Abstract
Agonists of a single G protein-coupled receptor (GPCR) may activate distinct signaling pathways. Functional selectivity, an emerging concept with therapeutic relevance for GPCRs, may be due to conformational selection or stabilization with respect to particular agonists, receptor dimerization, variable expression levels of GPCRs and downstream signaling molecules, and allosteric modulation. Allosteric modulators may have potential advantages over orthosteric ligands, including greater selectivity and safety. This review focuses on functional selectivity resulting from allosteric modulation.
Keywords: allosteric modulation, functional selectivity, GPCR, adenosine receptor, muscarinic receptor, metabotropic glutamate receptor, calcium sensing receptor, CCR5
Introduction
G protein-coupled receptors (GPCRs), also known as 7TM (seven transmembrane helical) receptors, represent ~4% of the human genome and are the largest class of druggable targets. Allosteric modulators of GPCRs may have a number of potential advantages over traditional orthosteric ligands (binding to the same site as the native agonist), including selectivity and pharmacokinetic properties [1-3]. The orthosteric agonist binding sites of many GPCRs are highly conserved across family members, making selectivity for a particular receptor subtype within one family hard to achieve. For example, selective orthosteric ligands of muscarinic receptors have been elusive, but selective modulators are now being developed successfully by targeting the less conserved allosteric sites [4]. Additionally, many natural ligands of GPCRs (e.g. mGlu, GLP-1 and P2Y receptors) are small amino acids, peptides and nucleotides, which have limitations in their pharmacokinetic properties; thus, their further pharmaceutical development is limited. Also, allosteric modulators have the capability to provide a more physiologically relevant effect by tuning receptor function only in the presence of the endogenous ligand. Positive allosteric modulators (PAMs) may also avoid the receptor desensitization and downregulation that commonly occur after chronic treatment with an orthosteric agonist. Allosteric modulators function by exerting cooperativity with orthosteric agonists to achieve a potential ‘ceiling effect’, which may avoid side effects due to overdose [1]. Recent studies suggest that allosteric modulators also induce functional selectivity. Allosteric agonists or positive allosteric modulators (PAMs) may selectively activate or enhance certain signaling pathways due to the stabilization of certain receptor conformations. Allosteric antagonists or negative allosteric modulators may selectively block some downstream signaling pathways, but not others induced by the same endogenous or orthosteric agonist. The present review will cover the functional selectivity induced by allosteric modulators and will briefly recapitulate the novel pharmacological methodology related to this topic.
Functional selectivity induced by PAMs and allosteric agonists
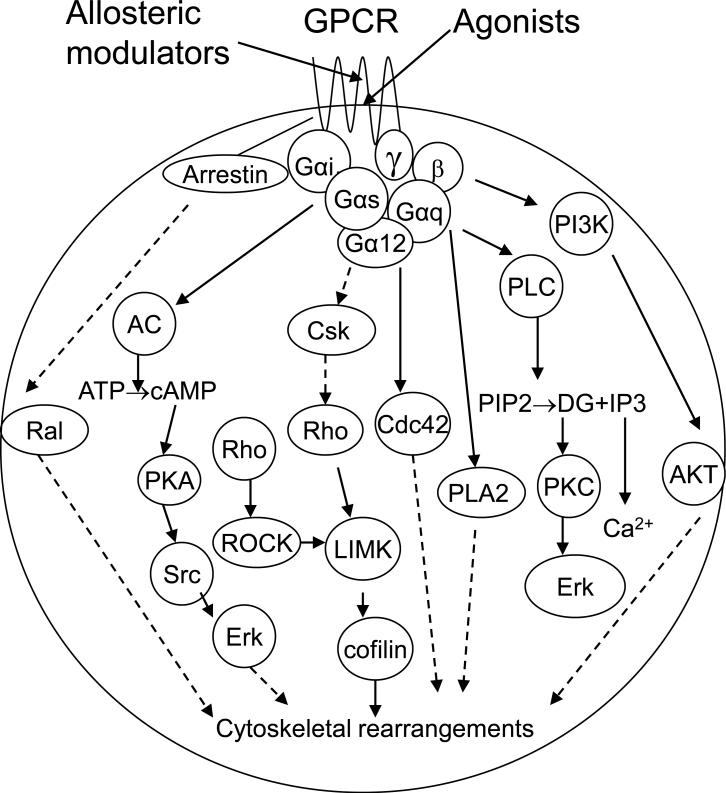
Recently, an increasing number of studies demonstrate that different agonists for a certain GPCR can activate distinct downstream signaling pathways, a phenomenon termed functional selectivity, which is presumably due to the receptor conformational stabilization or selection for a specific agonist [1]. In other words, a specific agonist activating one pathway over others via the same receptor can also be called biased agonism [5]. Functional selectivity or biased agonism has been reported among different G proteins and between G proteins and arrestins or other accessory signaling molecules (Figure 1).
Figure 1.
A generalized model of the signaling from a GPCR to the actin cytoskeleton. Not all of these pathways are present in each cell.
