Abstract
The health effects of exposure to household air pollution are gaining international attention. While the bulk of the known mortality estimates due to these exposures are derived from respiratory conditions, there is growing evidence of adverse cardiovascular health effects. Pulmonary hypertension and right heart failure are common conditions in low- and middle-income countries whose etiology may be related to common exposures in these regions such as schistosomiasis, human immunodeficiency virus, tuberculosis infections and other causes. While little is known of the interplay between exposure to household air pollution, right heart function and such conditions, the large burden of pulmonary hypertension and right heart failure in regions where there is significant exposure to household air pollution raises the possibility of a linkage. This review is presented in three parts. First, we explore what is known about pulmonary hypertension and right heart failure in low- and middle-income countries by focusing on eight common causes thereof. We then review what is known of the impact of household air pollution on pulmonary hypertension and posit that when individuals with one of these eight common comorbidities are exposed to household air pollution they may be predisposed to develop pulmonary hypertension or right heart failure. Lastly, we posit that there may be a direct link between exposure to household air pollution and right heart failure independent of pre-existing conditions which merits further investigation. Our overall aim is to highlight the multifactorial nature of these complex relationships and offer avenues for research in this expanding field of study.
Keywords: household air pollution, pulmonary hypertension, right heart failure, low- and middle-income countries
Introduction
Each day, over 3 billion people residing mostly in low- and middle-income countries (LMICs) prepare meals on inefficient cook stoves or heat their homes using biomass fuel exposing billions of people to household air pollution (HAP) [1]. In recent years, international attention has focused on the health effects of exposure to HAP in such countries [2-5]. The World Health Organization estimates that 2 million people die each year as a result of exposure to HAP with the bulk of this mortality estimate based on deaths due to childhood acute respiratory infections, chronic obstructive pulmonary disease and lung cancers [6].
Emerging evidence and research based on ambient air pollution also implicates HAP in the development of cardiovascular diseases such as myocardial ischemia, high blood pressure, and congestive heart failure as well as with elevated right heart pressures [7-9]. Cardiopulmonary diseases such as pulmonary hypertension (PH) and right heart failure (RHF) are common in LMICs and are often associated with HIV, schistosomiasis, parenchymal destruction from tuberculosis, chronic obstructive pulmonary diseases (COPD) and other conditions common in LMICs [5, 10]. Little is known, however, about the interplay between HAP, right heart function, and these common comorbidities in LMICs. Understanding these relationships is important so that the magnitude of the effect of HAP on PH and right heart function is understood and appropriate measures are taken to reduce the associated morbidity.
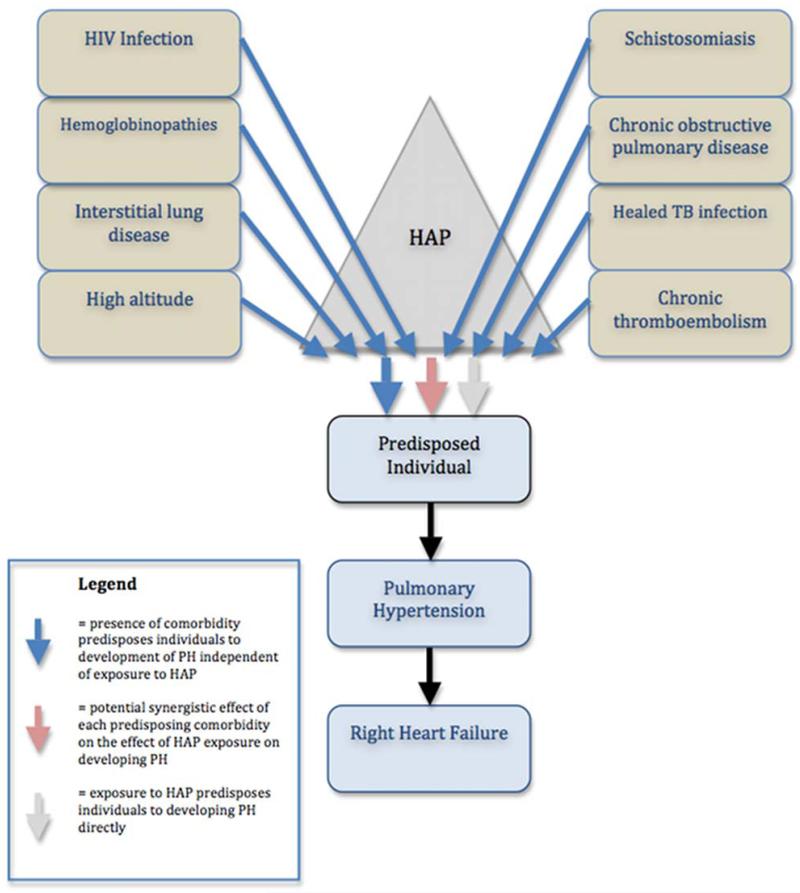
This review begins by highlighting what is known about the common causes of PH in LMICs using the international PH classification framework and highlighting conditions specific to LMICs. We then discuss HAP and its relationship to PH and RHF. Based on the disproportionate distribution of PH and RHF worldwide, we propose that the presence of certain comorbidities specific to LMICs may predispose individuals exposed to HAP to developing PH and RHF (Figure). Where data are available, we review the literature regarding the effects of pre-existing comorbidities on the impact of exposure to HAP on PH and subsequent RHF in LMICs. Where data are lacking, we present a conceptual framework of the unique co-morbid factors in LMICs that may predispose people to developing PH and RHF when exposed to HAP. Lastly, we suggest that a small proportion of patients exposed to HAP who develop isolated RHF may have no other predisposing factors and this group of patients deserves further study. By offering this conceptual framework on conditions specific to LMICs, HAP, PH and RHF, our ultimate aim is to highlight the multifactorial nature of these complex relationships and suggest ripe avenues for further investigation.
Figure.
Factors contributing to the burden of pulmonary hypertension and right heart failure in low- and middle-income countries.
Common comorbidities found in low- and middle-income countries LMICs (brown boxes) each can predispose individuals to developing pulmonary hypertension (PH) and subsequent right heart failure (RHF) (blue arrows). Household air pollution (HAP) (grey triangle) may also cause PH and RHF directly (grey arrow). The high burden of PH/RHF in LMICs may be due to a background predisposition related to the presence of these comorbidities and subsequent exposure to HAP (red arrow).
Pulmonary hypertension and right heart failure in LMICs
Most of what is known about the epidemiology of PH and RHF is based on registries from American and European populations; the actual prevalence in the LMICs remains to be determined [11]. Diagnostic challenges are a major limitation and while noninvasive methods of PH assessment (e.g. electrocardiography, echocardiography) may be more accessible and widespread in LMICs, they are not as accurate as invasive measurement of pulmonary pressures by cardiac catheterization [12]. Available data, however, suggest that 20 to 25 million people in LMICs have some form of pulmonary vascular disease, representing >97% of the global burden [13]. Right heart failure appears to be one of the leading types of heart failure in sub-Saharan Africa (SSA) if data from the Heart of Soweto study can be generalized [14]. In this study from South Africa, RHF was among the top three types (27%) of 844 de novo cases of heart failure seen in an urban setting, along with hypertensive heart disease (33%) and idiopathic dilated cardiomyopathy (28%) [14].
