Abstract
Background:
Mechanical ventilation used in patients with acute lung injury can damage pulmonary epithelial cells through production of inflammatory cytokines and excess deposition of the extracellular matrix protein lumican. Lumican participates in macrophage inflammatory protein (MIP)-2 and transforming growth factor-β1 (TGF-β1) signaling during the fibroproliferative phase of acute lung injury, which involves a process of epithelial-mesenchymal transition (EMT). The mechanisms regulating interactions between mechanical ventilation and lung injury are unclear. We hypothesized that lung damage and EMT by high tidal volume (Vt) mechanical stretch causes upregulation of lumican that modulates MIP-2 and TGF-β1 through the extracellular signal-regulated kinase (ERK) 1/2 pathway.
Methods:
Male C57BL/6 mice (either wild type or lumican null) aged 3 months and weighing between 25 and 30 g were exposed to low Vt (6 mL/kg) or high Vt (30 mL/kg) mechanical ventilation with room air for 2 to 8 h. Nonventilated mice were used as control subjects.
Results:
We found that high Vt mechanical ventilation increased microvascular permeability, neutrophil influx, production of free radicals, MIP-2 and TGF-β1 proteins, positive staining of α-smooth muscle actin and S100A4/fibroblast-specific protein-1, Masson trichrome staining and extracellular collagen, and activation of lumican and ERK1/2 in wild-type mice. Decreased staining of the epithelial marker E-cadherin was also observed. Mechanical stretch-augmented EMT was attenuated with lumican-deficient mice and pharmacologic inhibition of ERK1/2 activity by PD98059.
Conclusions:
The data suggest that lumican promotes high Vt mechanical ventilation-induced lung injury and EMT through the activation of the ERK1/2 pathway.
Acute lung injury (ALI) and its most severe manifestation, ARDS, are characterized by an initial inhomogeneous inflammatory reaction or epithelial injury that is followed by fibroblast proliferation and extracellular matrix (ECM) accumulation.1,2 Mechanical ventilation is required for life support in patients with ARDS; however, the potential for overdistension of the healthy parts of the lungs is great. Ventilator-induced lung injury (VILI) is characterized by noncardiogenic pulmonary edema; release of inflammatory cytokines; influx of neutrophils; and increased message for the ECM proteins, including proteoglycans.3,4 High tidal volume (Vt) mechanical ventilation can lead to the production of inflammatory cytokines, including tumor necrosis factor-α, IL-1β, macrophage inflammatory protein (MIP)-2, and transforming growth factor-β1 (TGF-β1).5,6
Chemokines are expressed in both the acute inflammatory response and the subsequent wound remodeling after lung injury. Previous studies demonstrated that MIP-2 induces neovascularization and promotes angiogenesis during VILI.5,7 As a major profibrogenic cytokine, TGF-β1 was also found in the pathogenesis of ALI related to mechanical ventilation and oxygen injury.8,9 Mechanical ventilation-induced oxidative stress may counter the protection of antioxidants in ALI and is a potent stimulus for the production of TGF-β1.6,9
Proteoglycans, a crucial component of pulmonary ECM, may contribute to ALI in response to excessive ventilation.10 Lumican belongs to the family of small leucine-rich repeat keratan sulfate proteoglycans (SLRPs) and is one of 10 members of SLRPs found in the lungs.11 Many members of SLRPs (eg, decorin, lumican, biglycan) play roles in tissue morphogenesis during embryonic development and in adults through their matricellular functions as regulator of collagen fibrillogenesis.12‐14 A previous study has shown that lumican increased human corneal epithelial cell migration through the extracellular signal-regulated kinase (ERK) 1/2 pathway.15 Epithelial-mesenchymal transition (EMT) is characterized by loss of epithelial markers (E-cadherin) and transition to a spindle-shaped morphology concurrent with the acquisition of mesenchymal markers (α-smooth muscle actin [α-SMA], S100A4).2,5,16 In addition to their roles in corneal transparency and EMT of lens epithelial cells,17 proteoglycans are involved in the alterations of lung mechanics after excessive mechanical ventilation; however, the mechanisms are unclear.2,18‐20 In the high Vt ventilation-induced ALI model in mice, we compared the effects among different Vts of mechanical ventilation, correlation of ALI to neutrophil influx, and production of MIP-2 and TGF-β1 using animals deficient in lumican and with the pharmacologic inhibitor of ERK1/2 PD98059. We hypothesized that lumican modulates the pathogenesis of high Vt ventilation-induced ALI characterized by excess neutrophil infiltration, production of free radicals, EMT, and MIP-2 and TGF-β1 production secondary to the activation of the ERK1/2 pathway.
Materials and Methods
Generation and Maintenance of Lumican-Deficient Mice
Male C57BL/6 (either wild-type or lumican deficient) mice aged 3 months and weighing between 25 and 30 g, were obtained from Chang Gung University Laboratory Animal Center (Taoyuan, Taiwan). Lumican-null mice were generated by targeted gene disruption as previously described.13,21 All procedures for handling mice conformed to Association of Research for Vision and Ophthalmology guidelines. Statements for the use of animals in research were approved by the Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital (permit number 2011093005). All surgery was performed under ketamine and xylazine anesthesia, and all efforts were made to minimize suffering.
Ventilator Protocol and Experimental Groups
We used our established mouse model of VILI as previously described.22 The experimental group of animals and procedures used in this study are summarized in Table 1.
Table 1.