Allosteric modulators may induce specific receptor conformational changes with functional implications. Therefore, PAMs and allosteric agonists (that activate the receptor in the absence of an orthosteric agonist) may activate only certain specific signaling pathways to provide a basis for functional selectivity. In the following sections, we will discuss functional selectivity that occurs mainly due to positive allosteric modulators and allosteric agonists, although in some cases functional selectivity of orthosteric agonists is also mentioned if they are closely related to this topic.
Adenosine receptors
Functional selectivity at the four subtypes of adenosine receptors (AR) has been recently reviewed [6]. Functional selectivity at the A1 AR has been reported for both orthosteric and allosteric agonists [7,8]. In CHO cells expressing the A1 AR, inhibition or potentiation of forskolin-stimulated cyclic AMP accumulation was used as readout for activation of Gαi and Gαs (in pertussis toxin (PTX)-treated cells), respectively. Stimulation of inositol phosphates production in PTX-treated cells was used as an index of Gαq activation. Cordeaux et al. (2004) found that the orthosteric agonist CHA activated Gαi, Gαs and Gαq proteins, but was more efficacious than CPA in activating the Gαs protein. CPeCA produced only a small stimulation (at 100 μM) at Gαq, but was a full agonist (compared with CPA) at Gαi and Gαs proteins. NECA has higher efficacy than CPA and R-PIA for Gαq and Gαs [7].
Valant et al. [8] screened a series of novel 2-amino-3-benzoylthiophenes using a functional assay of A1 AR -mediated phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) in CHO cells to identify allosteric agonists and allosteric modulators of the A1 AR. Two derivatives differing only in an electron-withdrawing group on the benzoyl moiety, were identified as biased allosteric agonists and PAMs at the A1 AR in two different functional assays. The authors suggested that subtle structural variations of that series of compounds can promote functionally distinct receptor conformational states. The potential functional selective property of allosteric modulators has also been explored in another series of compounds [9]. Many of those modulators are partial agonists in the ERK1/2 activation but are inactive in the calcium mobilization assays. Interestingly, the orthosteric agonist R-PIA was more potent in ERK1/2 activation than in inhibition of cyclic AMP accumulation, whereas the opposite was true for partial allosteric agonists, suggesting orthosteric agonists and allosteric agonists stabilize receptor conformations differently.
In the case of the A3 AR, both functionally selective (or biased) agonism [10] and functionally selective allosteric modulation [11] have been reported. Translocation of arrestin induced by A3 AR ligands in an engineered cell line expressing both the A3 AR and β-arrestin-2 was used for the comparison to cyclic AMP signaling pathway [10]. MRS542, MRS1760, and other adenosine derivatives, A3 AR antagonists in cyclic AMP assays, were found to be partial agonists in β-arrestin translocation, indicating biased agonism. The xanthine 7-riboside DBXRM, a full A3 AR agonist in the cyclic AMP pathway, was only partially efficacious in β-arrestin translocation. Non-nucleoside antagonists showed similar inhibitory potencies in these two signaling pathways.
In an assay of guanine nucleotide ([35S]GTPγS) binding to membranes from CHO cell expressing the A3 AR, a PAM of the A3 AR, LUF6000, was found to enhance the Emax of structurally diverse agonists, being more effective for low-Emax agonists than for high-Emax agonists. LUF6000 was demonstrated to convert an antagonist into an agonist [43], which represents the first example in GPCRs.
In a follow-up study, the agonist enhancing effects of LUF6000 in different signaling pathways were compared [11]. It is found that LUF6000 behaves differently in various signaling pathways. In the assay of cyclic AMP accumulation, LUF6000 enhanced the efficacy of all agonists examined, but in the membrane hyperpolarization assay, it only enhanced the efficacy of partial agonists. In calcium mobilization, LUF6000 did not affect the efficacy of the full agonist NECA but was able to switch the nucleoside antagonist MRS542 into a partial agonist. In translocation of β-arrestin2, the agonist-enhancing effect LUF6000 was not pronounced. In an assay of ERK1/2 phosphorylation LUF6000 did not show any effect on the efficacy of the A3 AR agonist Cl-IB-MECA. The fact that LUF6000 converts an antagonist into an agonist in the [35S]GTPγS binding assay [10] but not in the cyclic AMP pathway [11] indicates biased allosteric modulation, presumably due to the stabilization of the A3 AR by LUF6000 in a fixed conformation. The differential effects of LUF6000 on the efficacy and potency of the agonist Cl-IBMECA in various signaling pathway are interpreted quantitatively using a mathematical model [11]. Modeling results indicate that the intrinsic efficacy of orthosteric ligands is closely related to the enhancing effect of LUF6000.
In a recent study, Du et al. [12] found that another PAM of the A3 AR, LUF6096, was able to protect myocardial ischemia/reperfusion injury by enhancing the effect of the endogenous agonist adenosine in vivo. In vitro studies show that LUF6096 was found to exert potent enhancing activity with the canine A3 AR in a [35S]GTPγS binding assay. LUF6096 increased the maximal efficacy of Cl-IB-MECA and the native agonist adenosine more than 2-fold. In the dog studies, administration of LUF6096 had no effect on any hemodynamic parameter measured, but produced a marked reduction in infarct size (50% reduction) compared with vehicle-treated dogs, which represents the first study to demonstrate efficacy of an A3 AR allosteric enhancer in an in vivo model of infarction [12]. The study suggests that A3 AR enhancers may lack effects on their own under normal physiological conditions; however, they may enhance the cardioprotective effects under stress conditions.