The current classification system for PH (the Dana Point Classification) was adopted in 2008 by the World Symposium on PH [15]. The causes of PH are divided into 5 major groups, and a total of 28 subgroups based on shared pathophysiologic mechanisms, clinical presentations and therapeutic approaches. As such, the causes of PH are many, but only a few are thought to be responsible for the disproportionately high burden of PH in LMICs. Left-sided heart diseases (Group 2) are commonly associated with developing PH and by the time PH is present, left-sided heart diseases are usually severe and not clinically or echocardiographically silent. In many cases, however, the clinician caring for a patient with seemingly “idiopathic” PH needs to have a high clinical suspicion for causes of PH in other groups where local epidemiology is often the basis of a presumptive diagnosis. We focus on the relationship between HAP and causes of PH from Groups 1 (pulmonary arterial hypertension associated with specific conditions), Group 3 (pulmonary hypertension owing to lung diseases and/or hypoxia) and Group 4 (chronic thromboembolic PH) because despite the fact that the majority of cases worldwide are thought to fall into these categories, these conditions are perhaps less often considered and may represent more of a diagnostic challenge [13].
Common causes of PH in LMICs
Human immunodeficiency virus
Considering the global number of HIV infected individuals, 67% (22.1 million) reside in SSA with the majority of deaths globally due to HIV occurring in this region [16]. HIV-associated PH is included under Group 1.4.2 of the PH classification system [15]. This condition is a rare noninfectious pulmonary complication associated with HIV, estimated to occur in 0.6% to 5% of patients with HIV in LMICs [17]. The prevalence of PH is 6 to 12 times greater in HIV seropositive (HIV+) persons compared to HIV seronegative (HIV-) persons [18]. Echocardiography based studies of HIV+ persons suggest that as many as 35% have preclinical PH, but only 27% of persons with PH suspected by echocardiography will meet diagnostic criteria based on right heart catheterization [19]. The mean age of presentation with HIV-associated PH is 38 to 41 years, but may range from infancy to old age, affecting men more than women in a ratio of 1.2:1 [20]. Studies have not found any significant correlation between HIV-associated PH and viral load or CD4 T-cell count, although HIV-associated PH tends to be severe in the presence of AIDS [20].
HIV+ persons with PH exhibit increased mortality compared with counterparts who are HIV+ without PH with a 3-year survival rate of 70% [20, 21]. Fifty percent of deaths reported in patients with HIV-associated PH are related to non-infectious causes such as right ventricular failure or sudden cardiac death and not to HIV/AIDS itself [20]. The impact of combined antiretroviral (cART) on PH is still debated. Study results range from cART having no effect to accelerating the development of PH [22, 23].
The mechanism of PH in HIV+ patients is thought to be related to chronic inflammation and immune activation [17]. The HIV virus has not been shown to infect lung tissue directly. The histopathologic characteristics of HIV-PAH are similar to those of idiopathic PH, manifesting as obliterated pulmonary vasculature with medial hypertrophy, endothelial cell proliferation, increased expression of fibroblast growth factors and perivascular inflammatory cells [24]. The resulting clinical manifestation is characterized by progressive shortness of breath and fatigue, which is similar among patients in LMICs as well as developed countries [24].
Schistosomiasis
Schistosomiasis is an important neglected flatworm tropical disease, estimated to afflict more than 200 million people globally. Pulmonary hypertension due to schistosomiasis infection falls under Group 1.4.5 causes of PH and is found in 1-5% of individuals chronically infected [15, 25]. Schistosomiasis is endemic in 74 countries, with a reported 85% of the infected individuals living in SSA [26]. After being ingested, the adult worms stay in the superior mesenteric vein, producing about 300 ova per day. Ova which are not excreted are transported to the liver via the portal vein, where they lodge and die, evoking a granulomatous reaction around the hepatic veins that develops into fibrosis [13]. This results in portal hypertension that diverts blood from the mesenteric veins to pulmonary circulation. When this conduit matures, the ova are able to reach pulmonary circulation, where they cause vascular changes that lead to PH. Pulmonary disease is seldom seen in the absence of hepatosplenic disease [27]. After treatment, likelihood of recovery from acute pulmonary schistosomiasis (Katayama fever) without any residual pulmonary disease is good. In contrast, chronic pulmonary schistosomiasis, a major long-term consequence of schistosomiasis infestation in endemic areas, leads to vascular remodeling and pulmonary hypertension accounting for up to 30% of cases of PH in South America [28]. There is also important overlap between HIV and schistosomiasis in terms of the high risk and rate of co-infection [29] and the effect of schistosomiasis on the host-response to HIV [30].
Hemoglobinopathies
Hemoglobinopathies are an important cause of PH, and are included in category 1.4.6 of the updated PH classification system [15]. Several hemoglobinopathies disproportionately burden individuals from LMICs, and therefore are likely candidate co-morbidities that may exacerbate the impact of HAP on PH and RHF. Sickle cell disease (SCD) is an autosomal recessive Mendelian disease that disproportionately burdens populations of SSA [31]. Beta thalassemia, a group of inherited blood disorders characterized by reduced or absent synthesis of the beta chains of hemoglobin, is analogously more common among southeast Asian populations [32].
The mechanisms of PH and RHF in individuals with hemoglobinopathies have been described [33]. In these conditions, hemolysis releases cell-free hemoglobin which then oxidizes NO to nitrate, causing vasoconstriction and subsequent pulmonary vasculopathy [34]. Other mechanisms for PH include functional asplenia, thromboembolism, pulmonary fibrosis, and hypoxemia [35]. Notably, since well-treated beta thalassemia major patients (regular transfusion and chelation therapy) do not generally develop PH, it appears that hemolysis and its effects on tissue hypoxia play a key role in the development of PH in these patients [36].
Up to one-third of individuals with SCD have PH defined by echocardiography or by right heart catheterization [37]. SCD can cause RHF in the absence of PH, but PH exacerbates or triggers RHF in the majority of cases [34]. During an acute chest syndrome, pulmonary pressures can acutely rise and trigger RHF in about 13% of patients, particularly among patients with pre-existing baseline PH or right ventricular dysfunction [38]. The prevalence of PH and RHF among patients with beta thalassemia depends on the severity of disease (thalassemia major versus intermedia), on adequacy of treatment and modality of PH assessment [39]. Untreated thalassemia major patients nearly universally develop PH [40]. Notably, thalassemia intermedia patients are more likely to develop PH than treated thalassemia major patients, likely due to the protective effects of transfusion and chelation therapy in thalassemia major individuals [36]. In one series of thalassemia intermedia patients, nearly 60% were found to have PH [41]. Given the high prevalence of hemoglobinopathies in SSA and Southeast Asia and the strong association between hemoglobinopathies and PH, a large number of people in LMICs appear to be at risk for developing PH.
