Abstract
Lipoproteins are natural nanoparticles composed of phospholipids and apolipoproteins that transport lipids throughout the body. As key effectors of lipid homeostasis, the functions of lipoproteins have been demonstrated to be crucial during the development of cardiovascular diseases. Therefore various strategies have been used to study their biology and detect them in vivo. A recent approach has been the production of lipoprotein biomimetic particles loaded with diagnostically active nanocrystals in their core. These include, but are not limited to: quantum dots, iron oxide or gold nanocrystals. Inclusion of these nanocrystals enables the utilization of lipoproteins as probes for a variety of imaging modalities (computed tomography, magnetic resonance imaging, fluorescence) while preserving their biological activity. Furthermore as some lipoproteins naturally accumulate in atherosclerotic plaque or specific tumor tissues, nanocrystal core lipoprotein biomimetics have been developed as contrast agents for early diagnosis of these diseases.
Keywords: Lipoproteins, Quantum dots, Iron oxide nanoparticles, Gold nanoparticles, Atherosclerosis, Cancer
Introduction
Lipoproteins are a family of natural nanostructures whose surface is composed of a phospholipid layer, into which is embedded apolipoproteins and cholesterol. The main function of these lipoproteins has been shown to be the transport of lipids (mainly cholesterol and triglycerides) between organs and tissues through the circulatory system, thus contributing to the control of lipid homeostasis. Due to their key roles in lipid metabolism and cardiovascular diseases, new methodologies to investigate lipoprotein biology, for example with biomimetic systems that can be imaged and tracked, have attracted growing interest. As several lipoprotein fractions naturally target atherosclerotic plaques and tumors, further strategies have focused on the development of lipoprotein biomimetics for biomedical imaging strategies.
Various methods have been developed to incorporate labeling moieties into lipoprotein structures. One of the methods commonly employed is the incorporation of lipids labeled with radioisotopes [1, 2], paramagnetic chelates (magnetic resonance imaging, MRI) or fluorescent molecules [3-6]. However, as lipid exchange occurs naturally between lipoprotein fractions in the blood, labeling agents may be transferred to other lipoprotein fractions or cells, thereby complicating the interpretation of the data [7•]. An alternative strategy is to label the apolipoprotein portion of the lipoproteins with moieties such as radioisotopes [1, 2, 8]. In this review we focus on a recently developed third approach, which entails the inclusion of contrast-generating nanocrystals within the lipid core of the lipoprotein (Fig. 1a).
Fig. 1.
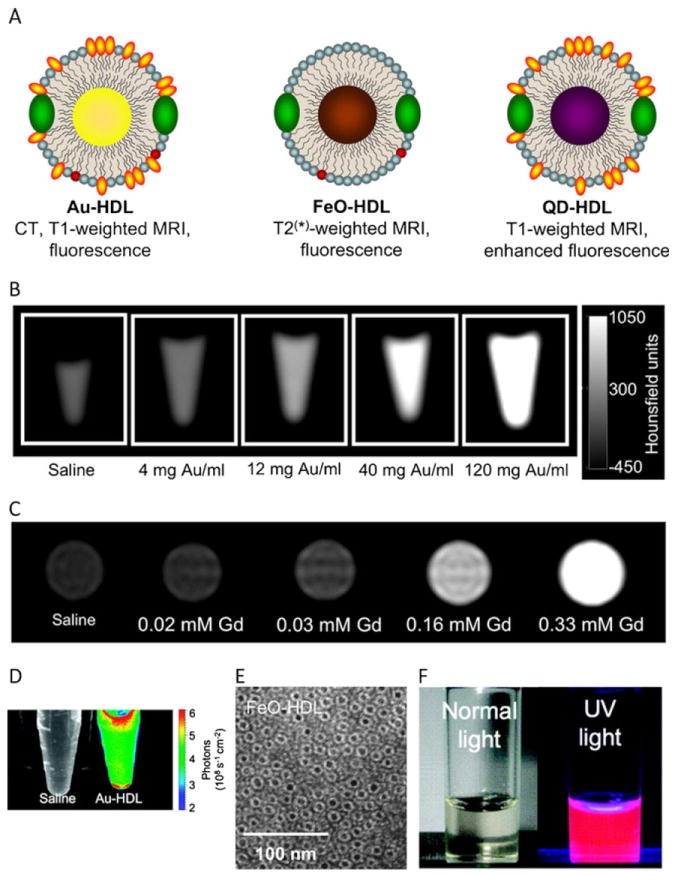
a Schematic depiction of nanocrystal core HDL biomimetics containing a gold, iron oxide and quantum dot core, respectively. b–d Phantoms of Gd-DTPA and fluorescently labeled Au-HDL imaged using (b) CT, (c) MRI and (d) optical imaging. e Negative stain TEM images of FeOHDL. f QD-HDL biomimetics emit a strong fluorescence signal under UV illumination. From reference [43•], adapted and used with permission
Lipoproteins
After extraction from blood, lipoproteins can be separated based on density, according to the method developed by Havel et al. [9], into five distinct fractions: high density lipoprotein (HDL; 7–13 nm size range), low density lipoprotein (LDL; 22–27 nm), intermediate density lipoprotein (IDL; 27–30 nm), very low density lipoprotein (VLDL; 35–80 nm) and chylomicron (80–1,200 nm). Each of these classes has a distinct lipid and apolipoprotein composition. Apolipoproteins, proteins present in lipoprotein nanoparticles, can be divided into two main subclasses based on their secondary structure. Apolipoproteins A, C, D and H are composed of an amphipathic α-helix and can be exchanged between lipoproteins, while apolipoprotein B contains a β-structure, which prevents such exchange [10]. As each apolipoprotein binds to specific cell membrane receptors and can trigger different pathways, it is believed that the apolipoprotein composition is the main determinant of lipoprotein function.
