In addition to allowing immune cells to signal tissue damage, HMBG1 is secreted by senescent cells to initiate inflammatory cytokine secretion.
Abstract
Cellular senescence irreversibly arrests proliferation in response to potentially oncogenic stress. Senescent cells also secrete inflammatory cytokines such as IL-6, which promote age-associated inflammation and pathology. HMGB1 (high mobility group box 1) modulates gene expression in the nucleus, but certain immune cells secrete HMGB1 as an extracellular Alarmin to signal tissue damage. We show that nuclear HMGB1 relocalized to the extracellular milieu in senescent human and mouse cells in culture and in vivo. In contrast to cytokine secretion, HMGB1 redistribution required the p53 tumor suppressor, but not its activator ATM. Moreover, altered HMGB1 expression induced a p53-dependent senescent growth arrest. Senescent fibroblasts secreted oxidized HMGB1, which stimulated cytokine secretion through TLR-4 signaling. HMGB1 depletion, HMGB1 blocking antibody, or TLR-4 inhibition attenuated senescence-associated IL-6 secretion, and exogenous HMGB1 stimulated NF-κB activity and restored IL-6 secretion to HMGB1-depleted cells. Our findings identify senescence as a novel biological setting in which HMGB1 functions and link HMGB1 redistribution to p53 activity and senescence-associated inflammation.
Introduction
Cellular senescence suppresses cancer by arresting the proliferation of cells at risk for malignant transformation (Campisi, 2001; Prieur and Peeper, 2008). Many potential oncogenic events initiate senescence, including telomere dysfunction, severe DNA damage, oncogenes, and disrupted chromatin. These events activate tumor suppressor pathways governed by p53 and pRB, transcriptional regulators that establish and maintain the senescence arrest (Herbig et al., 2006; Campisi and d’Adda di Fagagna, 2007). p53 arrests the cells primarily by inducing the p21 gene, which inhibits cyclin-dependent kinases and cell cycle progression; pRB imposes the arrest primarily by assembling repressive chromatin at pro-proliferative genes.
The senescence arrest was long considered a cell-intrinsic process (Hayflick, 1965). However, recent findings identified secreted proteins that partly reinforce the arrest in an autocrine manner (Kortlever et al., 2006; Acosta et al., 2008; Kuilman et al., 2008; Wajapeyee et al., 2008). These proteins comprise a larger senescence-associated secretory phenotype (SASP), featuring growth factors, proteases, and inflammatory cytokines that affect neighboring cells in a paracrine manner (Parrinello et al., 2005; Bavik et al., 2006; Coppé et al., 2008, 2010b). The senescence response might be antagonistically pleiotropic (Campisi, 2003). That is, it protects organisms from cancer early in life, but the accumulation of senescent cells in late life can drive age-related disease, including, ironically, cancer. Because both cancer and aging are fueled by inflammation (Coussens and Werb, 2002; Franceschi, 2007), the SASP can, in principle, account for the antagonistic pleiotropy.
It is not clear how the nuclear events that regulate the senescence arrest relate to the SASP. Neither pRB nor p53 are required for the SASP, and p53 deficiency amplifies the SASP (Coppé et al., 2008, 2011). However, DNA damage response (DDR) proteins that act upstream of p53 (ATM, NBS1, CHK2, H2AX) are required for the SASP (Rodier et al., 2009, 2011). Still, it is not known how senescence-inducing stimuli cause the widespread changes in gene expression required for the SASP.
HMGB1 is an abundant, evolutionarily conserved nonhistone protein (Goodwin et al., 1973) that bends DNA to provide transcription factor access to promoter regions (Grosschedl et al., 1994). One such factor is p53 for which HMGB1 increases DNA binding and transactivation activity (Jayaraman et al., 1998; Banerjee and Kundu, 2003). HMGBI has a surprising additional function—as a secreted protein (Wang et al., 1999a) with a central role in inflammation caused by cell or tissue damage (Bianchi, 2007; Yamada and Maruyama, 2007). HMGB1 passively leaks from necrotic, but not apoptotic, cells, is actively secreted by pathogen-stimulated macrophages, and mediates the potentially lethal inflammation caused by endotoxins (Gardella et al., 2002). Extracellular HMGB1 binds cell surface RAGE (receptor for advanced glycation end-products) and TLRs (toll-like receptors) to initiate signaling that culminates in the expression of inflammatory cytokines such as interleukin (IL)-6 (Park et al., 2003, 2006; Lotze and Tracey, 2005; Schiraldi et al., 2012). Recent findings suggest HMGB1 complexes with extracellular molecules such as single-stranded DNA or IL-1β to promote inflammation (Ivanov et al., 2007; Tian et al., 2007; Sha et al., 2008).
HMGB1 is an Alarmin family member. Alarmins function intracellularly, but upon cellular stress or damage are actively secreted, whereupon they provoke an innate immune response. HMGB1 is also a component of damage-associated molecular patterns (DAMPs), which act and reside intracellularly but are secreted upon cellular damage. Extracellular DAMPs induce a sterile inflammatory response (Bianchi, 2007, 2009; Andersson and Tracey, 2011).
We show that HMGB1 is secreted by senescent cells very early after a senescence-inducing stimulus, before development of the SASP. In contrast to the SASP, HMGB1 secretion depended on p53, but not ATM, and disrupted HMGB1 levels induced a p53-dependent arrest. Importantly, secreted HMGB1 was essential for optimal secretion of IL-6 and MMP-3, prominent SASP components.
Results
Senescent human cells secrete HMGB1
We examined normal human fibroblasts (WI-38) before (PRE) and after we induced them to senesce (SEN) by X-irradiation (10 Gy; XRA), replicative exhaustion (REP), overexpression of the p16INK4a tumor suppressor (p16; which activates pRB), and oncogenic RASV12 (RAS). Each stimulus caused a striking loss of intracellular HMGB1, determined by Western blotting (Fig. 1 A). As expected (Dimri et al., 1995; Coppé et al., 2008), each stimulus induced senescence, determined by enlarged morphology (not depicted), low BrdU incorporation (growth arrest), and senescence-associated β-galactosidase (SA-β-Gal) activity (Fig. 1 B).
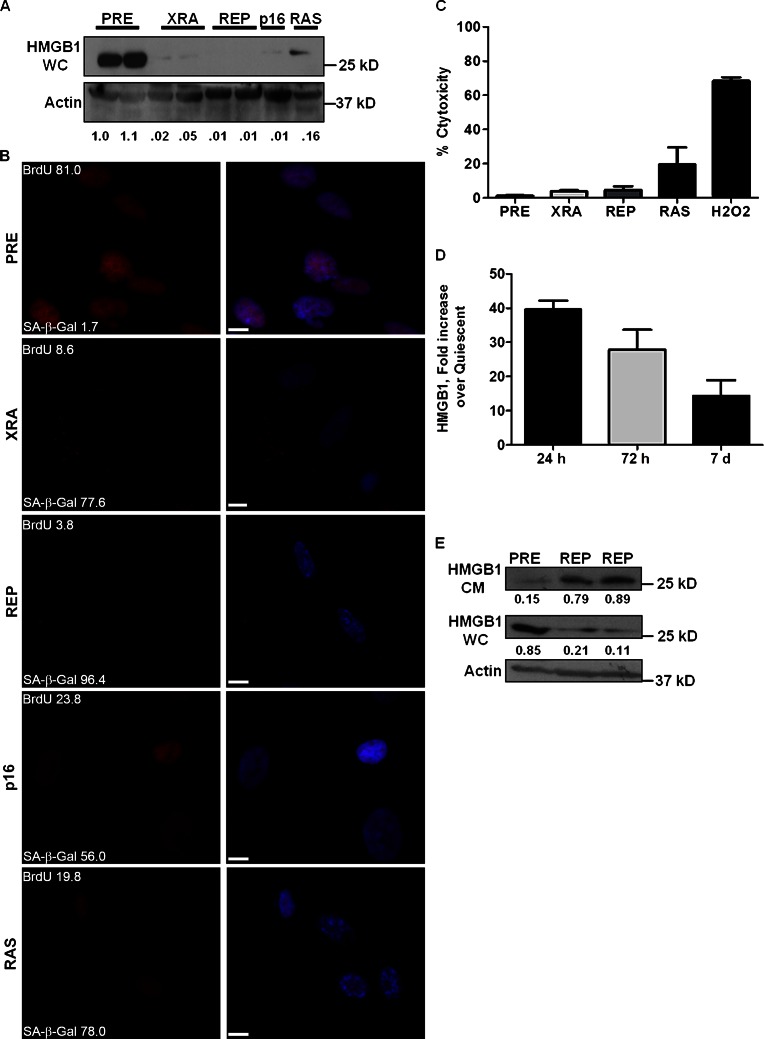
Figure 1.
HMGB1 relocalizes in SEN human cells. (A) Whole-cell lysates (WC) of 105 WI-38 human fibroblasts, PRE or made SEN by X-irradiation (XRA), replicative exhaustion (REP), p16INK4a overexpression (p16) or oncogenic RAS, were analyzed for HMGB1 and actin (control) by Western blotting. (B) IMR-90 fibroblasts described in A were immunostained for HMGB1 (red). Nuclei were stained with DAPI (blue). Parallel cells were labeled with BrdU (48 h) and stained for SA-β-Gal activity; values shown are percentage of cells. Bar, 10 μM. (C) Cytotoxicity was determined by lactate dehydrogenase in CM from IMR90 cells. Error bars are the standard deviation of triplicate samples. (D) At the indicated time after XRA, we analyzed CM from IMR90 cells for HMGB1 by ELISA. Shown is the fold increase over cells made quiescent by culture in 0.2% serum for 3 d. Error bars are the SEM of triplicate samples. (E) CM and whole-cell lysates (WC) were collected from PRE and duplicate REP IMR-90 cells and analyzed by Western blotting for HMGB1 and actin. Expression relative to actin is shown below each lane.
By immunostaining, HMGB1 was abundant in PRE nuclei (Fig. 1 B), as reported for most cells (Falciola et al., 1997). However, SEN cells had barely detectable nuclear HMGB1 (some had faint cytoplasmic staining; Fig. 1 B). Reduced nuclear HMGB1 occurred in several SEN human fibroblast strains (fetal lung, neonatal foreskin, placenta), as well as mammary and prostate epithelial cells (Fig. S1, A–D), indicating it is not strain- or cell type–specific. Further, it was not due to necrosis. SEN cells are viable and metabolically active (Campisi and d’Adda di Fagagna, 2007), and no senescence inducer caused cell loss or deterioration, as determined by visual inspection and release of lactate dehydrogenase into the culture media (Fig. 1 C). Thus, loss of nuclear HMBG1 is a hallmark of SEN cells, regardless of inducer or tissue of origin. In this regard, HMGB1 differs from high mobility group A (HMGA) proteins, which accumulate on chromatin in SEN cells (Narita et al., 2006).