Chronic obstructive pulmonary disease
COPD is a leading cause of morbidity and mortality worldwide and a recognized cause of PH (Group 3.1 causes) [15]. While precise data are lacking due to variable definitions of the disease and inconsistent data reporting, the World Health Organization estimates the world COPD mortality, incidence, and prevalence rates per 100,000 to be 44.2, 92.1, and 1013, respectively. The highest rates were seen in the Western Pacific (mostly China) (79.8, 176.6, 1675) and the lowest rates in Africa (18.1, 29.8, and 179). For Africa, these lower COPD rates may reflect a lack of cigarette smoking, younger age of the population, or the result of a paucity of reported data [42]. However, with the rapid rise in cigarette smoking rates reported in LMICs (2% annually), especially in Africa (3.2% annually), COPD rates are predicted to greatly increase in these countries over the next decade [43].
COPD can be complicated by the development of PH and RHF, worsening both patient quality of life and survival. Hypoxemia induced vasoconstriction and destruction of the pulmonary vascular bed are responsible mechanisms. Short-term hypoxemia leads to precapillary arteriole vasoconstriction in an attempt to lessen ventilation/perfusion mismatch with complete reversibility upon correction of the hypoxemia. In contrast, chronic hypoxemia associated with advanced COPD causes the release or suppression of mediators responsible for vascular tone and vascular cell proliferation. The resulting alteration in vascular function leads to vessel remodeling and destruction, and onset of persistent PH that is refractory to correction with supplemental oxygen. The prevalence of PH among patients with COPD varies widely among reports and this is likely due to the populations studied and the methodology used to define PH. In general the magnitude of PH is modest with severe PH being uncommon [44].
Interstitial lung disease
There are over 200 diseases that result in interstitial lung disease (ILD). In LMICs, inhalation of inorganic dusts (e.g., silica, coal, asbestos) [45], sarcoidosis [46] and HIV associated lymphocytic interstitial pneumonia in children [47] are common causes of ILD. Unfortunately no large epidemiologic studies are available to estimate the prevalence or incidence of ILD in LMICs. However, several small series suggest that the disease entity may be a significant contributor to lung morbidity and mortality [48]. As ILD slowly progresses over many years, respiratory failure and PH with RHF often develop. Parenchymal and vascular remodeling are the primary mechanisms responsible for the relatively high prevalence of PH in ILD [49]. Effective treatment for ILD remains elusive, even in developed nations. For those living in LMICs, limiting exposure and instituting appropriate therapy of the causative conditions may be the most successful interventions to lessen the severity of ILD and subsequent PH and RHF. The mortality rate from ILD remains high as diagnostic, referral, and therapeutic options are limited in many LMICs.
Healed tuberculosis infection
Tuberculosis (TB) remains a leading cause of disease, mortality and morbidity in the world with World Health Organization reporting an estimated 8.8 million cases occurring in 2010 and 1.4 million resultant deaths [50]. While not a unique subgroup within the PH classification system, TB-associated PH is most similar to Group 3 PH disorders [15]. Although curable in the majority of cases with prolonged antibiotic courses (minimum of 6 months), TB may result in marked lung destruction (fibrosis, bronchiectasis, bronchovascular distortion and/or emphysema) with consequent pulmonary physiological effects. These effects range to as high as over 50% of post TB patients demonstrating physiological impairment [51]. One study of South African miners demonstrated progressive loss of function associated with each recurrent case of TB, progressing from 18% following one episode to 35% after three episodes of disease [52]. The amount of destruction may also be related to the length of time preceding diagnosis and institution of effective therapy [53]. Thus, resultant long-term pulmonary disability related to tuberculosis is likely more common in resource limited regions where diagnosis and effective therapy are often delayed and where both pulmonary physiological assessments as well as effective pulmonary management is also limited.
Multiple studies from regions as diverse as Indonesia [54], USA [55], South Africa [51] and Latin America [56] have all demonstrated lung function impairment at the end of TB therapy. Impaired pulmonary physiology with resultant gas exchange abnormalities have clearly been shown to be a risk factor for secondary PH. One study in Ethiopia has examined the occurrence of PH in “healed” tuberculosis patients [57]. In this small series (n=14) all patients had not only physiologic pulmonary impairments but also radiologic pulmonary abnormalities in association with their pulmonary hypertension (estimated pulmonary artery systolic pressures 40 to >80 mm/Hg). The relationship of tuberculosis to pulmonary hypertension appears to be related not through a direct effect of the infectious agent but rather through the degree of parenchymal damage left in the wake of curative therapy.
High altitude
High-altitude is a well known risk factor for PH and is a designated subset of PH in Group 3.5 of the updated classification system [15]. High altitude causes chronic hypoxemia, which then causes hypoxia-induced pulmonary vasoconstriction and vascular remodeling of pulmonary arterioles. This ultimately leads to increased pulmonary vascular resistance and PH. Many regions of LMICs are located at high altitude, particularly in Asia, Africa, and Latin America, and a significant number of people live in high-altitude areas. In fact, an estimated more than 140 million people live 2,500 meters above sea level, and the majority of these individuals live in LMICs [58].
Epidemiological studies of high-altitude PH have revealed a prevalence of between 5 and 25%, with notable geographic diversity [59]. In high-altitude regions of Kyrgyzstan, men were more likely to have electrocardiographic evidence of right heart failure than women (23% vs. 6%) [60]. In the Andean region, prevalence of PH has been reported as between 5 and 18% [59]. In the Qinghai Province of China, high-altitude PH was more common among children than among adults [61]. Notably, Tibetan populations generally have less PH and exercise-induced PH than other high-altitude populations for unknown reasons [62]. RHF caused by exposure to PH at high altitude also appears to be region-specific. While high-altitude RHF is common among individuals with high-altitude PH in China and Tibet, severe RHF is relatively less common in the Andean region among those with PH[59].
Chronic thromboembolic pulmonary hypertension
Chronic thromboembolic pulmonary hypertension (CTEPH) is Group 4 of the PH classification system [15]. CTEPH is traditionally thought to occur in less than 1% of survivors of acute pulmonary embolism (PE) [63], however, that estimate could be much higher owing to substantial under-diagnosis. For example, a prospective, long-term study to assess the incidence of symptomatic CTEPH among consecutive index cases of acute PE in Italy described an incidence close to 4% after two years of follow-up [64]. In China, as the magnitude of pulmonary embolism has increased dramatically (10-fold increase in number of cases between 1997-2008), there has been a commensurate heightened national awareness of the importance of CTEPH as a cause of PH [11]. It is the remodeling of the pulmonary resistance vessels rather than recurrence of pulmonary embolism that is thought to lead to the development of pulmonary arterial hypertension and RHF [65]. Even then the exact pathophysiologic mechanisms leading to vascular remodeling remain poorly understood.