Muscarinic receptors
A growing number of allosteric agonists including clozapine, N-desmethylclozapine, AC-42 and 77-LH-28-1 were found to have a profile of receptor activation different from those traditional orthosteric agonists such as acetylcholine (ACh) and carbachol [13,14].
Avlani et al. [15] explored the mechanism of action of two novel agonists, 77-LH-28-1 and AC-42, at the wild-type M1 mAChR and three mutant M1 mAChRs. It was found that both agonists inhibited the binding of the orthosteric antagonist [3H]NMS in a manner consistent with orthosteric competition or high negative cooperativity. Functional interaction studies between 77-LH-28-1 and ACh also indicated a competitive mechanism. Dissociation kinetics assays revealed that the agonists could bind allosterically. Mutation of the key orthosteric site residues Y381A and W101A reduced the affinity of orthosteric agonists but increased the affinity of these two novel agonists. Divergent effects were also noted on agonist signaling efficacies at these mutants. The authors suggested that a possible “bitopic” binding mode may best explain the mechanism of action 77-LH-28-1 and AC-42.
Thomas et al [16] have investigated the signaling pathways activated by allosteric agonists AC-42, its analog 77-LH-28-1, and a range of orthosteric agonists oxotremorine-M (oxo-M), arecoline, and pilocarpine in CHO cells expressing the human M1 AChRs. The authors found that each agonist was able to activate Gαq/11-dependent signaling, as demonstrated by an increase in [35S]GTPγS binding to Gαq/11 proteins and total [3H]inositol phosphate accumulation assays in intact cells. All three orthosteric agonists caused significant enhancements in [35S]GTPγS binding to Gαi1/2 subunits over basal, whereas allosteric agonist did not produce significant response. However, both orthosteric and allosteric agonists are able to enhance forskolin-stimulated cAMP accumulation. The results suggest that both allosteric and orthosteric agonists stabilize receptor conformations associated with Gαq/11- and Gαs-dependent signaling. Allosteric agonists do not seem to promote M1 AChR-Gαi1/2 coupling, indicating that allosteric agonists have the potential to activate distinct downstream effectors to produce functional selectivity [16].
Butcher et al. [17], using tryptic phosphopeptide maps, mass spectrometry, and phospho-specific antibodies, determined that the prototypical Gαq/11-coupled M3 AChR was differentially phosphorylated in various cell and tissue types supporting a role for differential receptor phosphorylation in directing tissue-specific signaling. The phosphorylation profile of the M3 AChR was also dependent on the agonists used. Full and partial agonists of the receptor were observed to direct phosphorylation preferentially to specific sites. The authors propose that capacity of ligands to promote receptor phosphorylation at specific sites may be the result of functional selectivity of these agonists [17]. It should be interesting to further explore the differences between orthosteric agonists and allosteric agonists in this aspect.
Stewart et al. [18] probed for G protein-biased allosteric modulation using a yeast assay system. It is revealed that brucine was a partial allosteric agonist and positive modulator of carbachol when coupled to Gpa1/Gαq proteins, a positive modulator (no agonism) when coupled to Gpa1/Gα12 proteins, and a neutral modulator when coupled to Gpa1/Gαi proteins. These results were validated in a mammalian CHO cell background by determination of calcium mobilization and membrane ruffling as surrogate measures of activation of Gαq and G12 proteins, respectively. The authors indicated that the yeast platform could be used to identify functional selectivity of allosteric ligands and to facilitate dissection of convergent signaling pathways.
Recently, Nakajima and Wess [19] reported that a weak allosteric modulator CNO at the a reengineered M3 AChR was able to produce arrestin-biased signaling in this mutant M3 AChR and to induce insulin-release in MIN-6 pancreatic β-cells expressing the reengineered M3 AChR.
mGlu receptors
Recent data revealed that mGlu allosteric modulators tend to produce functional selectivity toward particular signal transduction cascades downstream of an individual mGlu subtype [20].
The mGlu4 is predominantly coupled to Gαi/o proteins. Yin et al. [21] recently reported that histamine induces substantial glutamate-activated calcium mobilization in mGlu4-expressing cells, an effect that is observed in the absence of co-expressed chimeric G proteins. The induction of calcium signaling depends upon the presence of H1 histamine receptors. Interestingly, the potentiating effect of histamine activation does not extend to other mGlu4-mediated signaling events downstream of Gαi/o proteins, such as cyclic AMP inhibition, suggesting that the presence of Gαq-coupled receptors such as the H1 histamine receptor may bias normal mGlu4-mediated Gαi/o signaling events. When the activity induced by small molecule PAMs of mGlu4 is assessed, the potentiated signaling of mGlu4 is further biased by histamine toward calcium-dependent pathways. These results suggest that Gαi/o-coupled mGlus may induce substantial, and potentially unexpected, calcium-mediated signaling events if stimulation occurs concomitantly with activation of Gq-coupled receptors. The authors also suggest that signaling induced by small molecule PAMs may be substantially biased when Gq-coupled receptors are activated.
