Abstract
Background:
Airway pepsin has been increasingly used as a potentially sensitive and quantifiable biomarker for gastric-to-pulmonary aspiration, despite lack of validation in normal control subjects. This study attempts to define normal levels of airway pepsin in adults and distinguish between pepsin A (exclusive to stomach) and pepsin C (which can be expressed by pneumocytes).
Methods:
We performed a prospective study of 51 otherwise healthy adult patients undergoing elective extremity orthopedic surgery at a single tertiary-care academic medical center. Lower airway samples were obtained immediately following endotracheal intubation and just prior to extubation. Total pepsin and pepsin A concentrations were directly measured by an enzymatic activity assay, and pepsin C was subsequently derived. Pepsinogen/pepsin C was confirmed by Western blot analyses. Baseline characteristics were secondarily compared.
Results:
In all, 11 (22%; 95% CI = 9.9%-33%) had detectable airway pepsin concentrations. All 11 positive specimens had pepsin C, without any detectable pepsin A. Pepsinogen/pepsin C was confirmed by Western blot analyses. In a multivariate logistic regression, men were more likely to have airway pepsin (OR, 12.71, P = .029).
Conclusions:
Enzymatically active pepsin C, but not the gastric-specific pepsin A, is frequently detected in the lower airways of patients who otherwise have no risk for aspiration. This suggests that nonspecific pepsin assays should be used and interpreted with caution as a biomarker of gastropulmonary aspiration, as pepsinogen C potentially expressed from pneumocytes may be detected in airway samples.
Pulmonary aspiration of gastric contents associated with anesthesia remains a serious complication in modern practice.1 Although the presence of witnessed or “macroaspiration” is a known risk for pneumonitis, pneumonia, and acute lung injury, extensive literature linking gastroesophageal reflux disease with a variety of pulmonary disorders, including obstructive pulmonary diseases,2,3 chronic cough,4 interstitial lung disease,5 as well as critical illness,6 have suggested that unwitnessed or “microaspiration” may also be an important determinant of patient outcomes.7,8 To determine whether microaspiration is indeed the link between reflux and pulmonary disorders, investigators have used the gastric enzyme pepsin as a putative biomarker of gastric-to-pulmonary aspiration.9‐16
Importantly, multiple pepsin isoforms17 exist, and the relationship between these isoforms and respiratory complications is unclear. To address this knowledge gap, we aimed to determine whether and which specific pepsin isoforms can be detected in the lower airways of patients who otherwise have no risk factors for aspiration. Secondarily, we aimed to determine the normal range for pepsin assays and to explore for any clinical correlates. Based on pilot data, we hypothesized that pepsin will be detected in a minority of healthy control subjects but that none will have the gastric-specific pepsin A.
Materials and Methods
Study Design and Population
Following approval from the Mayo Clinic Institutional Review Board (IRB #10-008019), we performed a prospective cohort study on consenting adult patients undergoing elective orthopedic upper and/or lower extremity surgery at a single tertiary-care academic medical center between March and May 2011. The target population for this investigation was otherwise healthy patients without known risk factors for aspiration. To help reduce confounding, the study protocol specifically excluded patients with chronic lung disease and potential risk factors for aspiration.18‐21 A full list of eligibility criteria is found in Table 1. Using pilot data, we calculated a sample size of 49 to achieve at least 80% power to detect pepsin in 11% of subjects (one-sample test of proportions, using exact calculations based on the binomial distribution, with a one-sided α level of 0.05).
Table 1.