To determine whether SEN cells secrete HMGB1, we used ELISAs and Western blotting to analyze whole-cell (WC) lysates and conditioned media (CM) from cells induced to senesce by XRA or REP (Fig. 1, D and E). In the case of XRA, which induces senescence synchronously, we detected high levels of HMGB1 in CM 24 h after irradiation (Fig. 1 D). In contrast to certain other cells in which secreted HMGB1 is in exosomes or insoluble fractions (Liu et al., 2006), HMGB1 secreted by SEN cells was predominantly soluble (Fig. S1 E). Pellet fractions of CM from SEN and PRE cells contained similar HMGB1 levels, even after treatment with detergent to disrupt possible exosomes (Fig. S1 E). Similar results were obtained using CM from REP SEN cells (Fig. 1 E). Thus, nuclear HMGB1 loss and its secretion occurred 1 d after XRA, preceding the SASP and SA-β-Gal expression, which initiate after ∼3–5 d and peak after 7–10 d after XRA (Coppé et al., 2008). A lower XRA dose (0.5 Gy), which causes modest DNA damage but not senescence (Rodier et al., 2009), did not cause HMGB1 relocalization (not depicted).
We conclude that HMGB1 redistribution from the nucleus to the extracellular space is a common, early response to senescence-causing stimuli.
Senescent mouse cells lose nuclear HMGB1 in culture and in vivo
HMGB1 activities are conserved throughout mammalian evolution (Dumitriu et al., 2005), so we asked whether SEN mouse cells also secrete HMGB1. Immunostaining showed abundant nuclear HMGB1 in PRE mouse embryo fibroblasts (MEFs); upon senescence induced by XRA, nuclear HMGB1 was barely detectable (Fig. 2 A). Further, Western blotting showed WC lysates from XRA SEN MEFs contained little HMGB1 compared with PRE MEFs (Fig. 2 B), whereas CM from SEN MEFs contained abundant HMGB1 (Fig. 2 C).
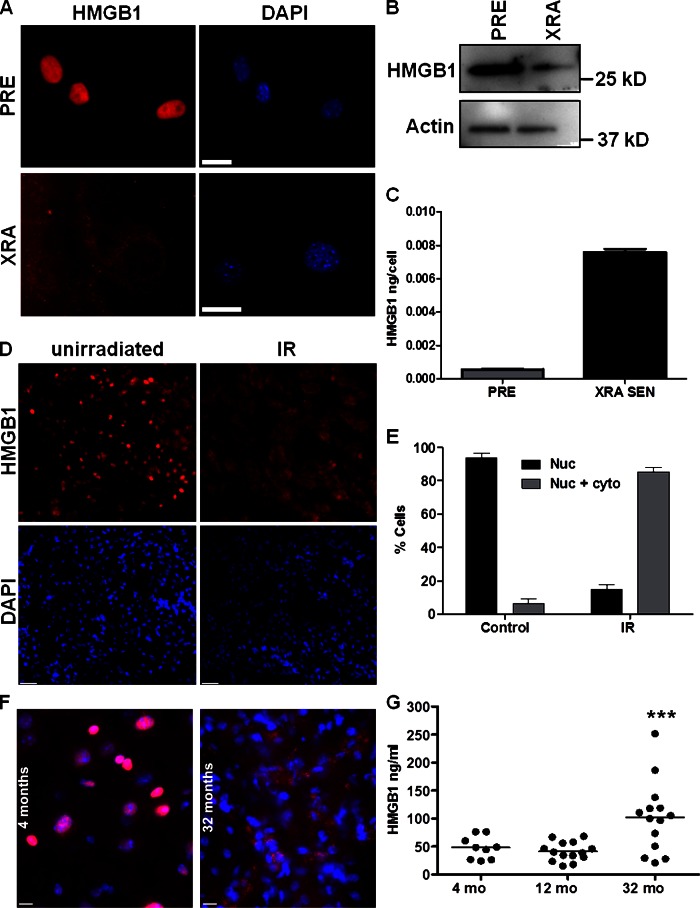
Figure 2.
HMGB1 relocalizes in SEN mouse cells. (A) PRE or SEN (XRA) MEFs were immunostained for HMGB1 (red). Nuclei were stained with DAPI (blue). Bar, 10 μM. (B) Lysates from PRE or SEN (XRA) MEFs were analyzed by Western blotting for HMGB1 and actin (control). (C) CM from PRE or XRA cells were analyzed for HMGB1 by ELISA. Error bars are the average of duplicate samples. (D) 6–8-wk-old C57BL/6 mice were unirradiated (control) or sublethally irradiated (IR). Kidneys collected 1 wk later were immunostained for HMGB1 (red); nuclei were stained with DAPI (blue). Bar, 25 μM. (E) Kidneys from mice in B were immunostained for nuclear (Nuc), or nuclear + cytoplasmic (Nuc + Cyto) HMGB1. More than 200 nuclei were scored blinded from 2–3 control or irradiated (IR) mice; error bars are SEM. (F) Kidneys from 4- and 32-mo-old C57BL/6 mice were immunostained for HMGB1 (red); nuclei were counterstained with DAPI (blue). Bars, 10 μM. (G) Sera from mice at the indicated ages were assayed for HMGB1 by ELISA. Kruskal-Wallis test was used to determine significance (***, P < 0.001).
To determine whether senescence depleted nuclear HMGB1 in vivo, we immunostained tissues from unirradiated mice and mice given whole-body XRA (9 Gy), which causes persistent hallmarks of senescence (DNA damage foci; p16INK4a expression) in several mouse tissues (Rodier et al., 2009; Le et al., 2010). HMGB1 was largely nuclear in unirradiated kidney cells, with <10% showing no or only faint nuclear staining (Fig. 2 D). 1 wk after XRA, ∼80% of kidney cells had faint nuclear and/or detectable cytoplasmic HMGB1 staining (Fig. 2, D and E). We also examined the brain after XRA. As reported (Müller et al., 2004), unirradiated brain stained weakly for HMGB1, but the staining was mostly nuclear; after XRA, nuclear staining diminished (Fig. S1 F).
Because SEN cells increase with age in mammalian tissues (Dimri et al., 1995; Herbig et al., 2006; Wang et al., 2009), we examined kidneys from young (4 mo) and old (32 mo) mice for nuclear HMGB1 (Fig. 2 F). Qualitatively, kidneys from old mice appeared to contain more cells that lacked nuclear HMGB1. Quantitatively, sera from old mice contained on average significantly more HMGB1 than sera from younger animals (Fig. 2 G; 4 and 12 mo; P < 0.001).
Our data support the idea that SEN cells increase with age, and validate loss of nuclear HMGB1 as a conserved feature of SEN mouse cells in culture and in vivo.
Senescent cells secrete acetylated HMGB1
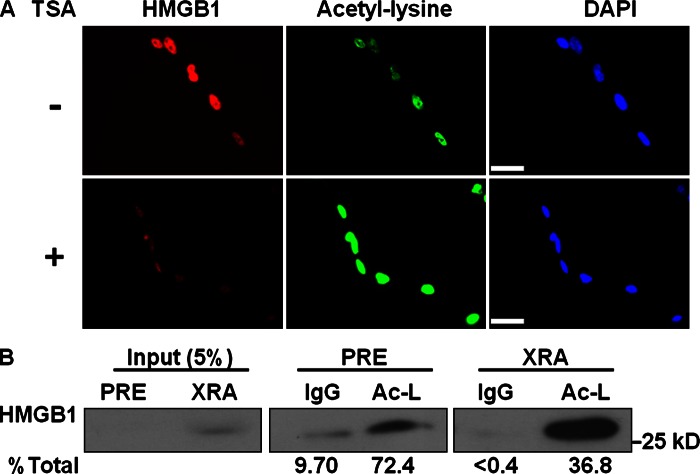
HMGB1 secretion by macrophages depends on acetylation, which prevents nuclear reentry and allows packaging into secretory lysosomes (Gardella et al., 2002; Raucci et al., 2007). To determine whether acetylation regulates HMGB1 localization in SEN cells, we incubated PRE human fibroblasts with trichostatin A (TSA), a protein deacetylase inhibitor that promotes rapid loss of nuclear HMGB1 in mouse macrophages (Bonaldi et al., 2003), and immunostained with pan-acetyl-lysine (Ac-Lys) and HMGB1 antibodies 4 h later. Control cells showed prominent nuclear HMGB1 and moderate Ac-Lys staining, as expected; TSA increased Ac-Lys staining and markedly reduced nuclear HMGB1 staining (Fig. 3 A). To determine whether HMGB1 secreted by SEN cells was acetylated, we immunoprecipitated CM from PRE or SEN cells with the Ac-Lys antibody, and analyzed precipitates for HMGB1 by Western blotting. HMGB1 in CM was acetylated, whether secreted at low levels by PRE cells or high levels by SEN cells (Fig. 3 B).
Figure 3.
Senescent cells secrete acetylated HMGB1. (A) WI-38 cells were given PBS (−, control) or 200 ng/ml trichostatin A (TSA) (+) for 4 h, and immunostained with pan-acetyl-lysine (green)– and HMGB1 (red)–specific antibodies. Nuclei were stained with DAPI (blue). Bar, 20 μM. (B) CM from PRE and SEN (XRA) IMR-90 cells were immunoprecipitated by mouse IgG (control) or acetyl-lysine (Ac-L) antibody. Precipitates and 5% of unprecipitated (Input) CM were analyzed by Western blotting for HMGB1. % total = amount of acetylated HMGB1 immunoprecipitated compared with total HMGB1 in CM.
Senescent cells actively export nuclear HMGB1
Acetylated HMGB1 is actively exported from the nucleus in monocytes and macrophages (Gardella et al., 2002; Raucci et al., 2007). To determine whether this was true in SEN cells, we used leptomycin B (LMB) to inhibit CRM1-dependent nuclear export. LMB prevented HMGB1 nuclear export in monocytes (Bonaldi et al., 2003). Untreated SEN cells immunostained weakly for nuclear HMGB1, but strongly for persistent DNA damage (53BP1) foci (Rodier et al., 2009) (Fig. 4, A and B). After a 4-h exposure to low-dose LMB (20 nM), the nuclei retained 53BP1 foci and gained robust nuclear HMGB1 staining (Fig. 4, A and B). These data indicate that SEN cells continually synthesize HMGB1, which transiently occupies the nucleus, but then is actively exported.
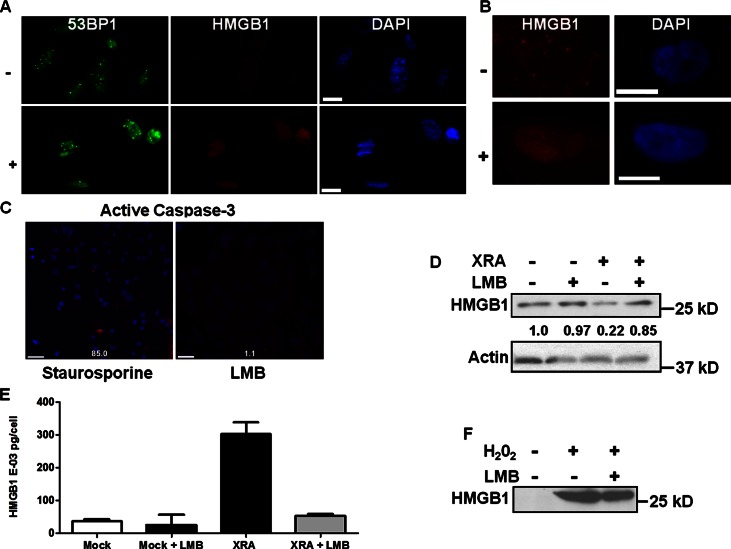
Figure 4.
