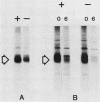
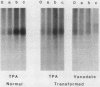
Abstract
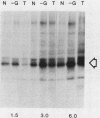
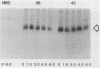
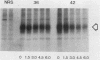
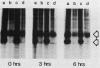
The rate of glucose transport in cultured fibroblasts is regulated to a number of physiological variables, including malignant transformation by src, glucose starvation, and stimulation with mitogens. Much of this transport regulation can be accounted for by variations in the amount of transporter protein in the cells. To determine the mechanisms by which levels of the transporter are regulated, we measured the rates of synthesis and degradation of the transporter by pulse-chase experiments and immunoprecipitation of the transporter. We found that transformation by the src oncogene results in a large decrease in the rate at which the transporter protein is degraded but that it does not appreciably increase the rate of transporter biosynthesis. On the other hand, glucose starvation and mitogen stimulation increase the rate of transporter biosynthesis, although a role for control of degradation is possible in these circumstances also. Variations in the rate of glucose transport or the amount of the transporter are not associated with phosphorylation of the transporter protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard W. J., Lienhard G. E. Monoclonal antibodies to the glucose transporter from human erythrocytes. Identification of the transporter as a Mr = 55,000 protein. J Biol Chem. 1985 Jul 25;260(15):8668–8675. [PubMed] [Google Scholar]
- Bader J. P., Brown N. R., Ray D. A. Increased glucose uptake capacity of Rous-transformed cells and the relevance of deprivation derepression. Cancer Res. 1981 May;41(5):1702–1709. [PubMed] [Google Scholar]
- Bader J. P. Temperature-dependent transformation of cells infected with a mutant of Bryan Rous sarcoma virus. J Virol. 1972 Aug;10(2):267–276. doi: 10.1128/jvi.10.2.267-276.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Colowick S. P. Stimulation of sugar uptake in cultured fibroblasts by epidermal growth factor (EGF) and EGF-binding arginine esterase. J Cell Physiol. 1976 Dec;89(4):633–639. doi: 10.1002/jcp.1040890420. [DOI] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Weber M. J., Blackshear P. J., Beatty S., Lim R., Herschman H. R. Protein phosphorylation in a tetradecanoyl phorbol acetate-nonproliferative variant of 3T3 cells. Mol Cell Biol. 1985 Sep;5(9):2231–2237. doi: 10.1128/mcb.5.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Pessin J. E., Mora R., Gitomer W., Czech M. P. Photoaffinity labeling of the human erythrocyte D-glucose transporter. J Biol Chem. 1982 May 25;257(10):5419–5425. [PubMed] [Google Scholar]