Chylomicrons, VLDL and IDL are triglyceride-rich lipoproteins secreted by the intestine to transport triglycerides to tissues and the liver. Apolipoprotein E (ApoE), which is present in the aforementioned lipoproteins as well as in HDL, has been shown to contribute to triglyceride homeostasis through binding to receptors present in peripheral tissues and liver cell membranes. It induces cellular uptake of lipoproteins and thereby facilitates triglyceride tissue delivery and hepatic removal [11]. As a consequence, ApoE deficiencies have been associated in both humans and animal models with hyperlipidemia and related diseases such as atherosclerosis [11, 12]. Additionally, a recent in vitro study has demonstrated ApoE’s capacity to drive macrophage plasticity towards antiinflammatory phenotypes [13].
Once in the bloodstream, VLDL triglycerides are hydrolyzed to fatty acids by lipoprotein lipases while remnants of VLDL are converted into LDL. Nearly 50 % of the weight of these LDL is from cholesterol esters. LDL is circulated to the peripheral tissues where it is internalized via LDL receptor-mediated endocytosis [14]. LDL can also cross the vascular endothelial cell barrier and accumulate within the intimal layer of the arterial walls where they are modified by free radical oxidation. Oxidized LDL interacts with the endothelial cells of arteries and induces surface expression of intercellular adhesion molecule 1 (ICAM-1/CD54) and vascular cell adhesion molecule 1 (VCAM-1/CD106) leading to monocyte recruitment [15]. Once in the arterial wall, monocytes can differentiate into macrophages. These invading macrophages can then engulf oxidized LDL and become foam cells that start producing inflammatory cytokines, which promote plaque development and, at advanced plaque stages, instability [16]. In addition to the atherosclerotic plaque, LDL has been shown to accumulate in certain tumors through direct binding to the LDL receptor. This receptor is overexpressed by a variety of cancers, including leukemia, and cervical and brain cancers [17, 18]. This high expression of the LDL receptor has been linked to an elevated need for cholesterol to synthesize plasma membranes as a result of the high proliferation rate of tumor cells.
HDL is synthesized by the liver and in the intestine as disk-shaped cholesterol-free complexes of phospholipids and primarily apolipoprotein A-1 (ApoA1). HDL has been shown to play a major role in lipid relocation and excretion [19], a function mediated by the interaction of ApoA1 with ATP binding cassette transporter A-1 (ABCA-1), ATP binding cassette transporter G-1 (ABCG-1) and Scavenger receptor class B member 1 which are expressed on cell membranes. Interactions between ApoA1 and ABCA-1/ABCG-1 trigger cholesterol efflux from the cells to the lipoprotein in a mechanism known as reverse cholesterol transport [20, 21]. Cholesterol-loaded HDL then migrates to the liver where the cholesterol is recycled or excreted by the bile [21]. In the bloodstream, HDL has been shown to interact with other lipoprotein fractions (VLDL/LDL) through lipid transfer [6]. High HDL blood concentrations exert a protective effect against atherosclerosis, which is thought to be due in part to its key role in cholesterol excretion from the body. Furthermore, HDL has been shown to naturally target atherosclerotic plaques, initiating reverse cholesterol transport from arterial wall to the liver. HDL’s presence in arterial plaques also causes antiinflammatory and antioxidative protective effects through inhibition of proinflammatory cytokines (tumor necrosis factor-alpha and interleukin 6), chemokines (monocyte chemotactic protein-1, MCP-1), and production of adhesion molecules (VCAM-1/ICAM-1) while inducing activation of endothelial nitric oxide synthase release [22, 23]. Using an ApoE–/– atherosclerosis mouse model, Nicholls and coworkers demonstrated that the infusion of reconstituted HDL induced a decrease in endothelial expression of VCAM-1, ICAM-1 and MCP-1 leading to a significant reduction of neutrophil recruitment [24].
There are several genetic conditions that cause dysfunctional apolipoprotein/lipoprotein function, leading to lipid accumulation in peripheral tissues and hyperlipidemia-related conditions. For example, Tangier disease has been characterized by defective ABCA-1 transporter expression which prevents cholesterol efflux between cells and HDL [25]. This leads to cholesterol accumulation in producing cells and symptoms such as eye abnormalities, enlarged spleen, liver and tonsils, as well as an increased risk of arteriosclerosis.
Nanocrystals
Gold Nanoparticles
The application of gold in medicine has been documented since the 1930s for the treatment of rheumatoid arthritis [26] and has been subsequently the subject of therapeutic and medical imaging strategies. Due to their high atomic number and extinction cross-section, gold nanoparticles (Au-NP) are attractive contrast agents for computed tomography (CT) [27] (Fig. 1b). Au-NP can also interact with visible / infrared light and produce contrast for a variety of optical imaging methods such as light scattering or Raman imaging, and can be detected at very low (10−16M) nanoparticle concentrations [28]. Another attractive phenomenon is the emission of heat by infrared-irradiated gold nanostructures, which can be used to selectively destroy surrounding tissue or trigger drug release [29].