Allosteric modulators of the mGlu5R have emerged as a novel approach for the treatment of schizophrenia and other central nervous system (CNS) disorders. A number of allosteric modulators were found in the recent years [22]. Similar to the allosteric modulators for the A1 AR, some mGlu5 PAMs are allosteric agonists in addition to their modulatory property. However, Noetzel et al. [23] recently suggested that the level of receptor expression influences the ability of mGlu5 PAMs to act as allosteric agonists in vitro and that allosteric agonist activity observed in cell-based assays may not be important for in vivo efficacy. Thus, the positive allosteric modulatory property seems play a major role in vivo. This may also suggest that other PAMs might activate the receptors directly under certain conditions, especially when the receptors or G proteins and signaling molecules are highly expressed. Interestingly, a number of mGlu5 PAMs were found to enhance agonist potency without affecting agonist binding affinity [2].
Functional selective antagonism
In addition to functional selective agonism, there is increasing number of reports on functional selective antagonism [24]. Allosteric antagonists may produce functionally selective antagonism by binding to the allosteric site on the receptor and allow orthosteric agonists to bind to the same receptor in the mean time. This can produce inhibition of some down-stream signaling pathways, leaving others unblocked. Such effects are referred to as functional selective or biased antagonism.
Muniz-Medina et al. [25] compared the effects six allosteric inhibitors of the chemokine receptor 5 (CCR5) as inhibitors of HIV-1 entry and antagonists of internalization of CCR5 mediated by chemokine (C-C motif) ligand 3-like 1 [CCL3L1]. These modulators produced 58-fold (HOS cells) and 282-fold (PBMC) difference in relative potency for blockade of CCL3L1-mediated internalization versus HIV-1 entry. Thus, allosteric inhibitors of the CCR5 receptor have the potential of functional selective antagonism related to HIV-1 entry and CCR5 internalization.
EMMCA, an allosteric antagonist of the chemoattractant receptor-homologous molecule on T helper 2 cells (CRTH2), is inactive against prostaglandin D2-induced G protein-mediated signaling pathways but is a potent antagonist of β-arrestin coupling to the same receptor [26].
The calcimimetic, Cinacalcet, for calcium-sensing receptor (CaSR) has proven clinically useful in the treatment of chronic kidney disease patients with secondary hyperparathyroidism. Davey et al. [27] recently determined the effects of the calcimimetics, NPS-R568 or cinacalcet, and the calcilytic, NPS-2143, on Cao2+-mediated intracellular Ca2+ mobilization, ERK1/2 phosphorylation, and plasma membrane ruffling in a stably transfected HEK293-TREx c-myc-CaSR cell line. The authors found the generation of stimulus bias by both positive and negative allosteric modulators of the CaSR, manifested as greater allosteric modulation of intracellular Ca2+ mobilization relative to ERK1/2 phosphorylation, and a higher affinity of the modulators for the state of the CaSR mediating plasma membrane ruffling relative to the other two pathways.
The negative allosteric modulator, CPPHA, was shown to have different effects on calcium mobilization and ERK1/2 phosphorylation, although both signaling pathways were coupled to the mGlu5 receptor [2]. Another negative allosteric modulator MPEP only produced partial inhibition on mGlu5-mediated signaling [2].
Fay and Farrens [28] recently showed that an allosteric modulator of the CB1 receptor, Org 27569, which increases agonist binding but blocks agonist-induced CB1 signaling, was able to stabilize the CB1 receptor at a unique conformation and thus producing an intriguing effect on agonist binding and activation.
Selected examples of allosteric modulation-related functional selective agonism and functional selective antagonism are summarized in Table 1.
Table 1.
Selected examples of functional selectivity induced by allosteric modulators
| Receptor | Modulator | Bias | Reference |
|---|---|---|---|
| Allosteric agonists and positive allosteric modulators | |||
| A1AR | R-PIA (orthorsteric)a | ERK1/2 vs. cAMP | [9] |
| A1AR | allosteric agonists | cAMP vs. ERK1/2 | [9] |
| M1 AChR | arecoline (orthosteric)a | GTPγS and cAMP | [16] |
| M1 AChR | allosteric agonists | cAMP but not GTPγS | [16] |
| M3 AChRb | CNO | arrestin vs. G protein | [19] |
| A3AR | LUF6000 | GTPγS binding, | [43] |
| cAMP, Ca, MP, ERK1/2, arrestin | [11] | ||
| mGlu5 | DFB | Potency vs. affinity | [2] |
| Allosteric antagonists or negative allosteric modulators | |||
| mGlu5 | CPPHA | Ca vs. ERK1/2 | [2] |
| mGlu5 | MPEP | partial inhibition | [2] |
| CCR5 | TAK652 | HIV entry vs. internal. | [25] |
| CRTH2R | EMMCA | Arrestin vs. G protein | [25] |
| CaSR | NPS-2143 | Ca vs. ERK1/2 | [27] |
| CB1 | Org27569 | binding vs. signaling | [28] |
These two orthosteric agonists were included in order to compare with allosteric agonists. Ca, calcium mobilization; MP, membrane hyperpolarization; Internal., Internalization
reengineered M3 AChR
Considering the complexity of GPCR signaling and the recently emerged concepts such as allosteric modulation, probe dependence and functional selectivity, it is important to develop assays that are probe- and pathway- independent for screening purpose. However, once the desired molecules are discovered, it should be desirable to test using relatively novel pharmacological assays to dissect their effects on various major signaling pathways.
