Abstract
The increase in total cross-sectional area in the distal airways of the human lung enhances the mixing of each tidal breath with end-expiratory gas volume by slowing bulk flow and increasing gas diffusion. However, this transition also favors the deposition of airborne particulates in this region because they diffuse 600 times slower than gases. Furthermore, the persistent deposition of toxic airborne particulates stimulates a chronic inflammatory immune cell infiltration and tissue repair and remodeling process that increases the resistance in airways <2 mm in diameter four to 40-fold in COPD. This increase was originally attributed to lumen narrowing because it increases resistance in proportion to the change in lumen radius raised to the fourth power. In contrast, removal of one-half the number of tubes arranged in parallel is required to double their resistance, and approximately 90% need to be removed to explain the increase in resistance measured in COPD. However, recent reexamination of this problem based on micro-CT imaging indicates that terminal bronchioles are both narrowed and reduced to 10% of the control values in the centrilobular and 25% in the panlobular emphysematous phenotype of very severe (GOLD [Global Initiative for Chronic Obstructive Lung Disease] grade IV) COPD. These new data indicate that both narrowing and reduction in numbers of terminal bronchioles contribute to the rapid decline in FEV1 that leads to severe airway obstruction in COPD. Moreover, the observation that terminal bronchiolar loss precedes the onset of emphysematous destruction suggests this destruction begins in the very early stages of COPD.
COPD is caused by the persistent inhalation of toxic particles and gases primarily but not exclusively derived from tobacco smoke.1 These types of repetitive lung tissue injuries stimulate a chronic inflammatory immune response in everyone who smokes and, in a susceptible minority, this process increases in both extent and severity as FEV1 declines and persists long after the person has stopped smoking tobacco.2 This response to repetitive injury differs substantially from that observed following a single injury where the inflammatory immune cell infiltration begins to disappear as fibroblasts migrate into the site of injury to lay down a mature scar in the absence of infiltrating inflammatory cells . In that repetitive injury is associated with persistent inflammatory immune cell infiltration and abnormal scar tissue formation (ie, fibrosis) that persists long after the inhalation exposure has stopped.3 It is also of interest that different regions respond in different ways. For example, the chronic bronchitis that develops in cartilaginous airways is associated with enlarged bronchial mucus glands and goblet metaplasia of the airway epithelial lining.4-6 The small conducting airways <2 mm in diameter that become the major site of airway obstruction in COPD7 show progressive thickening of their walls, infiltration of the wall tissue by inflammatory immune cells, and occlusion of the airway lumen by inflammatory exudates containing mucus as FEV1 declines.2 In contrast, dilatation and destruction of the gas-exchanging tissue beyond the terminal bronchioles defines emphysematous destruction of the lung. The purpose of this brief review is to discuss the relationship between small airways obstruction and emphysematous destruction based on new information gained using micro-CT imaging to obtain a better understanding of the three-dimensional anatomy of the peripheral lung and MRI imaging of hyperpolarized helium 3 (He3) to obtain a clearer insight into the effect of the changes in anatomy on gas distribution in the centrilobular emphysematous phenotype of emphysema commonly observed in smokers.
Peripheral Lung Anatomy
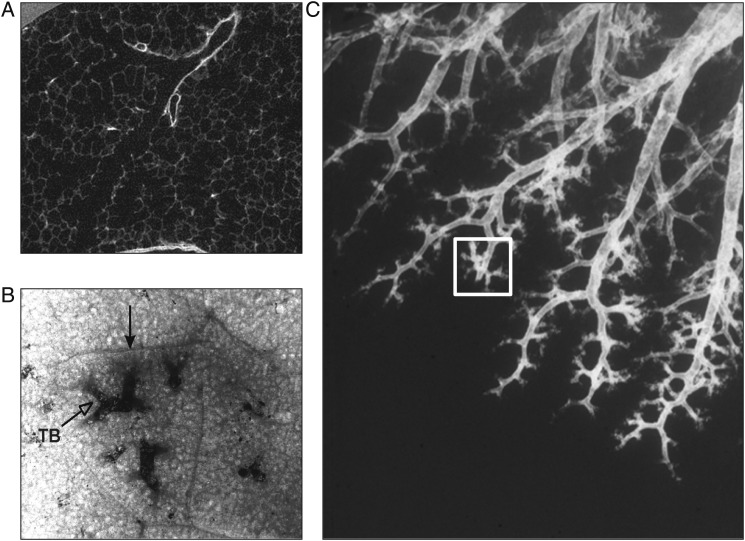