Iron Oxide Nanoparticles
Iron oxide nanoparticles (FeO-NP), which refer mainly to superparamagnetic iron oxide nanoparticles, have been extensively used in medical applications as MRI contrast agents [30-32]. In the presence of a magnetic field, FeO-NP’s strong magnetic moment causes local signal dephasing on T2*-weighted images, resulting in signal voids and in an enhancement in image contrast. FeO-NP formulations are clinically used as contrast agents for liver imaging, and additional studies have investigated their potential use in cancer and cardiovascular disease with promising results [33]. In one study, FeO-NP were shown to accumulate in atherosclerotic plaques rich in macrophages when injected into patients with severe internal carotid artery stenosis. This accumulation leads to local reduction in T2*-weighted signals and enhancement of MRI contrast [34]. FeO-NP can also produce heat when placed in an oscillating magnetic field and can thus be used in thermal therapies [35].
Quantum Dots
Quantum dots (QD) are fluorescent semiconductor nanocrystals typically made of cadmium selenide covered by a zinc sulfide shell. QD exhibit broad excitation bands and narrow (20–30 nm) emission spectra, and are therefore attractive agents for biological imaging (Fig. 1f), especially for multiplex/multicolor purposes. A variety of emission wavelengths can be obtained by using different crystal sizes or compositions. In comparison with conventional molecular dyes, QD have longer fluorescence life-times, higher extinction coefficients and negligible photobleaching. QD have been extensively used as a tool for bioimaging procedures such as in vivo cell tracking [36], the visualization of biomarker expression [37] and membrane transport studies [38]. Furthermore, due to their narrow emission spectra and the ability to excite them at a well-separated absorption wavelength, QD have been shown to be very useful as Förster resonance energy transfer (FRET) donors [7•]. FRET is a process during which a donor fluorophore is first excited; the energy is then transferred to a neighboring acceptor fluorophore leading to the emission at the acceptor fluorophore’s characteristic wavelength. As the FRET signal is highly dependent on the distance between the donor and the acceptor fluorophores, this technique enables the precise measurement of binding or dissociation mechanisms [39].
Nanocrystal Lipoprotein Formulations
Various methods have been used to synthesize nanocrystal lipoproteins. A method developed by Thaxton and coworkers consists of a procedure where Au-NP is first mixed with modified ApoA1 (bearing sulfhydryl groups). Their association is facilitated by an electrostatic interaction between the two species. Those Au-NP-ApoA1 complexes are then added to a water/chloroform solution containing an excess of phospholipids. The mixture is subsequently heated to induce chloroform evaporation and lipid aggregation around the Au-NP-ApoA1 complexes to form nanocrystal core HDL [40•, 41, 42•]. An alternative method (Fig. 2a) consists of first dissolving nanocrystals (gold, iron oxide or QD) with an excess of phospholipids in a chloroform/methanol solvent solution. This mixture is then added dropwise to hot deionized water, which results in an instantaneous evaporation of chloroform leading to the formation of nanocrystal core micelles. The micelles are then incubated overnight with ApoA1, which naturally translocates embeds within the micelles to form nanocrystal core HDL and empty HDL. The resulting mixture is finally purified to obtain a homogeneous population of single core nanocrystal HDL [43•]. Alternatively, triglyceride-rich QD-NP and FeO-NP have been produced by mixing the nanocrystals with triglycerides, cholesterol and phospholipids extracted from human lipoproteins in an aqueous solution to form nanocrystal nano-some vesicles. After sonication and purification, these nano-somes are incubated with ApoE and lipoprotein lipase to obtain functional triglyceride-rich lipoproteins for in vitro studies [44•]. In vivo, the naked nanosomes were intravenously administered and suggested to be spontaneously decorated with functional apolipoproteins.
Fig. 2.