Novel technologies related to the study of allosteric modulation and functional selectivity
To identify and classify novel drug effects at GPCRs, the key is the reliable pharmacological assays. Radioligand binding has been traditionally the principal assay for ligand-GPCR binding studies. A number of non-radioactive assays, including fluorescent polarization (FP) [29], TR-FRET [30] and Surface Plasmon Resonance assays (SPR) [31] are now available. Flow cytometry-based fluorescent ligand binding assays have also been applied to receptor binding in intact cells [32], the throughput of which can now be increased by installing a 96-well autosampler.
Traditional functional assays to analyze GPCR-mediated signaling pathways mainly rely on cyclic AMP assays based on protein binding methods for Gαi- or Gαs-coupled receptors, and measurement of inositol phosphates using anion-exchange column chromatography or calcium mobilization using FLIPR for Gαq/11-coupled receptor [33]. A number highly improved functional GPCR assays developed in the past few years for more systematic measurement of various signaling pathways include Amplified Luminescent Proximity Homogeneous Assay (Alpha, Perkin Elmer), Homogeneous Time Resolved Fluorescence (HTRF) technology (Cisbio), arrestin translocation assays (DiscoveRx; Life Technologies) and Label-Free assays, etc. (Table 2).
Table 2.
Selected pharmacological assays for screening or studying allosteric modulation and functional selectivity.
| Measurement | Method or Product | application | reference |
|---|---|---|---|
| Ligand-receptor binding or interactions | FP | membr. prep. | [29] |
| TR-FRET | cells | [30] | |
| SPR | solubil. receptors | [31] | |
| FACS | cells | [32] | |
| Receptor activation & signaling | HTRF (IP-One) | Gq (or some Gi) | [35] |
| FLIPR | Gq (or some Gi) | [33] | |
| Alpha (cAMP) | Gs or Gi | [34] | |
| TR-FRET (cAMP) | Gs or Gi | [30] | |
| PathHunter | arrestin | [36] | |
| Tango | arrestin | [37] | |
| Optical based label-free (BIND, EPIC, Enspire) cytoskeleton | [38] | ||
| Impedance based label-free (ECIS, xCELLigene cytoskeleton CellKey) | [38] | ||
The luminescence-based AlphaScreen is a sensitive and homogeneous assay that allows the screening of a large range of GPCR signaling pathways. Every AlphaScreen assay contains two bead types, donor beads and acceptor beads. The proximity-dependent energy transfer is the basis for AlphaScreen's homogeneous nature. Proximity of acceptor to donor beads depends on the biological interaction of the molecules bound to them. The most common AlphaScreen assay is constructed by capturing one binding partner onto the donor beads and the other partner onto the acceptor beads. When the partners interact, energy is transferred from donor to acceptor beads and a signal is produced. The Alpha-based measurements of signaling molecules, such as cyclic AMP and ERK1/2, have been widely used in the GPCR field [34].
HTRF combines fluorescence resonance energy transfer (FRET) with time-resolved measurement (TR). In TR-FRET assays, a signal is generated through fluorescent resonance energy transfer between two fluorophores, a donor and an acceptor, when in close proximity. Molecular interactions between biomolecules can be assessed by coupling each partner with a fluorescent label and by detecting the level of energy transfer. When two entities come close enough to each other, excitation of the donor by an energy source (e.g. a flash lamp or a laser) triggers an energy transfer towards the acceptor, which in turn emits specific fluorescence at a given wavelength. Europium cryptate or terbium cryptate is often used as a donor. The HTRF-based IP-one assay has been is available from Cisbio for the study of Gαq-coupled receptor. IP1 is a downstream metabolite of IP3, accumulates in cells following Gαq activation, making it an ideal functional assay for Gαq-coupled receptors [35].
DiscoveRx now offers a number of pre-validated arrestin GPCR assays for both known and orphan GPCRs. Because arrestin recruitment occurs independent of G-protein coupling, the luminescence-based PathHunter β-Arrestin assays offer a useful screening and profiling platform that can be used for many GPCRs [36]. The Tango™ arrestin ssay system for GPCRs is available from Life Technologies [37].
The emerging concepts in drug discovery, such as allosteric modulation and functional selectivity, create opportunities and possibilities for novel GPCR drugs. However, they also highlight some limitations of current GPCR screening assays. For example, some allosteric ligands selectively modulate receptor internalization or phosphorylation which may not be detected by many functional assays that only measure a single signaling pathway. The label-free assays, which can be used to detect very complex behavior of receptors, are mature to be applied to the study GPCR ligands, especially the screening and detection of allosteic modulators [24,38,39].