The boundary between purely conducting airways and gas-exchanging tissue is formed by the division of approximately 44,510±15,574 (mean±SD) terminal bronchioles into transitional bronchioles or first-order respiratory bronchioles where alveolar openings first appear (Fig 1).8,9 The alveolar openings steadily increase with successive generations of branching until they occupy the entire luminal surface of alveolar ducts and ducts branch for several more generations before terminating in closed alveolar sacs.8 The term “acinus” refers to a unit of lung supplied by a single terminal bronchiole; the term secondary lobule refers to groups of acini identified by either a surrounding connective tissue septa (Fig 1B) or a shift in bronchiolar branching pattern (Fig 1C) from one where the prelobular bronchioles branch roughly every centimeter to intralobular bronchioles that cluster only millimeters apart.8,10 The variation in pathway length from the trachea to the gas-exchanging surface distributes 2-mm airways from the fourth to the 14th generation of airway branching and the intralobular bronchioles between the eighth and 22nd generation of airway branching.11,12 The color-coded map (Fig 2B) illustrates that single generations of branching contain airways of different size by replotting Weibel’s data concerning the location of airways of different size.9,11 One effect of the rapid increase in the total cross-sectional area as branching generation increases (Figs 3A, 3B) is slowing the velocity of bulk flow and enhancing the diffusion of gas to the alveolar surface. Although this transition facilitates mixing between inspired air and end-expiratory gas volume, it also exposes the transitional region of the lung to excess deposition of particles suspended in the air because they diffuse much slower than gas. For example, the 0.5-μm diameter airborne particles that have the greatest probability of reaching the transitional zone of the lung are roughly 1,000 times larger and have a diffusion constant approximately 1 million times smaller than gas molecules.15 Moreover, because their root-mean-square displacement per second by diffusion is approximately 600 times smaller than oxygen (ie, 10 μm/s vs 6,000 μm/s), the particles are able to settle on the surface of the transitional zone of the lung between breaths.15 Furthermore, because the surface area of the preterminal, terminal, and transitional bronchioles is relatively small compared with the surface area of more distal air spaces, these airways receive the greatest dose of inhaled particles per unit of surface area.11
Figure 1.
A microCT scan of a terminal bronchiole branching into two first-order respiratory bronchioles (ie, transitional bronchioles) where alveolar openings first appear (reproduced with permission from McDonough et al9). B, Bronchogram of a postmortem human lung where a cluster of terminal bronchioles are surrounded by a fibrous connective tissue septa to form a secondary lung lobule.8 C, A lobule as defined by Reid10 where the branching pattern switches from one where the branches are cm apart to one where the branches within the square are only mm apart. TB=terminal bronchiole.
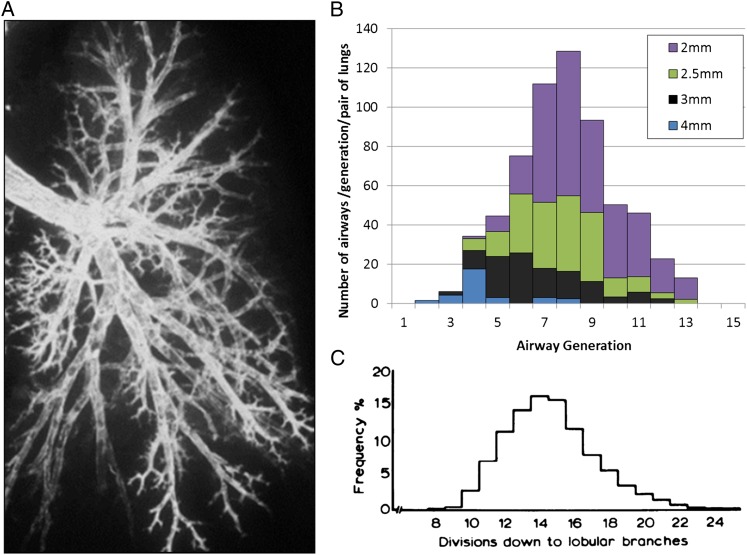
Figure 2.
A, Bronchogram from a normal human lung to demonstrate that the pathways from the main stem bronchus to the alveoli vary substantially in length. B, A color-coded map of data replotted from Weibel11 to demonstrate the distribution of airways of different sizes within each generation of airway branching. C, Data from Horsfield and Cumming12 demonstrating that the intralobular branches (see Fig 1 for a description of a lobule) can be reached in as few as eight generations of branching when the shortest pathways are followed, that the mean number of branches required to reach the interlobular airways is approximately 14 and that it may take as many as 24 generations of branching when the longest pathways are followed.