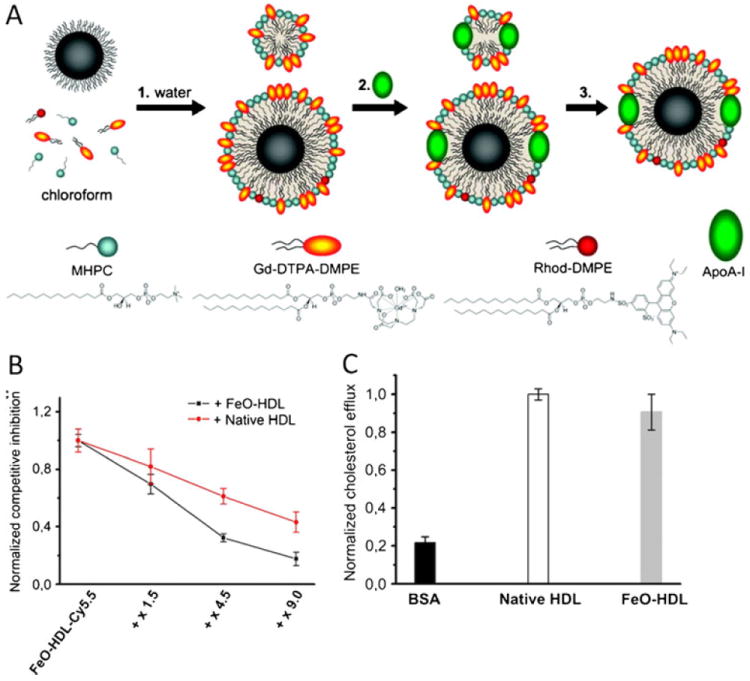
a Schematic depiction of the nanocrystal core HDL biomimetics formulation procedure developed by Cormode and co-workers. 1 The phospholipids and nanocrystals are solubilized in chloroform and infused dripped in hot water to form nanocrystal core micelles. 2 ApoA-I is then added to functionalize the micelles and produce nanocrystal core HDL particles. 3 Nanocrystal core HDL is subsequently purified by density gradient centrifugation. b Uptake of fluorescently labeled FeO-HDL by macrophages is inhibited by coincubation with native HDL or nonlabeled FeO-HDL, demonstrating that native HDL and nanocrystal core biomimetics show similar biological behavior. c Macrophage cholesterol efflux assay with BSA, native HDL and FeO-HDL showing that FeO-HDL is a potent cholesterol acceptor. a From reference [43•], adapted and used with permission; b, c from reference [46], adapted and used with permission
Nanocrystal Lipoprotein Characterization
Nanocrystal entrapment can be characterized using transmission electronic microscopy (TEM) or specific assays based on the nanocrystal characteristics such as fluorescence for QD [7•], x-ray attenuation for gold [45] and relaxation measurements for iron oxide [44•]. The HDL particle’s protein composition can be assessed using classic biochemistry assays such as bicinchoninic acid, Lowry colorimetric assays [42•, 45] or western blotting [44•]. Using fluorescent dyes or the imaging properties of nanocrystals, several studies have demonstrated that nanocrystal core lipoprotein bio-mimetics interact similarly to native lipoproteins with macrophages [42•, 43•]. In a competition assay, Skajaa and coworkers incubated macrophages with fluorescent FeO-HDL-Cy5.5 and various concentrations of native HDL. Addition of native HDL induced a decrease in the FeO-HDL-Cy5.5 fluorescence signal, indicative of decreased cell uptake, demonstrating that both particles compete for the same uptake pathway [46] (Fig. 2b).
Various assays have also been developed to show that nanocrystal HDL retain their native function as potent cholesterol acceptors. Using fluorescent cholesterol, Luthi and coworkers demonstrated that cholesterol binds to Au-HDL [41]. Subsequent experiments demonstrated that HDL bio-mimetics are able to induce cholesterol efflux and accept cholesterol from macrophages in vitro [41, 46] (Fig. 2c). Moreover, competition assays with macrophages that were incubated with both Au-HDL and native HDL demonstrated that both lipoproteins compete for cellular cholesterol and therefore operate through the same mechanism [41].
Lipoprotein Biology Studies Using Nanocrystal Core Biomimetics
As discussed above, lipoproteins are major factors in several diseases. Therefore, a number of studies have been performed using nanocrystal lipoprotein biomimetics to investigate lipoprotein biology, circulation dynamics and tissue–cell interactions. Skajaa and co-workers studied lipoprotein trafficking using FeO-NP-loaded HDL injected intravenously into mice [46]. Electronic microscopy examinations of tissue sections, made possible by the iron oxide core, demonstrated that HDL accumulated in the liver in Kupffer cells and hepatocytes localized around the bile canaliculi. FeO-HDL was also detected in bile and feces, revealing that HDL follows a similar excretion route to that of cholesterol. Bruns and coworkers studied the biology of triglyceride-rich lipoproteins using FeO-NP-loaded triglyceride nanosomes injected intravenously into mice. Using MRI, they were able to monitor in real-time FeO-NP biomimetic distribution and clearance from the bloodstream to the liver.
Quantitative monitoring of FeO-NP-labeled nanosome hepatic uptake in different mouse models (wild-type, ApoE–/– and LDL-receptor–/–) revealed a reduction in liver uptake in both knockout mice models, indicative of the importance of the interaction between the ApoE and LDL receptor in triglyceride-rich lipoprotein hepatic clearance mechanisms [44•]. In a subsequent study, Bartelt and coworkers used similar triglyceride-rich nanosomes labeled with 59Fe-FeO-NP and QD to assess the function of brown adipose tissue in triglyceride metabolism in mice [47]. Using gold nanocrystals as backbones, Luthi and coworkers produced a library of HDL with different sizes, lipid and protein compositions, and monitored the cholesterol binding and cholesterol efflux characteristics of their different HDL biomimetics. As expected, cholesterol efflux efficiency was found to be mainly influenced by ApoA1. The results also revealed that the surface curvature and phospholipid composition affect the cholesterol efflux characteristics [41].