The biological basis for label-free measurement is the utilization of morphological and adhesive characteristics of cells as readout, which is physiological, noninvasive and unbiased [38,39]. One of the major determinants of cell morphology and adhesion is actin cytoskeleton, which serves as a scaffold for the organization and localization of various signaling molecules including receptors and downstream signaling effectors. Activation of different G proteins has been shown to lead to distinct actin cytoskeletal dynamics.
Schroder et al. [40] compared the optical sensor based DMR label-free technology with traditional second-messenger assays that are currently used in GPCR drug discovery and suggested that this label-free technique can: 1. probe GPCR functionality along all four G-protein signaling pathways, beyond reach of most other assay platforms; 2. dissect complex GPCR signaling patterns in primary human cells with unprecedented accuracy; 3. define heterotrimeric G proteins as triggers for the complex optical fingerprints; 4. disclose previously undetected features of GPCR behavior. The authors suggested that label-free technology will have a substantial impact on the discovery of drugs with novel mechanisms.
Dodgson et al. [41] have evaluated the optical biosensor-based Epic label-free system for the purpose of identifying antagonists of the muscarinic M3 receptor. The authors compared the data generated from the label-free technology with data for the same compounds in a calcium flux assay. It was shown that this technology can be used for high-throughput screening (HTS) of GPCRs. A number of compounds have been identified which were not found in a functional HTS measuring the output from a single pathway. Peters et al. [42] compared effects known agonists at three different GPCRs, M1, M2 and CRF1, and found three label-free techniques (Cellkey, BIND and Epic) produced similar results, indicating the reliability of these novel techniques.
Navratilova et al. [31] have developed a GPCR biosensor assay protocol that offers the opportunity for label-free HTS that directly measures GPCR-ligand interactions. The surface plasmon resonance-based direct screening method identifies the interaction of both orthosteric and allosteric ligands with solubilized, native GPCRs, in a label-free and cell-free environment, thus overcoming the limitations of the traditional assays. The authors exemplify the method by the discovery of novel ligands for the chemokine receptor, CCR5.
Conclusions
Allosteric modulators may have many advantages over traditional orthosteric drugs. A growing number of functional selective agonism and biased antagonism induced by allosteric modulators have been reported. The current drug discovery process needs to be modified to accommodate the emerging concepts in the GPCR fields, such as allosteric modulation, probe dependence and functional selectivity. Label-free technology seems to be a promising approach for the detection of GPCR behavior and screening of allosteric modulators. A number highly improved cellular assays such as AlphaScreen, HTRF, and arrestin GPCR assays have been developed in the last few years should be useful for dissecting signaling pathways.
Acknowledgements
Supported by the NIDDK Intramural Research Program, National Institutes of Health.
Abbreviations
- AC-42
4-n-butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl]-piperidine
- ACh
acetylcholine
- alpha
Amplified Luminescent Proximity Homogeneous Assay
- AR
adenosine receptor
- cyclic AMP
adenosine 3′,5′-cyclic phosphate
- CaSR
calcium-sensing receptor
- CCPA
2-chloro-N6-cyclopentyladenosine
- CHO
Chinese hamster ovary
- Cl-IB-MECA
2-chloro-N6-(3-iodobenzyl)-5′-N-methylcarboxamidoadenosine
- CNO
clozapine N-oxide
- CPA
N6-cyclopentyladenosine
- CPeCA
5′-N-cyclopentyl-carboxamidoadenosine
- CPPHA
DBXRM, 5’-N-methyl 1,3-dibutylxanthine 7-β-D-ribofuronamide
- DMR
dynamic mass redistribution
- EMMCA
1-(4-ethoxyphenyl)-5-methoxy-2-methylindole-3-carboxylic acid
- ERK
extracellular signal-regulated kinases
- FLIPR
Fluorometric Imaging Plate Reader
- FP
fluorescence polarization
- FRET
fluorescence resonance energy transfer
- GPCR
G protein-coupled receptor
- GTPγS
guanosine 5′-[γ-thio]triphosphate
- HEK
human embryonic kidney
- HOS
human osteosarcoma
- HTRF
Homogeneous Time Resolved Fluorescence
- HTS
high-throughput screening
- LUF6000
N-(3,4-dichloro-phenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine
- LUF6096
N-{2-[(3,4-dichlorophenyl)amino]quinolin-4-yl}cyclohexane-carboxamide
- MRS542
2-chloro-N6-(3-iodobenzyl)-adenosine
- MPEP
2-methyl-6-(phenylethynyl)-pyridine
- MRS1760
(1'S,2'R,3'S,4'R,5'S)-4’-{2-chloro-6-[(3-iodophenylmethyl)amino]purin-9-yl}-1-(hydroxymethyl)bicycle-[3.1.0]hexane-2,3-diol
- NAM
negative allosteric modulator
- NECA
5′-N-ethylcarboxamidoadenosine
- NMS
N-methylscopolamine
- NPSR568
2-chloro-N-[(1R)-1-(3-methoxyphenyl)ethyl]-benzenepropanamine
- NPS-2143
2-chloro-6-[(2R)-3-[[1,1-dimethyl-2-(2-naphthalenyl)ethyl]amino-2-hydroxypropoxy]benz-onitrile