Where resources are limited, CTEPH represents a diagnostic challenge and under-diagnosis is expected to be more common. In LMICs, history and physical examination are especially important in diagnosing CTEPH because the symptoms and signs may be quite subtle. Progressive exertional dyspnea is the most consistent presenting complaint. Chest pain, near-syncope or syncope, and lower extremity edema are late symptoms. Many patients provide a history consistent with an acute pulmonary embolic event, although documentation of prior thromboembolism may be absent [66]. Physical examination remains unremarkable until late in the disease progression. It is not unusual in LMICs for patients to present with overt signs of right-sided heart failure. In many centers, basic laboratory tests (e.g., D-dimer) and chest X-ray may be available while more advanced investigations (e.g., brain natriuretic peptide, transthoracic echocardiography, computed tomography scanning, spirometry) are usually found in referral centers highlighting the significant diagnostic challenges. In western Kenya, for example, the most common diagnostic approach begins with a clinical and echocardiographic evaluation. Subsequent investigations supported by Doppler ultrasonography of extremity vasculature, chest radiography (X-ray and/or CT scan) and spirometry may lead to a working diagnosis of CTEPH. It should be noted that even these seemingly basic tests can only be performed at the national referral hospital in the region and are not universally accessible due to cost implications.
Do common causes of PH potentiate the adverse effects of HAP?
We now turn our attention to the interplay between the aforementioned conditions, HAP, PH and RHF. In most instances, the pathway suggested in the Figure has not been demonstrated empirically but appears plausible based on similarities in geographic distribution, pathophysiology and research from other forms of air pollution. In parts of the world where HAP is prevalent, the burden of HIV, schistosomiasis, hemoglobinopathies and tuberculosis are also common. In Malawi, for example, the HIV prevalence rate is among the top 10 in the world and >95% of households burn biomass fuels indoors [67]. There is also substantial overlap between high-altitude regions of the world and regions where there is significant exposure to HAP, due to the use of biomass fuels for heating as well as cooking. In the Himalaya mountains of northern India, measures of HAP were greater in the winter than the summer, due to increased use of biomass fuels for heating in the winter months [68], contributing to altitude-specific respiratory morbidity [69]. Similarly, in Peru, HAP measures were greater during the morning meal preparation time when both dung and wood were used as cooking fuels [70].
Pathophysiologic similarities also lend credibility to the potentiating effects of HAP on other forms of PH. HIV infection can impair lung function directly [71] as well as increase the host’s susceptibility to lung infections [72], both of which may predispose to PH. HIV and HAP exposure are also both associated with an increased risk of pneumonia and have similar effects on pulmonary inflammation [73]. HAP has been associated with bronchitis and asthma in the highland regions of Bolivia [74] and Guatemala [75] suggesting that high altitude may potentiate or exacerbate the impact of HAP on PH and RHF.
While the exposures and dose are different between HAP and tobacco smoking [7], parallel relationships also support the potentiating effect of other forms of PH on HAP. HIV+ tobacco smokers are at higher risk than non-smokers to develop pulmonary emphysema [76] and tobacco smoking is posited to exacerbate the effects of HIV on lung function [77]. With regard to hemoglobinopathies, tobacco smoke has been associated with increased risk of sickle cell crises [78]. Likewise, poor outdoor air quality has been associated with increased hospitalizations for acute sickle cell pain [52]. Smoking tobacco is a moderate risk factor for venous thromboembolism while higher levels of ambient air pollution (particulate matter <10μm in aerodynamic diameter or PM10) are associated with a greater risk of deep venous thrombosis [79, 80]. CTEPH has not specifically been studied in this regard and less is known with regard to schistosomiasis and tuberculosis and any potential synergy with HAP.
The literature is slightly more robust for COPD and ILD. HAP appears to be an important risk factor for the development of COPD in LMICs. Numerous population-based, cross-sectional, and case-control studies have found that indoor cooking with biomass fuels is a major risk factor for the development of COPD in resource poor countries [5, 81]. Women living in rural areas seem to be especially vulnerable [82]. For example, Smith and colleagues report that women exposed to HAP from wood burning were 3.2 (95% CI, 2.3-4.8) times as likely to develop COPD as those who cook and heat with electricity, gas, and other cleaner fuels [4]. In a study of 5,539 subjects from Colombia, cooking for 10 years or more with a wood stove was found to be an independent risk factor for the development of COPD even after adjusting for factors such as age, gender, active and passive smoking, education level, history of tuberculosis, and exposure to charcoal or dust at work [83]. Noteworthy in this study, men were also at risk for COPD but to a lesser degree than women (men: OR 1.53 vs. women: OR 1.84), suggesting a high level of pollutants in the home cooking environment.
Whether a direct link exists between HAP exposure and right heart function independent of chronic obstructive lung disease is unclear but it is noteworthy that the burden of PH seen in patients with COPD is modest compared to the degree of PH seen in LMICs. The attributable risk of RHF due to HAP separate from the impact of COPD will be difficult to assess as HAP exposure contributes simultaneously to RHF via direct and pulmonary pathways. (FIGURE) Further research is needed to determine if reducing HAP can lessen the development of COPD and associated PH and RHF. Reducing HAP, however, can reduce the incidence of COPD in LMICs. Since the 1980’s, the Chinese National Improved Stoves Program has installed over 180 million improved stoves in rural households. In one retrospective Chinese cohort study, stove improvement was associated with a reduction in COPD incidence [84]. Further, in a community intervention trial in Mexico, forced expiratory volume in 1 second (FEV1) declined by 31 ml/year in women using a fuel efficient, low emission stove compared to 62 ml/year in women cooking over open wood fires [85]. Non-HAP associated COPD (i.e., due to tobacco smoking) is a growing potential source of underlying lung disease in LMICs [43] and relatively little is known of its interaction with HAP to contribute to PH/RHF.
HAP has been associated with ILD in numerous case series from several different countries and this association forms the bulk of the literature on HAP and cardiopulmonary pathology. Restrepo and colleagues found 22 cases of pneumoconiosis related to the inhalation of wood smoke fly ash with silicates in the mountain area of Colombia; lung biopsies revealed fibrosis and anthracosis (deposition of carbon, silica, and quartz particles in the macrophages, mucosa, and submucosa) [86]. Similarly, Sandoval et al. reported 22 women from rural Mexico who had micronodular or reticular opacities on chest roentgen scans associated with wood smoke [87]. The anthracotic deposits were thought to result not so much from the wood burning itself but from the combination of the type of stoves used, the local cooking practices, and the floor materials in the cooking area. This complex combination of risk factors is also illustrated by the documentation of “hut lung”, found in South African women. With this disease, a combination of respirable quartz during maize grinding, non-quartz dust, and smoke from dung were all responsible for the interstitial disease, with the latter two the more important risk factors [88]. Finally, Hassan and coworkers found that chronic exposure to pinewood smoke in India resulted in “Gujjar lung”, an interstitial disease that was characterized by reticulonodular opacities on chest X-ray and anthracotic nodules, carbon laden macrophages, and fibrosis on lung biopsy [89].