- Christopher C. W., Colby W. W., Ullrey D. Derepression and carrier turnover: evidence for two distinct mechanisms of hexose transport regulation in animal cells. J Cell Physiol. 1976 Dec;89(4):683–692. doi: 10.1002/jcp.1040890427. [DOI] [PubMed] [Google Scholar]
- Christopher C. W., Kohlbacher M. S., Amos H. Transport of sugars in chick-embryo fibroblasts. Evidence for a low-affinity system and a high-affinity system for glucose transport. Biochem J. 1976 Aug 15;158(2):439–450. doi: 10.1042/bj1580439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher C. W., Morgan R. A. Are lysosomes involved in hexose transport regulation? Turnover of hexose carriers and the activity of thiol cathepsins are arrested by cyanate and ammonia. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4416–4420. doi: 10.1073/pnas.78.7.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher C. W., Ullrey D., Colby W., Kalckar M. Paradoxical effects of cycloheximide and cytochalasin B on hamster cell hexose uptake. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2429–2433. doi: 10.1073/pnas.73.7.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Bishop J. M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
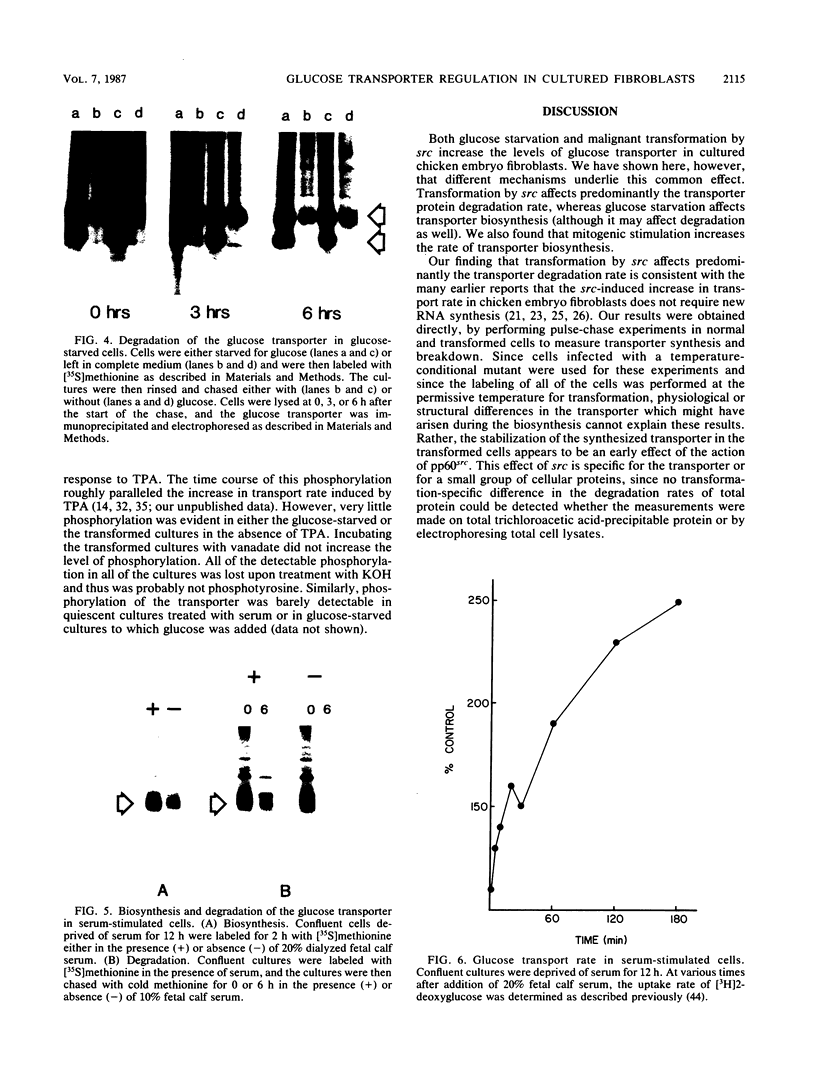
- Haspel H. C., Birnbaum M. J., Wilk E. W., Rosen O. M. Biosynthetic precursors and in vitro translation products of the glucose transporter of human hepatocarcinoma cells, human fibroblasts, and murine preadipocytes. J Biol Chem. 1985 Jun 25;260(12):7219–7225. [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Inui K. I., Tillotson L. G., Isselbacher K. J. Hexose and amino acid transport by chicken embryo fibroblasts infected with temperature-sensitive mutant of Rous sarcoma virus. Comparison of transport properties of whole cells and membrane vesicles. Biochim Biophys Acta. 1980 Jun 6;598(3):616–627. doi: 10.1016/0005-2736(80)90041-3. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kitagawa K., Nishino H., Iwashima A. Tumor promoter-stimulated translocation of glucose transport system in mouse embryo fibroblast Swiss 3T3 cell. Biochem Biophys Res Commun. 1985 May 16;128(3):1303–1309. doi: 10.1016/0006-291x(85)91082-4. [DOI] [PubMed] [Google Scholar]
- Kleitzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. 3. Evidence for post-transcriptional and post-translational regulation of transport following serum addition. J Biol Chem. 1974 Jun 10;249(11):3383–3387. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Induction of sugar transport in chick embryo fibroblasts by hexose starvation. Evidence for transcriptional regulation of transport. J Biol Chem. 1975 Jan 25;250(2):593–600. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Regulation of sugar transport in chick embryo fibroblasts and in fibroblasts transformed by a temperature-sensitive mutant of the Rous sarcoma virus. J Cell Physiol. 1976 Dec;89(4):723–728. doi: 10.1002/jcp.1040890432. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. II. Alterations in transport following transformation by a temperature-sensitive mutant of the Rous sarcoma virus. J Biol Chem. 1974 Jun 10;249(11):3375–3382. [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Blevins T. L., Ezaki O. Evidence that translocation of the glucose transport activity is the major mechanism of insulin action on glucose transport in fat cells. J Biol Chem. 1982 Sep 25;257(18):10942–10947. [PubMed] [Google Scholar]
- Kono T., Suzuki K., Dansey L. E., Robinson F. W., Blevins T. L. Energy-dependent and protein synthesis-independent recycling of the insulin-sensitive glucose transport mechanism in fat cells. J Biol Chem. 1981 Jun 25;256(12):6400–6407. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanks K. W., Kasambalides E. J., Chinkers M., Brugge J. S. A major cytoplasmic glucose-regulated protein is associated with the Rous sarcoma virus pp60src protein. J Biol Chem. 1982 Aug 10;257(15):8604–8607. [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Membrane effects of tumor promoters: stimulation of sugar uptake in mammalian cell cultures. J Cell Physiol. 1979 Jun;99(3):451–460. doi: 10.1002/jcp.1040990319. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G. Hexose transport in undifferentiated and differentiated BALB/c 3T3 preadipose cells: effects 12-O-tetradecanoylphorbol-13-acetate and insulin. J Cell Physiol. 1982 Jan;110(1):63–71. doi: 10.1002/jcp.1041100111. [DOI] [PubMed] [Google Scholar]
- Oren M. The p53 cellular tumor antigen: gene structure, expression and protein properties. Biochim Biophys Acta. 1985 Nov 12;823(1):67–78. doi: 10.1016/0304-419x(85)90015-0. [DOI] [PubMed] [Google Scholar]
- Salter D. W., Baldwin S. A., Lienhard G. E., Weber M. J. Proteins antigenically related to the human erythrocyte glucose transporter in normal and Rous sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1540–1544. doi: 10.1073/pnas.79.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. W., Weber M. J. Glucose-specific cytochalasin B binding is increased in chicken embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1979 May 10;254(9):3554–3561. [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Stimulation of glucose transport in cultures of density-inhibited chick embryo cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3154–3157. doi: 10.1073/pnas.68.12.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. F. Cytochalasin B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J Biol Chem. 1982 Jul 10;257(13):7290–7293. [PubMed] [Google Scholar]