HMGB1 is actively exported from senescent cell nuclei. (A) SEN (XRA) HCA2 cells were cultured for 3 h with DMSO (−, control) or 20 nM leptomycin B (LMB) (+) and stained for HMGB1 (red) and 53BP1 (green). Nuclei were stained with DAPI (blue). Bar, 10 μM. (B) REP SEN HCA2 cells were cultured as in A and stained for HMGB1 (red). Nuclei were stained with DAPI (blue). Bar, 10 μM. (C) HCA2 cells were given 5 nM staurosporine or 20 nM LBM for 6 h and stained for active caspase-3 (red; % positive cells shown). Nuclei were stained with DAPI (blue). Bar, 10 μM. (D) PRE (XRA−) or SEN (XRA+) IMR-90 cells were given DMSO (−) or 20 nM LMB (+) for 3 h. Lysates were analyzed for HMGB1 and actin (control) by Western blotting. Expression normalized to actin and relative to PRE cells (1.0) is indicated. (E) CM from D were analyzed for HMGB1 by ELISA. Error bars = SEM of triplicate samples. (F) HCA2 cells were cultured without or with 0.5 mM H2O2 for 2 h, washed, and incubated for 16 h with DMSO or LMB. CM were analyzed by Western blotting for HMGB1. Data are representative of at least two independent experiments.
High (2 µM) LMB doses and long exposures increase nuclear p53 and induce senescence (Smart et al., 1999). The low (20 nM) dose used here eventually increased nuclear p53 (Fig. S2 A); after 6 d, cells senesced, as assessed by arrested growth and SA-β-Gal activity (Fig. S2 B). Growth arrest and SA-β-Gal occurred despite strongly retained nuclear HMGB1 (Fig. S2 A), indicating these phenotypes do not depend on nuclear HMGB1 loss.
To rule out the possibility that LMB induced apoptosis, which could account for HMGB1 nuclear retention, we immunostained cells treated with LMB or staurosporine (positive control) for active caspase-3, a hallmark of apoptosis (Fig. 4 C). Greater than 80% of staurosporine-treated cells stained positive (left panel), in contrast to <2% of LMB-treated cells (right panel). Thus, LMB-treated cells retained nuclear HMGB1 owing to inhibited nuclear export, not apoptosis. Finally, western analysis and ELISAs showed that LMB-treated SEN cells failed to secrete HMGB1 (Fig. 4, D and E). In contrast, H2O2 at a dose (0.5 mM) that caused necrosis also caused HMGB1 release, as expected (Raucci et al., 2007), which was unaffected by LMB (Fig. 4 F).
HMGB1 depletion or overexpression induces senescence
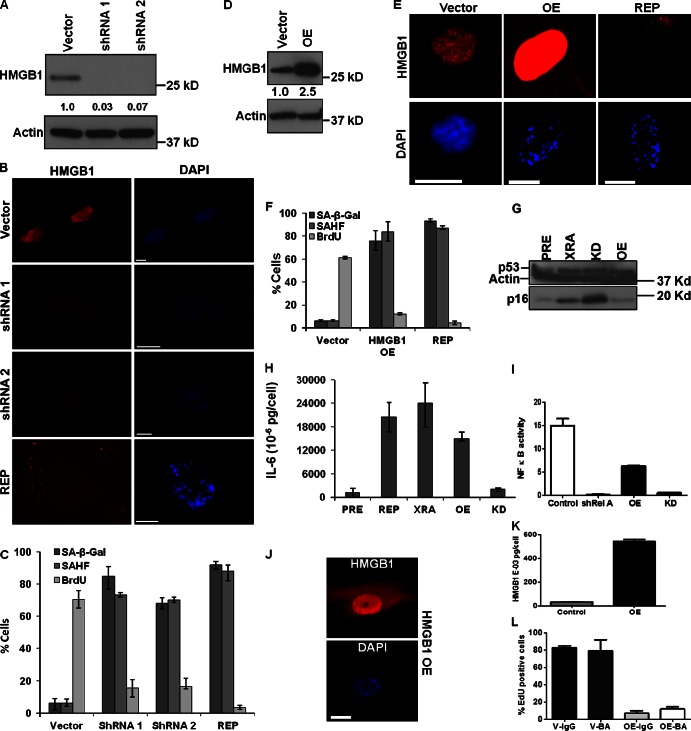
To determine the significance of HMGB1 relocalization, we depleted HMGB1 in human fibroblasts (IMR-90, HCA2) by RNAi using lentiviruses carrying no insert or either of two short hairpin RNAs (shRNAs) that reduced intracellular HMGB1 protein levels by >90% (Fig. 5, A and B; Fig. S3, A and B). HMGB1-depleted cells underwent senescence, as judged by arrested growth (Fig. 5 C), senescence-associated heterochromatin foci (SAHF) formation (Narita et al., 2003), and SA-β-Gal activity (Fig. 5, B and C; Fig. S3 B). HMGB1 depletion also caused senescence in human mammary epithelial cells (HMECs) and MEFs (Fig. S4, A–D), indicating the response was not restricted to human fibroblasts.
Figure 5.
HMGB1 depletion or overexpression induces senescence. (A) Lysates from IMR-90 cells infected with lentiviruses carrying no insert (Vector) or HMGB1 shRNAs (shRNA1, 2) were analyzed for HMGB1 and actin (control) by Western blotting. Shown is expression level relative to Vector. (B) Cells in A were immunostained for HMGB1 (red). Nuclei were stained with DAPI (blue). REP SEN cells are shown for comparison. Bar, 10 µM. (C) Cells in B were scored for SA-β-Gal, BrdU (24 h pulse), and SAHF. Error bars = SEM of two independent experiments. (D) Lysates from IMR-90 cells infected with lentivirus expressing no insert (Vector) or HMGB1 (OE) were analyzed for HMGB1 and actin (control) by Western blotting. Shown is expression level relative to Vector. (E) Cells in D were immunostained for HMGB1 (red). Nuclei were stained with DAPI (blue). REP SEN cells are shown for comparison. Bar, 10 µM. (F) Cells in E were scored for SA-β-Gal, BrdU (24 h pulse), and SAHFs. REP SEN cells are shown for comparison. Error bars = SEM of two independent experiments. (G) Lysates from PRE IMR-90 cells or cells induced to senesce by XRA (XRA), HMGB1-overexpression (OE), or HMGB1-depletion (KD) were analyzed for p53, p16INK4a, and actin (control) by Western blotting. (H) CM from PRE or SEN (REP, XRA, and HMGB1 OE or KD) IMR-90 cells were analyzed for IL-6 by ELISA. Shown is a representative of two experiments. Error bars = SEM of duplicate determinations. (I) HCA2 cells expressing an NF-κB luciferase reporter were infected with lentiviruses carrying GFP shRNA (control), RelA shRNA, HMGB1 cDNA (OE), or HMGB1 shRNA (KD). 7 d after selection, shGFP and shRel A cells were given TNF for 24 h in serum-free media. Cells were lysed and luciferase activity measured (fold change over cells given BSA (control) protein). One-way ANOVA was used to analyze groups; P < 0.001. (J) Cells infected with HMGB1 OE lentivirus were stained for HMGB1 (red) and nuclei (DAPI; blue). Bar, 10 μM. (K) CM from cells infected with insertless (control) or HMGB1-expressing (OE) lentiviruses were assayed for HMGB1 by ELISA. Error bars = SEM of duplicate determinations. (L) IMR-90 cells infected with lentiviruses carrying no insert (V) or overexpressing HMGB1 (OE) were cultured for 5 d with mouse IgG or HMGB1 blocking antibody (BA) and assessed for EdU incorporation. Error bars = SEM of duplicate determinations.
These findings are significant for two reasons. First, although IMR-90 cells senesce with SAHF (Narita et al., 2003), these structures are not a universal feature of SEN cells (Kosar et al., 2011). Indeed, HCA2 cells senesce without SAHF (Fig. S3 B; REP). Thus, HMGB1 depletion induced SAHF in cells that do not form these structures. Second, the consequence of HMGB1 depletion differed markedly from that of HMGA depletion, which reduced the senescence response to oncogenic RAS or p16INK4a overexpression (Narita et al., 2006).
Many tumor cell lines and tumors express high levels of HMGB1 (Brezniceanu et al., 2003; Ellerman et al., 2007), suggesting HMGB1 might be an oncogene that allows bypass of senescence signals. To test this idea, we used lentiviruses to overexpress HMGB1 in IMR-90 (Fig. 5, D and E) and HCA2 (Fig. S3, C, D, and F) cells. Surprisingly, overexpression also induced senescence, as determined by arrested growth, SAHF, and SA-β-Gal (Fig. 5, E and F; Fig. S3 E). Overexpression similarly induced senescence in HMECs and MEFs (Fig. S4, B and D). HMGB1 depletion or overexpression stimulated p53 expression to levels found in cells induced to senesce by XRA or REP (Fig. 5 G; Fig. S3 G).
Senescence is a stress response, although not all stressors induce senescence (Suzuki and Boothman, 2008). To examine whether disrupted HMGB1 levels caused a general stress response, we monitored heat shock protein 70 (HSP70). Although heat shock (42°C) robustly increased HSP70 expression, disrupted HMGB1 stoichiometry failed to do so (Fig. S3 H). Further, heat shock did not stimulate HMGB1 secretion (Fig. S3 I). Thus, altered HMGB1 expression, whether reduced or elevated, caused senescence.
IL-6 secretion by SEN cells requires HMGB1 expression
Because extracellular HMGB1 stimulates inflammatory cytokine secretion by immune cells (Andersson et al., 2002; Dumitriu et al., 2005; Yamada and Maruyama, 2007), and such cytokines are SASP components (Coppé et al., 2008, 2010b), we asked whether senescence caused by altered HMGB1 expression increased cytokine secretion. Despite inducing growth arrest, SAHF, SA-β-Gal (Fig. 5 C), and p53 expression (Fig. 5 G), HMGB1-depleted SEN cells secreted little IL-6 (Fig. 5 H), a key SASP component. By contrast, HMGB1-overexpressing SEN cells secreted both HMGB1 (Fig. 5 K; Fig. S4 E) and IL-6 (Fig. 5 H).
Because SEN cells express IL-6 owing primarily to NF-κB transcriptional activity (Freund et al., 2011), we measured NF-κB transcriptional activity in HMGB1-overexpressing or -depleted HCA2 cells, with or without shRNA against RelA, an essential NF-κB subunit. As a positive control, TNF strongly induced RelA-dependent NF-κB activity (Fig. 5 I). Consistent with the IL-6 ELISA results (Fig. 5 H), NF-κB activity was robust in HMGB1-overexpressing cells, but not HMGB1-depleted cells (Fig. 5 I). HMGB1 overexpression increased cytoplasmic HMGB1 immunostaining and secretion (Fig. 5, J and K). Secreted HMGB1 had no effect on the growth arrest because cells remained arrested in the presence of an HMGB1-blocking antibody (Fig. 5 L). Thus, altered HMGB1 expression induced senescence, but only depletion, not overexpression, suppressed NF-κB activity and IL-6 secretion.