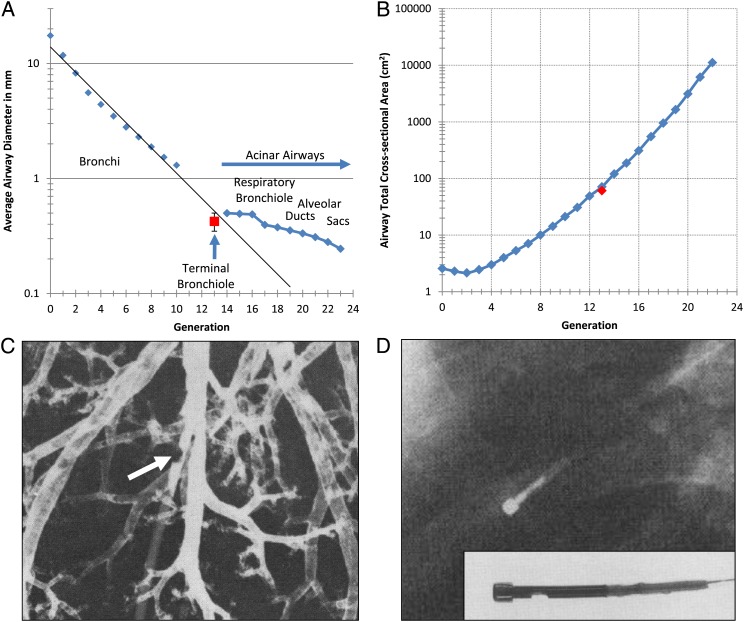
Figure 3.
A, Data from Weibel11 showing the average airway diameter at each generation of branching measured directly from an airway cast. The red square with error bars shows the mean diameter of the terminal bronchioles measured by McDonough et al9 using microCT scan. B, Comparison of the total cross-sectional area of all the airways in each generation of airway branching measured by Weibel11 to the total cross-sectional area of the terminal bronchioles measured from microCT images by McDonough et al.9 C, A retrograde catheter (arrow) placed in a small airway approximately 2 mm in diameter in the manner described in References 7 and 13. D, Chest radiograph from a living human with normal lung function where the bronchial pressure-measuring device developed by Yanai and colleagues14 was put in place through a bronchoscope. The direct measurement of the resistance of the small airways <2 mm in diameter provided in both postmortem human lungs7,13 and in living humans14 are consistent with Weibel’s measurements of a rapidly increasing total cross sectional area of the distal airways.
Peripheral Airways Resistance in Normal Lungs
The older paradigm that the small conducting airways offer the most resistance to airflow in normal human lungs put forward by Rohrer in the 1920s16,17 began to change in the 1960s when Green repeated Rohrer’s calculations using Weibel’s new data and found that the smaller airways offered much less resistance than Rohrer’s calculations indicated because both the total number and cross-sectional area of the smaller conducting airways were underestimated.11,16-18 However, the major shift in this paradigm occurred when Macklem and Mead13 provided the first direct measurements showing that airways <2 mm in diameter accounted for <20% of total lower airway resistance in both anesthetized animal and postmortem human lungs. In a subsequent editorial, Mead19 referred to this region of the lung as a “quiet zone” within the lung where disease might accumulate over many years without coming to the attention of either patient or physician. This new paradigm initiated a period of intense investigation into abnormalities in the peripheral lung during the transition between health and disease.20 However, this effort was eventually abandoned because the tests designed to detect small airways disease were unable to consistently identify the minority of smokers who would go on to develop COPD.21 Even though cross-sectional studies showed that pathology was present in the small airways when the FEV1 was normal and that the pathology progressed as FEV1 declined.22
A Belgian group subsequently challenged the theory that small airways resistance was low in normal lungs using a technique similar to the one developed by Macklem and Mead (Fig 3C) to obtain data that was more consistent with Rohrer’s calculations than with Green’s.13,23 However, three subsequent reports on living humans with normal lung function from two different laboratories using different methods supported Macklem and Mead’s original findings.14,24,25 In one of these studies, Yanai et al14 introduced a small catheter with a pressure-sensing device at its tip (Fig 3D) into the small airways of living humans with normal lung function and subtracted the resistance from mouth to catheter tip from measurements of total airways resistance measured during quiet breathing. In two other studies, Wagner and colleagues24,25 created pressure-flow curves in the peripheral airways by wedging a double-lumen catheter and delivering a constant flow of air at different rates though one lumen while measuring driving pressure through the other. The slope of the pressure flow curves produced by this procedure was then measured to obtain peripheral airway resistance. Table 1 summarizes the data from all of these sources and shows that total resistance in centimeters of water per liter per second of 0.18±0.04 (25% of total resistance) measured in control lungs confirmed Macklem and Mead’s original findings and compare favorably to the 0.15±0.03 and 0.18±0.05 measured by Wagner and associates.24,25 Although the values reported by Yanai et al14 (0.70±0.30) are somewhat higher, they still accounted for only 25% of the total resistance when it included the upper airways and larynx. This leaves the 1.24±0.27 (77% of total resistance) measured by the Belgian group as a single outlier that is not easy to explain.23
Table 1.
