuckermann MJ. What’s so special about cholesterol? Lipids. 2004;39:1101–1113. doi: 10.1007/s11745-004-1336-x. [DOI] [PubMed] [Google Scholar]
- 22.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Morell P, Jurevics H. Origin of cholesterol in myelin. Neurochem Res. 1996;21:463–470. doi: 10.1007/BF02527711. [DOI] [PubMed] [Google Scholar]
- 24.Vance JE, Hayashi H, Karten B. Cholesterol homeostasis in neurons and glial cells. Semin Cell Dev Biol. 2005;16:193–212. doi: 10.1016/j.semcdb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Dietschy JM. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem. 2009;390:287–293. doi: 10.1515/BC.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 27.Andersson M, Elmberger PG, Edlund C, et al. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Lett. 1990;269:15–18. doi: 10.1016/0014-5793(90)81107-y. [DOI] [PubMed] [Google Scholar]
- 28.Nieweg K, Schaller H, Pfrieger FW. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem. 2009;109:125–134. doi: 10.1111/j.1471-4159.2009.05917.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 30.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 31.Wolozin B, Kellman W, Ruosseau P, et al. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 32.Solomon A, Kivipelto M, Wolozin B, et al. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mielke MM, Zandi PP, Shao H, et al. The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology. 2010;75(21):1888–1895. doi: 10.1212/WNL.0b013e3181feb2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Kirkland S, Hogan DB, et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 36.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40:567–572. doi: 10.1016/s0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 37.Mihos CG, Santana O. Pleiotropic effects of the HMG-CoA reductase inhibitors. Int J Gen Med. 2011;4:261–271. doi: 10.2147/IJGM.S16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellosta S, Ferri N, Bernini F, et al. Non-lipid-related effects of statins. Ann Med. 2000;32:164–176. doi: 10.3109/07853890008998823. [DOI] [PubMed] [Google Scholar]
- 39.Ostrowski SM, Wilkinson BL, Golde TE, et al. Statins reduce amyloid-beta production through inhibition of protein isoprenylation. J Biol Chem. 2007;282:26832–26844. doi: 10.1074/jbc.M702640200. [DOI] [PubMed] [Google Scholar]
- 40.Biondi E. Prescription of lipophilic statins to Alzheimer’s disease patients: some controversies to consider. Neurol Sci. 2011;32:195–201. doi: 10.1007/s10072-010-0440-0. [DOI] [PubMed] [Google Scholar]
- 41.Zandi PP, Sparks DL, Khachaturian AS, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 42.Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 44.Haag MD, Hofman A, Koudstaal PJ, et al. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80:13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 45.Simons M, Keller P, De Strooper B, et al. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frears ER, Stephens DJ, Walters CE, et al. The role of cholesterol in the biosynthesis of [beta]-amyloid. Neuroreport. 1999;10(8):1699–705. doi: 10.1097/00001756-199906030-00014. [DOI] [PubMed] [Google Scholar]
- 47.Harris JR. Cholesterol binding to amyloid fibrils: a TEM study. Micron. 2008;39:1192–1196. doi: 10.1016/j.micron.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Beel AJ, Sakakura M, Barrett PJ, Sanders CR. Direct binding of cholesterol to the amyloid precursor protein: An important interaction in lipid-Alzheimer’s disease relationships? Biochim Biophys Acta. 2010;1801(8):975–982. doi: 10.1016/j.bbalip.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehehalt R, Keller P, Haass C, et al. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Refolo LM, Malester B, LaFrancois J, et al. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;4:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 51.Burns M, Gaynor K, Olm V, et al. Presenilin redistribution associated with aberrant cholesterol transport enhances beta-amyloid production in vivo. J Neurosci. 2003;23:5645–5649. doi: 10.1523/JNEUROSCI.23-13-05645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hooijmans CR, Rutters F, Dederen PJ, et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD) Neurobiol Dis. 2007;28:16–29. doi: 10.1016/j.nbd.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Fitz NF, Cronican A, Pham T, et al. LXR agonist treatment ameliorates amyloid pathology and memory deficits caused by high fat diet in APP23 mice. J Neurosci. 2010;30(20):6862–6872. doi: 10.1523/JNEUROSCI.1051-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sparks DL, Kuo YM, Roher A, et al. Alterations of Alzheimer’s disease in the cholesterol-fed rabbit, including vascular inflammation. Preliminary observations. Ann NY Acad Sci. 2000;903:335–344. doi: 10.1111/j.1749-6632.2000.tb06384.x. [DOI] [PubMed] [Google Scholar]
- 55.Ghribi O, Golovko MY, Larsen B, et al. Deposition of iron and beta-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol-enriched diets. J Neurochem. 2006;99:438–449. doi: 10.1111/j.1471-4159.2006.04079.x. [DOI] [PubMed] [Google Scholar]