—Experimental Design and Numbers of Animals per Group
| Variable | EBD, Lung Water (8 h) | MIP-2, TGF-β1, MPO (8 h) | Lumican and ERK1/2 Protein, Lumican mRNA (2 h) | Collagen, MDA (8 h) | IF, Trichrome Stain, EM (8 h) |
| Control, wild type | 5 | 5 | 5 | 5 | 5 |
| Control, lum−/− | 5 | 5 | 5 | 5 | 5 |
| Vt 6 mL/kg, wild type | 5 | 5 | 5 | 5 | 5 |
| Vt 10 mL/kg, wild type | 4 | 4 | 4 | 0 | 0 |
| Vt 30 mL/kg, wild type | 5 | 5 | 5 | 5 | 5 |
| Vt 30 mL/kg, wild type with PD98059 | 5 | 5 | 5 | 5 | 5 |
| Vt 30 mL/kg, lum−/− | 5 | 5 | 5 | 5 | 5 |
Control = spontaneously breathing, nonventilated mice; EBD = Evans blue dye; EM = electron microscopy; ERK = extracellular signal-regulated kinase; IF = immunofluorescence; lum2212/2212 = lumican null; MDA = malondialdehyde; MIP-2 = macrophage-inflammatory protein-2; MPO = myeloperoxidase; TGF-β1 = transforming growth factor-β1; Vt = tidal volume.
Pharmacologic Inhibitor
ERK1/2 inhibitor PD98059 2 mg/kg (Calbiochem; EMD Chemicals Inc) was given subcutaneously 30 min before ventilation. This was based on previous in vivo studies.5
Statistical Analysis
Western blots and lumican mRNA were quantified using National Institutes of Health image analyzer ImageJ version 1.27z and are presented as relative ERK1/2 phosphorylation and lumican-to-glyceraldehyde 3-phosphate dehydrogenase ratio in arbitrary units. Values are expressed as the mean ± SD from at least five separate experiments. Lung wet weight-to-dry weight ratio, MIP-2 and TGF-β1, free radicals, lung collagen, myeloperoxidase (MPO), Evans blue dye (EBD) staining, and immunofluorescent labeling were analyzed using StatView 5.0 (SAS Institute Inc) software. All data of Western blot and lumican mRNA were normalized to control nonventilated wild-type mice breathing room air. Analysis of variance was used to assess the statistical significance of the differences followed by multiple comparisons with Scheffé test. P < .05 was considered statistically significant. Additional details about the experimental animals, ventilator protocol, analysis of lung water content, EBD, measurement of MIP-2 and TGF-β1, MPO, Masson trichrome stain and fibrosis scoring, collagen assay, measurement of malondialdehyde (MDA), immunoblot analysis, immunofluorescent labeling, reverse transcription-polymerase chain reaction, and statistical analysis are provided in e-Appendix 1 (3.9MB, pdf) .
Results
Reduction of VILI in Lumican-Deficient Mice
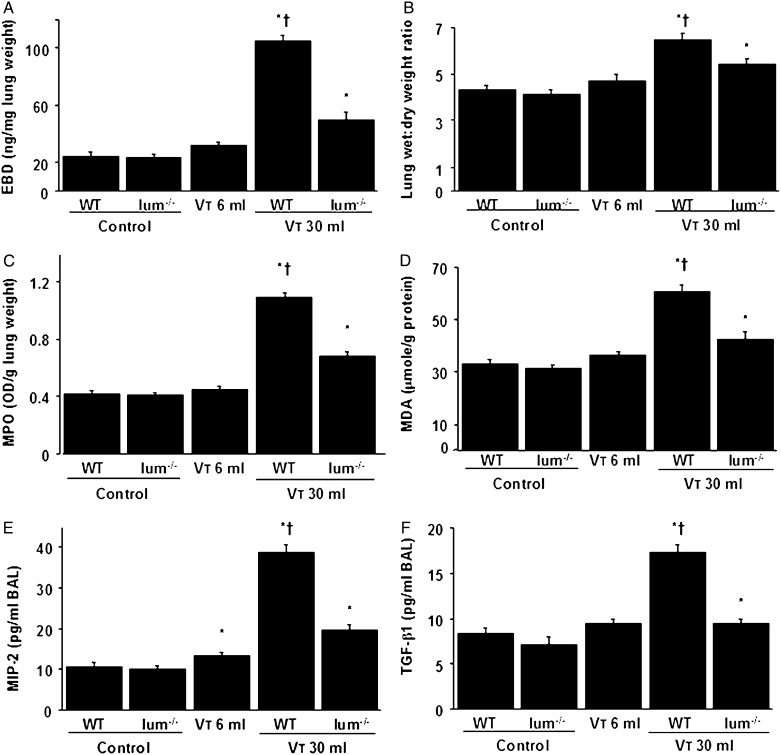
We measured lung EBD and wet weight-to-dry weight ratio to determine the effects of mechanical ventilation on changes of microvascular permeability and lung water content in VILI (Figs 1A, 1B). MPO assay was used to quantitate total lung neutrophils (Fig 1C). We measured MDA level, which is an aldehydic secondary product of lipid peroxidation used as a marker of oxidative stress9 and TGF-β1 and MIP-2 production, to determine the level of oxidant stress and chemoattractants associated with VILI (Figs 1D‐F). The levels of EBD, wet weight-to-dry weight ratio, MPO, MDA, and TGF-β1 and MIP-2 protein significantly increased in mice ventilated at Vt 30 mL/kg compared with those of at Vt 6 mL/kg or nonventilated control mice. No significant elevation was observed in mice ventilated at Vt 6 mL/kg compared with control mice. The increases of lung inflammation with Vt 30 mL/kg mechanical ventilation were significantly lower in lumican-deficient mice. Furthermore, the decreases of gas exchange (Pao2/Fio2 ratio) in mice receiving Vt 30 mL/kg mechanical ventilation were significantly restored in lumican-deficient mice (wild type, 405.2 ± 7.4; lumican deficient, 437.1 ± 7.9; P = .001) (Table 2).
Figure 1.
A-F, Lum−/− mice showed reduced lung stretch-induced microvascular leak, lung edema, neutrophil sequestration, oxygen radicals, and TGF-β1 and MIP-2 production. EBD analysis (A), lung wet weight-to-dry-weight ratio (B), MPO assay (C), MDA assay (D), and MIP-2 (E) and TGF-β1 (F) production in BAL fluid were from nonventilated control mice and mice ventilated at Vt 6 mL/kg or 30 mL/kg for 8 h with room air (n = 5 per group). *P < .05 vs nonventilated control mice; †P < .05 vs all other groups. EBD = Evans blue dye; lum−/− = lumican null; MDA = malondialdehyde; MIP-2 = macrophage inflammatory protein-2; MPO = myeloperoxidase; OD = optical density; TGF-β1 = transforming growth factor-β1; WT = wild type; Vt = tidal volume.