— Summary of Direct Measurements of Total and Peripheral Airways Resistance in Human Lungs
| Control |
Emphysema |
Chronic Bronchitisa |
||||
| Source | Total | Peripheral | Total | Peripheral | Total | Peripheral |
| Hogg et al7 | 0.69±0.04 | 0.18±0.04 | 3.66±0.99 | 2.97±0.93 | ... | ... |
| Van Brabandt et al23 | 1.52±0.27 | 1.24±0.27 | 10.47±3.79 | 9.44±3.42 | ... | ... |
| Yanai et al14 | ||||||
| Inspiration | 3.1±0.1 | 0.7±0.3 | 5.9±0.8 | 2.8±0.4 | 8.7±1.1 | 4.6±0.5 |
| Expiration | 4.0±0.3 | 1.1±0.4 | 7.3±1.1 | 3.7±0.6 | 10.6±1.8 | 5.9±0.8 |
| Wagner et al24/1990 | ... | 0.15±0.03 | ... | ... | ... | ... |
| Wagner et al25/1998 | ... | 0.18±0.05 | ... | ... | ... | ... |
Mean±SE. All units are cm H2O/L/s. The original Macklem and Mead data can be found in Reference 13. References in the Source column refer to studies where direct measurements of peripheral airways resistance have been reported.
Although the authors referred to these cases as chronic bronchitis, the obstruction was most likely attributable to the bronchiolitis present in some but not all cases of chronic bronchitis.14
Peripheral Airway Resistance in COPD
In contrast to the disagreement concerning the resistance to flow offered by small airways <2 mm in diameter in normal lungs, all three groups that have made direct measurements of small airways resistance in COPD (including the Belgian group) reported that the small conducting airways <2 mm in diameter become the major site of increased resistance in COPD.7,14,23
On theoretical grounds, the substantial increase in peripheral airways resistance measured in COPD is easier to explain by generalized narrowing of most of small airways lumens rather than by a reduction in their number because lumen narrowing increases resistance in proportion to the reduction in the radius raised to the fourth power. However, in a system of tubes arranged in parallel, total resistance at each generation is obtained by addition according to the equation 1/RT=1/R111/R211/R3+...11/Rn meaning that removal of one-half of the tubes arranged in parallel is required to double their resistance. Therefore, if we accept the theory on the behavior of tubes arranged in parallel summarized by the above equation and begin with the value 0.70±0.26 cm H2O/L/s measured by Yanai et al14 in living humans with normal lung function, removal of one-half of the small airways will only increase their resistance to 1.4 cm H2O/L/s. A value well below the 2.78 and 4.59 cm H2O/L/s measured in living people with COPD in the same study (Table 1).24 Furthermore, removal of one-half of the remaining airways (ie, a reduction to 25% of their starting numbers) would only increase small airways resistance to 2.8 cm H2O/L/s which is barely within the lower range of the resistance they measured in COPD (Table 1). A reduction to 12.5% of their starting number is required to increase small airways resistance to 5.6 cm H2O/L/s which falls in the upper range of the values measured in COPD by Yanai et al.14 Bignon et al26 provided the first histologic evidence in support of the theory that the airways narrowed in COPD by showing that the proportion of bronchioles <400 μm in diameter increased in lungs from patients that died of respiratory failure caused by severe emphysema. Although Matsuba and Thurlbeck27 reported a similar trend in people with less severe emphysema that did not reach statistical significance, they added the caveat that complete destruction of the smaller bronchioles might have buffered the downward shift in small airways diameters measured in their study. Importantly, both of these studies were done long before the stereology-based methodology for counting numbers of structures in three-dimensional space became available and subsequently became accepted in guidelines published in a joint American Thoracic Society/European Respiratory Society statement.28