—Eligibility Criteria
| Inclusion Criteria | Exclusion Criteria |
| Age ≥ 18 y | Gastroesophageal reflux disease, prior esophageal surgery, or other esophageal disorders |
| Elective orthopedic surgery of the extremity | Hiatal hernia or prior gastric surgery |
| American Society of Anesthesiologists’ physical classification ≤ 2 | Dysphagia or prior head/neck surgery |
| General anesthesia with endotracheal intubation | History of stroke, dementia, neurodegenerative disorders, or depressed mental status |
| Planned extubation at the end of the surgery | Chronic pulmonary symptoms/disease, history of recurrent pulmonary infections, or abnormal pulmonary examination |
| Difficult intubation, endotracheal tube cuff failure, witnessed aspiration, emesis | |
| Medical hospitalization within 6 mo | |
| Pregnancy | |
| Morbid obesity (BMI > 35) | |
| Established diagnosis of OSA | |
| Nonfasting status | |
| Surgery for multiple trauma |
Patients identified for elective orthopedic surgery of the extremity were identified at their preoperative medical evaluation. Eligibility criteria were aimed at eliminating patients at risk for aspiration and those with known or suspected gastroesophageal or pulmonary disorders. OSA =obstructive sleep apnea.
Outcomes
The primary outcomes are the detection rates of total pepsin and pepsin A in the lower airways. Descriptive and exploratory analyses were performed to identify any potential associations with age, sex, BMI (kg/m2), obstructive sleep apnea (OSA) predictor score,22 diabetes, and the preoperative use of opioids or nonsteroidal antiinflammatory drugs (NSAIDs).
Sample Collection
For each subject, airway secretions were obtained at two time points: immediately following endotracheal intubation and shortly before extubation. To facilitate collection of lower airway secretions, up to two 5-mL aliquots of sterile saline were instilled through the endotracheal tube to achieve a minimum collection volume of 0.5 mL.11,23 After administering two to three tidal ventilations, secretions were collected using a 14F suction catheter. The specimen was immediately placed on dry ice for transport and frozen at −80°C.
Detection of Total Pepsin, Pepsin A, and Pepsin/Pepsinogen C
Pepsin enzymatic activity and concentrations were measured using a well-established enzymatic assay.24 Notably, this assay does not distinguish pepsin from its zymogen pepsinogen, as any pepsinogen that is present in the samples is digested to pepsin. All assays were performed in duplicate. Each subject sample was run concurrently with known concentrations of porcine pepsin standards from 12.5 to 400 ng/mL to allow computation of pepsin concentrations in the sample, positive human control subjects from gastric fluid of deidentified patients, and blank samples containing inactivated pepsin from which net fluorescent units were calculated (by subtracting the fluorescent units from the blank sample). For each sample, 50 μL were mixed with 23 μL of 129 mM hydrochloric acid to convert any pepsinogen to active pepsin and to inactivate the competing protease, lysosomal acid hydrolase (cathepsin D). In a replicate set of assays, pepstatin was included to selectively inhibit pepsin A.25,26 Next, the samples were incubated with 20 μL 0.5% fluorescein isothiocyanate casein as a substrate for 3 h at 37°C. The enzymatic reaction was stopped by heat inactivation at 100°C, followed by trichloroacetic acid precipitation and centrifugation. The supernatant was transferred to a microplate and mixed with 500 mM Tris, pH 8.5. The plate was read in a spectrofluorometer (Cytofluor Plate Reader 4000; PerSeptive Biosystem, Inc) with excitation at 485 nm and emission at 530 nm. The net fluorescence unit of the total activity in a sample was calculated by subtracting values from the blank sample. Total pepsin concentrations were determined from the pepsin standards curve, and Pepsin A concentrations were determined from loss of enzymatic activity from the pepstatin-inhibited assays. Although the measures from the assay run to zero, the assay’s cutoff for the presence of total pepsin and pepsin A was previously determined and set at the assay’s upper 95th CI for blanks (negative controls) at 12.5 and 25 ng/mL, respectively.24 These cutoffs were used to define the presence or absence of pepsin in the binary analyses. The concentration of pepsin C was determine by subtracting pepsin A from the total pepsin concentration.17
Western Blot Analysis for Pepsinogen/Pepsin C:
In a selection of pepsin-positive and pepsin-negative samples in which adequate samples were available, a Western blot was performed to test for pepsin/pepsinogen C. Samples containing 20 μg of protein along with negative and positive controls were separated on 12% polyacrylamide gel in a discontinuous pH electrophoresis system and then transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Inc) using a Bio-Rad Transblot at 400 mA constant current for 50 min. Briefly, the membrane was blocked with 5% nonfat milk in Tris-buffered saline (pH 7.3) containing 0.1% Tween-20 for 1 h and probed with 1:2,000 diluted sheep anti-human pepsin C antibody (Abcam plc). The immunoreaction was detected using HRP-conjugated rabbit anti-sheep antibody (Abcam plc) and enhanced chemiluminescence (ECL) plus Western blot detection reagents (GE Healthcare). The blot was then developed in an x-ray developer.