Table 2.
—Physiologic Conditions at the Beginning and End of Ventilation
| Condition | Control Nonventilated Wild Type | Control Nonventilated Lum−/− | Vt 6 mL/kg Wild Type | Vt 10 mL/kg Wild Type | Vt 30 mL/kg Wild Type | Vt 30 mL/kg PD98059 | Vt 30 mL/kg Lum−/− |
| pH | 7.42 ± 0.03 | 7.40 ± 0.05 | 7.36 ± 0.05 | 7.36 ± 0.07 | 7.35 ± 0.06 | 7.34 ± 0.09 | 7.36 ± 0.04 |
| Pao2, mm Hg | 96.1 ± 0.2 | 96.8 ± 0.1 | 92.7 ± 1.2 | 90.1 ± 1.3 | 85.1 ± 1.4 | 91.2 ± 1.5 | 91.8 ± 1 |
| Pao2/Fio2 ratio | 457.6 ± 9.2 | 461 ± 9.1 | 441.4 ± 7.1 | 429.1 ± 6.8 | 405.2 ± 7.4a | 434.3 ± 6.6 | 437.1 ± 7.9 |
| Paco2, mm Hg | 39.1 ± 0.1 | 38.9 ± 0.1 | 41.3 ± 0.6 | 38.3 ± 0.7 | 35.1 ± 1.2 | 35.4 ± 1.6 | 35.6 ± 1.3 |
| MAP, mm Hg | |||||||
| Start | 84 ± 1.1 | 83 ± 0.9 | 83.2 ±± 1.2 | 83.1 ± 1.4 | 82.8 ± 1.6 | 82.6 ± 1.5 | 82.5 ± 1.3 |
| End | 83 ± 0.5 | 82 ± 0.4 | 80.4 ± 1.7 | 79.6 ± 2.1 | 76.6 ± 5.2 | 77.1 ± 4.8 | 77.9 ± 2.8 |
| PIP, mm Hg | |||||||
| Start | … | … | 9.7 ± 1.2 | 15.2 ± 1.3 | 23.4 ± 1.5 | 23.5 ± 1.7 | 23.2 ± 1.4 |
| End | … | … | 11.2 ± 0.8 | 16.7 ± 1.6 | 27.9 ± 2.4 | 26.2 ± 3.1 | 25.9 ± 2.1 |
Data are presented as mean ± SD. Arterial blood gas levels and mean arterial pressure were obtained from nonventilated control mice and mice ventilated at a Vt of 6 mL/kg, 10 mL/kg, or 30 mL/kg for 8 h (n = 10 per group). MAP = mean arterial pressure; PIP = peak inspiratory pressure. See Table 1 legend for expansion of other abbreviations.
P < .05 vs all other groups. The physiologic data of the control groups were similar during the experiment and were used as the beginning data of ventilation.
Reduced Production of Collagen Fibers in the ECM of Lumican-Deficient Mice
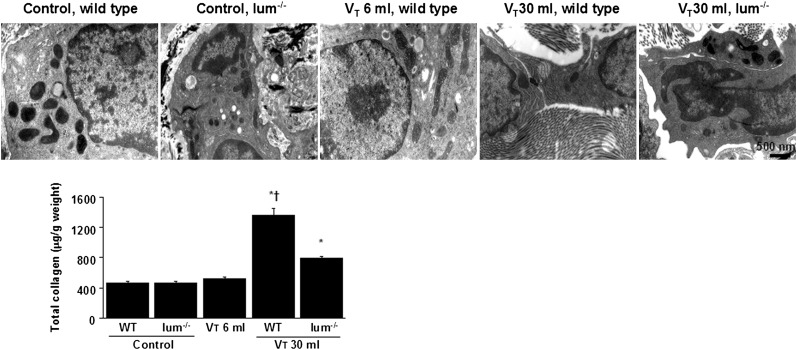
Electron microscopy was used to determine the effects of mechanical ventilation on the ultrastructures of peribronchiolar and parenchymal collagen fibers. Increases of intercellular space of fibroblasts and collagen fibers in the ECM were observed in mice subjected to Vt 30 mL/kg compared with mice subjected to Vt 6 mL/kg or nonventilated control mice (Fig 2). The increases of collagen fibers were significantly lower in lumican-deficient mice as measured quantitatively by total lung collagen content.
Figure 2.
Ultrastructural attenuation of lung stretch-induced production of collagen in lum−/− mice. Representative micrographs (× 35,000, n = 3 per group) of the lung sections and total collagen of lung tissue were from nonventilated control mice and mice ventilated at Vt 30 mL/kg for 8 h with room air (n = 5 per group). Control mice and mice ventilated at Vt 6 mL/kg have no increase of intercellular collagen fibers; WT mice ventilated at Vt 30 mL/kg have increased intercellular space and deposition of collagen fibers; and lum−/− mice ventilated at Vt 30 mL/kg show decreased collagen accumulation. *P < .05 vs nonventilated control mice; †P < .05 vs all other groups. See Figure 1 legend for expansion of abbreviations.