The introduction of micro-CT imaging greatly simplified the application of the American Thoracic Society/European Respiratory Society guidelines because it made it possible to identify terminal bronchioles anatomically, counting their number per milliliter in known volumes of a representative lung tissue sample that allowed the total number of terminal bronchioles per lung pair to be computed. As the product of the mean number of terminal bronchioles per milliliter of sampled lung tissue and total lung volume measured from the multidetector CT scan of the intact lung specimen.9 In addition, the product of the total number of terminal bronchioles per lung pair and direct measurements of the mean lumen cross-sectional area provided the total cross-sectional area of the terminal bronchioles in the lung.9 Comparison of these new data to the mean of four published datasets of the individual lung casts showed excellent agreement between total numbers of terminal bronchioles per lung pair (44,510±15,574 vs 44,500±18,574 mean±SD).11,12,17,29 Moreover, terminal bronchiolar diameter (424±76 μm mean±SD) and total cross-sectional area (3,050.3±576.6 mm2) were almost exactly the same (see Figs 3C, 3D) as those in Weibel’s classic report.11 In sharp contrast, lungs donated by patients with very severe COPD (GOLD [Global Initiative for Chronic Obstructive Lung Disease] grade IV) treated by lung transplantation (Table 2) showed the number of terminal bronchioles per lung pair was reduced to approximately 10% of control values in the centrilobular emphysematous phenotype and to 25% of the control values in the panlobular emphysematous phenotype of COPD observed in α-1 antitrypsin deficiency. In addition, the mean diameter of surviving terminal bronchioles was reduced from 424±76 μm in the control subjects to 52±30 μm in centrilobular emphysema and 210±48 μm in the panlobular emphysematous phenotypes of COPD.9 The combination of micro-CT imaging and histology also showed this massive reduction in total number of terminal bronchioles is associated with thickening of the walls of the surviving bronchioles.9 More importantly, they also showed that the number of terminal bronchioles per milliliter lung was reduced before the alveolar dimensions measured as the mean linear intercept increased into the emphysematous range.9 These observations established that the primary cause of small airways narrowing is related to the pathology in the small airways themselves and that any further narrowing caused by emphysematous destruction lung elastic recoil on the supporting structure of the small airways must occur as a secondary phenomenon associated with the later onset of emphysematous destruction. Based on these findings, we speculate that the gradual decline in FEV1 with age is explained by a parallel subtraction of terminal and perhaps preterminal bronchioles over time that has little effect on total resistance until large numbers of airways are destroyed. Moreover, we speculate that a similar mechanism of tissue remodeling and destruction could be responsible for both the bronchiolar and alveolar tissue in COPD.
Table 2.
—Comparison of Control to Patient Data
| Characteristics | Control | CLE | PLE |
| No. of patients (No. of lungs) | 4 (4) | 4 (4) | 8 (10) |
| Sex, female (male) | 0 (4) | 2 (2) | 3 (5) |
| Age, y | 53.8±4.3 | 60.0±1.6 | 49.6±3.8 |
| Pack-y | 31.5±7.5, n=2 | 43.0±5.5 | 17.9±3.2 |
| FEV1, % pred | N/A | 18.0±2.7 | 19.0±1.6 |
| FEV1/FVC | N/A | 26.8±2.9 | 32.6±2.3 |
| Total lung volume, % predicted | N/A | 137.0±3.6 | 140.1±4.1 |
| Lung volume, mL | 3,251±261 | 3,456±602 | 3,794±595 |
| Lung mass, g | 332±11 | 358±27 | 394±41 |
| No. of terminal bronchioles/mL | 6.9±1.2 | 0.7±0.1 | 1.6±0.5 |
| Total no. of terminal bronchioles | 22,300±3,900 | 2,400±600 | 6,200±2,100, n=7 |
| Average terminal bronchioles cross-sectional area, mm2 | 0.145±0.025 | 0.004±0.002 | 0.047±0.012 |
| Minimum terminal bronchiole lumen diameter, μm | 424.0±48.0 | 51.8±30.0 | 210.2±48.0 |