- 56.Ghribi O, Larsen B, Schrag M, Herman MM. High cholesterol content in neurons increases BACE, β-amyloid, and phosphorylated tau levels in rabbit hippocampus. Exp Neurol. 2006;200:460–467. doi: 10.1016/j.expneurol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Jaya Prasanthi RP, Schommer E, Thomasson S, et al. Regulation of beta-amyloid levels in the brain of cholesterol-fed rabbit, a model system for sporadic Alzheimer’s disease. Mech Ageing Dev. 2008;129:649–655. doi: 10.1016/j.mad.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fassbender K, Simons M, Bergmann C, et al. Simvastatin strongly reduces levels of Alzheimer’s disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 60.Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- 61.Schipper HM. Apolipoprotein E: implications for AD neurobiology, epidemiology and risk assessment. Neurobiology of Aging. 2011;32(5):778–790. doi: 10.1016/j.neurobiolaging.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 62.Xu Q, Bernardo A, Walker D, et al. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Namba Y, Tomonaga M, Kawasaki H, et al. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- 64.Sheng JG, Mrak RE, Griffin WS. Apolipoprotein E distribution among different plaque types in Alzheimer’s disease: Implications for its role in plaque progression. Neuropathol Appl Neurobiol. 1996;22:334–341. doi: 10.1111/j.1365-2990.1996.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 65.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carter DB, Dunn E, McKinley DD, et al. Human apolipoprotein E4 accelerates beta-amyloid deposition in APPsw transgenic mouse brain. Ann Neurol. 2001;50:468–475. doi: 10.1002/ana.1134. [DOI] [PubMed] [Google Scholar]
- 67.Bales KR, Verina T, Cummins DJ, et al. Apolipoprotein E is essential for amyloid deposition in the APP (V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wahrte SE, Holtzman DM. Apolipoprotein E, amyloid b peptide, and Alzheimer’s disease. In: Dawbarn D, Allen SJ, editors. Neurobiology of Alzheimer’s Disease. New York: Oxford University Press, Inc; 2007. pp. 161–172. [Google Scholar]
- 69.Deane R, Sagare A, Hamm K, et al. ApoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bjorkhem I. Rediscovery of cerebrosterol. Lipids. 2007;42:5–14. doi: 10.1007/s11745-006-1003-2. [DOI] [PubMed] [Google Scholar]
- 71.Meaney S, Hassan M, Sakinis A, et al. Evidence that the major oxysterols in human circulation originate from distinct pools of cholesterol: a stable isotope study. J Lipid Res. 2001;42:70–78. [PubMed] [Google Scholar]
- 72.Lund EG, Guileyardo JM, Russell DW. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc Natl Acad Sci USA. 1999;96:7238–7243. doi: 10.1073/pnas.96.13.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bjorkhem I, Lutjohann D, Diczfalusy U, et al. Cholesterol homeostasis in human brain: Turnover of 24s-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res. 1998;39:1594–1600. [PubMed] [Google Scholar]
- 74.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Dietschy JM, Turley SD. Thematic review series: Brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Andersson S, Davis DL, Dahlback H, et al. Cloning, structure, and expression of the mitochondrial cytochrome p-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- 77.Reiss AB, Martin KO, Rojer DE, et al. Sterol 27-hydroxylase: Expression in human arterial endothelium. J Lipid Res. 1997;38:1254–1260. [PubMed] [Google Scholar]
- 78.Heverin M, Meaney S, Lutjohann D, et al. Crossing the barrier: Net flux of 27-hydroxycholesterol into the human brain. J Lipid Res. 2005;46:1047–1052. doi: 10.1194/jlr.M500024-JLR200. [DOI] [PubMed] [Google Scholar]
- 79.Sharma S, Prasanthi RPJ, Schommer E, et al. Hypercholesterolemia-induced Abeta accumulation in rabbit brain is associated with alteration in IGF-1 signaling. Neurobiol Dis. 2008;32:426–32. doi: 10.1016/j.nbd.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bjorkhem I, Heverin M, Leoni V, et al. Oxysterols and Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:43–49. doi: 10.1111/j.1600-0404.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 81.Brown J, 3rd, Theisler C, Silberman S, et al. Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J Biol Chem. 2004;279:34674–34681. doi: 10.1074/jbc.M402324200. [DOI] [PubMed] [Google Scholar]
- 82.Kim WS, Chan SL, Hill AF, et al. Impact of 27-hydroxycholesterol on amyloid-beta peptide production and ATP-binding cassette transporter expression in primary human neurons. J Alzheimers Dis. 2009;16(1):121–131. doi: 10.3233/JAD-2009-0944. [DOI] [PubMed] [Google Scholar]
- 83.Prasanthi JRP, Huls A, Thomasson S, et al. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on beta-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol Neurodegener. 2009;4:1. doi: 10.1186/1750-1326-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halford RW, Russell DW. Reduction of cholesterol synthesis in the mouse brain does not affect amyloid formation in alzheimer’s disease, but does extend lifespan. Proc Natl Acad Sci USA. 2009;106:3502–3506. doi: 10.1073/pnas.0813349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie C, Lund EG, Turley SD, et al. Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J Lipid Res. 2003;44:1780–1789. doi: 10.1194/jlr.M300164-JLR200. [DOI] [PubMed] [Google Scholar]