Reduction of Mechanical Ventilation-Induced Fibrogenic Markers in Lumican-Deficient Mice
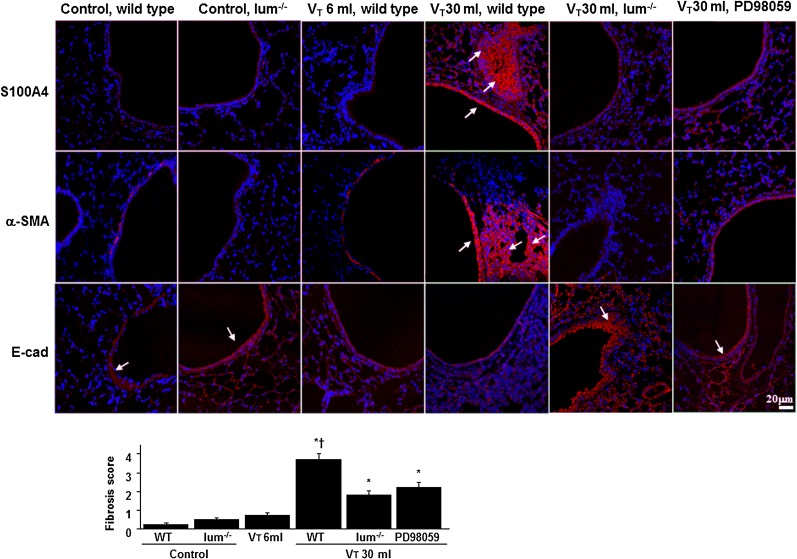
We measured the expression of E-cadherin, S100A4, and α-SMA using immunofluorescent labeling to identify the cells types involved in the lung stretch-induced EMT (Fig 3). The downregulation of E-cadherin but upregulation of S100A4 and α-SMA in bronchiolar epithelium and peribronchiolar lung parenchyma of mice ventilated at Vt 30 mL/kg compared with nonventilated control mice and those ventilated at Vt 6 mL/kg indicated the presence of transition from epithelial cells to fibroblasts. Increased expression of E-cadherin but decreased expression of S100A4 and α-SMA were observed in lumican-deficient mice. To further determine the effects of mechanical ventilation on ECM accumulation, we performed fibrosis scoring using Masson trichrome staining (Fig 3, e-Fig 1). The extent of peribronchiolar ECM deposition increased in mice ventilated at Vt 30 mL/kg compared with Vt 6 mL/kg and control mice. No significant elevation of collagen accumulation was observed in mice ventilated at Vt 6 mL/kg compared with control mice. The fibrosis score of ventilation-induced lung fibrogenesis was significantly lower in lumican-deficient mice.
Figure 3.
Lum−/− mice and PD98059 reduced lung stretch-induced fibrogenic biomarkers. Representative photomicrographs (magnification × 400) with S100A4 (red), α-SMA (red), E-cad (red), and Hoechst (blue) immunofluorescent labeling of paraffin lung sections were from nonventilated control mice and mice ventilated at Vt 6 mL/kg or Vt 30 mL/kg for 8 h with room air (n = 5 per group). PD98059 2 mg/kg was given subcutaneously 30 min before ventilation. Positive red staining in the lung epithelium and interstitium is identified by arrows (n = 5 per group). The fibrotic scoring was quantified as the average number of 10 nonoverlapping fields in Masson trichrome staining of paraffin lung sections (bar graph, n = 5 per group). *P < .05 vs nonventilated control mice; †P < .05 vs all other groups. α-SMA = α-smooth muscle actin; E-cad = E-cadherin. See Figure 1 legend for expansion of other abbreviations.
Reduction of Mechanical Ventilation-Induced Lumican Expression by Lumican-Deficient Mice
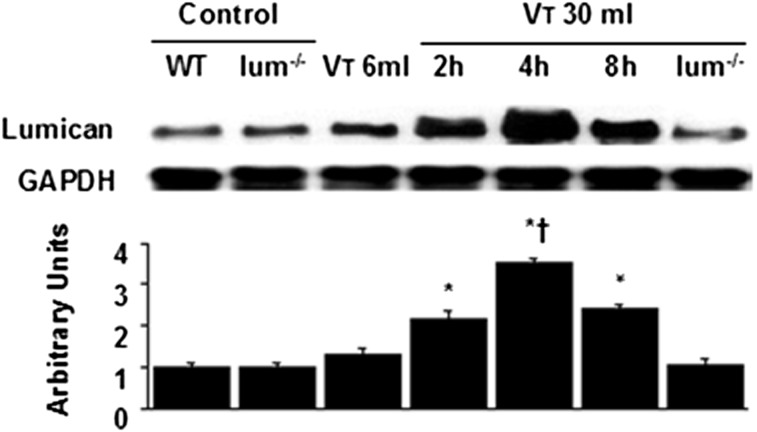
To determine the time courses of stretch-induced lumican expression, we measured the level of lumican in the lung after 2 to 8 h of mechanical ventilation (Fig 4). The level of lumican increased at 2 h of mechanical ventilation and remained elevated 2.5-fold for up to 8 h compared with nonventilated control mice and mice ventilated at Vt 6 mL/kg. No significant elevation was observed in mice ventilated with Vt 6 mL/kg compared with control mice.
Figure 4.
Lum−/− mice reduced lung stretch-induced lumican expression. Western blot was performed using an antibody that recognizes the lumican expression and an antibody that recognizes GAPDH expression in lung tissue from nonventilated control mice and mice ventilated at Vt 6 mL/kg for 2 h or Vt 30 mL/kg at indicated time periods. Arbitrary units were expressed as the ratio of lumican to GAPDH (n = 5 per group). *P < .05 vs nonventilated control mice; †P < .05 vs all other groups. GAPDH = glyceraldehyde 3-phosphate dehydrogenase. See Figure 1 legend for expansion of other abbreviations.