| Total terminal bronchiole cross-sectional area, mm2 | 3,050.3±576.6 | 7.7±5.1 | 514.1±181.9, n=7 |
CLE=centrilobular emphysema; N/A=not available; PLE=panlobular emphysema associated with α1-antitrypsin deficiency. (Adapted with permission from McDonough et al.9)
The Sequence of Tissue Destruction in COPD
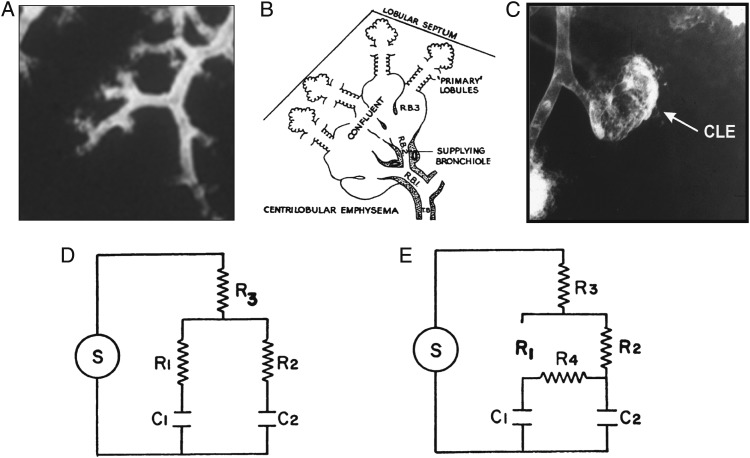
Figure 4 was constructed from existing data where Figure 4A shows normal terminal bronchioles and Figure 4B shows Leopold and Gough’s30 original diagram of a primary centrilobular lesion that develops beyond the terminal bronchiole that was also described by McLean31 at about the same time. Figure 4C shows a fully developed centrilobular lesion outlined by radio-opaque dust in a study that showed these lesions are on the flat part of their pressure volume curve at about 80% of their total gas volume and have a reduced compliance in relation to normal alveoli.32 Figure 4D represents the electrical analogue of the normal lung lobule where terminal bronchioles R1 and R2 supply normal alveoli C1 and C233 to a diseased situation (Fig 4E) where the normal alveoli beyond a destroyed R1 are collaterally ventilated from the centrilobular emphysematous lesion forming the surviving bronchiole R2 and the resistance between the centrilobular lesion and surrounding normal lung falls to very low levels. Although collateral flow resistance is difficult to measure within individual lobules, it has been measured across incomplete lobar fissures in postmortem lungs and within lobes of lungs in living people where both groups of investigators showed that collateral flow resistance falls from very high levels in normal lungs to very low levels in lungs from patients with emphysema.34,35 MRI imaging studies of the distribution of hyperpolarized He3 gas provided new information by demonstrating both large and small filling defects following the inspiration of He3 gas.36,37 In addition, Marshall and colleagues37 demonstrated collateral movement of gas into the larger defects from the surrounding lung tissue during a 20-s breath hold following the inhalation of hyperpolarized gas. Based on these reports, we postulate that, within a normal lobule (Fig 4A), destruction of a terminal bronchiole is followed by: (1) the spread of the destructive process along surviving bronchioles to initiate the primary lesions of centrilobular emphysema (Fig 4B), (2) coalescence of several primary lesions forming larger centrilobular emphysematous spaces (Fig 4C), and (3) enlarged collateral channels formed by this centrilobular destruction ventilating the normal alveoli isolated by the initial destruction of the terminal bronchiole.34 In contrast, the larger bullous lesions formed by coalescence of completely destroyed lobules fill through collateral channels formed at their boundary with the surrounding normal lung because nearly all of the supplying bronchioles are completely destroyed within these bullous lesions.
Figure 4.
A, Normal lung lobule containing six terminal bronchioles. B, Leopold and Gough’s30 original diagram of a primary centrilobular emphysematous lesion described almost simultaneously by themselves in the UK and by McLean31 in Australia. C, Bronchogram of a centrilobular emphysematous space formed by the coalescence of several primary lesions.32 D, The electrical analog of the peripheral lung used by Otis et al,33 where S is the power source, R is resistance, and C is capacitance. E, Modification of the normal electrical analogue to include collateral channels R4, that have very high resistance in the normal lung and fall to very low levels in emphysematous regions of lungs affected by COPD.34,35 We postulate that the primary centrilobular lesions develop beyond surviving terminal bronchioles and that the fall in collateral resistance associated with emphysematous destruction provides ventilation to the normal alveoli located beyond destroyed terminal bronchioles. CLE=centrilobular emphysematous space.