A similar Au-HDL biomimetic platform was exploited by the same group to efficiently deliver antisense DNA sequences to cells expressing HDL receptors [42•]. Using the contrast properties of gold, transmission electron microscopy (TEM) data 16 hours after transfection revealed the presence of Au-HDL within the cell cytosol where antisense DNA is effective (Fig. 3a). Using QD-HDL biomimetics containing lipids conjugated with the near-infrared fluorophore Cy5.5, Skajaa and coworkers monitored HDL lipid exchange with cells and other lipoproteins by FRET analyses [7•]. Incubation of these QD-HDL-Cy5.5 with THP-1 macrophages led to a significant decrease in the Cy5.5:QD intensity ratio, indicative of a decrease in Cy5.5 lipids in the HDL corona and lipid exchange with the cells (Fig. 3b–d). In a second set of experiments, macrophages were first loaded with Cy5.5 lipids and subsequently incubated with Cy5.5-free QD-HDL. Fluorescence data revealed a time-dependent decrease in QD fluorescent intensity signal due to FRET-facilitated signal quenching, indicative of exchange of Cy5.5 lipids from the cells to the QD-HDL. This result confirms that lipid exchange between lipoproteins and cells is bidirectional. When the experiments were performed using QD micelles, i.e. lipid-coated QDs without ApoA1, the lipid exchange rates observed were significantly higher than with QD-HDL, indicative of the stabilizing function of ApoA1. While these results demonstrate the possibility of using nanocrystal-loaded lipoproteins as tools to study lipoprotein biology at both the molecular and macro scale, these biomimetics may also be used as contrast agents for imaging specific diseases in which lipoproteins are involved.
Fig. 3.
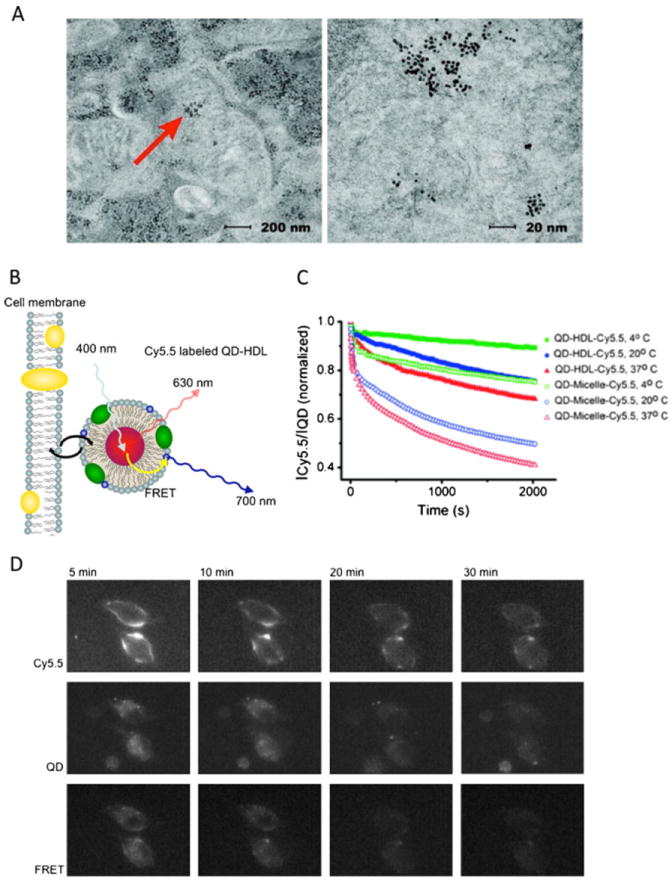
a TEM images of human prostate cancer cells transfected for 16 h with Au-HDL. Gold core enabled TEM localization of the lipoproteins within the cell cytosol (arrows Au-NPs). b Schematic depiction of lipid exchange analysis by FRET. As Cy5.5 lipids exchange from QD-HDL to the cell membrane, the FRET signal decreases. c Time evolution of FRET signal emitted by QD-HDL-Cy5.5 and QD-micelle-Cy5.5 incubated with macrophages at different temperatures. HDL lipid exchange rates are slower than the micelle exchange rates, indicating the stabilizing effect of ApoA1. d Fluorescence microscopy images of macrophages incubated with QD-HDL-Cy5.5. a From reference [42•], adapted and used with permission; b–d from reference [7•], adapted and used with permission
Nanocrystal Biomimetics for Medical Imaging
The development of diagnostic strategies that could lead to early treatment and better prognosis has been a major objective in cancer and atherosclerosis research [48•, 49, 50]. While the development of contrast or imaging agents has aided the detection of pathological sites, early detection of such sites remains a challenge.
Due to their natural affinity for tumors and the arterial plaque, lipoproteins have attracted increasing interest as contrast agent vectors [3, 5, 51-56]. Cormode and coworkers imaged the abdominal aortas of ApoE–/– mice (a model of atherosclerosis) before and 24 h after intravenous injection of nanocrystal HDL or nanocrystal PEG micelles [43•] (Fig. 4). As shown by MRI, administration of Au-HDL and QD-HDL labeled with gadolinium-labeled lipid to ApoE–/– mice led to significant brightening of the aortic wall on T1-weighted MR images. In animals injected with FeO-HDL, a decrease in signal was observed in T2*-weighted MR images of the vessel wall. As further ex vivo analyses revealed, while there was no significant fluorescent signal enhancement in animals injected with QD-PEG, a single injection of QD-HDL induced a significant fluorescent signal at plaque sites (Fig. 4b). Parallel experiments using gold nanocrystal particles showed a similar pattern on CT imaging. Collectively, these results demonstrate the efficacy of using nanocrystal HDL as atherosclerotic plaque contrast agents. In a follow-up study, the same Au-HDL nanoparticle platform was injected into ApoE–/– mice in combination with an iodine contrast agent. Using spectral CT (also known as multicolor CT), signals originating from gold, iodine and calcium-rich material were discriminated in a single scan. This methodology potentially allows the characterization of complex stenotic plaques that are composed of macrophages and are rich in calcifications within the coronary arteries [47].