- Shanahan M. F., Olson S. A., Weber M. J., Lienhard G. E., Gorga J. C. Photolabeling of glucose-sensitive cytochalasin B binding proteins in erythrocyte, fibroblast and adipocyte membranes. Biochem Biophys Res Commun. 1982 Jul 16;107(1):38–43. doi: 10.1016/0006-291x(82)91666-7. [DOI] [PubMed] [Google Scholar]
- Shelton R. L., Jr, Langdon R. G. Reconstitution of glucose transport using human erythrocyte band 3. Biochim Biophys Acta. 1983 Aug 24;733(1):25–33. doi: 10.1016/0005-2736(83)90087-1. [DOI] [PubMed] [Google Scholar]
- Taverna R. D., Langdon R. G. Reversible association of cytochalasin B with the human erythrocyte membrane. Inhibition of glucose transport and the stoichiometry of cytochalasin binding. Biochim Biophys Acta. 1973 Oct 11;323(2):207–219. doi: 10.1016/0005-2736(73)90145-4. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Evans P. K., Johnson M. A., McNair T. F., Nakamura K. D., Salter D. W. Transport of potassium, amino acids, and glucose in cells transformed by Rous sarcoma virus. Fed Proc. 1984 Jan;43(1):107–112. [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]
- White M. K., Bramwell M. E., Harris H. Kinetic parameters of hexose transport in hybrids between malignant and nonmalignant cells. J Cell Sci. 1983 Jul;62:49–80. doi: 10.1242/jcs.62.1.49. [DOI] [PubMed] [Google Scholar]
- Wice B. M., Reitzer L. J., Kennell D. The continuous growth of vertebrate cells in the absence of sugar. J Biol Chem. 1981 Aug 10;256(15):7812–7819. [PubMed] [Google Scholar]
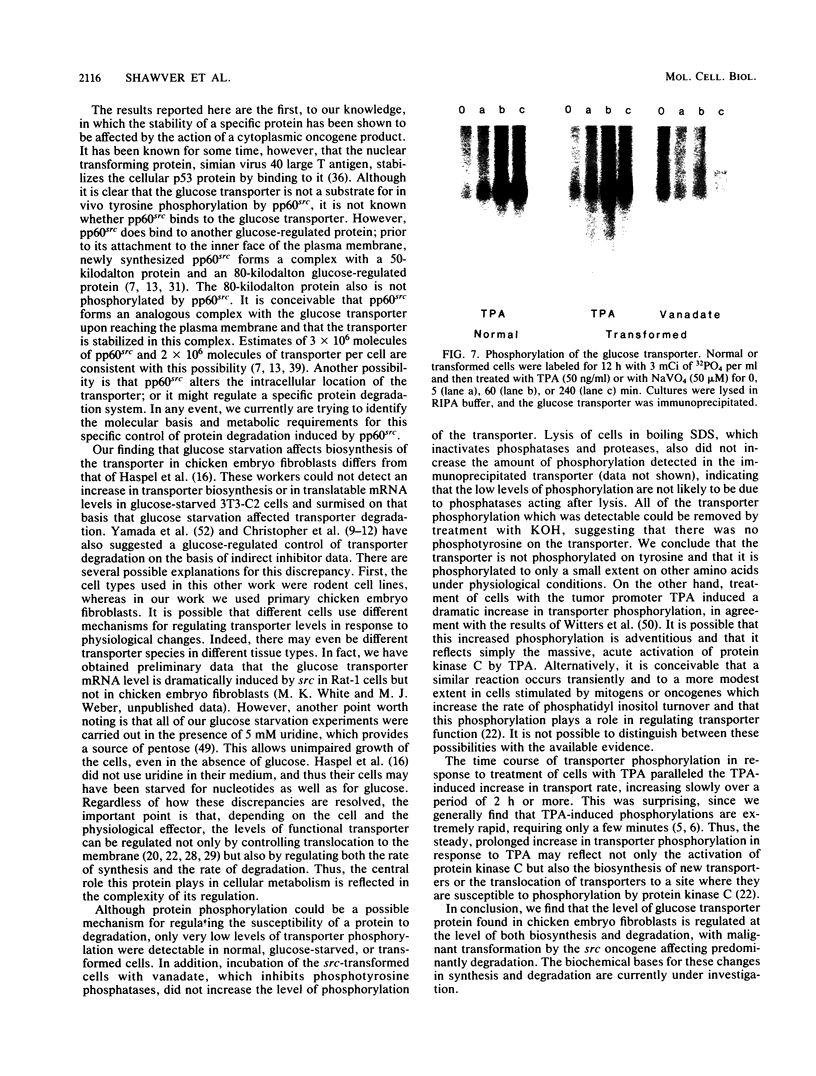
- Witters L. A., Vater C. A., Lienhard G. E. Phosphorylation of the glucose transporter in vitro and in vivo by protein kinase C. 1985 Jun 27-Jul 3Nature. 315(6022):777–778. doi: 10.1038/315777a0. [DOI] [PubMed] [Google Scholar]
- Wu J. S., Kwong F. Y., Jarvis S. M., Young J. D. Identification of the erythrocyte nucleoside transporter as a band 4.5 polypeptide. Photoaffinity labeling studies using nitrobenzylthioinosine. J Biol Chem. 1983 Nov 25;258(22):13745–13751. [PubMed] [Google Scholar]
- Yamada K., Tillotson L. G., Isselbacher K. J. Regulation of hexose carriers in chicken embryo fibroblasts. Effect of glucose starvation and role of protein synthesis. J Biol Chem. 1983 Aug 25;258(16):9786–9792. [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M., Robins M. J., Paterson A. R. Photoaffinity labeling of the human erythrocyte nucleoside transporter by N6-(p-Azidobenzyl)adenosine and nitrobenzylthioinosine. Evidence that the transporter is a band 4.5 polypeptide. J Biol Chem. 1983 Feb 25;258(4):2202–2208. [PubMed] [Google Scholar]