Is there a direct link between HAP and RHF?
As Pandey describes in this issue, over 50 years of research, mostly in the form of case series, has shown a relationship between exposure to HAP and right-sided cardiac pathology in LMICs [87, 90-93]. HAP has also been implicated as a cause of isolated RHF in epidemiological studies. In the Heart of Soweto study, most of the cases of RHF were young women with isolated RHF and idiopathic was the most common etiology of PH (34% and 66% of cases for women and men, respectively) after an exhaustive search for other causes [10]. Based on common exposures in this region, HAP from burning solid fuels has been postulated to be an important contributor to the otherwise idiopathic RHF in South Africa but a definitive link has not been specifically examined.
There is increasing evidence that PH and RHF occur at an increased rate in settings where HAP is an ongoing problem. While direct causality has not been proven, it is highly likely that a link between HAP, PH and subsequent RHF exists. This exploratory review suggests that HAP may play an indirect role in the development of RHF and pulmonary HTN. For instance, HAP is a known risk factor for the development of COPD that then predisposes patients to the occurrence of PH via hypoxemia induced vasoconstriction and increased airway inflammation. Alternatively, HAP may exacerbate underlying lung disease thus causing increased inflammation and vascular remodeling that subsequently causes RHF [5]. However, several case reports indicate the presence of a unique cohort of patients in whom PH exists in spite a lack of obvious secondary causes [90, 93]. In western Kenya, isolated PH has been reported in middle age and older women without any underlying lung disease and no other known risk factors of PH [90]. It is these cases of PH that are especially intriguing. It is plausible that HAP plays an integral role in the development of isolated PH via various mechanisms including the promotion of vascular remodeling. Whether this condition is reversible by the removal of continued exposure to biomass byproducts remains to be seen.
Exposure to household air pollutants such as wood smoke particulate matter results in increased oxidative stress with production of reactive oxygen species, increased secretion of pro-inflammatory cytokines including TNF-alpha and alterations in endothelin receptor expression [94]. Endothelin-1, for example, is a vasoactive peptide that acts as a potent vasoconstrictor in the lungs. In animal models, inhalation of urban particles (Ottawa dust) and ozone increases endothelin-1 expression [95]. In a study of children living in Mexico City, elevations in serum endothelin-1 levels were correlated with exposure to outdoor air pollutants (7 day cumulative levels of PM2.5) and the presence of elevated pulmonary pressures [96]. These findings suggest that inhalation of air pollutants could result in an increased risk of vascular remodeling and thus the development of PH.
There is a paucity of research focused on isolated RHF in LMICs despite mounting evidence supporting the significant morbidity associated with this disease in otherwise healthy individuals. There is a desperate need for well-characterized studies. There are significant barriers to research in this area the most important of which is a lack of research funding that impedes progress in discovering novel mechanisms of disease and subsequently the discovery of appropriate therapies for patients afflicted with PH and RHF. The lack of resources stems from poor recognition of the magnitude of the problem and indeed studies are required to determine the exact prevalence of seemingly isolated RHF in populations where the use of biomass fuel is common. Additional barriers include poor access to medical care and a lack of the technological tools required to completely evaluate patients presenting with RHF. The importance and complexity of determining the contribution of various factors to the ultimate development of RHF cannot be overstated. Increased awareness of this disease would lead to a determination of appropriate therapy for these patients and would undoubtedly have a significant impact on the lives of a large population.
Conclusions
The burden of PH and RHF in settings where HAP is prevalent is greater than the burden of PH and RHF seen in chronic lung diseases such as COPD [44]. There has been little attention paid to the association between exposure to HAP and cardiovascular diseases independent of apparent lung disease [4]. This may be related due to a perceived lack of evidence. The only HAP-cardiovascular disease relationship supported by randomized trial evidence and observational studies is that of blood pressure [97-100]. Ambient air pollution has been linked to myocardial ischemia, heart failure exacerbations and overall cardiovascular mortality [8].
Relatively little attention has been paid to assessing PH or right ventricular dysfunction in patients with HAP exposure in the setting of co-morbidities affecting cardio-pulmonary health, especially in LMICs. Nevertheless, clinical experience and small studies lend credence to the utility of systematically exploring these relationships.
We suggest that more consideration should be given to the impact of pre-existing, and perhaps subclinical, comorbidities on the likelihood of HAP causing PH and RHF. The challenges inherent in establishing this proposed linkage rest in research methodology but are also compounded by the fact that these types of conditions are most common in settings where diagnostic and therapeutic resources are limited. Nevertheless, given the large number of individuals who have or are at risk for developing PH in LMICs, a concerted effort to address these relationships is certainly warranted. This is especially pertinent and timely given the growing international attention in HAP and its health effects, and large-scale efforts to implement solutions to reduce or eliminate it. By reviewing and positing these relationships, our goal is to spark a dialogue and further investigation into conditions that disproportionately affect the most disadvantaged.