Fig. 4.
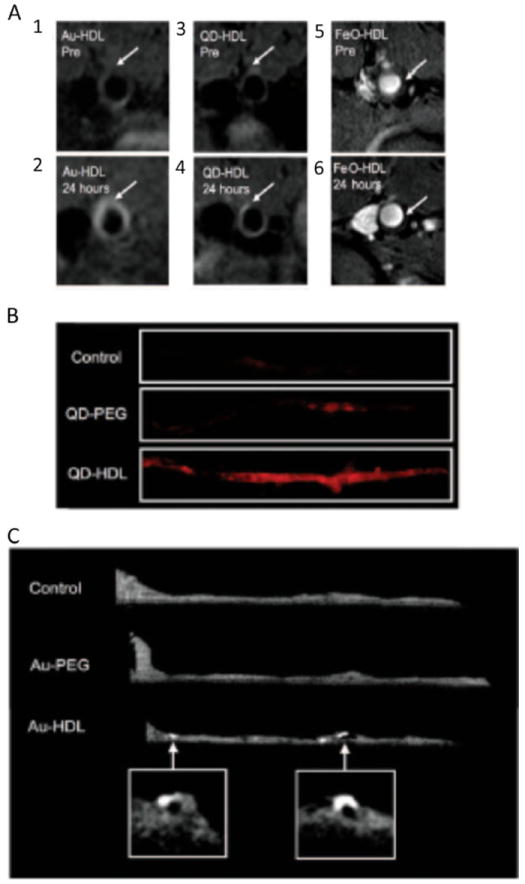
a T1-weighted MR images of the aortas of apoE–/– mice before (1, 3) and 24 h after (2, 4) injection of Au-HDL and QD-HDL, respectively, and T2*-weighted images of the aorta of an apoE–/– mouse before (5) and 24 h (6) after injection of FeO-HDL. The images acquired after injection show increased signal intensities in the aorta walls of animals injected with the Au-HDL and QD-HDL, and a clear decrease in the animal injected with FeO-HDL (arrows areas enhanced in the postinjection images). b Ex vivo fluorescence images of the aortas of mice injected with saline, QD-PEG or QD-HDL. The images reveal a much stronger signal in the aorta following QD-HDL injection than following saline or QD-PEG injection. c Ex vivo CT images of the aortas of mice injected with saline, Au-PEG or gold Au- HDL. The aortas of mice injected with Au-HDL exhibit bright spots, not observed in the aortas of mice injected with saline or Au-PEG, indicating HDL-dependent accumulation of the contrast agent in the aorta wall. Adapted and used with permission from [43•]
Conclusion
Nanocrystal core lipoprotein biomimetics have proved to be powerful tools to study and image lipoprotein biology and related diseases both in vitro and in small animal models. Owing to the variety of nanocrystals that can be incorporated into the lipoprotein core, a large spectrum of imaging techniques can be employed to visualize these HDL-based reporters. To date nanocrystal core lipoprotein production methods involve a succession of multiple independent steps, hampering the production of large batches. Therefore, before extensive in vivo preclinical studies can be performed, production processes need to be improved, scaled up and standardized in order to mass-produce well-defined and consistent lipoprotein biomimetics using good manufacturing practices.
Acknowledgments
This work was funded in part by National Heart, Lung, and Blood Institute (NHLBI) and the US National Institutes of Health (NIH), as a Program of Excellence in Nanotechnology (PEN) Award (Contract no. HHSN268201000045C), as well as by the NIH grants R00 EB012165 (D.P.C.), R01 EB009638 (Z.A.F.) and R01 CA155432 (W.J.M.M.).
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Francois Fay, Translational and Molecular Imaging, Institute, Mount Sinai School of Medicine, New York, NY, USA.
Brenda L. Sanchez-Gaytan, Translational and Molecular Imaging, Institute, Mount Sinai School of Medicine, New York, NY, USA
David P. Cormode, Department of Radiology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA
Torjus Skajaa, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Edward A. Fisher, Department of Medicine, Division of Cardiology, Marc and Ruti Bell Program in Vascular Biology and Disease, New York University School of Medicine, New York, NY, USA
Zahi A. Fayad, Translational and Molecular Imaging, Institute, Mount Sinai School of Medicine, New York, NY, USA
Willem J. M. Mulder, Translational and Molecular Imaging Institute, Mount Sinai School of Medicine, New York, NY, USA willem.mulder@mssm.edu Department of Vascular Medicine, Academic Medical Center, Amsterdam, The Netherlands.