Summary
In the normal lung, the increase in total cross-sectional area of the conducting airways with each generation of branching facilitates gas mixing by slowing the bulk flow of gas and increasing its diffusion into the distal airapaces.11,15 However, this transition also favors the accumulation of airborne particles within this region of the lung because these particles diffuse approximately 600 times slower than gas.15 The accumulation of toxic particles in this lung region stimulates a persistent inflammatory immune response and tissue repair and remodeling process that sequentially destroys terminal followed by respiratory bronchioles to form centrilobular lesions. This is followed by the destruction of the entire lung lobule, and the coalescence of many destroyed lobules forms the larger bullous lesions. The observation that terminal bronchiolar destruction precedes emphysematous in COPD is consistent with recent reports showing that the early appearance of emphysematous destruction predicts a more rapid decline in FEV138-41 because it is consistent with widespread destruction of terminal and possibly preterminal bronchioles before emphysematous destruction appears. Collectively, these observations suggest that the reason treatment trials have failed to reverse the decline in FEV1 is that large numbers of terminal bronchioles have already been destroyed.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Hogg serves as the principal investigator (PI) or co-investigator of grants to The University of British Columbia from the US National Institutes of Health, Canadian Institutes of Health Research, and the British Columbia Lung Association. Additionally, Dr Hogg serves as the PI on a contract between The University of British Columbia and Boehringer Ingleheim GmbH (BI) and Merck Canada Inc, a subsidiary of Merck Sharp & Dohme Corp. Portions of the salary paid to Dr Hogg from The University of British Columbia come from these grants. Dr Hogg has served as both a consultant and speaker for Novartis AG and BI. Drs McDonough and Suzuki have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- He3
helium 3.
Footnotes
Dr McDonough is currently pursuing postdoctoral studies at Imperial College London, London, England. Dr Suzuki is currently affiliated with Hokkaido University, Saporro, Japan.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Rabe KF, Hurd S, Anzueto A, et al. ; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555. [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645-2653. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Abbas A, Fausto N. Tissue renewal and repair: regeneration, healing and fibrosis. In: Robbins & Cotran Pathologic Basis of Disease. 7th ed Elsevier Saunders; 2005:87-118. [Google Scholar]
- 4.Reid L. Measurement of the bronchial mucous gland layer: a diagnostic yardstick in chronic bronchitis. Thorax. 1960;15:132-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullen JB, Wright JL, Wiggs BR, Pare PD, Hogg JC. Reassessment of inflammation of airways in chronic bronchitis. Br Med J (Clin Res Ed). 1985;291(6504):1235-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saetta M, Di Stefano A, Maestrelli P, et al. Activated T-lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitis. Am Rev Respir Dis. 1993;147(2):301-306. [DOI] [PubMed] [Google Scholar]
- 7.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355-1360. [DOI] [PubMed] [Google Scholar]
- 8.Miller WS. The Lung. Springfield, IL: C. C. Thomas; 1947:222. [Google Scholar]
- 9.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid L. The secondary lobule in the adult human lung, with special reference to its appearance in bronchograms. Thorax. 1958;13(2):110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weibel ER. Morphometry of the Human Lung. New York, NY: Academic Press Inc; 1963. [Google Scholar]
- 12.Horsfield K, Cumming G. Morphology of the bronchial tree in man. J Appl Physiol. 1968;24(3):373-383. [DOI] [PubMed] [Google Scholar]
- 13.Macklem PT, Mead J. Resistance of central and peripheral airways measured by a retrograde catheter. J Appl Physiol. 1967;22(3):395-401. [DOI] [PubMed] [Google Scholar]
- 14.Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol. 1992;72(3):1016-1023. [DOI] [PubMed] [Google Scholar]
- 15.Altshuler B, Palmes ED, Yarmus L, Nelson N. Intrapulmonary mixing of gases studied with aerosols. J Appl Physiol. 1959;14(3):321-327. [DOI] [PubMed] [Google Scholar]