Analyses
Dichotomous variables are presented as counts and percentages with their 95% CIs. Continuous data are presented as median and interquartile ranges (IQRs). Because of the skewed distribution of the pepsin values, with the majority of participants having no detectable pepsin identified, the continuous pepsin variable was transformed to a binary (present/absent) variable for all evaluations detailing the associations between baseline characteristics and pepsin concentrations. More specifically, subjects were dichotomized into “total pepsin present” or “total pepsin absent” based on the total pepsin assay cutoff of 12.5 ng/mL. Multivariate logistic regression was then used to identify statistically significant baseline predictors of airway total pepsin being present or absent. Of note, there were no subjects with pepsin A present using the assay cutoff of 25 ng/mL. Therefore, no analyses evaluating the influence of baseline characteristics on pepsin A concentrations were performed. For all statistical tests, a P value of < .05 was considered statistically significant. JMP software (SAS Institute Inc) was used for all data analyses.
Results
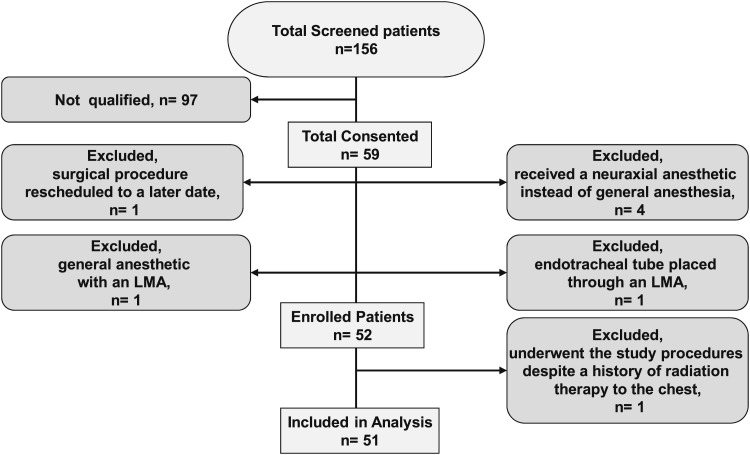
Of the 156 eligible patients screened, 59 consented. Of these 59 initial study participants, a single patient had the surgical procedure rescheduled to a later date, four received a neuraxial anesthetic instead of general anesthesia, one had a general anesthetic with a laryngeal mask airway (LMA) rather than an endotracheal tube, and one had an endotracheal tube placed through an LMA. These seven patients were excluded from the study. A single patient who underwent study procedures had a distant history of radiation therapy that was subsequently identified and was also excluded. (Of note, this study participant was also noted to have the highest levels of airway pepsin at 149.5 ng/mL, without any detectable pepsin A.) In all, 51 subjects completed study procedures and were analyzed (Fig 1). The median age was 58 years (IQR, 42-68), and 57% were women. The median BMI was 25.9 kg/m2 (IQR, 23.9-29.2). The primary surgical procedures were total knee arthroplasties (n = 7), total hip arthroplasties (n = 9), shoulder arthroplasties (n = 13), and shoulder arthroscopies (n = 13). The median volume of saline instillation was 5.0 mL (IQR, 4.0-8.0), and the median specimen collection volume was 1.0 mL (IQR, 1.0-1.5).
Figure 1.
Study flow and baseline characteristics. LMA = laryngeal mask airway.