Acknowledgements
The authors report no conflicts of interest. This work was supported by cooperative agreement 623-A-00-08-00003-00 between the United States Agency for International Development (USAID) as part of the President’s Emergency Plan for AIDS Relief (PEPFAR) and the USAID-Academic Model Providing Access to Healthcare (AMPATH) Partnership, contract HHSN268200900031C with the National Heart Lung and Blood Institute Global Health Initiative’s AMPATH Cardiovascular and Pulmonary Disease Center of Excellence (to SK), and grant 5K01TW008407-02 from the Fogarty International Center (to GSB) of the National Institutes of Health. The authors are solely responsible for drafting and editing of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].World Health Organization Indoor Air Pollution. 2011 WHO Fact Sheet No. 292. [Google Scholar]
- [2].Martin WJ, Glass RI, Balbus JM, Collins FS. Public health. A major environmental cause of death. Science. 2011;334(6053):180–1. doi: 10.1126/science.1213088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mendis S, Alwan A, editors. Prioritized research agenda for prevention and control of noncommunicable diseases. World Health Organization; Geneva: 2011. [Google Scholar]
- [4].Smith KR, Mehta S, Maeusezahl-Feuz M. Indoor air pollution from household use of solid fuels. In: Ezzati M RA, Lopez A, Murray C, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease due to Selected Major Risk Factors. 1st ed World Health Organization; 2004. pp. 1435–93. [Google Scholar]
- [5].Bruce N, Perez-Padilla R, Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ. 2000;78(9):1078–92. [PMC free article] [PubMed] [Google Scholar]
- [6].WHO . Global Health Risks. Mortality and burden of disease attributable to selected major risks. World Health Organization; Geneva: 2009. [Google Scholar]
- [7].Smith KR, Peel JL. Mind the gap. Environ Health Perspect. 2010;118(12):1643–5. doi: 10.1289/ehp.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- [9].Rich DQ, Freudenberger RS, Ohman-Strickland P, Cho Y, Kipen HM. Right heart pressure increases after acute increases in ambient particulate concentration. Environ Health Perspect. 2008;116(9):1167–71. doi: 10.1289/ehp.11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stewart S, Mocumbi AO, Carrington MJ, Pretorius S, Burton R, Sliwa K. A not-so-rare form of heart failure in urban black Africans: pathways to right heart failure in the Heart of Soweto Study cohort. Eur J Heart Fail. 2011;13(10):1070–7. doi: 10.1093/eurjhf/hfr108. [DOI] [PubMed] [Google Scholar]
- [11].Zhai Z, Wang J, Zhao L, Yuan JX-J, Wang C. Pulmonary hypertension in China: pulmonary vascular disease: the global perspective. Chest. 2010;137(6 Suppl):69S–77S. doi: 10.1378/chest.09-2802. [DOI] [PubMed] [Google Scholar]
- [12].Galié N, Hoeper MM, Humbert M, Torbicki A, Vachiery J-L, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) European Heart Journal. 2009;30(20):2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- [13].Butrous G, Ghofrani HA, Grimminger F. Pulmonary vascular disease in the developing world. Circulation. 2008;118(17):1758–66. doi: 10.1161/CIRCULATIONAHA.107.727289. [DOI] [PubMed] [Google Scholar]
- [14].Stewart S, Wilkinson D, Hansen C, Vaghela V, Mvungi R, McMurray J, et al. Predominance of heart failure in the Heart of Soweto Study cohort: emerging challenges for urban African communities. Circulation. 2008;118(23):2360–7. doi: 10.1161/CIRCULATIONAHA.108.786244. [DOI] [PubMed] [Google Scholar]
- [15].Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- [16].Joint United Nations Programme on HIV/AIDS (UNAIDS) World AIDS Day Report 2011. Geneva, Switzerland: 2011. [Google Scholar]
- [17].Mette SA, Palevsky HI, Pietra GG, Williams TM, Bruder E, Prestipino AJ, et al. Primary pulmonary hypertension in association with human immunodeficiency virus infection. A possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis. 1992;145(5):1196–200. doi: 10.1164/ajrccm/145.5.1196. [DOI] [PubMed] [Google Scholar]
- [18].Cicalini S, Chinello P, Cicini MP, Petrosillo N. Pulmonary arterial hypertension and HIV infection. AIDS. 2008;22(16):2219–20. doi: 10.1097/QAD.0b013e328310161f. author reply 20. [DOI] [PubMed] [Google Scholar]
- [19].Sitbon O, Lascoux-Combe C, Delfraissy J-F, Yeni PG, Raffi F, De Zuttere D, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1):108–13. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- [20].Degano B, Guillaume M, Savale L, Montani D, Jaïs X, Yaici A, et al. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24(1):67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- [21].Zuber J-P, Calmy A, Evison JM, Hasse B, Schiffer V, Wagels T, et al. Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004;38(8):1178–85. doi: 10.1086/383037. [DOI] [PubMed] [Google Scholar]
- [22].Pellicelli AM, D'Ambrosio C, Vizza CD, Borgia MC, Tanzi P, Pino P, et al. HIV-related pulmonary hypertension. From pathogenesis to clinical aspects. Acta Cardiol. 2004;59(3):323–30. doi: 10.2143/AC.59.3.2005190. [DOI] [PubMed] [Google Scholar]
- [23].Reinsch N, Buhr C, Krings P, Kaelsch H, Kahlert P, Konorza T, et al. Effect of gender and highly active antiretroviral therapy on HIV-related pulmonary arterial hypertension: results of the HIV-HEART Study. HIV Med. 2008;9(7):550–6. doi: 10.1111/j.1468-1293.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- [24].Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-Related pulmonary hypertension: analytic review of 131 cases. Chest. 2000;118(4):1133–41. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- [25].Fernandes CJCDS, Jardim CVP, Hovnanian A, Hoette S, Morinaga LK, Souza R. Schistosomiasis and pulmonary hypertension. Expert Rev Respir Med. 2011;5(5):675–81. doi: 10.1586/ers.11.58. [DOI] [PubMed] [Google Scholar]
- [26].Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- [27].Cheever AW, Andrade ZA. Pathological lesions associated with Schistosoma mansoni infection in man. Trans R Soc Trop Med Hyg. 1967;61(5):626–39. doi: 10.1016/0035-9203(67)90125-3. [DOI] [PubMed] [Google Scholar]
- [28].Lapa MS, Ferreira EVM, Jardim C, Martins BdCdS, Arakaki JSO, Souza R. Clinical characteristics of pulmonary hypertension patients in two reference centers in the city of Sao Paulo. Rev Assoc Med Bras. 2006;52(3):139–43. doi: 10.1590/s0104-42302006000300012. [DOI] [PubMed] [Google Scholar]
- [29].Stoever K, Molyneux D, Hotez P, Fenwick A. HIV/AIDS, schistosomiasis, and girls. Lancet. 2009;373(9680):2025–6. doi: 10.1016/S0140-6736(09)61111-9. [DOI] [PubMed] [Google Scholar]
- [30].Erikstrup C, Kallestrup P, Zinyama-Gutsire RBL, Gomo E, van Dam GJ, Deelder AM, et al. Schistosomiasis and infection with human immunodeficiency virus 1 in rural Zimbabwe: systemic inflammation during co-infection and after treatment for schistosomiasis. Am J Trop Med Hyg. 2008;79(3):331–7. [PubMed] [Google Scholar]
- [31].Stuart MJ, Nagel RL. Sickle-cell disease. The Lancet. 2004;364(9442):1343–60. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- [32].Angastiniotis M, Modell B. Global epidemiology of hemoglobin disorders. Ann N Y Acad Sci. 1998;850:251–69. doi: 10.1111/j.1749-6632.1998.tb10482.x. Epub 1998/07/21. [DOI] [PubMed] [Google Scholar]