HMGB1 relocalization does not depend on p16INK4a
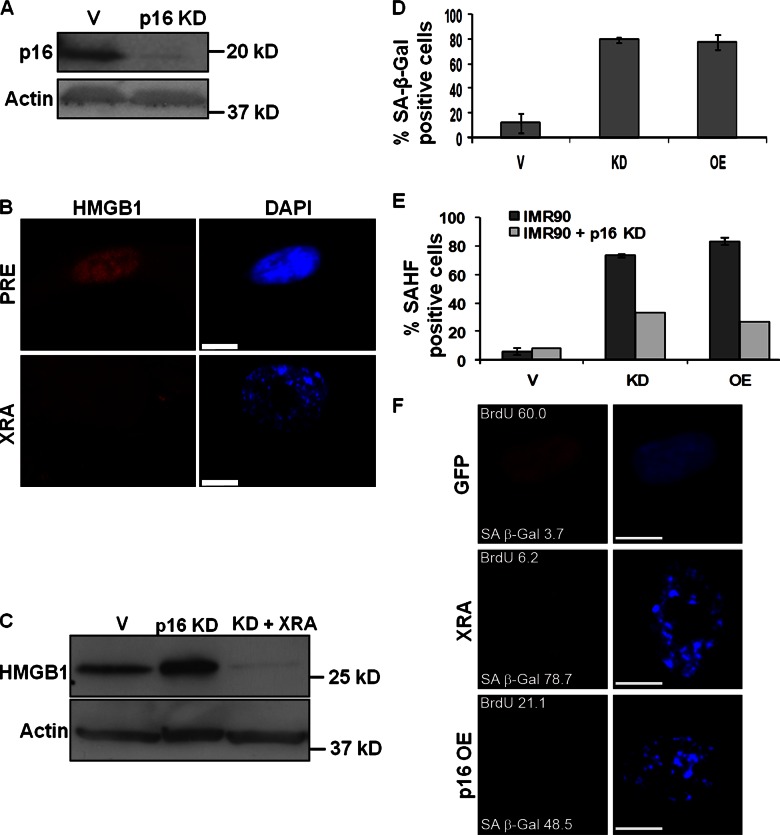
Both HMGB1 overexpression and depletion increased p53 to SEN levels (Fig. 5 G; Fig. S3 G). However, only HMGB1 depletion induced p16INK4a, and only in high p16-expressing IMR-90 (Fig. 5 G), not low p16-expressing HCA2 cells (Fig. S3 G). Further, altered HMGB1 expression did not induce SAHF in p16INK4a-deficient HMECs, despite altering heterochromatin in MEFs (Fig. S4 C). Thus, p16INK4a may have little or no role in the senescence response to perturbed HMGB1 expression.
To test this possibility, we depleted IMR-90 cells of p16INK4a by RNAi (Fig. 6 A). When induced to senesce by XRA, the cells lost nuclear/intracellular HMGB1 (Fig. 6, B and C), expressed SA-β-Gal, and arrested growth in response to HMGB1 overexpression or depletion (Fig. 6 D; and unpublished data). As observed for RAS-induced senescence (Narita et al., 2003), efficient SAHF formation required p16INK4a. Altered HMGB1 expression induced SAHF in 50–70% fewer p16INK4a-depleted cells compared with control cells (Fig. 6 E). Further, loss of nuclear HMGB1 and HMGB1 secretion occurred when senescence was induced by p16INK4a overexpression (Fig. 6 F; Fig. S4 E), which does not cause a SASP (Coppé et al., 2011), suggesting that HMGB1 relocalization and the SASP are regulated differently.
Figure 6.
p16INK4a does not regulate HMGB1 in SEN cells. (A) Lysates from IMR-90 cells infected with lentiviruses carrying no insert (V) or p16INK4a shRNA (p16 KD) were analyzed for p16INK4a and actin (control) by Western blotting. (B) PRE and SEN (XRA) IMR-90 cells depleted of p16INK4a were immunostained for HMGB1 (red). Nuclei were stained with DAPI (blue). Bars, 10 µM. (C) Lysates from PRE IMR-90 cells or cells infected with lentiviruses carrying no insert (V) or p16INK4a shRNA (p16 KD). p16 KD cells were made senescent by X-irradiation (XRA) and analyzed 10 d later. Cells were analyzed for HMGB1 and actin (control) by Western blotting. (D) IMR90 cells depleted of p16INK4a were infected with lentiviruses carrying no insert (V), HMGB1 cDNA (OE), or HMGB1 shRNA (KD) and stained for SA-β-Gal. Error bars = SEM of two independent experiments. (E) IMR-90 cells expressing or depleted of p16INK4a were infected with lentiviruses carrying no insert (V), HMGB1 shRNA (KD), or HMGB1 cDNA (OE). After 10 d, cells were scored for SAHFs. Error bars = SEM of two independent experiments. (F) IMR-90 cells infected with lentiviruses carrying GFP (control) or p16 (OE) were scored for SA-β-Gal and BrdU incorporation (24 h pulse) and immunostained for HMGB1 (red). Nuclei were stained with DAPI (blue). XRA-induced SEN cells are shown for comparison. Bars, 10 µM.
HMGB1 relocalization requires p53 activity
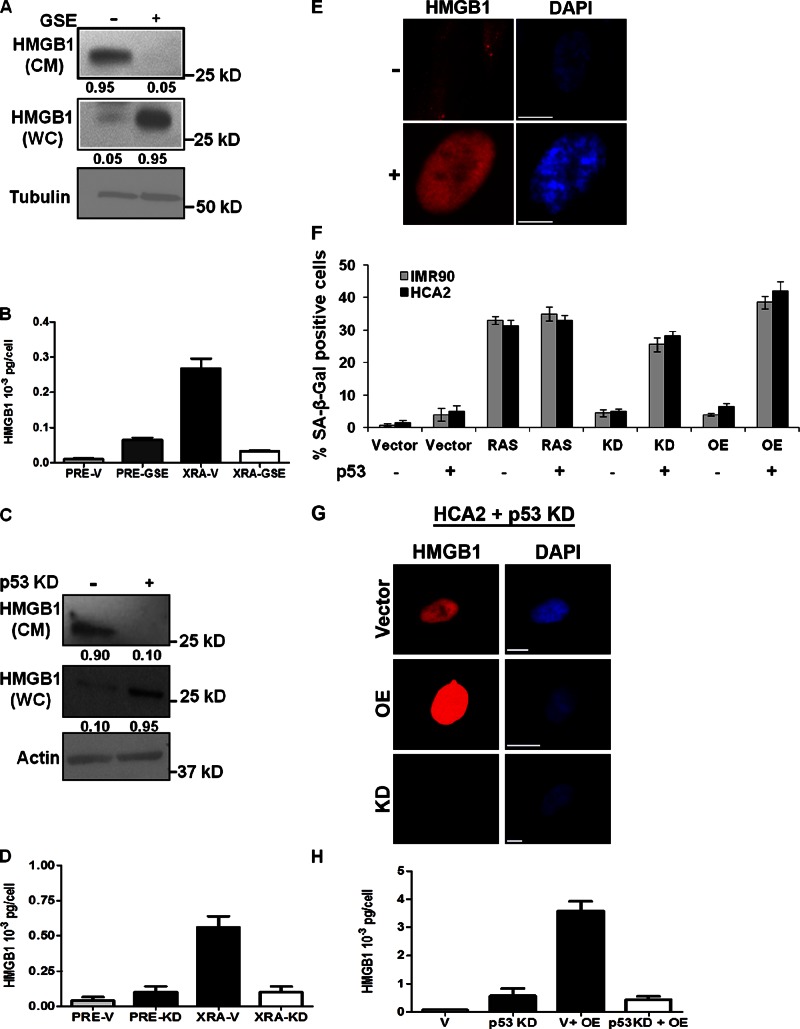
To determine the role of p53, we expressed a dominant-negative p53 peptide (GSE22; Ossovskaya et al., 1996) or depleted p53 by RNAi. HMGB1 was abundant in CM from HCA2 fibroblasts expressing p53, but low upon GSE22 expression, mirrored by a commensurate increase in HMGB1 in WC lysates (Fig. 7 A). HMGB1 ELISA confirmed that GSE attenuated HMGB1 secretion in XRA SEN cells (Fig. 7, B and E). Low p16INK4a-expressing cells such as HCA2 resume growth (revert) upon p53 inactivation (Beauséjour et al., 2003). To test whether HMGB1 retention upon p53 loss was due simply to resumed proliferation, we depleted p53 in cells expressing high levels of p16. Depletion of p53 produced retention of HMGB1 after senescence induced by X-irradiation, which was confirmed using an HMGB1 ELISA of CM (Fig. 7 D). Thus, senescence-associated nuclear HMGB1 loss and secretion required p53 function, in contrast to the SASP.
Figure 7.
HMGB1 relocalization and senescence caused by altered HMGB1 expression requires p53. (A) Lysates (WC) and CM from XRA SEN HCA2 cells infected with control (−) or GSE (+) expressing lentiviruses were analyzed for HMGB1 and tubulin (control) by Western blotting. The fraction of detectable protein is given below each lane. (B) CM from PRE or SEN (XRA) HCA2 cells expressing control (V) or GSE-expressing lentiviruses were analyzed for HMGB1 by ELISA. (C) Lysates (WC) or CM from XRA SEN IMR90 cells infected with control (−) or shp53 (+) expressing lentiviruses were analyzed for HMGB1 and actin (control) by Western blotting. The fraction of detectable protein is given below each lane. (D) CM from PRE or SEN (XRA) IMR90 cells expressing control (V) or shp53 (KD) lentiviruses were analyzed for HMGB1 by ELISA. (E) REP SEN HCA2 cells infected with control (GFP; −) or GSE (+) expressing lentivirus were immunostained for HMGB1 (red). Nuclei were stained with DAPI (blue). Bars, 10 µM. (F) IMR-90 and HCA2 cells expressing (+) or depleted of p53 (−) were infected with a lentivirus carrying no insert (Vector), oncogenic RAS, HMGB1 shRNA (KD), or HMGB1 (OE), and scored for SA-β-Gal 3 d later. Error bars = SEM of two independent experiments. (G) Cells in B were infected with lentiviruses carrying no insert (Vector), HMGB1 (OE), or HMGB1 shRNA (KD). 6 d after infection, cells were immunostained for HMGB1 (red). Nuclei were stained with DAPI (blue). Bars, 10 µM. (H) CM from cells infected with lentiviruses carrying no insert (V), shp53 (p53 KD), and/or HMGB1 (OE) were analyzed for HMGB1 by ELISA. Error bars = SEM of triplicate samples.
Senescence caused by altered HMGB1 expression is p53-dependent
We confirmed that some cancer cells overexpress HMGB1 (Brezniceanu et al., 2003) using a panel of normal and tumor-derived human breast cells (unpublished data), which appears at odds with HMGB1 overexpression inducing senescence. However, the cancer lines we tested were all p53 defective. To determine whether senescence caused by altered HMGB1 expression was p53 dependent, we overexpressed or depleted HMGB1 in cells in which p53 was unaltered (+) or depleted by RNAi (−) (Fig. 7 F). As reported (Narita et al., 2003), RAS-induced senescence was p53 independent (Fig. 7 F). However, senescence caused by HMGB1 overexpression or depletion was p53 dependent, as determined by SA-β-Gal activity (Fig. 7 F), growth arrest (not depicted), and SAHF formation (Fig. 7 G). Further, in the absence of p53 function, cells that overexpress HMGB1 secreted very little HMGB1 (Fig. 7 H), in contrast to the SASP, which is more robust in p53-deficient cells (Coppé et al., 2008).