Inhibition of ERK1/2 Activation by PB98059 Reduced Mechanical VILI
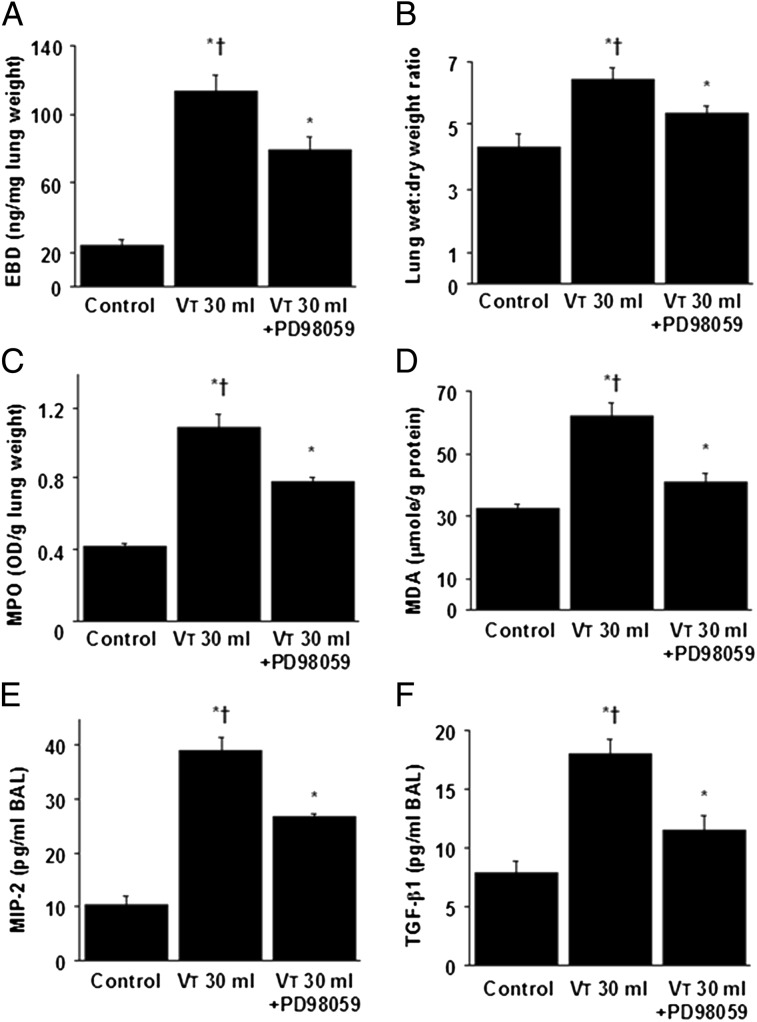
To further explore the roles of ERK1/2 activation in VILI, we measured lung injury parameters and chemoattractants (Fig 5). The increases of microvascular permeability, lung water content, MDA, neutrophil infiltration, and MIP-2 and TGF-β1 production were significantly lowered by pharmacologic inhibition with PD98059, suggesting the involvement of ERK1/2 pathway in the regulation of VILI.
Figure 5.
A-F, PD98059 reduced lung stretch-induced microvascular leak, lung edema, neutrophil influx, oxidative stress, and TGF-β1 and MIP-2 production. EBD analysis (A), lung wet weight-to-dry weight ratio (B), MPO assay (C), MDA assay (D), and MIP-2 (E) and TGF-β1 (F) production were from nonventilated control mice and mice ventilated at Vt 30 mL/kg for 8 h with room air (n = 5 per group). PD98059 2 mg/kg was given subcutaneously 30 min before ventilation. P < .05 vs nonventilated control mice; †P < .05 vs PD98059 group. See Figure 1 for expansion of abbreviations.
Inhibition of ERK1/2 Activation by PB98059 Reduced Mechanical Ventilation-Induced Fibrogenic Markers
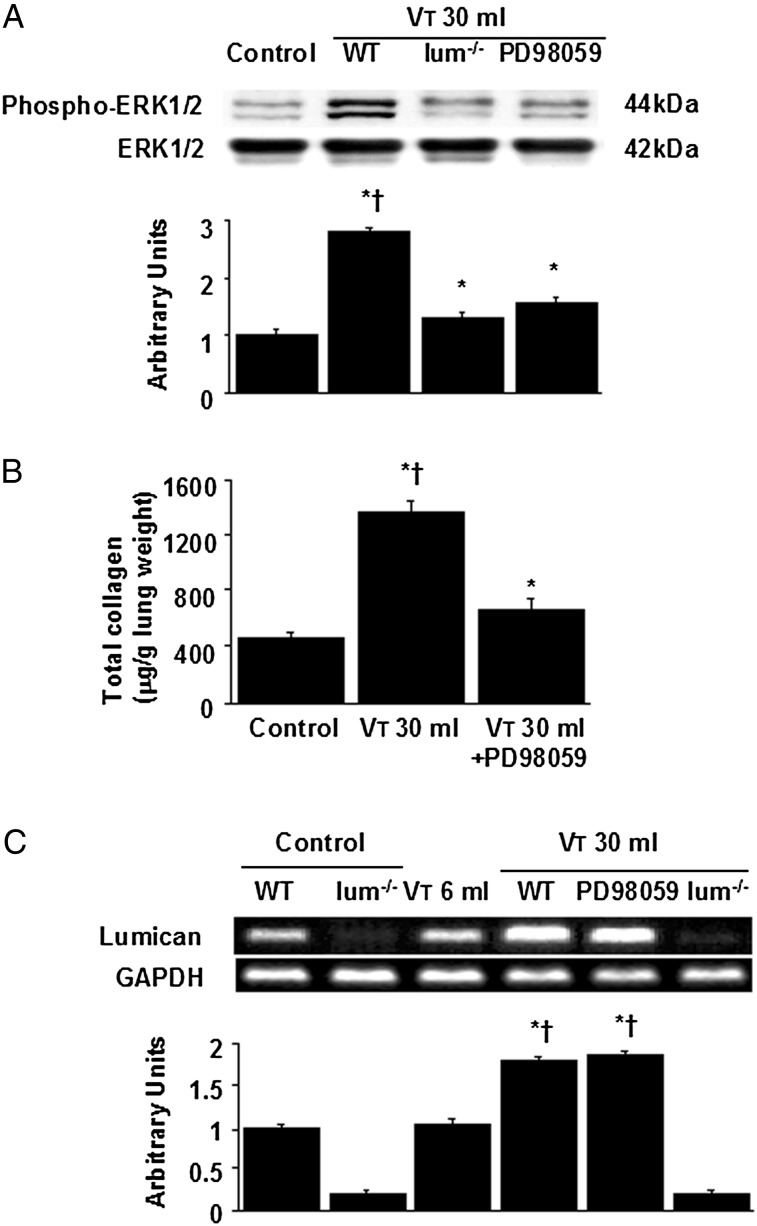
Our previous study demonstrated that activation of ERK1/2 was involved in the bleomycin-induced fibrogenesis of VILI.5 We measured phosphorylation of ERK1/2, biomarkers of EMT, and level of collagen to determine the role of ERK1/2 in lumican-related VILI (Figs 3, 6A, 6B). Increased expression of E-cadherin but decreased expression of S100A4 and α-SMA was observed by pharmacologic inhibition with PD98059. The elevation of stretch-induced ERK1/2 activation and total collagen content were significantly lowered in lumican-deficient mice. We measured lumican mRNA expression to further determine whether the increased neutrophil influx in mice ventilated at Vt 30 mL/kg was associated with the upregulation of chemotactic factors for neutrophils (Fig 6C). The increases of lumican mRNA expression after mechanical ventilation were significantly lowered in lumican-deficient mice but not inhibited by PD98059, suggesting that lumican was the upstream regulator of ERK1/2 signaling involved in VILI.