References
Papers of particular interest, published recently, have been highlighted as follows:
Of importance
- 1.Singaraja RR, Stahmer B, Brundert M, et al. Hepatic ATP-binding cassette transporter A1 is a key molecule in high-density lipoprotein cholesteryl ester metabolism in mice. Arterioscler Thromb Vasc Biol. 2006;26:1821–7. doi: 10.1161/01.ATV.0000229219.13757.a2. [DOI] [PubMed] [Google Scholar]
- 2.Brundert M, Ewert A, Heeren J, et al. Scavenger receptor class B type I mediates the selective uptake of high-density lipoprotein-associated cholesteryl ester by the liver in mice. Arterioscler Thromb Vasc Biol. 2005;25:143–8. doi: 10.1161/01.ATV.0000149381.16166.c6. [DOI] [PubMed] [Google Scholar]
- 3.Cormode DP, Chandrasekar R, Delshad A, et al. Comparison of synthetic high density lipoprotein (HDL) contrast agents for MR imaging of atherosclerosis. Bioconjug Chem. 2009;20:937–43. doi: 10.1021/bc800520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zanotti I, Reilly MP, et al. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–3. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 5.Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc. 2004;126:16316–7. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J Lipid Res. 2004;45:1594–607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 7•.Skajaa T, Zhao Y, van den Heuvel DJ, et al. Quantum dot and Cy5.5 labeled nanoparticles to investigate lipoprotein biointeractions via Förster resonance energy transfer. Nano Lett. 2010;10:5131–8. doi: 10.1021/nl1037903. This paper describes the development of FRET QD-HDL to study interactions between lipoproteins and lipid exchange dynamics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaish A, Keren G, Chouraqui P, Levkovitz H, Harats D. Imaging of aortic atherosclerotic lesions by (125)I-LDL, (125)I-oxidized-LDL, (125)I-HDL and (125)I-BSA. Pathobiology. 2001;69:225–9. doi: 10.1159/000055947. [DOI] [PubMed] [Google Scholar]
- 9.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–53. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segrest JP, Jones MK, De Loof H, et al. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992;33:141–66. [PubMed] [Google Scholar]
- 11.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–71. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 12.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–37. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 13.Baitsch D, Bock HH, Engel T, et al. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1160–8. doi: 10.1161/ATVBAHA.111.222745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- 15.Lehr HA, Becker M, Marklund SL, et al. Superoxide-dependent stimulation of leukocyte adhesion by oxidatively modified LDL in vivo. Arterioscler Thromb. 1992;12:824–9. doi: 10.1161/01.atv.12.7.824. [DOI] [PubMed] [Google Scholar]
- 16.Seneviratne AN, Sivagurunathan B, Monaco C. Toll-like receptors and macrophage activation in atherosclerosis. Clin Chim Acta. 2012;413:3–14. doi: 10.1016/j.cca.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Firestone RA. Low-density lipoprotein as a vehicle for targeting antitumor compounds to cancer cells. Bioconjug Chem. 1994;5:105–13. doi: 10.1021/bc00026a002. [DOI] [PubMed] [Google Scholar]
- 18.Maletinska L, Blakely EA, Bjornstad KA, Deen DF, Knoff LJ, Forte TM. Human glioblastoma cell lines: levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000;60:2300–3. [PubMed] [Google Scholar]
- 19.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oram JF. HDL apolipoproteins and ABCA1: partners in the removal of excess cellular cholesterol. Arterioscler Thromb Vasc Biol. 2003;23:720–7. doi: 10.1161/01.ATV.0000054662.44688.9A. [DOI] [PubMed] [Google Scholar]
- 21.Wang MD, Franklin V, Marcel YL. In vivo reverse cholesterol transport from macrophages lacking ABCA1 expression is impaired. Arterioscler Thromb Vasc Biol. 2007;27:1837–42. doi: 10.1161/ATVBAHA.107.146068. [DOI] [PubMed] [Google Scholar]
- 22.Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 23.Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem. 2003;278:9142–9. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls SJ, Dusting GJ, Cutri B, et al. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–50. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 25.Nofer JR, Remaley AT. Tangier disease: still more questions than answers. Cell Mol Life Sci. 2005;62:2150–60. doi: 10.1007/s00018-005-5125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merchant B. Gold, the noble metal and the paradoxes of its toxicology. Biologicals. 1998;26:49–59. doi: 10.1006/biol.1997.0123. [DOI] [PubMed] [Google Scholar]
- 27.Galper MW, Saung MT, Fuster V, et al. Effect of computed tomography scanning parameters on gold nanoparticle and iodine contrast. Invest Radiol. 2012;47:475–81. doi: 10.1097/RLI.0b013e3182562ab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yguerabide J, Yguerabide EE. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications. Anal Biochem. 1998;262:157–76. doi: 10.1006/abio.1998.2760. [DOI] [PubMed] [Google Scholar]
- 29.You J, Zhang R, Xiong C, et al. Effective photothermal chemotherapy using doxorubicin-loaded gold nanospheres that target EphB4 receptors in tumors. Cancer Res. 2012;72:4777–86. doi: 10.1158/0008-5472.CAN-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yilmaz A, Rosch S, Klingel K, et al. Magnetic resonance imaging (MRI) of inflamed myocardium using iron oxide nanoparticles in patients with acute myocardial infarction—preliminary results. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Kimura K, Tanigawa N, Matsuki M, et al. High-resolution MR lymphography using ultrasmall superparamagnetic iron oxide (USPIO) in the evaluation of axillary lymph nodes in patients with early stage breast cancer: preliminary results. Breast Cancer. 2010;17:241–6. doi: 10.1007/s12282-009-0143-7. [DOI] [PubMed] [Google Scholar]
- 32.Tang TY, Howarth SP, Miller SR, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–50. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Chandrasekharan P, Maity D, Yong CX, Chuang KH, Ding J, Feng SS. Vitamin E (D-alpha-tocopheryl-co-poly(ethylene glycol) 1000 succinate) micelles-superparamagnetic iron oxide nanoparticles for enhanced thermotherapy and MRI. Biomaterials. 2011;32:5663–72. doi: 10.1016/j.biomaterials.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi RA, Mallawarachi C, U-King-Im JM, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–6. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 35.Bruners P, Braunschweig T, Hodenius M, et al. Thermoablation of malignant kidney tumors using magnetic nanoparticles: an in vivo feasibility study in a rabbit model. Cardiovasc Intervent Radiol. 2010;33:127–34. doi: 10.1007/s00270-009-9583-x. [DOI] [PubMed] [Google Scholar]
- 36.Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking meta-static tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med. 2004;10:993–8. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Lau SK, Varma VA, Kairdolf BA, Nie S. Multiplexed detection and characterization of rare tumor cells in Hodgkin’s lymphoma with multicolor quantum dots. Anal Chem. 2010;82:6237–43. doi: 10.1021/ac101065b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Liu Y, Liu S, Pang DW, Xiao G. Clathrin-mediated endocytosis in living host cells visualized through quantum dot labeling of infectious hematopoietic necrosis virus. J Virol. 2011;85:6252–62. doi: 10.1128/JVI.00109-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasuhn DE, Feltz A, Blanco-Canosa JB, et al. Quantum dot peptide biosensors for monitoring caspase 3 proteolysis and calcium ions. ACS Nano. 2010;4:5487–97. doi: 10.1021/nn1016132. [DOI] [PubMed] [Google Scholar]
- 40•.Thaxton CS, Daniel WL, Giljohann DA, Thomas AD, Mirkin CA. Templated spherical high density lipoprotein nanoparticles. J Am Chem Soc. 2009;131:1384–5. doi: 10.1021/ja808856z. This paper describes the synthesis of Au-HDL which are able to bind cholesterol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luthi AJ, Zhang H, Kim D, et al. Tailoring of biomimetic high-density lipoprotein nanostructures changes cholesterol binding and efflux. ACS Nano. 2012;6:276–85. doi: 10.1021/nn2035457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.McMahon KM, Mutharasan RK, Tripathy S, et al. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 2011;11:1208–14. doi: 10.1021/nl1041947. This paper describes the development of an Au-HDL platform to deliver nucleic acids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Cormode DP, Skajaa T, van Schooneveld MM, et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8:3715–23. doi: 10.1021/nl801958b. This paper describes the development of nanocrystal core HDL (Au-HDL, FeO-HDL, QD-HDL) as multimodality imaging platforms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Bruns OT, Ittrich H, Peldschus K, et al. Real-time magnetic resonance imaging and quantification of lipoprotein metabolism in vivo using nanocrystals. Nat Nanotechnol. 2009;4:193–201. doi: 10.1038/nnano.2008.405. This paper describes the development of QD/FeO core triglyceride-rich lipoproteins to study their metabolism. [DOI] [PubMed] [Google Scholar]
- 45.Cormode DP, Roessl E, Thran A, et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology. 2010;256:774–82. doi: 10.1148/radiol.10092473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skajaa T, Cormode DP, Jarzyna PA, et al. The biological properties of iron oxide core high-density lipoprotein in experimental atherosclerosis. Biomaterials. 2011;32:206–13. doi: 10.1016/j.biomaterials.2010.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–5. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 48•.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–7. doi: 10.1038/nature06803. This review describes the different techniques and strategies to image arteriosclerotic vascular disease. [DOI] [PubMed] [Google Scholar]
- 49.Lindsay AC, Choudhury RP. Form to function: current and future roles for atherosclerosis imaging in drug development. Nat Rev Drug Discov. 2008;7:517–29. doi: 10.1038/nrd2588. [DOI] [PubMed] [Google Scholar]
- 50.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–9. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng G, Li H, Zhang M, Lund-Katz S, Chance B, Glickson JD. Low-density lipoprotein reconstituted by pyropheophorbide cholesteryl oleate as target-specific photosensitizer. Bioconjug Chem. 2002;13:392–6. doi: 10.1021/bc025516h. [DOI] [PubMed] [Google Scholar]
- 52.Wu SP, Lee I, Ghoroghchian PP, et al. Near-infrared optical imaging of B16 melanoma cells via low-density lipoprotein-mediated uptake and delivery of high emission dipole strength tris[(porphinato)zinc(II)] fluorophores. Bioconjug Chem. 2005;16:542–50. doi: 10.1021/bc0497416. [DOI] [PubMed] [Google Scholar]
- 53.Corbin IR, Li H, Chen J, et al. Low-density lipoprotein nanoparticles as magnetic resonance imaging contrast agents. Neoplasia. 2006;8:488–98. doi: 10.1593/neo.05835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamakoshi Y, Qiao H, Lowell AN, et al. LDL-based nanoparticles for contrast enhanced MRI of atheroplaques in mouse models. Chem Commun (Camb) 2011;47:8835–7. doi: 10.1039/c1cc10924c. [DOI] [PubMed] [Google Scholar]
- 55.Chen W, Vucic E, Leupold E, et al. Incorporation of an apoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast Media Mol Imaging. 2008;3:233–42. doi: 10.1002/cmmi.257. [DOI] [PubMed] [Google Scholar]
- 56.Frias JC, Ma Y, Williams KJ, Fayad ZA, Fisher EA. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006;6:2220–4. doi: 10.1021/nl061498r. [DOI] [PubMed] [Google Scholar]