ATM is dispensable for HMGB1 secretion
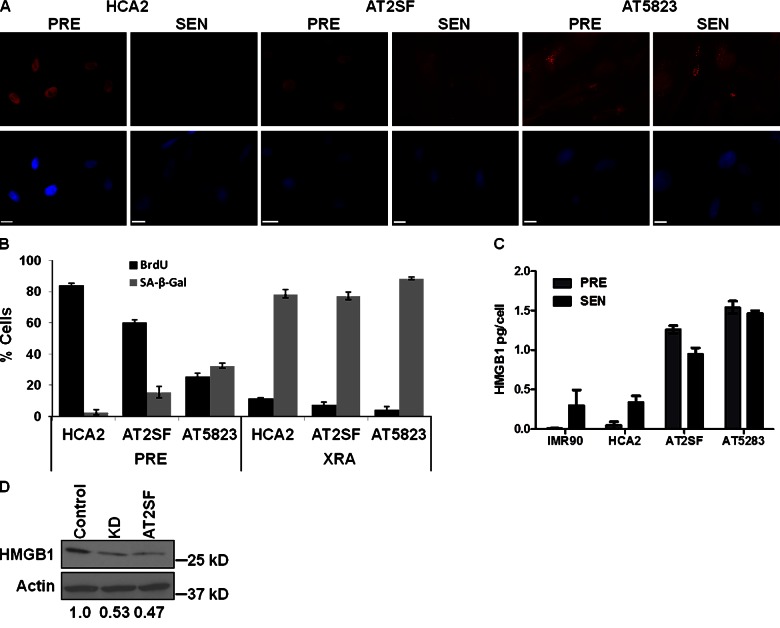
Although the SASP does not require p53 activity, it requires DDR signaling (Coppé et al., 2008; Bhaumik et al., 2009; Rodier et al., 2009, 2011; Le et al., 2010), and cells lacking ATM, a key DDR component, senesce with much reduced cytokine secretion (Rodier et al., 2009). We therefore examined HMGB1 localization in HCA2 and ATM-deficient fibroblasts (AT2SF, AT5823). PRE AT cells had less nuclear HMGB1 immunostaining than HCA2 cells (Fig. 8 A), which LMB prevented (not depicted). After XRA-induced senescence, HCA2 cells lost nuclear staining as expected, but AT cells stained similarly to unirradiated cells (Fig. 8 A). Unirradiated AT cells incorporated less BrdU and expressed more SA-β-Gal than HCA2 cells, yet both cell types responded to XRA by reduced BrdU incorporation and increased SA-β-Gal expression (Fig. 8 B). Consistent with the immunostaining, PRE AT cells secreted more HMGB1 than PRE HCA2 cells. Further, SEN AT cells secreted more HMGB1 than SEN HCA2 cells, which was unaltered by senescence (Fig. 8 C). We also expressed shRNAs against GFP (control) or ATM in HCA2 cells. ATM depletion reduced intracellular HMGB1 to levels present in AT cells (Fig. 8 D). Although reasons for the high HMGB1 secretion and low intracellular HMGB1 in AT cells are unknown, ATM deficiency clearly did not prevent loss of nuclear HMGB1 or its secretion.
Figure 8.
ATM is dispensable for HMGB1 secretion. (A) PRE or SEN (XRA) HCA2 cells and two A-T derived fibroblast strains (AT2SF, AT5283) were immunostained for HMGB1 (red). Nuclei were stained with DAPI (blue). Bars,10 µM. (B) Cells in A were scored for SA-β-Gal and BrdU incorporation (24-h pulse). Error bars = SEM of two experiments. (C) CM from cells in A were assessed for HMGB1 secretion by ELISA. Error bars = SEM of two experiments. (D) Lysates from HCA2 cells infected with lentiviruses carrying GFP (Control) or ATM (KD) shRNA and lysates from A-T fibroblasts (AT2SF) were analyzed for HMGB1 and actin by Western blotting. HMGB1 signals were normalized to actin, and are shown relative to shGFP.
Intracellular and extracellular HMGB1 regulate the SASP
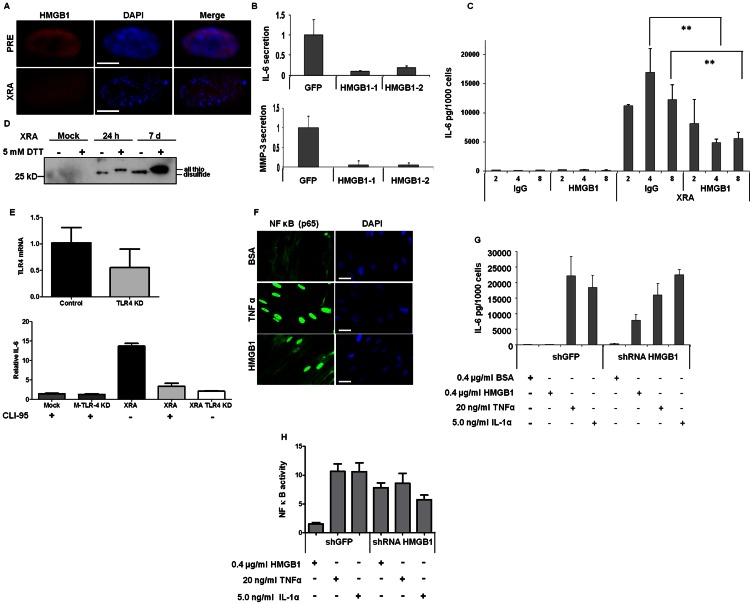
Stressed cells secrete DAMPs, including HMGB1 (Bianchi, 2007; Lotze et al., 2007), which also function intracellularly. HMGB1 preferentially binds euchromatin (Falciola et al., 1997). Although SEN cells secreted HMGB1, we detected low levels in the nucleus (Fig. 9 A). In all SEN cells examined, residual nuclear HGMB1 localized to areas of light DAPI staining, indicative of euchromatin, and was excluded from SAHF (Fig. 9 A). This result, and our finding that >95% HMGB1 depletion (Fig. 5 A) caused senescence without a SASP, suggested that low levels of nuclear HMGB1 were necessary for the SASP. To test this idea, we expressed shRNAs against GFP or HMGB1 in SEN cells, and measured secretion of IL-6 and MMP3, two SASP factors. HMGB1 depletion reduced IL-6 and MMP3 secretion (>90%; Fig. 9 B). Thus, the SASP requires HMGB1 expression, but not high levels in the nucleus.
Figure 9.
HMGB1 regulates SASP factors. (A) PRE and SEN (XRA) WI-38 cells were immunostained for HMGB1 (red). DAPI stained the nuclei (blue). Images were uniformly enhanced to detect HMGB1 in SEN cells. Bar, 10 µM. (B) XRA SEN WI-38 cells were infected with lentiviruses carrying GFP or either of two HMGB1 (HMGB1-1, HMGB1-2) shRNAs. 4 d later, CM was collected and analyzed by ELISA for IL-6 and MMP-3. Shown are fold changes relative to shGFP. Error bars = SEM of two experiments. (C) PRE or SEN (XRA) HCA2 cells were incubated with 2, 4, and 8 µg/ml mouse IgG or HMGB1 blocking antibody. CM were analyzed by ELISA for IL-6. Error bars = SEM of triplicate samples. **, P < 0.02. (D) CM was collected from IMR90 cells that were mock irradiated, or 24 h or 7 d after irradiation. CM were heated in the presence (+) or absence (−) of 5 mM DTT, then analyzed by Western blotting. Positions of reduced (all thiol) and oxidized (disulfide) HMGB1 are indicated. (E) IMR90 cells were infected with control or shTLR-4 expressing lentiviruses and assessed for relative TLR-4 mRNA and IL-6 secretion. IL-6 secretion was assessed in mock (M) or X-irradiated (XRA) SEN cells expressing no insert or TLR-4 shRNA (TLR4 KD), or cultured in the presence (+) or absence (−) of CLI-95, a TLR-4 inhibitor. Shown is the fold expression relative to mock-irradiated cells without CLI-95. Error bars = SEM of triplicate samples. (F) IMR90 cells were cultured with 400 ng/ml BSA, 20 ng/ml TNF, or 400 ng/ml recombinant HMGB1 for 20 min and immunostained for NF-κB (p65 subunit; green). DAPI stained the nuclei (blue). Bar, 10 µM. (G) HCA2 cells infected with shGFP or shHMGB1-expressing lentiviruses were selected for 7 d and cultured for 24 h with indicated concentrations of BSA, recombinant HMGB1, TNF, or IL-1α. CM were analyzed by ELISA for IL-6. Error bars = SEM of triplicate samples. (H) HCA2 cells expressing an NF-κB luciferase reporter were infected with lentiviruses expressing shGFP or shHMGB1. 7 d after selection, cells were cultured for 24 h with the indicated proteins in serum free media at the concentrations in G. Cells were lysed and luciferase was measured. Luciferase levels are fold changes over cells incubated with BSA. One-Way ANOVA was used to analyze the groups; P < 0.001.
To test whether the SASP requires extracellular HMGB1, we cultured PRE or SEN cells with a control or HMGB1 blocking antibody. At a low antibody concentration (2 µg/ml), IL-6 secretion was unaffected (Fig. 9 C). However, at higher concentrations (4 and 8 µg/ml), the blocking, but not control, antibody reduced IL-6 secretion two- to threefold, indicating that optimal IL-6 secretion by SEN cells also requires extracellular HMGB1.
The redox status of reactive HMGB1 cysteines distinguishes its cytokine-inducing and chemokine activity (Venereau et al., 2012; Yang et al., 2012). To determine the redox status of HMGB1 secreted by SEN cells, we collected CM from PRE and SEN cells 24 h and 7 d after XRA, and analyzed CM before (−) or after (+) addition of dithiothreitol (DTT). As expected, PRE CM contained very little HMGB1. In the absence of DTT, we detected a rapidly migrating (oxidized) form, which migrated more slowly after DTT reduction (Fig. 9 D). Thus, SEN cells secreted oxidized HMGB1, the form that stimulates cytokine secretion (Venereau et al., 2012), consistent with our finding that an HMGB1-blocking antibody attenuated IL-6 secretion by SEN cells.
Extracellular HMGB1 signals via TLR4 and NF-κB, and is inhibited by intracellular HMGB1
HMGB1 released by immune cells signals primarily through the RAGE and TLR4 receptors (Klune et al., 2008; Tang et al., 2010). RAGE-blocking antibodies did not alter IL-6 secretion by SEN cells (unpublished data). However, lentiviral-delivered shRNA against TLR-4, which reduced TLR4 mRNA ∼50%, as well as CLI-95, a small molecule inhibitor of TLR-4 signaling (Kawamoto et al., 2008), both significantly reduced IL-6 secretion by SEN cells (Fig. 9 E). Thus, in SEN cells, extracellular HMGB1 signals IL-6 secretion primarily through TLR4.