Figure 6.
Lum−/− mice and PD98059 reduced lung stretch-induced ERK1/2 activation, total collagen content, and lumican mRNA expression. A, Western blot was performed using an antibody that recognizes phosphorylated ERK1/2 and an antibody that recognizes total ERK1/2 expression in lung tissue from nonventilated control mice and mice ventilated at Vt 30 mL/kg for 2 h with room air. Arbitrary units were expressed as relative ERK1/2 phosphorylation (n = 5 per group). B, Total collagen level was from control mice and mice ventilated at Vt 30 mL/kg for 8 h with room air (n = 5 per group). P < .05 vs nonventilated control mice; †P < .05 vs PD98059 group. C, Reverse transcription-polymerase chain reaction assay was performed for lumican mRNA, GAPDH mRNA, and arbitrary units from nonventilated control mice and mice ventilated at Vt 6 mL/kg or Vt 30 mL/kg for 2 h with room air (n = 5 per group). Arbitrary units were expressed as the ratio of lumican mRNA to GAPDH. PD98059 2 mg/kg was given subcutaneously 30 min before ventilation. *P < .05 vs nonventilated control mice; †P < .05 vs lum−/− group. ERK = extracellular signal-regulated kinase. See Figure 1 legend for expansion of other abbreviations.
Discussion
High Vts in normal animals have been used to mimic the overdistension of the less-injured and, thus, more-compliant areas of lung found in ARDS. Previous studies demonstrated that hyperexpansion of the lung was the cause of noncardiogenic pulmonary edema in VILI.1,5,22,23 A previous study of an acid-induced lung injury model in mice showed that 2 h of mechanical stretch induced lung fibrogenesis.2 Our previous study of hyaluronan synthase knockout mice showed that 5 h of high Vt mechanical ventilation induced hyaluronan synthase 3 mRNA and hyaluronan production in fibroblasts, contributing to ECM-induced inflammatory changes.24 In the present VILI model of mice, we found that mechanical ventilation of healthy lungs increased microvascular permeability, hypoxemia, neutrophil infiltration, free radicals, and MIP-2 and TGF-β1 production. Upregulation of lumican is associated with the increase of lung injury. We further explored the roles of neutrophils, alveolar epithelia, fibroblasts, and ERK1/2 activation in fibrogenesis of VILI.
Neutrophils are the main inflammatory cells involved in the process of ALI through the secretion of chemotactic factor MIP-2 and play a vital role in the generation of reactive oxygen species.22 The reactive oxygen species can enhance TGF-β1 production by inflammatory cells and alveolar epithelial cells.6 Mechanical stretch is a crucial mechanism that underlies fibrogenesis through matrix-induced activation of myofibroblasts. TGF-β1 promotes the EMT of the damaged epithelium and stimulates fibroblast proliferation, which may account for the progression of the fibroproliferative phase seen in ARDS.25,26 The expressions of S100A4/fibroblast-specific protein-1 and α-SMA further supported the presence of an ongoing angiogenetic program determining mesenchymal phenotype in VILI.16,17 High Vt ventilation increased neutrophil infiltration; production of MDA, MIP-2, and TGF-β1; accumulation of collagen around bronchioles; and positive staining of S100A4 and α-SMA in bronchial epithelium and peribronchiolar parenchyma.
The pulmonary ECM comprises several crucial components, including collagens, elastin, fibronectin, proteoglycans, hyaluronan, and laminin.18 Proteoglycan comprises a central core protein and glycosaminoglycan side chains, including chondroitin sulfate, keratan sulfate, heparan sulfate, dermatan sulfate, and hyaluronic acid.4 In addition to the effects of stabilizing the collagen network in connective tissue, proteoglycans also have proinflammatory effects, including binding to TGF-β and toll-like receptors and activation of ERK1/2.4,15,18 Mechanical ventilation can increase production and fragmentation of proteoglycans and affect the transmission of stress among the ECM, cellular membrane, and cytoskeleton.4,27 Previous studies of injurious mechanical ventilation in rats demonstrated that excessive mechanical ventilation for 2 h results in increased message for the proteoglycans.27 Moriondo et al10 found heparan sulfate fragmentation after mechanical ventilation, indicating ventilation-induced plasma membrane disruption. The lesional effect of mechanical ventilation on the ECM may depend on several factors, such as increased transpulmonary pressure, heterogeneous distribution of ventilation and increased tissue stretch, and reduction of pulmonary lymphatic drainage.
Lumican may affect the collagen deposition in connective tissues by binding to the surface of collagen fibrils.28 The lumican is expressed by dermal fibroblasts, cardiac muscle cells, kidney glomerular cells, alveolar epithelial cells, endothelial cells, and fibroblasts in the lung.29 In addition to regulating the collagen fibril architecture within the ECM of lung, previous studies of mice demonstrated that lack of lumican reduced neutrophil infiltration into damaged tissue because of the absence of chemokine gradient and a scaffold for neutrophil extravasation.30,31 We found that mechanical ventilation downregulated expression of epithelial marker E-cadherin but upregulated expression of mesenchymal markers S100A4/fibroblast-specific protein-1 and α-SMA. Using lumican-deficient mice, we observed a decrease of MIP-2 and TGF-β1 production and expression of TGF-β1-inducible EMT markers, suggesting the involvement of lumican in the regulation of fibrogenesis of VILI.