- Org27569
5-chloro-3-ethyl-1H-indole-2-carboxylic acid [2-(4-piperidin-1-ylphenyl)ethyl]amide
- PAM
positive allosteric modulator
- PBMC
peripheral blood mononuclear cells
- PIA
N6-phenylisopropyladenosine
- SPR
Surface Plasmon Resonance
- TAK652
(5E)-8-[4-(2-butoxyethoxy)phenyl]-1,2,3,4-tetrahydro-1-(2-methylpropyl)-N-[4-[[(1-propyl-1H-imidazol-5-yl)methyl]sulfinyl]phenyl]-1-benzazocine-5-carboxamide
- 77-LH-28-1
1-[3-(4-Butyl-1-piperidinyl)propyl]-3,4-dihydro-2(1H)-quinolinone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- 1.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev. 2010;62(2):265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conn PJ, et al. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nature Reviews Drug Discovery. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao ZG, Jacobson KA. Keynote review: allosterism in membrane receptors. Drug Disc. Today. 2006;11:191–202. doi: 10.1016/S1359-6446(05)03689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conn PJ, et al. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol. Sci. 2009;30:148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajagopal S, et al. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80(3):367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verzijl D, IJzerman AP. Functional selectivity of adenosine receptor ligands. Purinergic Signal. 2011;7(2):171–192. doi: 10.1007/s11302-011-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordeaux Y, et al. Coupling of the human A1 adenosine receptor to different heterotrimeric G proteins: evidence for agonist-specific G protein activation. Br. J. Pharmacol. 2004;143(6):705–714. doi: 10.1038/sj.bjp.0705925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valant C, et al. Synthesis and characterization of novel 2-amino-3-benzoylthiophene derivatives as biased allosteric agonists and modulators of the adenosine A1 receptor. J Med Chem. 2012;55(5):2367–75. doi: 10.1021/jm201600e. [DOI] [PubMed] [Google Scholar]
- 9.Valant C, et al. Delineating the mode of action of adenosine A1 receptor allosteric modulators. Mol Pharmacol. 2010;78(3):444–455. doi: 10.1124/mol.110.064568. [DOI] [PubMed] [Google Scholar]
- 10.Gao ZG, Jacobson KA. Translocation of arrestin induced by human A(3) adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways. Pharmacol Res. 2008;57(4):3033–11. doi: 10.1016/j.phrs.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao ZG, et al. Functionally biased modulation of A3 adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem. Pharmacol. 2011;82(6):658–668. doi: 10.1016/j.bcp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du L, et al. Protection from ischemia/reperfusion injury by the positive allosteric modulator of the A3 adenosine receptor LUF6096. J. Pharm. Exp. Therap. 2012;340:210–7. doi: 10.1124/jpet.111.187559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson MA, et al. The M1 muscarinic receptor allosteric agonists AC-42 and 1-[1'-(2-methylbenzyl)-1,4'-bipiperidin-4-yl]-1,3-dihydro-2H-benzimidazol-2-one bind to a unique site distinct from the acetylcholine orthosteric site. Mol Pharmacol. 2010;78(4):648–657. doi: 10.1124/mol.110.065771. [DOI] [PubMed] [Google Scholar]
- 14.Spalding TA, et al. Structural requirements of transmembrane domain 3 for activation by the M1 muscarinic receptor agonists AC-42, AC-260584, clozapine, and N-desmethylclozapine: evidence for three distinct modes of receptor activation. Mol Pharmacol. 2006;70(6):1974–1983. doi: 10.1124/mol.106.024901. [DOI] [PubMed] [Google Scholar]
- 15.Avlani VA, et al. Orthosteric and allosteric modes of interaction of novel selective agonists of the M1 muscarinic acetylcholine receptor. Mol Pharmacol. 2010;78(1):94–104. doi: 10.1124/mol.110.064345. [DOI] [PubMed] [Google Scholar]
- 16.Thomas RL, et al. G protein coupling and signaling pathway activation by m1 muscarinic acetylcholine receptor orthosteric and allosteric agonists. J. Pharmacol. Exp. Ther. 2008;327(2):365–374. doi: 10.1124/jpet.108.141788. [DOI] [PubMed] [Google Scholar]
- 17.Butcher AJ, et al. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J. Biol. Chem. 2011;286(13):11506–18. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart GD, et al. Prediction of functionally selective allosteric interactions at an M3 muscarinic acetylcholine receptor mutant using Saccharomyces cerevisiae. Mol Pharmacol. 2010;78(2):205–14. doi: 10.1124/mol.110.064253. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima KI, Wess J. Design and Functional Characterization of a Novel, Arrestin-Biased Designer G Protein-Coupled Receptor. Mol Pharmacol. 2012 doi: 10.1124/mol.112.080358. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheffler DJ, et al. Allosteric modulation of metabotropic glutamate receptors. Adv Pharmacol. 2011;62:37–77. doi: 10.1016/B978-0-12-385952-5.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin S, et al. Functional selectivity induced by mGlu4 receptor positive allosteric modulation and concomitant activation of Gq coupled receptors. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.03.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stauffer SR. Progress toward Positive Allosteric Modulators of the Metabotropic Glutamate Receptor Subtype 5 (mGlu(5)). ACS Chem Neurosci. 2011;2(8):450–470. doi: 10.1021/cn2000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noetzel MJ, et al. Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol Pharmacol. 2012;81(2):120–133. doi: 10.1124/mol.111.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenakin TP. Cellular assays as portals to seven-transmembrane receptor-based drug discovery. Nat Rev Drug Discov. 2009;8(8):617–626. doi: 10.1038/nrd2838. [DOI] [PubMed] [Google Scholar]