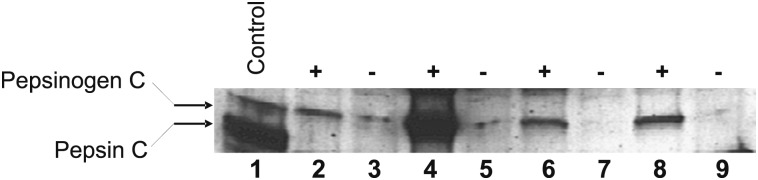
In all, 11 of the 51 subjects (22%; 95% CI, 9.9-33) had detectable airway pepsin (total) immediately following uncomplicated intubation, with a median concentration of 0.0 ng/mL (IQR, 0.0-9.4). However, as there was no detectable pepsin A in any of these samples, the 11 positive samples with pepsin activity could only be attributed to pepsin C. In a selection of four available samples that had pepsin activity and four available samples that did not, Western blot analyses qualitatively confirmed that all four samples that were positive for pepsin were due to pepsinogen C, whereas the negative samples had little or no pepsin/pepsinogen C (Fig 2).
Figure 2.
Western blot to detect pepsinogen/pepsin C. A Western blot probed with anti-pepsinogen/pepsin C antibody is shown. Lane 1 is purified human pepsinogen/pepsin C. Lanes 2, 4, 6, and 8 are from patients positive for pepsin C enzymatic activity. In all these patients, the pepsin C enzymatic activity is shown to be predominantly from pepsinogen C. Lanes 3, 5, 7, and 9 are from patients negative for measureable pepsin C enzymatic activity, showing little or no pepsinogen/pepsin C by Western blot.
On secondary exploratory analyses, pepsin detection was increased in men (OR, 12.7; 95% CI, 1.52-169.61), whereas obesity, age, the OSA predictor score, use of opiates or NSAIDS, smoking history, and diabetes were not significantly associated with pepsin concentrations or its detection (Table 2). When comparing pepsin concentrations between the first postintubation and the second preextubation samples, there was no significant difference (mean difference in pepsin concentrations = 2.9 ng/mL; 95% CI, −1.1 to 6.9). The mean time between the collections was 120 min (95% CI, 104-136 min), and duration between the two sample collections did not correlate with the change in pepsin concentrations.
Table 2.
—Clinical Variables and Total Pepsin Detection (Present/Absent)
| Variable | OR | 95% CI | P Value |
| BMI | 1.09 | 0.77-1.60 | .62 |
| Age | 1.03 | 0.94-1.16 | .59 |
| OSA predictor score | 0.98 | 0.78-1.22 | .87 |
| Diabetes | 0.18 | 0.0041-3.75 | .31 |
| Preoperative opioids | 0.75 | 0.088-5.65 | .77 |
| Preoperative NSAIDs | 1.08 | 0.15-8.87 | .94 |
| Sex (Male) | 12.71 | 1.52-169.61 | .029 |
Logistic regression was used to determine the multivariate OR of having pepsin detected in the airways for each of the clinical variables explored. Only male sex was significantly associated with pepsin detection, with an OR of 12.71. NSAID = nonsteroidal antiinflammatory drug. See Table 1 legend for expansion of other abbreviation.
Discussion
Although the link between reflux and pulmonary disorders has been well established in cross-sectional studies,7,8,27 it is unclear whether this is an association or whether reflux actually contributes to lung pathology via microaspiration. Toward a more causal mechanism, an increasing number of investigators have independently developed their own pepsin assays and successfully identified pepsin in the airways of a variety of lung pathologies: lung transplant patients,28,29 interstitial lung disease,30 pediatric patients,10 and the critically ill.6,11 Recognizing that pepsin itself is directly cytotoxic not just to the esophagus but also to the respiratory epithelium,31 it may help to finally link the relationship between reflux and lung pathology and explain why gastric acid-suppressive therapies have largely been ineffective in the treatment of presumed pulmonary manifestations of reflux.27,32 Unfortunately, controls in these airway pepsin studies have not consistently been reported, and, to our knowledge, only one study has tried to specifically examine for the presence of airway pepsin in a controlled setting using a qualitative pepsin assay in normal adults.33
However, there are additional important limitations to the assumption that the detection of pepsin in the lower airways is always indicative of gastropulmonary aspiration. First, the means of obtaining the airway specimen itself is flawed. For example, a bronchoscope has to be introduced through the pharynx to access the lower airways, and based on studies of salivary amylase, bronchoscopy almost always induces some degree of oropharyngeal aspiration.34,35 As such, pepsin in the lower airways may simply reflect proximal reflux (laryngopharyngeal reflux). A second important consideration is that, unlike pepsinogen A, pepsinogen C has been identified in extragastric36 sites, including the lungs.37‐39 Specifically, it appears to be expressed by type 2 pneumocytes and is involved in surfactant B processing.40 As such, it is plausible that the detection of pepsin in the lower airways, if not specific for pepsin A, may be due to the detection of pepsinogen C in pathologic states in which surfactant B expression has been increased. This would potentially account for why a variety of otherwise unrelated pathologic conditions have demonstrated an increase in airway pepsin. Similarly, the detection of pepsin in the upper airways (pharynx) has often been attributed to laryngopharyngeal reflux,41,42 but as it may reflect lower respiratory secretions, similar considerations on whether the source of the pharyngeal pepsin is gastric or pulmonary in origin must also be made. Our investigation tried to address some of these prior limitations and specifically aimed to also distinguish between the two primary pepsin isoforms A and C in lower airway samples.
Our study demonstrates surprisingly that enzymatically active pepsin may be recovered from the lower airways of up to 22% of otherwise healthy patients. However, contrary to increasing reports using airway pepsin as a marker of gastric-to-pulmonary aspiration, we were unable to detect any of the gastric predominant pepsin A isoform.17 Rather, all the pepsin-positive specimens could be attributed to pepsin/pepsinogen C. Thus, as pepsinogen C can be expressed natively from the lungs,37,38 the detection of pepsin C alone without pepsin A suggests that airway pepsin in these otherwise healthy subjects originated from the lungs rather than from microaspiration of gastric secretions. As such, our investigation challenges the face validity of using total pepsin concentrations as a biomarker of gastric-to-pulmonary aspiration, and raises the importance of (1) identifying the specificity of pepsin assays that are being reported, and (2) clearly establishing their validity. This report is one of few that attempt to estimate the frequency of pepsin detection in adults (without apparent lung disease or aspiration risk factors) and is the first report to our knowledge to suggest that pepsinogen C may indeed be expressed in adult lungs adequately enough to be detected in lower airway secretions. Secondarily, of the clinical variables explored, we found that men were more likely to have pepsin detected from their lower airways, which is at least consistent with prior literature on sex/hormonal differences in gastric secretion of pepsin.43,44
A number of limitations with this study deserve note. One, the study subjects are not truly normal healthy individuals, as they had an extremity fracture undergoing elective surgery. Nonetheless, we have carefully tried to exclude patients with lung pathologies and risk factors for aspiration. Moreover, a perfect normal standard could only be achieved if healthy volunteers were recruited for intubation and testing. In this preliminary stage of investigations, we did not feel this approach was justified. A second limitation with the current study is the limited sample size. This limitation would have been more of a concern had the primary aim resulted in a pepsin detection rate of zero (ie, type 2 error), but notably we identified 22% of subjects as having pepsin. Regardless, the limited sample size does limit our ability to evaluate clinical correlates in the exploratory and hypothesis-generating secondary analyses. A third limitation relates to the methodology used to obtain pepsin samples from the lower airways. As indicated, bronchoscopy is flawed in truly reflecting lower airway samples because of its potential to contaminate the intended lower respiratory sample. This potential limitation was addressed by carefully selecting out patients who were fasting, who were without thoracic or GI pathologies, and who underwent an uncomplicated intubation. Short of lung biopsies, this is the most appropriate way to sample the lower airways with minimal contamination. Finally, we cannot conclusively state that the pepsin detected was not of gastric origin, since pepsinogen C and pepsin C are also found in the stomach. However, it should be recognized that 80% of pepsin17 found in the stomach is pepsin A, with the rest mostly composed of pepsin C. Therefore, the detection of airway pepsin with a gastric source should include pepsin A to a greater extent than pepsin C. Observing the converse in our investigation strengthens the argument that the pepsin/pepsinogen detected in our samples originated from the lungs. Moreover, in a subsample of specimens that were positive for total pepsin by the activity assay, we confirmed that this positivity was due to pepsinogen C, the particular zymogen known to be expressed from type 2 pneumocytes.
Conclusions
We demonstrate in this study that enzymatically active pepsin can be detected from the lower airways of adults who otherwise should not have aspirated and challenge the notion that pepsin can be used as an exclusive biomarker for gastric-to-pulmonary aspiration. Although 22% of these otherwise normal subjects had pepsin, the predominant pepsin detected was the isoform pepsin/pepsinogen C and not pepsin A. Given that pepsinogen C can be natively expressed from type 2 pneumocytes, this finding suggests the need for caution when interpreting the presence of airway pepsin as confirmation of gastropulmonary aspiration. Future studies should be explicit when distinguishing pepsin/pepsinogen A from pepsin/pepsinogen C when measured from either lower or upper airway samples. Further investigations will be required to determine the clinical relevance of detecting pepsin/pepsinogen C in the airways.
Acknowledgments
Author contributions: Dr Lee is the guarantor of the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Bohman: contributed to enrolling study subjects, performing data collection and management, analyzing the results, and assembling the manuscript.
Dr Kor: contributed to conceiving and organizing the project, designing the research and protocol, providing oversight on the study conduct, analyzing the results, assembling the manuscript, and revising the manuscript.
Dr Kashyap: contributed to enrolling study subjects, performing data collection and management, and revising the manuscript.
Dr Gajic: contributed to conceiving and organizing the project and revising the manuscript.
Dr Festic: contributed to conceiving and organizing the project and revising the manuscript.
Dr He: contributed to designing the research and protocol, performing the sample analyses, and revising the manuscript.
Dr Lee: contributed to conceiving and organizing the project, designing the research and protocol, providing oversight on the study conduct, analyzing the results, assembling the manuscript, and revising the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank our study coordinators, Laurie Meade, RN, and Lavonne Liedl, RRT, from the Anesthesia Clinical Research Unit at Mayo Clinic, Rochester, for their help in subject screening and enrollment. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the Mayo Clinic.
Abbreviations
- IQR
interquartile range
- LMA
laryngeal mask airway
- NSAID
nonsteroidal antiinflammatory drug
- OSA
obstructive sleep apnea
Footnotes
Funding/Support: This research was supported in part by the Mayo Clinic and by the National Institutes of Health/National Center for Research Resources/National Center for Advancing Translational Sciences Clinical and Translational Science Award [Grant KL2 RR024151].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Cook TM, Woodall N, Frerk C; Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 2011;106(5):617-631. [DOI] [PubMed] [Google Scholar]
- 2.García Rodríguez LA, Ruigómez A, Martín-Merino E, Johansson S, Wallander MA. Relationship between gastroesophageal reflux disease and COPD in UK primary care. Chest. 2008;134(6):1223-1230. [DOI] [PubMed] [Google Scholar]
- 3.Tsai MC, Lin HL, Lin CC, et al. Increased risk of concurrent asthma among patients with gastroesophageal reflux disease: a nationwide population-based study. Eur J Gastroenterol Hepatol. 2010;22(10):1169-1173. [DOI] [PubMed] [Google Scholar]
- 4.Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010;139(3):754-762. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Ryu JH, Elicker BM, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(12):1390-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nseir S, Zerimech F, Jaillette E, Artru F, Balduyck M. Microaspiration in intubated critically ill patients: diagnosis and prevention. Infect Disord Drug Targets. 2011;11(4):413-423. [DOI] [PubMed] [Google Scholar]
- 7.Kor DJ, Warner DO, Alsara A, et al. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology. 2011;115(1):117-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bice T, Li G, Malinchoc M, Lee AS, Gajic O. Incidence and risk factors of recurrent acute lung injury. Crit Care Med. 2011;39(5):1069-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decalmer S, Stovold R, Houghton LA, et al. Chronic cough: relationship between micro-aspiration, gastroesophageal reflux and cough frequency. Chest. 2012;142(4):958-964. [DOI] [PubMed]
- 10.Farrell S, McMaster C, Gibson D, Shields MD, McCallion WA. Pepsin in bronchoalveolar lavage fluid: a specific and sensitive method of diagnosing gastro-oesophageal reflux-related pulmonary aspiration. J Pediatr Surg. 2006;41(2):289-293. [DOI] [PubMed] [Google Scholar]
- 11.Gopalareddy V, He Z, Soundar S, et al. Assessment of the prevalence of microaspiration by gastric pepsin in the airway of ventilated children. Acta Paediatr. 2008;97(1):55-60. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan U, Mitchell JD, Messina I, Day AS, Bohane TD. Assay of tracheal pepsin as a marker of reflux aspiration. J Pediatr Gastroenterol Nutr. 2002;35(3):303-308. [DOI] [PubMed] [Google Scholar]
- 13.Meert KL, Daphtary KM, Metheny NA. Detection of pepsin and glucose in tracheal secretions as indicators of aspiration in mechanically ventilated children. Pediatr Crit Care Med. 2002;3(1):19-22. [DOI] [PubMed] [Google Scholar]
- 14.Stovold R, Forrest IA, Corris PA, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: a putative association with rejection. Am J Respir Crit Care Med. 2007;175(12):1298-1303. [DOI] [PubMed] [Google Scholar]
- 15.Ward C, Forrest IA, Brownlee IA, et al. Pepsin like activity in bronchoalveolar lavage fluid is suggestive of gastric aspiration in lung allografts. Thorax. 2005;60(10):872-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metheny NA, Chang YH, Ye JS, et al. Pepsin as a marker for pulmonary aspiration. Am J Crit Care. 2002;11(2):150-154. [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts NB. Review article: human pepsins - their multiplicity, function and role in reflux disease. Aliment Pharmacol Ther. 2006;24(suppl 2):2-9. [DOI] [PubMed] [Google Scholar]
- 18.Blitt CD, Gutman HL, Cohen DD, Weisman H, Dillon JB. “Silent” regurgitation and aspiration during general anesthesia. Anesth Analg. 1970;49(5):707-713. [PubMed] [Google Scholar]
- 19.Ng A, Smith G. Gastroesophageal reflux and aspiration of gastric contents in anesthetic practice. Anesth Analg. 2001;93(2):494-513. [DOI] [PubMed] [Google Scholar]
- 20.Olsson GL, Hallen B, Hambraeus-Jonzon K. Aspiration during anaesthesia: a computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986;30(1):84-92. [DOI] [PubMed] [Google Scholar]
- 21.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62. [DOI] [PubMed] [Google Scholar]
- 22.Gali B, Whalen FX, Schroeder DR, Gay PC, Plevak DJ. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology. 2009;110(4):869-877. [DOI] [PubMed] [Google Scholar]
- 23.Mirant-Borde MC, Tripathi A, Lee A. Feasibility of pepsin measurement in endotracheal secretion specimens as a marker of aspiration [abstract]. Am J Respir Crit Care Med. 2011;183:A5841 [Google Scholar]
- 24.He Z, O’Reilly RC, Bolling L, et al. Detection of gastric pepsin in middle ear fluid of children with otitis media. Otolaryngol Head Neck Surg. 2007;137(1):59-64. [DOI] [PubMed] [Google Scholar]
- 25.Kageyama T, Takahashi K. Pepsinogen C and pepsin C from gastric mucosa of Japanese monkey. Purification and characterization. J Biochem. 1976;80(5):983-992. [DOI] [PubMed] [Google Scholar]
- 26.Roberts NB, Taylor WH. Comparative pepstatin inhibition studies on individual human pepsins and pepsinogens 1,3 and 5(gastricsin) and pig pepsin A. J Enzyme Inhib Med Chem. 2003;18(3):209-217. [DOI] [PubMed] [Google Scholar]
- 27.Galmiche JP, Zerbib F, Bruley des Varannes S. Review article: respiratory manifestations of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2008;27(6):449-464. [DOI] [PubMed] [Google Scholar]
- 28.Blondeau K, Mertens V, Vanaudenaerde BA, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31(4):707-713. [DOI] [PubMed] [Google Scholar]
- 29.Henley P, Mallea D, Erasmus D, et al. Enzymatically active pepsin as a potential biomarker of gastropulmonary aspiration following lung transplantation [abstract]. Am J Respir Crit Care Med. 2012;185:A5331 [Google Scholar]
- 30.Lee JS, Song JW, Wolters PJ, et al. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39(2):352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bathoorn E, Daly P, Gaiser B, et al. Cytotoxicity and induction of inflammation by pepsin in acid in bronchial epithelial cells. Int J Inflam 2011; 2011:569416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan WW, Chiou E, Obstein KL, Tignor AS, Whitlock TL. The efficacy of proton pump inhibitors for the treatment of asthma in adults: a meta-analysis. Arch Intern Med. 2011;171(7):620-629. [DOI] [PubMed] [Google Scholar]
- 33.Ufberg JW, Bushra JS, Patel D, Wong E, Karras DJ, Kueppers F. A new pepsin assay to detect pulmonary aspiration of gastric contents among newly intubated patients. Am J Emerg Med. 2004;22(7):612-614. [DOI] [PubMed] [Google Scholar]
- 34.Tripathi A, Mirant-Borde MC, Lee A. Amylase in bronchoalveolar lavage as a potential marker of oropharyngeal-to-pulmonary aspiration [abstract]. Am J Respir Crit Care Med. 2011;183:A4616 [Google Scholar]
- 35.Rennard SI, Ghafouri M, Thompson AB, et al. Fractional processing of sequential bronchoalveolar lavage to separate bronchial and alveolar samples. Am Rev Respir Dis. 1990;141(1):208-217. [DOI] [PubMed] [Google Scholar]
- 36.Merino AM, Vázquez J, Rodríguez JC, et al. Pepsinogen C expression in tumors of extragastric origin. Int J Biol Markers. 2000;15(2):165-170. [DOI] [PubMed] [Google Scholar]
- 37.Moriyama A, Kageyama T, Takahashi K. Identification of monkey lung procathepsin D-II as a pepsinogen-C-like acid protease zymogen. Eur J Biochem. 1983;132(3):687-692. [DOI] [PubMed] [Google Scholar]
- 38.Foster C, Aktar A, Kopf D, Zhang P, Guttentag S. Pepsinogen C: a type 2 cell-specific protease. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L382-L387. [DOI] [PubMed] [Google Scholar]
- 39.Elabiad MT, Zhang J. Detection of pepsinogen in the neonatal lung and stomach by immunohistochemistry. J Pediatr Gastroenterol Nutr. 2011;53(4):401-403. [DOI] [PubMed] [Google Scholar]
- 40.Gerson KD, Foster CD, Zhang P, Zhang Z, Rosenblatt MM, Guttentag SH. Pepsinogen C proteolytic processing of surfactant protein B. J Biol Chem. 2008;283(16):10330-10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knight J, Lively MO, Johnston N, Dettmar PW, Koufman JA. Sensitive pepsin immunoassay for detection of laryngopharyngeal reflux. Laryngoscope. 2005;115(8):1473-1478. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Liu X, Liu YL, et al. Correlation of pepsin-measured laryngopharyngeal reflux disease with symptoms and signs. Otolaryngol Head Neck Surg. 2010;143(6):765-771. [DOI] [PubMed] [Google Scholar]
- 43.Adeniyi KO. Gastric acid secretion and parietal cell mass: effect of sex hormones. Gastroenterology. 1991;101(1):66-69. [DOI] [PubMed] [Google Scholar]
- 44.Hirschowitz BI. Apparent and intrinsic sensitivity to pentagastrin of acid and pepsin secretion in peptic ulcer. Gastroenterology. 1984;86(5 pt 1):843-851. [PubMed] [Google Scholar]