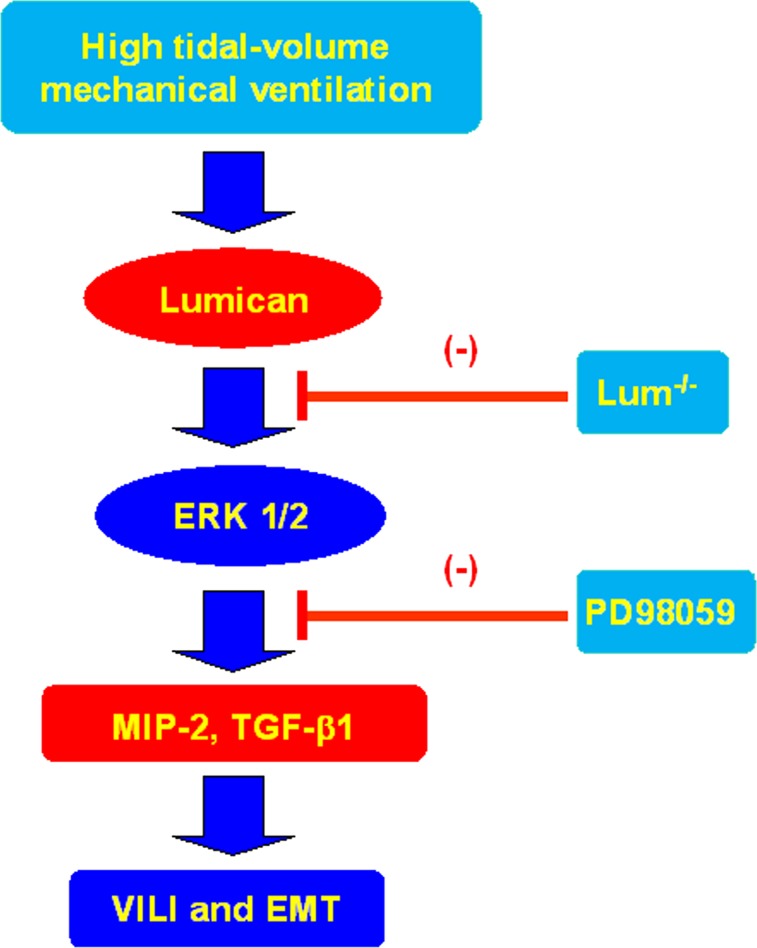
Our previous murine study of lung fibrosis after bleomycin exposure indicated that the ERK1/2 pathway may contribute to lung fibrogenesis induced by the production of MIP-2.5 In a study of corneal epithelial cells, ERK1/2 was also demonstrated as the downstream signaling pathway of lumican by regulating cell migration and expression of integrin β1.15 The present study demonstrated that mechanical ventilation increased expression of lumican and ERK1/2. Using lumican-deficient mice and EKR1/2 inhibitor, we observed reduced lung injury and the progression of EMT. Pharmacologic inhibition with ERK1/2 inhibitor cannot reduce the expression of lumican, indicating that ERK1/2 was the downstream signal of lumican (Fig 7).
Figure 7.
Schematic figure illustrating the signaling pathway activation with high tidal volume mechanical ventilation. Mechanical stretch-induced cytokine production and EMT were attenuated with lum−/− mice and pharmacologic inhibition of ERK1/2 activity by PD98059. EMT = epithelial-mesenchymal transition; VILI = ventilator-induced lung injury. See Figure 1 and 6 legends for expansion of other abbreviations.
The present study has some limitations. An in vitro study revealed that alveolar epithelial type 2 cells and primary human bronchial epithelial cells in response to TGF-β1 undergo EMT through a Smad-mediated pathway.32 In healthy mice, an increase of lung inflammation because of alveolar derecruitment and atelectasis but not overstretch was observed after 3 h of normal Vt mechanical ventilation (10 mL/kg)33 (see e-Appendix 1 (3.9MB, pdf) for details).
Conclusions
By using an in vivo mouse model of ARDS, this study demonstrated that high Vt mechanical VILI and EMT are associated with activation of ERK1/2 and the production of MIP-2, TGF-β1, and MDA. This process partly depends on the activation of lumican. Knowledge of the effect of mechanical forces on lumican allows clarification of the pathophysiologic mechanisms regulating the fibroproliferative phase of ARDS that may progress to irreversible pulmonary fibrosis and the need for long-term ventilator support. The inhibition of ECM lumican, a modulator of inflammation, may help in the development of improved treatment of lung injury in patients with ARDS.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Drs Li and Yang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Li: contributed to the coordination of the study and review of the manuscript.
Dr Chu: contributed to the coordination of the study and review of the manuscript.
Dr Hung: contributed to the coordination of the study and review of the manuscript.
Dr Kao: contributed to the coordination of the study and review of the manuscript.
Dr Lin: contributed to the coordination of the study and review of the manuscript.
Dr Liu: contributed to the coordination of the study and review of the manuscript.
Dr Yang: contributed to the coordination of the study and review of the manuscript.
Other contributions: We thank Wei-Han Lin, BS, and the Microscope Core Laboratory, Chang Gung Memorial Hospital, Linkou, for their help with the experiment.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendix and e-Figure can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- ALI
acute lung injury
- α-SMA
α-smooth muscle actin
- EBD
Evans blue dye
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal-regulated kinase
- MDA
malondialdehyde
- MIP
macrophage inflammatory protein
- MPO
myeloperoxidase
- SLRP
small leucine-rich repeat keratan sulfate proteoglycan
- TGF-β1
transforming growth factor-β1
- VILI
ventilator-induced lung injury
- Vt
tidal volume
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Funding/Support: The study was supported by the National Science Council [98-2314-B-182A-084-MY3 and 101-2314-B-182A-088-MY3], Chang Gung Research Project [3A0711 (to Dr Li) 97-2314-B-182-028-MY2, and 99-2314-B-182-042-MY3 (to Dr Chu)]; National Institutes of Health, National Eye Institute [Grant EY011845], Research to Prevent Blindness; and Ohio Lions Eye Research Foundation (to Dr Kao).