We also cultured cells depleted of HMGB1 with recombinant HMGB1 (rHMGB1). The rHMGB1 was active because immunostaining showed it promoted NF-κB nuclear localization to the same extent as TNF (Fig. 9 F), as demonstrated for mouse cells (Palumbo et al., 2007). We incubated HMGB1-depleted cells with BSA (negative control), TNF or IL-1α (positive controls), or HMGB1. After 24 h, TNF and IL-1α stimulated IL-6 secretion, regardless of HMGB1 status, as expected (Ammit et al., 2000; Orjalo et al., 2009). rHMGB1 failed to stimulate IL-6 secretion in PRE cells, despite inducing nuclear localization of NF-κB (Fig. S5). As expected, TNF and IL-1α induced both NF-κB translocation and IL-6 secretion by PRE cells. In contrast, rHMGB1 stimulated both NF-κB translocation and IL-6 secretion in HMGB1-depleted cells, as did TNF and IL-1α (Fig. 9 G). Further, using a luciferase reporter to measure NF-κB transcriptional activity (Freund et al., 2011), TNF and IL-1α stimulated transcription (Fig. 9 H) regardless of the presence of endogenous HMGB1, but rHMGB1 stimulated NF-κB transcriptional activity only in HMGB1-depleted cells (Fig. 9 H).
The data suggest that IL-6 secretion by SEN cells is stimulated by extracellular HMGB1, but inhibited by high HMGB1 levels in the nucleus. Thus, development of the SASP likely requires both loss of nuclear HMGB1 and gain of extracellular HMGB1.
Discussion
Cellular senescence prevents the proliferation of potential cancer cells (Campisi, 2001). Several markers identify senescent cells, although none are completely unique to the senescent state. These markers include an enlarged morphology, SA-β-Gal, SAHF (in some cells; Campisi and d’Adda di Fagagna, 2007), persistent DNA damage foci (Rodier et al., 2009, 2011) and the SASP (Coppé et al., 2008), all of which require 3–7 d to develop. We show that HMGB1 relocalization is an early response to senescence cues, occurring within 24–48 h of XRA, which delivers a synchronous senescence signal. Several human and mouse cells lost nuclear HMGB1 and secreted it upon entering senescence, suggesting that loss of nuclear HMGB1 can be used as another (imperfect) marker of senescent cells.
HMGB1 is a founding member of the Alarmin family. Alarmins function both inside and outside the cell, the latter in response to damage or stress (Bianchi, 2007). Necrotic cells passively leak HMBG1, but immune cells actively secrete this protein to amplify inflammatory stimuli such as those initiated by lipopolysaccharide, interferons, or nitric oxide (Wang et al., 1999a,b; Semino et al., 2005). Our finding that SEN cells also actively secrete HMGB1 before other well characterized senescence-associated changes suggests loss of nuclear HMGB1 and its secretion initiates the SASP and, hence, the inflammatory activity of senescent cells (Coppé et al., 2010a; Freund et al., 2010). HMGB1 secretion may also initiate the removal of senescent cells by innate immune cells, which has been described in mouse models of tumorigenesis and liver fibrosis (Xue et al., 2007; Krizhanovsky et al., 2008).
The redox state of HMGB1 determines whether it stimulates inflammatory cytokine secretion or acts as a chemoattractant to recruit immune cells (Venereau et al., 2012). In culture, senescent cells secreted oxidized HMGB1, the form that stimulates cytokine secretion. In vivo, senescent cells may initially secrete oxidized HMGB1, which then becomes reduced to promote immune cell recruitment. Although HMGB1 secretion and other mechanisms may stimulate removal of senescent cells by the immune system, senescent cells nonetheless accumulate with age and at sites of age-related pathology. Consistent with this finding, we detected elevated levels of circulating HMGB1 in old mice.
The SASP, a hallmark of many senescent cells (Coppé et al., 2008), is restrained by p53 (Coppé et al., 2008) and stimulated by ATM (Rodier et al., 2009). HMGB1 secretion differed from the SASP in several ways. First, HMGB1 secretion preceded the secretion of SASP components. This finding reinforces the idea that senescence entails a series of defined events (Rodier and Campisi, 2011), similar to apoptosis (Green, 2005). Second, unlike the SASP, loss of nuclear HMGB1 and HMGB1 secretion required p53 activity. p53-deficient cells did not secrete or lose nuclear HMGB1 in response to senescence-inducing stimuli, nor did they cease growth upon altered HMGB1 expression. Third, unlike the SASP, HMGB1 secretion did not require ATM activity, suggesting HMGB1 release is not a DNA damage response. Consistent with this idea, HMGB1 was secreted by cells induced to senescence by p16INK4a overexpression, which do not experience DNA damage or express a SASP (Rodier et al., 2009; Coppé et al., 2011). Thus, regulation of HMGB1 secretion is distinct from regulation of the SASP.
Early loss of an abundant chromatin architectural protein like HMGB1 might drive changes that lead to the senescence growth arrest and SASP. But it is also possible that earlier senescence-associated chromatin changes drive loss of nuclear HMGB1. For example, after 6 d in TSA, which alters chromatin, cells lost nuclear HMGB1, as reported for monocytes (Bonaldi et al., 2003), and ceased growth, but <15% of cells expressed SA-β-Gal and adopted a senescent morphology. Because HMGB1 binds DNA loosely and transiently (Scaffidi et al., 2002), early chromatin remodeling during senescence may release HMGB1. ATM-deficient cells also show chromatin alterations, as well as levels of reactive oxygen species (ROS; Kim and Wong, 2009) that can provoke loss of nuclear HMGB1 (Tang et al., 2010), which may explain why A-T fibroblasts secreted HMGB1 independent of senescence-inducing stimuli.
Our findings are consistent with a model in which HMGB1 regulates the SASP at two levels: secreted HMGB1 stimulates the SASP through TLR4 signaling and NF-κB transcriptional activity, whereas high levels of nuclear HMGB1 suppress the SASP and NF-κB transcriptional activity. Thus, HMGB1 depletion reduced both nuclear and secreted HMGB1 in senescent cells, and reduced the secretion of SASP components, similar to HMGB1 blocking antibodies. Further, although HMGB1 depletion and overexpression both induced growth arrest, SA-β-Gal, and SAHF, only overexpression caused HMGB1 secretion, NF-κB transcriptional activity, and IL-6 secretion. Further, exogenous HMGB1 stimulated NF-κB translocation but not transactivation activity or IL-6 secretion in PRE or HMGB1-depleted cells. Notably, PRE and HMGB1-depeleted cells retained the ability to secrete IL-6 in response to TNF and IL-1α, indicating that HMGB1 is not required for cells to respond to other inducers of cytokine secretion.
Extracellular HMGB1 is thought to contribute to age-related pathologies such as cardiovascular disease and cancer by stimulating chronic inflammation, a characteristic of aging tissues (Kohno et al., 2009; Tang et al., 2010; Andersson and Tracey, 2011). Likewise, the SASP is thought to contribute to a variety of age-related pathologies, also by driving the sterile inflammation that is hallmark of aging tissues (Campisi and d’Adda di Fagagna, 2007; Coppé et al., 2010a; Freund et al., 2010). At present, the precise relationships between secreted HMGB1, the SASP, and age-related pathology remain to be determined.
Materials and methods
Cells and cell culture
HCA2 (O. Pereira-Smith, University of Texas Health Science Center, San Antonio, TX), BJ (American Tissue Type Collection), IMR-90, WI-38, IMR91(L) and IMR91(S) (Coriell Repository, Camden, NJ), and placental fibroblasts (A. Krtolica, StemLifeLine, Inc.) were cultured in DMEM plus 10% FBS and penicillin/streptomycin at 3% oxygen and 10% CO2. AT2SF and AT5823 were cultured in MEM plus 15% FBS and penicillin/streptomycin at ambient oxygen and 5% CO2. Human mammary epithelial cells (M. Stampfer, Lawrence Berkeley National Laboratory, Berkeley, CA) were cultured in complete MEGM (Lonza) at ambient oxygen and 10% CO2. Prostate epithelial (Coriell Repository) cells were cultured in PrEC complete media (Lonza) at ambient oxygen and 5% CO2. MEFs were isolated from C57BL/6 13.5-d embryos, and cultured in 3% O2 (Parrinello et al., 2003).
Senescence induction, SA-β-Gal, and BrdU labeling
We induced senescence by exposing subconfluent cultures to 10 Gy x-rays; control cultures were mock irradiated. Alternatively, we used lentiviruses to express no insert (control), p16INK4a, Ha-RASV12, or HMGB1 cDNAs, or shRNAs against GFP (control) or HMGB1, or allowed cells to undergo replicative exhaustion. PRE cells were used 1–2 d after mock irradiation or infection; SEN cells were used 7–10 d after irradiation or infection. Cells were considered PRE if >70% incorporated BrdU over a 2-d interval and <10% expressed SA-β-Gal. Cells were considered SEN after 7–10 d; <10% incorporated BrdU and >80% expressed SA-β-Gal (Dimri et al., 1995). We detected SA-β-Gal as described previously (Dimri et al., 1995) or using a commercial kit (BioVision). In brief, cells were fixed in 4% paraformaldehyde, washed, and incubated with detection reagent (provided by manufacturer) at 37°C overnight. We measured DNA synthesis using a modified BrdU labeling kit (Roche) or the EdU proliferation assay (Life Technologies).
Immunofluorescence
Cells in 4- or 8-well chamber slides were immunostained as described previously (Davalos and Campisi, 2003). In brief, cells were fixed with 4% formaldehyde, permeabilized with 0.5% Triton, and blocked with 10% goat or donkey serum before incubating overnight at 4°C with primary antibody in 5 or 10% serum. After washes with PBS, we incubated samples with Alexa Fluor 488– or 594–conjugated secondary antibodies for 30 min at room temperature, followed by three washes with PBS. The final wash contained 0.1 mg/ml DAPI. We mounted slides in Vectashield and viewed by epifluorescence. Mouse tissue was processed in coplin jars using the same protocol. We viewed samples using a microscope (model BX60; Olympus) and either a 100× UPlanFl 1.3 NA lens with oil or a 40× UPlanFl 0.5 NA lens without oil. Images were acquired with a charge-coupled device camera (Spot Flex color camera; Diagnostic Instruments, Inc.) and captured using SPOT imaging software (Diagnostic Instruments, Inc.). Any image modifications were applied to the entire image and all relevant fields and samples processed with Photoshop CS2 (Adobe).
Antibodies and chemicals
Rabbit (BD or Proteintech) or mouse (R&D Systems) anti-HMGB1 antibodies were used for Western blots, and rabbit (BD or Abcam) antibodies or DPH1.1 (a kind gift from K.J. Tracey, Feinstein Institute for Medical Research, Manhasset, NY) for immunofluorescence. We also used mouse anti-actin (EMD Millipore), anti-tubulin (Sigma-Aldrich), anti–acetyl-lysine, anti-53BP1 (EMD Millipore), anti-p16 (Neomarkers), anti-p53 (Santa Cruz Biotechnology, Inc.), anti–NF-κB (Santa Cruz Biotechnology, Inc.), and rabbit anti-active caspase-3 (Cell Signaling Technology) primary antibodies. Secondary goat antibodies conjugated to Alexa Fluors (488, 594) were from Molecular Probes. We used DAPI to stain nuclear DNA. H2O2, trichostatin A, leptomycin B, staurosporine, recombinant HMGB1, BSA, and TNF were from Sigma-Aldrich. IL-1α was from R&D Systems.
ELISA and cytotoxicity
CM were collected from cells cultured in 0.2% serum for 24 h, filtered, and stored at −80°C. Cell number was determined and each sample was normalized for cell number. We used ELISA kits to measure IL-6 (R&D Systems) and HMGB1 (IBL International, Chondrex), and a lactate dehydrogenase kit (Takara Bio Inc.) to measure cytotoxicity, in each case following the manufacturer’s instructions.
Mouse sera
Young and old mouse (C57BL/6) tissue and sera were obtained from the National Institute on Aging rodent tissue bank.
Western analysis
Whole-cell lysates were prepared in 5% SDS or RIPA buffer (EMD Millipore; Davalos and Campisi, 2003). Cells were cultured in 0.2% serum before collecting CM. The medium was filtered and either precipitated with trichloroacetic acid or concentrated with Centricon centrifugal filter units (EMD Millipore) to 100×. Western analyses were performed as described previously, loading either 30 µg protein or concentrated medium equivalent to 3–6 × 105 cells per lane (Davalos and Campisi, 2003).
Irradiation
Cells were irradiated with a total dose of 10 Gy at 0.8 Gy/min using a Pantak x-ray generator (320 kV/10 mA with 0.5 mm copper filtration). 6–8-wk-old C57BL/6 mice (Charles River) were total body irradiated at the sublethal dose of 9 Gy (30 Gy/min) using a Gammacell 220 and 60Co as a source. Brain and kidney were collected 1 wk after irradiation.
NF-κB assay
Cells expressing the Cignal NF-κB Lentiviral Reporter (CLS-013L; SA Biosciences) were infected with insertless, HMGB1-overexpressing or HMGB1 shRNA-expressing lentiviruses, and incubated with recombinant BSA, TNF, IL-1α, or HMGB1 for 24 h in serum-free media. NF-κB activity was measured as described previously (Freund et al., 2011). In brief, cells were lysed and assayed for luciferase using the Luciferase Assay System (E1500; Promega). All values were normalized to cell number.
Expression vectors and siRNA
The following sequences were used to target the indicated proteins by RNAi: HMGB-#1, 5′-GAGGCCTCCTTCGGCCTTC-3′; HMGB1-#2, 5′-GTTGGTTCTAGCGCAGTTT-3′; p53, 5′-GACUCCATRGGUAAUCUAC-3′ (Nair et al., 2005); p16INK4a, 5′-CGCTGTGGCCCTCGTGCTGATGCTA3′ (Beauséjour et al., 2003; Narita et al., 2003); TLR-4, 5′-ATATTAAGGTAGAGAGGTGGC-3′ (OpenBiosystems, TRCN0000056894); RELA, 5′-AACTCATCATAGTTGATGGTG-3′ (OpenBiosystems, TRCN0000014686; Freund et al., 2011); and ATM, 5′-TGAAGATGGTGCTCATAAA-3′ (Open Biosystems, TRCN0000039948; Rodier et al., 2009). We also used a sequence verified HMGB1 cDNA (TC118802; Origene) cloned into a modified lentiviral vector (670–1) that conferred puromycin resistance (Campeau et al., 2009). To inactivate p53, we used the genetic suppressor element (GSE) 22 (Gudkov et al., 1993; Beauséjour et al., 2003).
Online supplemental material
Fig. S1 shows that HMGB1 relocalization occurred in senescent cells from multiple tissue types. Fig. S2 demonstrates that extended incubation of fibroblasts with leptomycin B induced senescence without HMGB1 secretion. Fig. S3 shows overexpression or depletion of HMGB1 induced senescence in low expressing p16 HCA2 fibroblasts. In addition to growth arrest and senescence-associated β-galactosidase activity, HMGB1-mediated senescence induced associated heterochromatin foci in HCA2 and senescence did not occur in response to stress. Fig. S4 shows that HMGB1 overexpression and deletion induced senescence in MEF and HMEC. p16-mediated senescence does not exhibit a SASP, but promoted HMGB1 secretion. Fig. S5 shows rHMGB1 induced NF-κB nuclear relocalization in the presence or absence of endogenous nuclear HMGB1. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201206006/DC1.
Supplementary Material
Acknowledgments
We thank R.-M. Laberge and M. Hararah for technical assistance and E. Campeau for access to lentiviral systems before publication; R. Hansen for assistance in generating graphs; K. Tracey for HMGB1 antibody; P. Desprez for critical review of the manuscript; and the Buck Institute Morphology and Imaging Core facility.
This work was supported by grants from the National Institutes of Health (AG09909, AG017242 to J. Campisi), California Breast Cancer Research Program (11IB-0153 to A.R. Davalos), and contract AC03-76SF00098 from the US Department of Energy and SENS Foundation (to N. Schaum).
Footnotes
Abbreviations used in this paper:
- A-T
- ataxia telangiectasia
- ATM
- ataxia-telangiectasia mutated
- CM
- conditioned medium
- DAMPs
- damage-associated molecular patterns
- DDF
- DNA damage foci
- DDR
- DNA damage response
- GSE
- genetic suppressor element
- HAT
- histone acetyl transferase
- HMEC
- human mammary epithelial cells
- HMGB1
- high mobility group box 1 protein
- IL-6
- interleukin-6
- LMB
- leptomycin B
- MEF
- mouse embryo fibroblast
- MMP-3
- matrix metalloproteinase 3
- PRE
- presenescent (non-senescent)
- REP
- replicative senescence
- SA-β-Gal
- senescence-associated β-galactosidase
- SAHF
- senescence-associated heterochromatin foci
- SASP
- senescence-associated secretory phenotype
- SEN
- senescent
- TLR
- toll-like receptor
- TSA
- trichostatin A
- XRA
- X-irradiation
References
- Acosta J.C., O’Loghlen A., Banito A., Guijarro M.V., Augert A., Raguz S., Fumagalli M., Da Costa M., Brown C., Popov N., et al. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 133:1006–1018 10.1016/j.cell.2008.03.038 [DOI] [PubMed] [Google Scholar]
- Ammit A.J., Hoffman R.K., Amrani Y., Lazaar A.L., Hay D.W., Torphy T.J., Penn R.B., Panettieri R.A., Jr 2000. Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth-muscle cells. Modulation by cyclic adenosine monophosphate. Am. J. Respir. Cell Mol. Biol. 23:794–802 10.1165/ajrcmb.23.6.4184 [DOI] [PubMed] [Google Scholar]
- Andersson U., Tracey K.J. 2011. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29:139–162 10.1146/annurev-immunol-030409-101323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U., Erlandsson-Harris H., Yang H., Tracey K.J. 2002. HMGB1 as a DNA-binding cytokine. J. Leukoc. Biol. 72:1084–1091 [PubMed] [Google Scholar]
- Banerjee S., Kundu T.K. 2003. The acidic C-terminal domain and A-box of HMGB-1 regulates p53-mediated transcription. Nucleic Acids Res. 31:3236–3247 10.1093/nar/gkg412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavik C., Coleman I., Dean J.P., Knudsen B., Plymate S., Nelson P.S. 2006. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 66:794–802 10.1158/0008-5472.CAN-05-1716 [DOI] [PubMed] [Google Scholar]
- Beauséjour C.M., Krtolica A., Galimi F., Narita M., Lowe S.W., Yaswen P., Campisi J. 2003. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 22:4212–4222 10.1093/emboj/cdg417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik D., Scott G.K., Schokrpur S., Patil C.K., Orjalo A.V., Rodier F., Lithgow G.J., Campisi J. 2009. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY). 1:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M.E. 2007. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81:1–5 10.1189/jlb.0306164 [DOI] [PubMed] [Google Scholar]
- Bianchi M.E. 2009. HMGB1 loves company. J. Leukoc. Biol. 86:573–576 10.1189/jlb.1008585 [DOI] [PubMed] [Google Scholar]
- Bonaldi T., Talamo F., Scaffidi P., Ferrera D., Porto A., Bachi A., Rubartelli A., Agresti A., Bianchi M.E. 2003. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 22:5551–5560 10.1093/emboj/cdg516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezniceanu M.L., Völp K., Bösser S., Solbach C., Lichter P., Joos S., Zörnig M. 2003. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J. 17:1295–1297 [DOI] [PubMed] [Google Scholar]
- Campeau E., Ruhl V.E., Rodier F., Smith C.L., Rahmberg B.L., Fuss J.O., Campisi J., Yaswen P., Cooper P.K., Kaufman P.D. 2009. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS ONE. 4:e6529 10.1371/journal.pone.0006529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. 2001. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11:S27–S31 [DOI] [PubMed] [Google Scholar]
- Campisi J. 2003. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Exp. Gerontol. 38:5–11 10.1016/S0531-5565(02)00152-3 [DOI] [PubMed] [Google Scholar]
- Campisi J., d’Adda di Fagagna F. 2007. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8:729–740 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- Coppé J.P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6:2853–2868 10.1371/journal.pbio.0060301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J.P., Desprez P.Y., Krtolica A., Campisi J. 2010a. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5:99–118 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J.P., Patil C.K., Rodier F., Krtolica A., Beauséjour C.M., Parrinello S., Hodgson J.G., Chin K., Desprez P.Y., Campisi J. 2010b. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE. 5:e9188 10.1371/journal.pone.0009188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J.P., Rodier F., Patil C.K., Freund A., Desprez P.Y., Campisi J. 2011. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 286:36396–36403 10.1074/jbc.M111.257071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. 2002. Inflammation and cancer. Nature. 420:860–867 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos A.R., Campisi J. 2003. Bloom syndrome cells undergo p53-dependent apoptosis and delayed assembly of BRCA1 and NBS1 repair complexes at stalled replication forks. J. Cell Biol. 162:1197–1209 10.1083/jcb.200304016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O., et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 92:9363–9367 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu I.E., Baruah P., Manfredi A.A., Bianchi M.E., Rovere-Querini P. 2005. HMGB1: guiding immunity from within. Trends Immunol. 26:381–387 10.1016/j.it.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Ellerman J.E., Brown C.K., de Vera M., Zeh H.J., Billiar T., Rubartelli A., Lotze M.T. 2007. Masquerader: high mobility group box-1 and cancer. Clin. Cancer Res. 13:2836–2848 10.1158/1078-0432.CCR-06-1953 [DOI] [PubMed] [Google Scholar]
- Falciola L., Spada F., Calogero S., Langst G., Voit R., Grummt I., Bianchi M.E. 1997. High mobility group 1 protein is not stably associated with the chromosomes of somatic cells. J. Cell Biol. 137:19–26 10.1083/jcb.137.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C. 2007. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr. Rev. 65:173–176 10.1301/nr.2007.dec.S173-S176 [DOI] [PubMed] [Google Scholar]
- Freund A., Orjalo A.V., Desprez P.Y., Campisi J. 2010. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 16:238–246 10.1016/j.molmed.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A., Patil C.K., Campisi J. 2011. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 30:1536–1548 10.1038/emboj.2011.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella S., Andrei C., Ferrera D., Lotti L.V., Torrisi M.R., Bianchi M.E., Rubartelli A. 2002. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 3:995–1001 10.1093/embo-reports/kvf198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G.H., Sanders C., Johns E.W. 1973. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 38:14–19 10.1111/j.1432-1033.1973.tb03026.x [DOI] [PubMed] [Google Scholar]