- 25.Muniz-Medina VM, et al. The relative activity of “function sparing” HIV-1 entry inhibitors on viral entry and CCR5 internalization: is allosteric functional selectivity a valuable therapeutic property? Mol Pharmacol. 2009;75(3):490–501. doi: 10.1124/mol.108.052555. [DOI] [PubMed] [Google Scholar]
- 26.Mathiesen JM, et al. Identification of indole derivatives exclusively interfering with a G protein-independent signaling pathway of the prostaglandin D2 receptor CRTH2. Mol. Pharmacol. 2005;68:393–402. doi: 10.1124/mol.104.010520. [DOI] [PubMed] [Google Scholar]
- 27.Davey AE, et al. Positive and negative allosteric modulators promote biased signaling at the calcium-sensing receptor. Endocrinology. 2012;153(3):1232–41. doi: 10.1210/en.2011-1426. [DOI] [PubMed] [Google Scholar]
- 28.Fay JF, Farrens DL. A key agonist-induced conformational change in the cannabinoid receptor CB1 is blocked by the allosteric ligand Org 27569. J. Biol. Chem. 2012 doi: 10.1074/jbc.M112.352328. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kecskés M, et al. Novel Alexa Fluor-488 labeled antagonist of the A2A adenosine receptor: Application to a fluorescence polarization-based receptor binding assay. Biochem. Pharmacol. 2010;80:506–511. doi: 10.1016/j.bcp.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degorce F, et al. HTRF: A technology tailored for drug discovery - a review of theoretical aspects and recent applications. Curr. Chem. Genomics. 2009;3:22–32. doi: 10.2174/1875397300903010022. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navratilova I, et al. Screening for GPCR Ligands Using Surface Plasmon Resonance. ACS Med Chem Lett. 2011;2(7):549–554. doi: 10.1021/ml2000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozma E, et al. Novel fluorescent antagonist as a molecular probe in A3 adenosine receptor binding assays using flow cytometry. Biochem Pharmacol. 2012;83(11):1552–1261. doi: 10.1016/j.bcp.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlton SJ, Vauquelin G. Elusive equilibrium: the challenge of interpreting receptor pharmacology using calcium assays. Br J Pharmacol. 2010;161(6):1250–1265. doi: 10.1111/j.1476-5381.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eglen RM, et al. The use of AlphaScreen technology in HTS: current status. Curr Chem Genomics. 2008 2008 Feb 25;1:2–10. doi: 10.2174/1875397300801010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinquet E, et al. Monitoring Gq-coupled receptor response through inositol phosphate quantification with the IP-One assay. Expert Opin Drug Discov. 2011;6(10):981–94. doi: 10.1517/17460441.2011.608658. Epub 2011 Sep 8. [DOI] [PubMed] [Google Scholar]
- 36.Olson KR, Eglen RM. Beta galactosidase complementation: a cell-based luminescent assay platform for drug discovery. Assay Drug Dev. Technol. 2007;5(1):137–44. doi: 10.1089/adt.2006.052. [DOI] [PubMed] [Google Scholar]
- 37.Allen JA, et al. Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A. 2011;108(45):18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang Y. Label-Free Receptor Assays. Drug Discov Today Technol. 2011;7(1):e5–e11. doi: 10.1016/j.ddtec.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott CW, Peters MF. Label-free whole-cell assays: expanding the scope of GPCR screening. Drug Discov Today. 2010;15(17-18):704–716. doi: 10.1016/j.drudis.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Schröder R, et al. Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat. Biotechnol. 2010;28(9):943–949. doi: 10.1038/nbt.1671. [DOI] [PubMed] [Google Scholar]
- 41.Dodgson K, et al. 100K well screen for a muscarinic receptor using the Epic label-free system--a reflection on the benefits of the label-free approach to screening seven-transmembrane receptors. J Recept Signal Transduct Res. 2009;29(3-4):163–172. doi: 10.1080/10799890903079844. [DOI] [PubMed] [Google Scholar]
- 42.Peters MF, et al. Comparing label-free biosensors for pharmacological screening with cell-based functional assays. Assay Drug Dev Technol. 2010;8(2):219–27. doi: 10.1089/adt.2009.0232. [DOI] [PubMed] [Google Scholar]
- 43.Gao ZG, et al. Flexible modulation of agonist efficacy at the human A3 adenosine receptor by the imidazoquinoline allosteric enhancer LUF6000. BMC Pharmacol. 2008;8:20. doi: 10.1186/1471-2210-8-20. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]