References
- 1.Held HD, Boettcher S, Hamann L, Uhlig S. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kappaB and is blocked by steroids. Am J Respir Crit Care Med. 2001;163(3 pt 1):711-716. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera-Benítez NE, Parotto M, Post M, et al. Mechanical stress induces lung fibrosis by epithelial-mesenchymal transition. Crit Care Med. 2012;40(2):510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J Suppl. 2003;2242):2s-9s. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig MS. Proteoglycans and pathophysiology. J Appl Physiol. 2007;103(3):735-736. [DOI] [PubMed] [Google Scholar]
- 5.Li LF, Liao SK, Huang CC, Hung MJ, Quinn DA. Serine/threonine kinase-protein kinase B and extracellular signal-regulated kinase regulate ventilator-induced pulmonary fibrosis after bleomycin-induced acute lung injury: a prospective, controlled animal experiment. Crit Care. 2008;12(4):R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahy RJ, Lichtenberger F, McKeegan CB, Nuovo GJ, Marsh CB, Wewers MD. The acute respiratory distress syndrome: a role for transforming growth factor-beta 1. Am J Respir Cell Mol Biol. 2003;28(4):499-503. [DOI] [PubMed] [Google Scholar]
- 7.Keane MP, Belperio JA, Moore TA, et al. Neutralization of the CXC chemokine, macrophage inflammatory protein-2, attenuates bleomycin-induced pulmonary fibrosis. J Immunol. 1999;162(9):5511-5518. [PubMed] [Google Scholar]
- 8.Li LF, Chen BX, Tsai YH, Kao WW, Yang CT, Chu PH. Lumican expression in diaphragm induced by mechanical ventilation. PLoS ONE. 2011;6(9):e24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HS, Kim SR, Lee YC. Impact of oxidative stress on lung diseases. Respirology. 2009;14(1):27-38. [DOI] [PubMed] [Google Scholar]
- 10.Moriondo A, Pelosi P, Passi A, et al. Proteoglycan fragmentation and respiratory mechanics in mechanically ventilated healthy rats. J Appl Physiol. 2007;103(3):747-756. [DOI] [PubMed] [Google Scholar]
- 11.Dolhnikoff M, Morin J, Roughley PJ, Ludwig MS. Expression of lumican in human lungs. Am J Respir Cell Mol Biol. 1998;19(4):582-587. [DOI] [PubMed] [Google Scholar]
- 12.Nikitovic D, Katonis P, Tsatsakis A, Karamanos NK, Tzanakakis GN. Lumican, a small leucine-rich proteoglycan. IUBMB Life. 2008;60(12):818-823. [DOI] [PubMed] [Google Scholar]
- 13.Kao WW, Funderburgh JL, Xia Y, Liu CY, Conrad GW. Focus on molecules: lumican. Exp Eye Res. 2006;82(1):3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao WW-Y. Ocular surface tissue morphogenesis in normal and disease states revealed by genetically modified mice. Cornea. 2006;25(suppl):S7-S19. [DOI] [PubMed] [Google Scholar]
- 15.Seomun Y, Joo CK. Lumican induces human corneal epithelial cell migration and integrin expression via ERK 1/2 signaling. Biochem Biophys Res Commun. 2008;372(1):221-225. [DOI] [PubMed] [Google Scholar]
- 16.Lawson WE, Polosukhin VV, Zoia O, et al. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(8):899-907. [DOI] [PubMed] [Google Scholar]
- 17.Saika S, Miyamoto T, Tanaka S, et al. Response of lens epithelial cells to injury: role of lumican in epithelial-mesenchymal transition. Invest Ophthalmol Vis Sci. 2003;44(5):2094-2102. [DOI] [PubMed] [Google Scholar]
- 18.Pelosi P, Rocco PR. Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med. 2008;34(4):631-639. [DOI] [PubMed] [Google Scholar]
- 19.Villar J, Cabrera NE, Valladares F, et al. Activation of the Wnt/β-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS ONE. 2011;6(9):e23914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011;286(20):17435-17444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saika S, Shiraishi A, Liu CY, et al. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275(4):2607-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li LF, Yang CT, Huang CC, Liu YY, Kao KC, Lin HC. Low-molecular-weight heparin reduces hyperoxia-augmented ventilator-induced lung injury via serine/threonine kinase-protein kinase B. Respir Res. 2011;12:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han B, Lodyga M, Liu M. Ventilator-induced lung injury: role of protein-protein interaction in mechanosensation. Proc Am Thorac Soc. 2005;2(3):181-187. [DOI] [PubMed] [Google Scholar]
- 24.Bai KJ, Spicer AP, Mascarenhas MM, et al. The role of hyaluronan synthase 3 in ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;172(1):92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agostini C, Gurrieri C. Chemokine/cytokine cocktail in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3(4):357-363. [DOI] [PubMed] [Google Scholar]
- 26.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136(5):1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Jamal R, Ludwig MS. Changes in proteoglycans and lung tissue mechanics during excessive mechanical ventilation in rats. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1078-L1087. [DOI] [PubMed] [Google Scholar]
- 28.Svensson L, Närlid I, Oldberg Å. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett. 2000;470(2):178-182. [DOI] [PubMed] [Google Scholar]
- 29.Ying S, Shiraishi A, Kao CW, et al. Characterization and expression of the mouse lumican gene. J Biol Chem. 1997;272(48):30306-30313. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi Y, Call MK, Chikama TI, et al. Lumican is required for neutrophil extravasation following corneal injury and wound healing. J Cell Sci. 2010;123(pt 17):2987-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Bowrin K, Hamad AR, Chakravarti S. Extracellular matrix lumican deposited on the surface of neutrophils promotes migration by binding to β2 integrin. J Biol Chem. 2009;284(35):23662-23669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Câmara J, Jarai G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-α. Fibrogenesis Tissue Repair. 2010;3(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson MR, Patel BV, Takata M. Ventilation with “clinically relevant” high tidal volumes does not promote stretch-induced injury in the lungs of healthy mice. Crit Care Med. 2012;40(10):2850-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement