Abstract
Background:
The presence of interstitial pneumonitis (IP) on surveillance lung biopsy specimens in lung transplant recipients is poorly described, and its impact on posttransplant outcomes is not established. The following study assessed the association of posttransplant IP with the development of bronchiolitis obliterans syndrome (BOS).
Methods:
We examined all recipients of primary cadaveric lung transplants at our institution between January 1, 2000, and December 31, 2007 (N = 145). Patients had bronchoscopies with BAL, and transbronchial biopsies performed for surveillance during posttransplant months 1, 3, 6, and 12 as well as when clinically indicated. Patients were given a diagnosis of IP if, in the absence of active infection and organizing pneumonia, they showed evidence of interstitial inflammation and fibrosis on two or more biopsy specimens.
Results:
IP was a significant predictor of BOS (OR, 7.84; 95% CI, 2.84-21.67; P < .0001) and was significantly associated with time to development of BOS (hazard ratio, 3.8; 95% CI, 1.93-7.39; P = .0001) within the first 6 years posttransplant. The presence of IP did not correlate with a significantly higher risk of mortality or time to death. There was no association between the presence of IP and the development of or time to acute rejection.
Conclusions:
The presence of IP on lung transplant biopsy specimens suggests an increased risk for BOS, which is independent of the presence of acute cellular rejection.
Lung transplantation remains the only curative option for end-stage lung diseases, such as cystic fibrosis and pulmonary fibrosis.1 Despite a growing understanding of lung transplantation, the 5-year mortality for transplant recipients remains about 50%.2 The majority of posttransplant complications occurring after the first posttransplant year are attributable to chronic allograft rejection.
Bronchiolitis obliterans syndrome (BOS) is the clinical manifestation of chronic allograft rejection. BOS is associated with numerous risk factors, including medication noncompliance,3 cytomegalovirus (CMV) infection,4,5 gastroesophageal reflex disease (GERD),6‐10 acute cellular rejection,11,12 and air pollution.13 The incidence of interstitial pneumonitis (IP) on lung biopsy specimen is poorly described, and its impact on outcomes is not established. This study assessed the association of posttransplant IP with the development of BOS.
Materials and Methods
Study Population
We examined all recipients of primary cadaveric lung transplants at our institution between January 1, 2000, and December 31, 2007 (N = 145), for evidence of IP on lung biopsy specimen. Clinical data were collected through December 31, 2010. Approval from our institutional review board (Partners Human Research Committee; protocol # 2007-P000334/3) was obtained prior to study initiation.
Care of Patients
Patients had scheduled bronchoscopies with BAL and transbronchial biopsies (TBBs) performed during posttransplant months 1, 3, 6, and 12. All attempts were made to adhere to current professional guidelines, which recommend that five or more specimens be obtained during bronchoscopy.14 All biopsy specimens obtained during the study period were included in the data set. Pulmonary function tests (Morgan Scientific, Inc) were obtained at every follow-up clinic visit using standards published by the American Thoracic Society.15 Additional pulmonary function tests and bronchoscopies were performed based on symptoms or clinical deterioration (as evidenced by desaturation and decline in FEV1, or forced expiratory flow, midexpiratory phase). The schedule of outpatient visits and surveillance bronchoscopies did not change over the study period.
Patients were given a diagnosis of BOS according to consensus criteria (Table 1).3 Patients were considered to have bacterial infection if bacterial cultures from either BAL or TBB lead to initiation of antibiotic therapy. Positive results from BAL or TBB viral cultures were considered clinically significant in all cases. Methods of assessment for CMV viremia changed at our institution over the study period. From 2000 to 2007 blood buffy coat was evaluated for CMV with the Digene Hybrid Capture System (Qiagen) DNA test. From 2008 to 2010, viremia was assessed through an in-house quantitative polymerase chain reaction technique. A positive test result for CMV viremia at any time point was considered significant in the analysis.
Table 1.
—Classification and Grading Criteria for BO and BOS
| Classification of BO | Classification of BOS |
| C0: Biopsy sample absent of BO | BOS 0: FEV1 > 90% of baseline and FEF25%-75% > 75% of baseline |
| C1: Biopsy sample showing BO | BOS 0-p: FEV1 81%-90% of baseline and FEF25%-75% ≤ 75% of baseline |
| BOS 1: FEV1 66%-80% of baseline | |
| BOS 2: FEV1 51%-65% of baseline | |
| BOS 3: FEV1 ≤ 50% of baseline | |
| Parameters for BO diagnosis | Parameters for BOS diagnosis |
| Presence defined by dense eosinophilic hyaline fibrosis in submucosa or bronchioles, resulting in partial or complete occlusion of lumina | Spirometry measurements made per ATS criteria |
| Diagnosis requires at least five pieces of well-aerated lung parenchyma retrieved from biopsy | Baseline value defined as the average of the two highest posttransplant measurements obtained at least 3 wk apart without bronchodilators |
| BOS is the preferred method for diagnosing chronic airway rejection | Changes present > 3 wk |
All patients received a standardized immunosuppressant regimen in accordance with our institutional protocols. Antithymocyte globulin induction therapy (ATGAM; Pfizer, Inc) and triple-agent maintenance immunosuppression were initiated in the immediate posttransplant period. All patients received empiric broad-spectrum peritransplant antibiotics tailored to respiratory tract cultures obtained in donors and recipients. A majority of patients received Pneumocystis jiroveci prophylaxis with trimethoprim-sulfamethoxazole. Prophylaxis for herpes virus was instituted according to donor and recipient CMV serostatus. CMV prophylaxis was changed from IV ganciclovir therapy to oral valganciclovir in 2001. No other changes were made to the immunosuppression or prophylactic antimicrobial protocols over the study period.
Specimen Processing
Biopsy specimens were fixed in formalin and embedded in paraffin. Six microscopic slides were produced; three were stained with hematoxylin and eosin for morphologic evaluation, and one (with intervening levels) was stained with Gram stain, methenamine silver stain, and acid-fast stain to identify microorganisms. The histologic slides were examined by local pathologists with expertise in lung transplant histology. All specimens were graded according to guidelines set forth by the International Society of Heart & Lung Transplantation.14
Definition of IP
IP was diagnosed in patients if, in the absence of active infection and organizing pneumonia on biopsy, the patient had evidence of diffuse interstitial chronic inflammation and fibrosis on two or more biopsy specimens (Fig 1). The mild, diffuse pattern of inflammation characterized as IP is distinct from that of cellular rejection in that it is a sparse (rather than dense) infiltrate not centered on parenchymal vessels or in bronchiolar walls. In addition to standard review of all samples by staff pathologists, including assessment of the presence or absence of IP, 10% of specimens were audited by a second, independent pathologist for diagnostic confirmation. The auditing pathologist was blinded to prior histologic evaluations. No inconsistencies with prior diagnoses were discovered during the course of the audit.
Figure 1.

Posttransplant transbronchial biopsy specimen demonstrating interstitial thickening and inflammation consistent with interstitial pneumonitis (hematoxylin and eosin stain, original magnification × 100).
Statistical Analysis
Categorical variables were analyzed using χ2 analysis or Fisher exact test. Continuous variables were evaluated using nonparametric assessment with the Wilcoxon rank sum test. Time to the development of BOS, death, and acute rejection was assessed using Kaplan-Meier survival curves. The proportional hazards assumption was tested by the application of the proportional hazards models. The assumption was violated in the survival analysis of time to BOS because there was an overlap of the survival curves in posttransplant year 6. Cox proportional hazard models were used to compare the risk for BOS in the first 6 years posttransplant, time to acute rejection, and time to death between study groups. IP was incorporated as a time-varying covariate in Cox models. The associations between IP and BOS, death, and acute rejection were analyzed with logistic regression models using generalized estimating equations. Correlations among outcome measurements within individual patients were evaluated. All statistical analyses were conducted using SAS versions 9.1 and 9.2 (SAS Institute Inc) software.
Results
Baseline clinical characteristics and demographic data appear in Table 2. Overall, 145 patients underwent 591 bronchoscopies over 8 years. Forty-four (30.3%) transplant recipients met criteria for inclusion into the IP group. The median time to the development of IP was 165 days from the date of transplant (interquartile range [IQR], 105-195 days). Of patients with evidence of IP, 26 (59%) were men compared with 50 (49%) without IP (P = .29). An equal distribution of patients with and without IP (60%) underwent single lung transplantation. Median patient age at the time of transplantation was similar in both groups. Pretransplant diagnoses among patients with and without IP showed similar distributions.
Table 2.
—Baseline Patient Characteristics
| Characteristic | IP Present (n = 44) | IP Absent (n = 101) | P Value |
| Male sex | 26 (59) | 50 (49) | .29 |
| Female sex | 18 (41) | 51 (51) | … |
| Age, y | 52.6 (41.8-59.9) | 55.7 (47.5-59.9) | .53 |
| Single lung transplant | 27 (61) | 61 (60) | 1.00 |
| Double lung transplant | 17 (39) | 40 (40) | … |
| Pretransplant pathology | |||
| Obstructive lung disease | 17 (39) | 38 (37) | .71 |
| Idiopathic pulmonary fibrosis | 11 (25) | 33 (33) | … |
| Cystic fibrosis | 11 (25) | 23 (23) | … |
| Other | 5 (11) | 7 (7) | … |
| Donor/recipient CMV status | |||
| D−/R− | 9 (21) | 23 (23) | .34 |
| D−/R+ | 6 (14) | 19 (19) | … |
| D+/R+ | 8 (19) | 27 (27) | … |
| D+/R− | 20 (46) | 31 (31) | … |
Data are presented as No. (%) or median (interquartile range). CMV = cytomegalovirus; D = donor; IP = interstitial pneumonitis; R = recipient.
Table 3 shows the frequency of histopathologic, bacterial, and virologic results of the bronchoscopies performed. A median of five (IQR, 4-5) bronchoscopies were performed in the IP group vs four (IQR, 2-5) in the non-IP group (P < .0001). This discrepancy was related to a higher number of clinically indicated bronchoscopies performed in the IP group (median, 3 [IQR, 2-4] vs 2 [IQR, 1-2] procedures; P < .0001). Patients meeting criteria for inclusion in the IP group had evidence of IP on a median of two (IQR, 2-3) samples, whereas patients in the non-IP group were noted to have evidence of IP on a median of one (IQR, 0-1) sample (P = .40).
Table 3.
—Pathologic, Bacterial, and Virologic Data From Bronchoscopic Evaluation of Transplant Recipients
| Variable | Recipients With IP (n = 44) | Recipients Without IP (n = 101) | P Value |
| No. biopsy specimens | 5 (4-5) | 4 (2-5) | < .0001 |
| Surveillance biopsiesa | 2 (1-3) | 2 (1-3) | .91 |
| Clinically indicated biopsiesb | 3 (2-4) | 2 (1-2) | < .0001 |
| Pathologic results | |||
| Presence of IP | 2 (2-3) | 1 (0-1) | .40 |
| Acute rejection | 0 (0-1) | 0 (0-1) | .84 |
| Severe acute rejectionc | 0 (0) | 0 (0) | .50 |
| Lymphocytic bronchiolitis | 0 (0-1) | 0 (0-1) | .18 |
| Microbiologic results | |||
| Bacterial infectiond | 0 (0-1) | 0 (0-1) | .58 |
| Viral infectione | 0 (0) | 0 (0) | .40 |
Data are presented as median (interquartile range). See Table 2 legend for expansion of abbreviation.
Routine transbronchial biopsy specimens obtained at 1, 3, 6, 12 wk per institutional protocols.
Transbronchial biopsy specimens obtained as a result of physician decisions regarding changes in clinical status, symptoms, or radiographic findings.
Defined as stage 3 or higher by the International Society of Heart & Lung Transplantation.14
Any positive bacterial culture result in the context of clinical or radiographic findings warranting treatment decisions by primary physicians.
Any recovery of viral agents from BAL or biopsy specimens.
A total of 71 bronchoscopy specimens with positive culture results led to the initiation of antibacterial treatment in the study cohort. The median frequency of treated bacterial infections was comparable between groups (0; IQR, 0-1; P = .58). The frequency of positive viral culture results on bronchoscopy was also comparable between groups (0; IQR, 0; P = .40), whereas posttransplant CMV viremia showed a trend toward higher frequency in the IP group (patients without IP, n = 33 [32%]; patients with IP, n = 24 [49%]; P = .07). No differences were observed in the frequency of acute rejection, severe acute rejection (defined as grade 3 or higher), or lymphocytic bronchiolitis between groups (Table 3).
Thirty-four (87%) patients without IP and 32 (91%) with IP met criteria for stage 1 BOS at the time of BOS diagnosis. Three (8%) patients without IP and two (6%) with IP met criteria for stage 2 BOS, whereas two (5%) patients without IP and one (3%) with IP met criteria for stage 3 (P = .82). Of the patients without IP who progressed in stage of BOS over the course of the study, 37% advanced to stage 2 and 63% to stage 3. In comparison, five (38%) and eight (61%) patients with IP progressed to stages 2 and 3, respectively (P = 1.0).
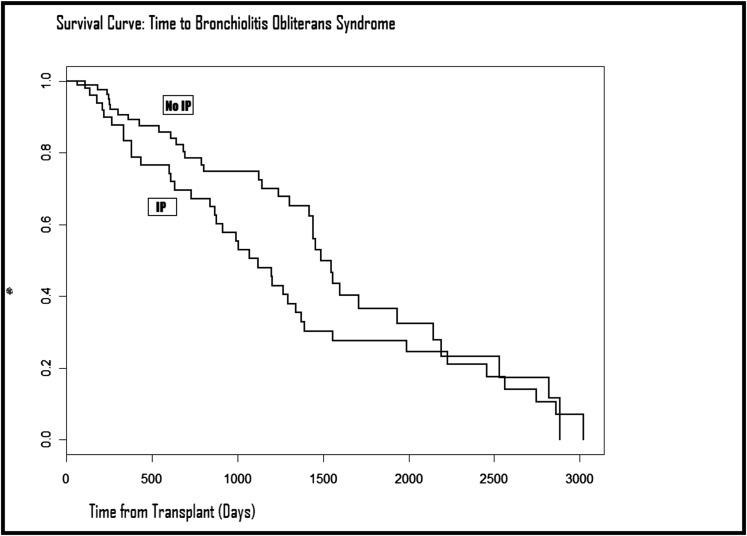
The median time from the diagnosis of IP to the development of BOS was 633.5 (IQR, 262.5-908) days. In a multivariate logistic regression model incorporating all baseline variables as well as transplant era, CMV viremia status, history of acute rejection, severe acute rejection, and lymphocytic bronchiolitis, IP was associated with a 7.8-fold increase in the odds of BOS development (OR, 7.84; 95% CI, 2.84-21.67; P < .0001). IP was significantly associated with time to BOS (hazard ratio [HR], 3.8; 95% CI, 1.93-7.39; P = .0001) within the first 6 years posttransplant. However, because of violations of the proportional hazards assumption (an intersection of the curves at posttransplant year 6), multivariate models for the entire follow-up period could not be assessed. Figure 2 shows the time from transplant to BOS for the two study groups. IP was not predictive of mortality (OR, 0.7; 95% CI, 0.30-1.63; P = .41) or time to death (HR, 0.67; 95% CI, 0.36-1.27; P = .22) in multivariate models. Moreover, IP was not predictive of acute rejection (OR, 0.96; 95% CI, 0.36-2.56; P = .93) or time to acute rejection (HR, 0.13; 95% CI, 0-1.75; P = .08) in multivariate models.
Figure 2.
Kaplan-Meier curves demonstrating time to the development of bronchiolitis obliterans syndrome in patients with and without IP. IP = interstitial pneumonitis.
Discussion
Our results suggest that the presence of IP on lung transplant biopsy specimen is a marker for increased risk of BOS development. This risk appears to be independent of the presence of acute cellular rejection. To the best of our knowledge, this study is the first to demonstrate a longitudinal association between IP and BOS.
Previously published studies examined the cross-sectional importance of pneumonitis in transplant recipients. McDonald et al16 noted that mixed IP accompanied 4.7% of allograft biopsy specimens across a 50-month sampling period. The authors of that study demonstrated that IP did not correlate with evidence of acute allograft rejection. While McDonald et al16 did not show an association with BOS, an association of IP with decreased survival was implied. In a cross-sectional retrospective study of parenchymal inflammatory changes in 2,697 posttransplant TBB specimens, Burton et al17 found evidence of IP in 288 samples (15%). IP was associated with an increased risk of bronchiolitis obliterans (OR, 2.2; P = .007). The present study further strengthens the existing evidence of a relationship between IP and the risk of BOS and time to BOS in the first 6 years posttransplant.
BOS is the primary cause of death after the first year in lung transplant recipients.2,18,19 The origins of chronic rejection are believed to be upstream events immediately following lung transplantation.20 In an update to the BOS classification system by the International Society of Heart & Lung Transplantation, greater credence is given to identifying patients at risk for BOS with the goal of establishing earlier interventions.3,21 The present findings suggest that awareness of IP has the potential to identify early BOS-prone patients at a stage where intervention may be beneficial. Identification of IP could allow for the early assessment and modification of potential risk factors for BOS while also providing a rationale for adjusting immunosuppressive medications.
There are several limitations to this study. The interpretive nature of biopsy specimen review has the potential to introduce bias into the results. To address this, an independent pathologist performed an audit of 10% of the biopsy samples and found no interpretive discrepancies. Patients with the finding of IP on only a single biopsy were included in the non-IP group, which could have introduced a bias. Additionally, the observation that an increased risk of BOS exists in patients with IP could have been confounded by ascertainment bias (ie, patients with IP had increased frequency of biopsy sampling and, therefore, more opportunities to show IP). An alternative interpretation may be that the presence of IP on lung biopsy specimen correlates with a higher rate of allograft injury, leading to a higher rate of lung biopsies performed.
Although we demonstrated that IP is associated with an increase risk of BOS in the first 6 years posttransplant, a proportional hazards model could not be used to assess the risk of IP with BOS overall. We postulate that the predictive value of IP is attenuated after posttransplant year 6 because BOS becomes more commonplace later in the posttransplant course. IP was, however, also strongly predictive of BOS development in a multivariate logistic regression model, supporting its predictive value in assessing the risk of BOS.
The data also demonstrate a trend toward increased CMV viremia in the IP group, raising the concern that the finding of IP in biopsy specimens reflects active CMV infection in the lung. However, no differences in viral infection rates on BAL were noted between the two groups, and pathologic changes suggestive of CMV infection were not identified in any of the samples ultimately classified as IP. In addition, IP remained a significant predictor of chronic allograft rejection after incorporation of all microbiologic data, including CMV viremia, into the multivariable model.
The study results do not incorporate radiographic findings of the patient population. High-resolution CT scanning and other chest radiography is not routinely obtained in transplant recipients at our institution and is not reported in other studies of IP. The changes seen in biopsy specimens were subtle and early, and we would not expect significant changes on standard CT images.
Finally, recognition of GERD and antibody-mediated rejection as risk factors for rejection and the methods used to assess these variables evolved significantly during the study period. As such, IP may represent a surrogate marker of the impact of these conditions on the development of BOS. We were unable to fully address this possibility through the study design. We suggest that recognition of the presence of IP, even if only as a surrogate for other conditions, should prompt an aggressive evaluation for modifiable risk factors for allograft dysfunction.
In summary, IP is a poorly understood and rarely discussed entity in the lung transplant population. To our knowledge, this study is the first to longitudinally assess patients with IP and correlate this finding with chronic allograft rejection. Early identification of patients at risk for chronic allograft rejection is critical to the preservation of allograft function, and we propose that posttransplant biopsy samples be assessed for the presence of IP. We also suggest further prospective studies to validate the finding of IP as either a marker of allograft injury or a risk factor for chronic rejection. Additional studies, preferably prospective, are needed to investigate the influence of GERD, infection, antibody-mediated rejection, and other potential confounding factors on the development of IP and rejection.
Acknowledgments
Author contributions: Dr Goldberg had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Mihalek: contributed to the data collection and writing and revision of the manuscript.
Dr Rosas: contributed to the study concept and design and writing and revision of the manuscript.
Dr Padera: contributed to the blinded audit of pathology specimens and writing and revision of the manuscript.
Dr Fuhlbrigge: contributed to the study concept and design and writing and revision of the manuscript.
Dr Hunninghake: contributed to the data interpretation and analysis and writing and revision of the manuscript.
Dr DeMeo: contributed to the study concept and design and writing and revision of the manuscript.
Dr Camp: contributed to the study concept and design and writing and revision of the manuscript.
Dr Goldberg: contributed to the study concept and design, data collection and analysis, and writing and revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The funding sources had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: The authors recognize the patients experiencing the myriad end-stage pulmonary diseases who have entrusted their medical care to our staff and institution.
Abbreviations
- BOS
bronchiolitis obliterans syndrome
- CMV
cytomegalovirus
- GERD
gastroesophageal reflux disease
- HR
hazard ratio
- IP
interstitial pneumonitis
- IQR
interquartile range
- TBB
transbronchial biopsy
Footnotes
Funding/Support: This study did not receive financial support from outside sources. Dr Rosas is supported by the National Institutes of Health [K23 HL087030]. Dr Fuhlbrigge is supported by the National Institutes of Health [5 U01 HL075419, N01 HR76183, 5 R01 HL094635, and 1 R01 HS019408]. Dr Hunninghake is supported by the National Institutes of Health [K08 HL092222]. Dr DeMeo is supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. This work was performed at Brigham and Women’s Hospital, Lung Transplant Program, Boston, MA.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351(9095):24-27. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-eight adult lung and heart-lung transplant report—2011. J Heart Lung Transplant. 2011;30(10):1104-1122. [DOI] [PubMed] [Google Scholar]
- 3.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297-310. [DOI] [PubMed] [Google Scholar]
- 4.Kroshus TJ, Kshettry VR, Savik K, John R, Hertz MI, Bolman RM., III Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg. 1997;114(2):195-202. [DOI] [PubMed] [Google Scholar]
- 5.Keller CA, Cagle PT, Brown RW, Noon G, Frost AE. Bronchiolitis obliterans in recipients of single, double, and heart-lung transplantation. Chest. 1995;107(4):973-980. [DOI] [PubMed] [Google Scholar]
- 6.Reid KR, McKenzie FN, Menkis AH, et al. Importance of chronic aspiration in recipients of heart-lung transplants. Lancet. 1990;336(8709):206-208. [DOI] [PubMed] [Google Scholar]
- 7.Rinaldi M, Martinelli L, Volpato G, et al. Gastro-esophageal reflux as cause of obliterative bronchiolitis: a case report. Transplant Proc. 1995;27(3):2006-2007. [PubMed] [Google Scholar]
- 8.Cantu E III, Appel JZ III, Hartwig MG, et al. J. Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Chest. 2004;78(4):1142-1151. [DOI] [PubMed] [Google Scholar]
- 9.D’Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129(5):1144-1152. [DOI] [PubMed] [Google Scholar]
- 10.Hartwig MG, Anderson DJ, Onaitis MW, et al. Fundoplication after lung transplantation prevents the allograft dysfunction associated with reflux. Ann Thorac Surg. 2011;92(2):462-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bando K, Paradis IL, Similo S, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995;110(1):4-13. [DOI] [PubMed] [Google Scholar]
- 12.Husain AN, Siddiqui MT, Holmes EW, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 1999;159(3):829-833. [DOI] [PubMed] [Google Scholar]
- 13.Nawrot TS, Vos R, Jacobs L, et al. The impact of traffic air pollution on bronchiolitis obliterans syndrome and mortality after lung transplantation. Thorax. 2011;66(9):748-754. [DOI] [PubMed] [Google Scholar]
- 14.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229-1242. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. [DOI] [PubMed] [Google Scholar]
- 16.McDonald JW, Keller CA, Ramos RR, Brunt EM. Mixed (neutrophil-rich) interstitial pneumonitis in biopsy specimens of lung allografts: a clinicopathologic evaluation. Chest. 1998;113(1):117-123. [DOI] [PubMed] [Google Scholar]
- 17.Burton CM, Iversen M, Carlsen J, Andersen CB. Interstitial inflammatory lesions of the pulmonary allograft: a retrospective analysis of 2697 transbronchial biopsies. Transplantation. 2008;86(6):811-819. [DOI] [PubMed] [Google Scholar]
- 18.Burke CM, Theodore J, Dawkins KD, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest. 1984;86(6):824-829. [DOI] [PubMed] [Google Scholar]
- 19.Glanville AR, Baldwin JC, Burke CM, Theodore J, Robin ED. Obliterative bronchiolitis after heart-lung transplantation: apparent arrest by augmented immunosuppression. Ann Intern Med. 1987;107(3):300-304. [DOI] [PubMed] [Google Scholar]
- 20.Neuringer IP, Chalermskulrat W, Aris R. Obliterative bronchiolitis or chronic lung allograft rejection: a basic science review. J Heart Lung Transplant. 2005;24(1):3-19. [DOI] [PubMed] [Google Scholar]
- 21.Bowdish ME, Arcasoy SM, Wilt JS, et al. Surrogate markers and risk factors for chronic lung allograft dysfunction. Am J Transplant. 2004;4(7):1171-1178. [DOI] [PubMed] [Google Scholar]