- [33].Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655–65. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- [34].Gladwin MT, Sachdev V. Cardiovascular abnormalities in sickle cell disease. J Am Coll Cardiol. 2012;59(13):1123–33. doi: 10.1016/j.jacc.2011.10.900. Epub 2012/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Minter KR, Gladwin MT. Pulmonary complications of sickle cell anemia. A need for increased recognition, treatment, and research. Am J Respir Crit Care Med. 2001;164(11):2016–9. doi: 10.1164/ajrccm.164.11.2104101. [DOI] [PubMed] [Google Scholar]
- [36].Aessopos A, Farmakis D. Pulmonary hypertension in beta-thalassemia. Ann N Y Acad Sci. 2005;1054:342–9. doi: 10.1196/annals.1345.041. [DOI] [PubMed] [Google Scholar]
- [37].Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(1):44–53. doi: 10.1056/NEJMoa1005565. Epub 2011/07/08. [DOI] [PubMed] [Google Scholar]
- [38].Mekontso Dessap A, Leon R, Habibi A, Nzouakou R, Roudot-Thoraval F, Adnot S, et al. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2008;177(6):646–53. doi: 10.1164/rccm.200710-1606OC. Epub 2008/01/05. [DOI] [PubMed] [Google Scholar]
- [39].Aessopos A, Farmakis D, Deftereos S, Tsironi M, Tassiopoulos S, Moyssakis I, et al. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127(5):1523–30. doi: 10.1378/chest.127.5.1523. Epub 2005/05/13. [DOI] [PubMed] [Google Scholar]
- [40].Derchi G, Fonti A, Forni GL, Galliera EO, Cappellini MD, Turati F, et al. Pulmonary hypertension in patients with thalassemia major. Am Heart J. 1999;138(2 Pt 1):384. doi: 10.1016/s0002-8703(99)70129-8. Epub 1999/07/30. [DOI] [PubMed] [Google Scholar]
- [41].Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, et al. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97(11):3411–6. doi: 10.1182/blood.v97.11.3411. Epub 2001/05/23. [DOI] [PubMed] [Google Scholar]
- [42].Chan-Yeung M, Aït-Khaled N, White N, Ip MS, Tan WC. The burden and impact of COPD in Asia and Africa. Int J Tuberc Lung Dis. 2004;8(1):2–14. [PubMed] [Google Scholar]
- [43].World Health Organization . Smoking Prevalence. In: World Health Organization, editor. Tobacco or Health: A Global Status Report. Geneva: 1997. pp. 10–8. [Google Scholar]
- [44].Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation. 2007;116(25):2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- [45].Jindal SK, Aggarwal AN, Gupta D. Dust-induced interstitial lung disease in the tropics. Curr Opin Pulm Med. 2001;7(5):272–7. doi: 10.1097/00063198-200109000-00004. [DOI] [PubMed] [Google Scholar]
- [46].Jindal SK, Gupta D, Aggarwal AN. Sarcoidosis in developing countries. Curr Opin Pulm Med. 2000;6(5):448–54. doi: 10.1097/00063198-200009000-00011. [DOI] [PubMed] [Google Scholar]
- [47].Zar HJ. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatr Pulmonol. 2008;43(1):1–10. doi: 10.1002/ppul.20676. [DOI] [PubMed] [Google Scholar]
- [48].Torres-Duque C, Maldonado D, Pérez-Padilla R, Ezzati M, Viegi G. Biomass fuels and respiratory diseases: a review of the evidence. Proc Am Thorac Soc. 2008;5(5):577–90. doi: 10.1513/pats.200707-100RP. [DOI] [PubMed] [Google Scholar]
- [49].Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J. 2008;31(6):1357–67. doi: 10.1183/09031936.00171307. [DOI] [PubMed] [Google Scholar]
- [50].World Health Organization . Global Tuberculosis Control. WHO; Geneva: 2011. [Google Scholar]
- [51].Plit ML, Anderson R, Van Rensburg CE, Page-Shipp L, Blott JA, Fresen JL, et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998;12(2):351–6. doi: 10.1183/09031936.98.12020351. [DOI] [PubMed] [Google Scholar]
- [52].Yallop D, Duncan ER, Norris E, Fuller GW, Thomas N, Walters J, et al. The associations between air quality and the number of hospital admissions for acute pain and sickle-cell disease in an urban environment. Br J Haematol. 2007;136(6):844–8. doi: 10.1111/j.1365-2141.2007.06493.x. Epub 2007/03/08. [DOI] [PubMed] [Google Scholar]
- [53].de Valliére S, Barker RD. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8(6):767–71. [PubMed] [Google Scholar]
- [54].Maguire GP, Anstey NM, Ardian M, Waramori G, Tjitra E, Kenangalem E, et al. Pulmonary tuberculosis, impaired lung function, disability and quality of life in a highburden setting. Int J Tuberc Lung Dis. 2009;13(12):1500–6. [PubMed] [Google Scholar]
- [55].Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, et al. Pulmonary impairment after tuberculosis. Chest. 2007;131(6):1817–24. doi: 10.1378/chest.06-2949. [DOI] [PubMed] [Google Scholar]
- [56].Menezes AMB, Hallal PC, Perez-Padilla R, Jardim JRB, Muiño A, Lopez MV, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J. 2007;30(6):1180–5. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- [57].Ahmed AEH, Ibrahim AS, Elshafie SM. Pulmonary hypertension in patients with treated pulmonary tuberculosis: analysis of 14 consecutive cases. Clin Med Insights Circ Respir Pulm Med. 2011;5:1–5. doi: 10.4137/CCRPM.S6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation. 2007;115(9):1132–46. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- [59].Xu X-Q, Jing Z-C. High-altitude pulmonary hypertension. Eur Respir Rev. 2009;18(111):13–7. doi: 10.1183/09059180.00011104. [DOI] [PubMed] [Google Scholar]
- [60].Aldashev AA, Sarybaev AS, Sydykov AS, Kalmyrzaev BB, Kim EV, Mamanova LB, et al. Characterization of high-altitude pulmonary hypertension in the Kyrgyz: association with angiotensin-converting enzyme genotype. Am J Respir Crit Care Med. 2002;166(10):1396–402. doi: 10.1164/rccm.200204-345OC. Epub 2002/10/31. [DOI] [PubMed] [Google Scholar]
- [61].Wu TY. An investigation on high altitude heart disease. Zhonghua Yi Xue Za Zhi. 1983;63(2):90–2. Epub 1983/02/01. [PubMed] [Google Scholar]
- [62].Groves BM, Droma T, Sutton JR, McCullough RG, McCullough RE, Zhuang J, et al. Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J Appl Physiol. 1993;74(1):312–8. doi: 10.1152/jappl.1993.74.1.312. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- [63].Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345(20):1465–72. doi: 10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- [64].Pengo V, Lensing AWA, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–64. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- [65].Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest. 1993;103(3):685–92. doi: 10.1378/chest.103.3.685. [DOI] [PubMed] [Google Scholar]
- [66].Monreal M, Ruíz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. A systematic study. Chest. 1992;102(3):677–81. doi: 10.1378/chest.102.3.677. [DOI] [PubMed] [Google Scholar]
- [67].Fullerton DG, Semple S, Kalambo F, Suseno A, Malamba R, Henderson G, et al. Biomass fuel use and indoor air pollution in homes in Malawi. Occupational and Environmental Medicine. 2009;66(11):777–83. doi: 10.1136/oem.2008.045013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Norboo T, Yahya M, Bruce NG, Heady JA, Ball KP. Domestic pollution and respiratory illness in a Himalayan village. Int J Epidemiol. 1991;20(3):749–57. doi: 10.1093/ije/20.3.749. Epub 1991/09/01. [DOI] [PubMed] [Google Scholar]