- 16.Rohrer F. Der Stromungswiderstand in der menschlichen Atemwegen und der Einfluss der unregelmässigen Verzweigung es Bronchial-systems auf der Atmungsverlauf in vershiedenen Lungenbezinken. Arch Ges Physiol. 1915;162:225-229. [Google Scholar]
- 17.Rohrer F. Physiologie der Atembewegung. In: Bethe A, von Bergmann G, Embden G, et al., eds. Handbuch der Normalen und Pathologischen Physiologie. Berlin, Germany: Springer; 1925:70-127. [Google Scholar]
- 18.Green M. How big are the bronchioles? St Thomas Hosp Gaz. 1965;63:136-139. [Google Scholar]
- 19.Mead J. The lung’s “quiet zone”. N Engl J Med. 1970;282(23):1318-1319. [DOI] [PubMed] [Google Scholar]
- 20.Macklem PT, Permutt S, eds. The Lung in the Transition Between Health and Disease. New York, NY: Marcel Dekker Inc.; 1979. [Google Scholar]
- 21.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1(6077):1645-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1978;298(23):1277-1281. [DOI] [PubMed] [Google Scholar]
- 23.Van Brabandt H, Cauberghs M, Verbeken E, Moerman P, Lauweryns JM, Van de Woestijne KP. Partitioning of pulmonary impedance in excised human and canine lungs. J Appl Physiol. 1983;55(6):1733-1742. [DOI] [PubMed] [Google Scholar]
- 24.Wagner EM, Liu MC, Weinmann GG, Permutt S, Bleecker ER. Peripheral lung resistance in normal and asthmatic subjects. Am Rev Respir Dis. 1990;141(3):584-588. [DOI] [PubMed] [Google Scholar]
- 25.Wagner EM, Bleecker ER, Permutt S, Liu MC. Direct assessment of small airways reactivity in human subjects. Am J Respir Crit Care Med. 1998;157(2):447-452. [DOI] [PubMed] [Google Scholar]
- 26.Bignon J, Khoury F, Even P, Andre J, Brouet G. Morphometric study in chronic obstructive bronchopulmonary disease. Pathologic, clinical, and physiologic correlations. Am Rev Respir Dis. 1969;99(5):669-695. [DOI] [PubMed] [Google Scholar]
- 27.Matsuba K, Thurlbeck WM. The number and dimensions of small airways in emphysematous lungs. Am J Pathol. 1972;67(2):265-275. [PMC free article] [PubMed] [Google Scholar]
- 28.Hsia CC, Hyde DM, Ochs M, Weibel ER; ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181(4):394-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Findeisen W. Uber das Absetzen kleiner, in dur Luft suspendierter Teilchen in der menschlichen Lunge bei der Atmung. Arch Ges Physiol. 1935;236:367-379. [Google Scholar]
- 30.Leopold JG, Gough J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax. 1957;12(3):219-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLean KH. The histology of generalized pulmonary emphysema. I. The genesis of the early centrolobular lesion: focal emphysema. Australas Ann Med. 1957;6(2):124-140. [DOI] [PubMed] [Google Scholar]
- 32.Hogg JC, Nepszy SJ, Macklem PT, Thurlbeck WM. Elastic properties of the centrilobular emphysematous space. J Clin Invest. 1969;48(7):1306-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otis AB, McKerrow CB, Bartlett RA, et al. Mechanical factors in distribution of pulmonary ventilation. J Appl Physiol. 1956;8(4):427-443. [DOI] [PubMed] [Google Scholar]
- 34.Hogg JC, Macklem PT, Thurlbeck WM. The resistance of collateral channels in excised human lungs. J Clin Invest. 1969;48(3):421-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terry PB, Traystman RJ, Newball HH, Batra G, Menkes HA. Collateral ventilation in man. N Engl J Med. 1978;298(1):10-15. [DOI] [PubMed] [Google Scholar]
- 36.Kirby M, Mathew L, Wheatley A, Santyr GE, McCormack DG, Parraga G. Chronic obstructive pulmonary disease: longitudinal hyperpolarized (3)He MR imaging. Radiology. 2010;256(1):280-289. [DOI] [PubMed] [Google Scholar]
- 37.Marshall H, Deppe MH, Parra-Robles J, et al. Direct visualisation of collateral ventilation in COPD with hyperpolarised gas MRI. Thorax. 2012;67(7):613-617. [DOI] [PubMed] [Google Scholar]
- 38.Yuan R, Hogg JC, Paré PD, et al. Prediction of the rate of decline in FEV(1) in smokers using quantitative computed tomography. Thorax. 2009;64(11):944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohamed Hoesein FA, de Hoop B, Zanen P, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66(9):782-787. [DOI] [PubMed] [Google Scholar]
- 40.Vestbo J, Edwards LD, Scanlon PD, et al. ; ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184-1192. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura M, Makita H, Nagai K, et al. ; Hokkaido COPD Cohort Study Investigators. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(1):44-52. [DOI] [PubMed] [Google Scholar]