- 86.Voloshyna I, Reiss AB. The ABC transporters in lipid flux and atherosclerosis. Prog Lipid Res. 2011;50(3):213–24. doi: 10.1016/j.plipres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 87.Schmitz G, Langmann T. Structure, function and regulation of the ABC1 gene production. Curr Opin Lipidol. 2001;12:129–140. doi: 10.1097/00041433-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 88.Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:1178–1184. doi: 10.1161/01.ATV.0000075912.83860.26. [DOI] [PubMed] [Google Scholar]
- 89.Koldamova R, Fitz NF, Lefterov I. The role of ATP-binding cassette transporter A1 in Alzheimer’s disease and neurodegeneration. Biochim Biophys Acta. 2010;1801(8):824–30. doi: 10.1016/j.bbalip.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim WS, Hill AF, Fitzgerald ML, et al. Wild Type and Tangier Disease ABCA1 Mutants Modulate Cellular Amyloid-β Production Independent of Cholesterol Efflux Activity. J Alzheimers Dis. 2011;27(2):441–452. doi: 10.3233/JAD-2011-110521. [DOI] [PubMed] [Google Scholar]
- 91.Wahrle SE, Jiang H, Parsadanian M, et al. ABCA1 is required for normal central nervous system APOE levels and for lipidation of astrocyte-secreted APOE. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 92.Wahrle SE, Jiang H, Parsadanian M, et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008;118 (2):671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loane DJ, Washington PM, Vardanian L, et al. Modulation of ABCA1 by an LXR agonist reduces beta-amyloid levels and improves outcome after traumatic brain injury. J Neurotrauma. 2011;28(2):225–236. doi: 10.1089/neu.2010.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Terwel D, Steffensen KR, Verghese PB, et al. Critical role of astroglial apolipoprotein E and liver X receptor-α expression for microglial Aβ phagocytosis. J Neurosci. 2011;11:31(19):7049–7059. doi: 10.1523/JNEUROSCI.6546-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schweinzer C, Kober A, Lang I, et al. Processing of Endogenous AβPP in Blood-Brain Barrier Endothelial Cells is Modulated by Liver-X Receptor Agonists and Altered Cellular Cholesterol Homeostasis. J Alzheimers Dis. 2011 Aug 2; doi: 10.3233/JAD-2011-110854. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 96.Donkin JJ, Stukas S, Hirsch-Reinshagen V, et al. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J Biol Chem. 2010;285(44):34144–34154. doi: 10.1074/jbc.M110.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cronican AA, Fitz NF, Pham T, et al. Proton pump inhibitor Lansoprazole is a nuclear liver X receptor agonist. Biochem Pharmacol. 2010;79(9):1310–1316. doi: 10.1016/j.bcp.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan SL, Kim WS, Kwok JB, et al. ATP-binding cassette transporter A7 regulates processing of amyloid precursor protein in vitro. J Neurochem. 2008;106:793–804. doi: 10.1111/j.1471-4159.2008.05433.x. [DOI] [PubMed] [Google Scholar]
- 99.Chang TY, Chang CCY, Cheng D. Acyl-coenzyme A: cholesterol acyltransferase. Annu Rev Biochem. 1997;66:613–633. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 100.Huttunen HJ, Peach C, Bhattacharyya R, et al. Inhibition of acyl-coenzyme A: cholesterol acyl transferase modulates amyloid precursor protein trafficking in the early secretory pathway. FASEB J. 2009;23:3819–3828. doi: 10.1096/fj.09-134999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hutter-Paier B, Huttunen HJ, Puglielli L, et al. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 102.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer Disease: II. Review of human trials and recommendations. Arch Neurol. 2011;68:1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee CY, Tse W, Landreth GE. ApoE promotes Aβ trafficking and degradation by modulating microglial cholesterol levels. J Biol Chem. 2011 doi: 10.1074/jbc.M111.295451. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12(5):284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ariga T, Miyatake T, Yu RK. Role of proteoglycans and glycosaminoglycans in the pathogenesis of Alzheimer’s disease and related disorders: amyloidogenesis and therapeutic strategies—a review. Journal of Neuroscience Research. 2010;88(11):2303–2315. doi: 10.1002/jnr.22393. [DOI] [PubMed] [Google Scholar]
- 106.Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2011 Oct 10; doi: 10.1001/archneurol.2011.1538. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 107.Iqbal K, Grundke-Iqbal I. Opportunities and challenges in developing Alzheimer disease therapeutics. Acta Neuropathol. 2011 Sep 30; doi: 10.1007/s00401-011-0878-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 108.Ben Halima S, Rajendran L. Membrane anchored and lipid raft targeted β-secretase inhibitors for Alzheimer’s disease therapy. J Alzheimers Dis. 2011;24 (Suppl 2):143–152. doi: 10.3233/JAD-2011-110269. [DOI] [PubMed] [Google Scholar]
- 109.Rajendran L, Schneider A, Schlechtingen G, et al. Efficient inhibition of the Alzheimer’s disease beta-secretase by membrane targeting. Science. 2008;320(5875):520–523. doi: 10.1126/science.1156609. [DOI] [PubMed] [Google Scholar]