- [69].Norboo T, Saiyed HN, Angchuk PT, Tsering P, Angchuk ST, Phuntsog ST, et al. Mini review of high altitude health problems in Ladakh. Biomed Pharmacother. 2004;58(4):220–5. doi: 10.1016/j.biopha.2004.02.003. Epub 2004/06/09. [DOI] [PubMed] [Google Scholar]
- [70].Pearce JL, Aguilar-Villalobos M, Rathbun SL, Naeher LP. Residential exposures to PM2.5 and CO in Cusco, a high-altitude city in the Peruvian Andes: a pilot study. Arch Environ Occup Health. 2009;64(4):278–82. doi: 10.1080/19338240903338205. Epub 2009/12/17. [DOI] [PubMed] [Google Scholar]
- [71].Diaz PT, Wewers MD, Pacht E, Drake J, Nagaraja HN, Clanton TL. Respiratory symptoms among HIV-seropositive individuals. Chest. 2003;123(6):1977–82. doi: 10.1378/chest.123.6.1977. [DOI] [PubMed] [Google Scholar]
- [72].Raju R, Peters BS, Breen RAM. Lung infections in the HIV-infected adult. Curr Opin Pulm Med. 2012;18(3):253–8. doi: 10.1097/MCP.0b013e32835213d3. [DOI] [PubMed] [Google Scholar]
- [73].Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102(9):843–51. doi: 10.1016/j.trstmh.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Albalak R, Frisancho AR, Keeler GJ. Domestic biomass fuel combustion and chronic bronchitis in two rural Bolivian villages. Thorax. 1999;54(11):1004–8. doi: 10.1136/thx.54.11.1004. Epub 1999/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schei MA, Hessen JO, Smith KR, Bruce N, McCracken J, Lopez V. Childhood asthma and indoor woodsmoke from cooking in Guatemala. J Expo Anal Environ Epidemiol. 2004;14(Suppl 1):S110–7. doi: 10.1038/sj.jea.7500365. Epub 2004/05/01. [DOI] [PubMed] [Google Scholar]
- [76].Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132(5):369–72. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- [77].Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. American Journal of Respiratory and Critical Care Medicine. 2011;183(3):388–95. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].West DC, Romano PS, Azari R, Rudominer A, Holman M, Sandhu S. Impact of environmental tobacco smoke on children with sickle cell disease. Arch Pediatr Adolesc Med. 2003;157(12):1197–201. doi: 10.1001/archpedi.157.12.1197. Epub 2003/12/10. [DOI] [PubMed] [Google Scholar]
- [79].Baccarelli A, Martinelli I, Zanobetti A, Grillo P, Hou L-F, Bertazzi PA, et al. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch Intern Med. 2008;168(9):920–7. doi: 10.1001/archinte.168.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Di Minno G, Mannucci PM, Tufano A, Palareti G, Moia M, Baccaglini U, et al. The first ambulatory screening on thromboembolism: a multicentre, cross-sectional, observational study on risk factors for venous thromboembolism. J Thromb Haemost. 2005;3(7):1459–66. doi: 10.1111/j.1538-7836.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- [81].Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138(1):3–6. doi: 10.1378/chest.10-0645. [DOI] [PubMed] [Google Scholar]
- [82].Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- [83].Caballero A, Torres-Duque CA, Jaramillo C, Bolívar F, Sanabria F, Osorio P, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study) Chest. 2008;133(2):343–9. doi: 10.1378/chest.07-1361. [DOI] [PubMed] [Google Scholar]
- [84].Zhang JJ, Smith KR. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environ Health Perspect. 2007;115(6):848–55. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, et al. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J. 1998;11(3):554–9. [PubMed] [Google Scholar]
- [86].Restrepo J, Reyes P, de Ochoa EP. Neumoconiosis por inhalacion del humo de lena. Acta Medica Colombiana. 1983;8(4):191–204. [Google Scholar]
- [87].Sandoval J, Salas J, Martinez-Guerra ML, Gómez A, Martinez C, Portales A, et al. Pulmonary arterial hypertension and cor pulmonale associated with chronic domestic woodsmoke inhalation. Chest. 1993;103(1):12–20. doi: 10.1378/chest.103.1.12. [DOI] [PubMed] [Google Scholar]
- [88].Gold JA, Jagirdar J, Hay JG, Addrizzo-Harris DJ, Naidich DP, Rom WN. Hut lung. A domestically acquired particulate lung disease. Medicine (Baltimore) 2000;79(5):310–7. doi: 10.1097/00005792-200009000-00004. [DOI] [PubMed] [Google Scholar]
- [89].Hassan G, Qureshi W, Kadri SM, Khan GQ, Sona-Ul-Lah, Rather RA, et al. Gujjar lung: a disease mimicking miliary tuberculosis. Int J Health Sci (Qassim) 2008;2(1):105–8. [PMC free article] [PubMed] [Google Scholar]
- [90].Opotowsky AR, Vedanthan R, Mamlin JJ. A case report of cor pulmonale in a woman without exposure to tobacco smoke: an example of the risks of indoor wood burning. Medscape J Med. 2008;10(1):22. [PMC free article] [PubMed] [Google Scholar]
- [91].Pandey MR. History of Household Smoke Pollution and Chronic Cor Pulmonale. Global Heart. 2012;7 doi: 10.1016/j.gheart.2012.06.011. [DOI] [PubMed] [Google Scholar]
- [92].Moran-Mendoza O, Pérez-Padilla JR, Salazar-Flores M, Vazquez-Alfaro F. Wood smokeassociated lung disease: a clinical, functional, radiological and pathological description. Int J Tuberc Lung Dis. 2008;12(9):1092–8. [PubMed] [Google Scholar]
- [93].Padmavati S, Pathak SN. Chronic cor pulmonale in Delhi: a study of 127 cases. Circulation. 1959;20:343–52. doi: 10.1161/01.cir.20.3.343. Epub 1959/09/01. [DOI] [PubMed] [Google Scholar]
- [94].Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674(1-2):45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [95].Thomson E, Goegan P, Kumarathasan P, Vincent R. Air pollutants increase gene expression of the vasoconstrictor endothelin-1 in the lungs. Biochim Biophys Acta. 2004;1689(1):75–82. doi: 10.1016/j.bbadis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [96].Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, Franco-Lira M, Henríquez-Roldán C, Barragán-Mejía G, et al. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect. 2007;115(8):1248–53. doi: 10.1289/ehp.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].McCracken JP, Smith KR, Díaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect. 2007;115(7):996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Dutta A, Mukherjee B, Das D, Banerjee A, Ray MR. Hypertension with elevated levels of oxidized low-density lipoprotein and anticardiolipin antibody in the circulation of premenopausal Indian women chronically exposed to biomass smoke during cooking. Indoor Air. 2011;21(2):165–76. doi: 10.1111/j.1600-0668.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- [99].Clark ML, Bazemore H, Reynolds SJ, Heiderscheidt JM, Conway S, Bachand AM, et al. A baseline evaluation of traditional cook stove smoke exposures and indicators of cardiovascular and respiratory health among Nicaraguan women. Int J Occup Environ Health. 2011;17(2):113–21. doi: 10.1179/107735211799030942. [DOI] [PubMed] [Google Scholar]
- [100].Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, et al. Indoor air pollution and blood pressure in adult women living in rural China. Environ Health Perspect. 2011;119(10):1390–5. doi: 10.1289/ehp.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]