- Green D.R. 2005. Apoptotic pathways: ten minutes to dead. Cell. 121:671–674 10.1016/j.cell.2005.05.019 [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Giese K., Pagel J. 1994. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 10:94–100 10.1016/0168-9525(94)90232-1 [DOI] [PubMed] [Google Scholar]
- Gudkov A.V., Zelnick C.R., Kazarov A.R., Thimmapaya R., Suttle D.P., Beck W.T., Roninson I.B. 1993. Isolation of genetic suppressor elements, inducing resistance to topoisomerase II-interactive cytotoxic drugs, from human topoisomerase II cDNA. Proc. Natl. Acad. Sci. USA. 90:3231–3235 10.1073/pnas.90.8.3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. 1965. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37:614–636 10.1016/0014-4827(65)90211-9 [DOI] [PubMed] [Google Scholar]
- Herbig U., Ferreira M., Condel L., Carey D., Sedivy J.M. 2006. Cellular senescence in aging primates. Science. 311:1257 10.1126/science.1122446 [DOI] [PubMed] [Google Scholar]
- Ivanov S., Dragoi A.M., Wang X., Dallacosta C., Louten J., Musco G., Sitia G., Yap G.S., Wan Y., Biron C.A., et al. 2007. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 110:1970–1981 10.1182/blood-2006-09-044776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman L., Moorthy N.C., Murthy K.G., Manley J.L., Bustin M., Prives C. 1998. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 12:462–472 10.1101/gad.12.4.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Ii M., Kitazaki T., Iizawa Y., Kimura H. 2008. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 584:40–48 10.1016/j.ejphar.2008.01.026 [DOI] [PubMed] [Google Scholar]
- Kim J., Wong P.K. 2009. Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling. Stem Cells. 27:1987–1998 10.1002/stem.125 [DOI] [PubMed] [Google Scholar]
- Klune J.R., Dhupar R., Cardinal J., Billiar T.R., Tsung A. 2008. HMGB1: endogenous danger signaling. Mol. Med. 14:476–484 10.2119/2008-00034.Klune [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T., Anzai T., Naito K., Miyasho T., Okamoto M., Yokota H., Yamada S., Maekawa Y., Takahashi T., Yoshikawa T., et al. 2009. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc. Res. 81:565–573 10.1093/cvr/cvn291 [DOI] [PubMed] [Google Scholar]
- Kortlever R.M., Higgins P.J., Bernards R. 2006. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 8:877–884 10.1038/ncb1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosar M., Bartkova J., Hubackova S., Hodny Z., Lukas J., Bartek J. 2011. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a). Cell Cycle. 10:457–468 10.4161/cc.10.3.14707 [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S.W. 2008. Senescence of activated stellate cells limits liver fibrosis. Cell. 134:657–667 10.1016/j.cell.2008.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T., Michaloglou C., Vredeveld L.C., Douma S., van Doorn R., Desmet C.J., Aarden L.A., Mooi W.J., Peeper D.S. 2008. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 133:1019–1031 10.1016/j.cell.2008.03.039 [DOI] [PubMed] [Google Scholar]
- Le O.N., Rodier F., Fontaine F., Coppe J.P., Campisi J., DeGregori J., Laverdière C., Kokta V., Haddad E., Beauséjour C.M. 2010. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 9:398–409 10.1111/j.1474-9726.2010.00567.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Stolz D.B., Sappington P.L., Macias C.A., Killeen M.E., Tenhunen J.J., Delude R.L., Fink M.P. 2006. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am. J. Physiol. Cell Physiol. 290:C990–C999 10.1152/ajpcell.00308.2005 [DOI] [PubMed] [Google Scholar]
- Lotze M.T., Tracey K.J. 2005. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5:331–342 10.1038/nri1594 [DOI] [PubMed] [Google Scholar]
- Lotze M.T., Zeh H.J., Rubartelli A., Sparvero L.J., Amoscato A.A., Washburn N.R., Devera M.E., Liang X., Tör M., Billiar T. 2007. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 220:60–81 10.1111/j.1600-065X.2007.00579.x [DOI] [PubMed] [Google Scholar]
- Müller S., Ronfani L., Bianchi M.E. 2004. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J. Intern. Med. 255:332–343 10.1111/j.1365-2796.2003.01296.x [DOI] [PubMed] [Google Scholar]
- Nair A.R., Schliekelman M., Thomas M.B., Wakefield J., Jurgensen S., Ramabhadran R. 2005. Inhibition of p53 by lentiviral mediated shRNA abrogates G1 arrest and apoptosis in retinal pigmented epithelial cell line. Cell Cycle. 4:697–703 10.4161/cc.4.5.1672 [DOI] [PubMed] [Google Scholar]
- Narita M., Nũnez S., Heard E., Narita M., Lin A.W., Hearn S.A., Spector D.L., Hannon G.J., Lowe S.W. 2003. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 113:703–716 10.1016/S0092-8674(03)00401-X [DOI] [PubMed] [Google Scholar]
- Narita M., Narita M., Krizhanovsky V., Nuñez S., Chicas A., Hearn S.A., Myers M.P., Lowe S.W. 2006. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 126:503–514 10.1016/j.cell.2006.05.052 [DOI] [PubMed] [Google Scholar]
- Orjalo A.V., Bhaumik D., Gengler B.K., Scott G.K., Campisi J. 2009. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA. 106:17031–17036 10.1073/pnas.0905299106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossovskaya V.S., Mazo I.A., Chernov M.V., Chernova O.B., Strezoska Z., Kondratov R., Stark G.R., Chumakov P.M., Gudkov A.V. 1996. Use of genetic suppressor elements to dissect distinct biological effects of separate p53 domains. Proc. Natl. Acad. Sci. USA. 93:10309–10314 10.1073/pnas.93.19.10309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo R., Galvez B.G., Pusterla T., De Marchis F., Cossu G., Marcu K.B., Bianchi M.E. 2007. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J. Cell Biol. 179:33–40 10.1083/jcb.200704015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.S., Arcaroli J., Yum H.K., Yang H., Wang H., Yang K.Y., Choe K.H., Strassheim D., Pitts T.M., Tracey K.J., Abraham E. 2003. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. Cell Physiol. 284:C870–C879 [DOI] [PubMed] [Google Scholar]
- Park J.S., Gamboni-Robertson F., He Q., Svetkauskaite D., Kim J.Y., Strassheim D., Sohn J.W., Yamada S., Maruyama I., Banerjee A., et al. 2006. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 290:C917–C924 10.1152/ajpcell.00401.2005 [DOI] [PubMed] [Google Scholar]
- Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. 2003. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 5:741–747 10.1038/ncb1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S., Coppe J.P., Krtolica A., Campisi J. 2005. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 118:485–496 10.1242/jcs.01635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur A., Peeper D.S. 2008. Cellular senescence in vivo: a barrier to tumorigenesis. Curr. Opin. Cell Biol. 20:150–155 10.1016/j.ceb.2008.01.007 [DOI] [PubMed] [Google Scholar]
- Raucci A., Palumbo R., Bianchi M.E. 2007. HMGB1: a signal of necrosis. Autoimmunity. 40:285–289 10.1080/08916930701356978 [DOI] [PubMed] [Google Scholar]
- Rodier F., Campisi J. 2011. Four faces of cellular senescence. J. Cell Biol. 192:547–556 10.1083/jcb.201009094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F., Coppé J.P., Patil C.K., Hoeijmakers W.A., Muñoz D.P., Raza S.R., Freund A., Campeau E., Davalos A.R., Campisi J. 2009. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11:973–979 10.1038/ncb1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F., Muñoz D.P., Teachenor R., Chu V., Le O., Bhaumik D., Coppé J.P., Campeau E., Beauséjour C.M., Kim S.H., et al. 2011. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 124:68–81 10.1242/jcs.071340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P., Misteli T., Bianchi M.E. 2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 418:191–195 10.1038/nature00858 [DOI] [PubMed] [Google Scholar]
- Schiraldi M., Raucci A., Muñoz L.M., Livoti E., Celona B., Venereau E., Apuzzo T., De Marchis F., Pedotti M., Bachi A., et al. 2012. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 209:551–563 10.1084/jem.20111739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semino C., Angelini G., Poggi A., Rubartelli A. 2005. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 106:609–616 10.1182/blood-2004-10-3906 [DOI] [PubMed] [Google Scholar]
- Sha Y., Zmijewski J., Xu Z., Abraham E. 2008. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J. Immunol. 180:2531–2537 [DOI] [PubMed] [Google Scholar]
- Smart P., Lane E.B., Lane D.P., Midgley C., Vojtesek B., Laín S. 1999. Effects on normal fibroblasts and neuroblastoma cells of the activation of the p53 response by the nuclear export inhibitor leptomycin B. Oncogene. 18:7378–7386 10.1038/sj.onc.1203260 [DOI] [PubMed] [Google Scholar]
- Suzuki M., Boothman D.A. 2008. Stress-induced premature senescence (SIPS)—influence of SIPS on radiotherapy. J. Radiat. Res. (Tokyo). 49:105–112 10.1269/jrr.07081 [DOI] [PubMed] [Google Scholar]
- Tang D., Kang R., Zeh H.J., III, Lotze M.T. 2010. High-mobility group box 1 and cancer. Biochim. Biophys. Acta. 1799:131–140 10.1016/j.bbagrm.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Avalos A.M., Mao S.Y., Chen B., Senthil K., Wu H., Parroche P., Drabic S., Golenbock D., Sirois C., et al. 2007. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8:487–496 10.1038/ni1457 [DOI] [PubMed] [Google Scholar]
- Venereau E., Casalgrandi M., Schiraldi M., Antoine D.J., Cattaneo A., De Marchis F., Liu J., Antonelli A., Preti A., Raeli L., et al. 2012. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 209:1519–1528 10.1084/jem.20120189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajapeyee N., Serra R.W., Zhu X., Mahalingam M., Green M.R. 2008. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 132:363–374 10.1016/j.cell.2007.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Jurk D., Maddick M., Nelson G., Martin-Ruiz C., von Zglinicki T. 2009. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 8:311–323 10.1111/j.1474-9726.2009.00481.x [DOI] [PubMed] [Google Scholar]
- Wang H., Bloom O., Zhang M., Vishnubhakat J.M., Ombrellino M., Che J., Frazier A., Yang H., Ivanova S., Borovikova L., et al. 1999a. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 285:248–251 10.1126/science.285.5425.248 [DOI] [PubMed] [Google Scholar]
- Wang H., Vishnubhakat J.M., Bloom O., Zhang M., Ombrellino M., Sama A., Tracey K.J. 1999b. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 126:389–392 10.1016/S0039-6060(99)70182-0 [DOI] [PubMed] [Google Scholar]
- Xue W., Zender L., Miething C., Dickins R.A., Hernando E., Krizhanovsky V., Cordon-Cardo C., Lowe S.W. 2007. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 445:656–660 10.1038/nature05529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Maruyama I. 2007. HMGB1, a novel inflammatory cytokine. Clin. Chim. Acta. 375:36–42 10.1016/j.cca.2006.07.019 [DOI] [PubMed] [Google Scholar]
- Yang H., Lundbäck P., Ottosson L., Erlandsson-Harris H., Venereau E., Bianchi M.E., Al-Abed Y., Andersson U., Tracey K.J., Antoine D.J. 2012. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol. Med. 18:250–259 10.2119/molmed.2011.00389 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.