Abstract
Human embryonic stem cell-derived neural precursors (hESC NPs) are considered to be a promising tool for cell-based therapy in central nervous system injuries and neurodegenerative diseases. The Ca2+ ion is an important intracellular messenger essential for the regulation of various cellular functions. We investigated the role and physiology of Ca2+ signaling to characterize the functional properties of CCTL14 hESC NPs during long-term maintenance in culture (in vitro). We analyzed changes in cytoplasmic Ca2+ concentration ([Ca2+]i) evoked by high K+, adenosine-5′-triphosphate (ATP), glutamate, γ-aminobutyric acid (GABA), and caffeine in correlation with the expression of various neuronal markers in different passages (P6 through P10) during the course of hESC differentiation. We found that only differentiated NPs from P7 exhibited significant and specific [Ca2+]i responses to various stimuli. About 31% of neuronal-like P7 NPs exhibited spontaneous [Ca2+]i oscillations. Pharmacological and immunocytochemical assays revealed that P7 NPs express L- and P/Q-type Ca2+ channels, P2X2, P2X3, P2X7, and P2Y purinoreceptors, glutamate receptors, and ryanodine (RyR1 and RyR3) receptors. The ATP- and glutamate-induced [Ca2+]i responses were concentration-dependent. Higher glutamate concentrations (over 100 μM) caused cell death. Responses to ATP were observed in the presence or in the absence of extracellular Ca2+. These results emphasize the notion that with time in culture, these cells attain a transient period of operative Ca2+ signaling that is predictive of their ability to act as stem elements.
Introduction
Human embryonic stem cells (hESCs) are pluripotent cells derived from the inner cell mass of a preimplantation embryo [1]. In vitro, these cells are able to maintain a normal euploid kariotype, differentiate into derivatives of all 3 germ layers, and proliferate extensively [2,3]. These properties make them unique candidates for cell transplantation, research into growth factors and early human development, and for drug discovery. Substantial progress has been made recently in the differentiation of hESCs into a neuronal phenotype [3–5], this being a promising strategy for cell-based therapy of central nervous system injuries and neurodegenerative diseases. However, to date, several important questions remain unanswered: (1) when and at what stage of differentiation should cells be transplanted; (2) what are the functional properties (ion channels, receptors, and second messengers) of these cells and how are they regulated; and (3) how compatible are these properties with the physiological or pathological environment at the site of transplantation and treatment? Hitherto, with a few exceptions, the quality of stem cells is generally evaluated by determining the expression of various genes and key proteins during the process of differentiation; these however, although being present in the cell, may be physiologically inactive. Therefore, the aim of this study was, for the first time, to determine and characterize Ca2+ signals activated by physiological stimulation of neural precursors (NPs) derived from hESCs.
Ca2+ is a ubiquitous second messenger involved in the regulation of almost all known cellular processes and, above all, in defining the life and death of every cell [6–10]. Signals mediated by Ca2+ are fundamental for fertilization, cell differentiation, proliferation [11], intercellular signaling, transcription factor activation, and various death programs including necrosis and apoptosis [12]. Ca2+ can enter the cytoplasm from 2 sources: either by an influx via plasmalemmal voltage-operated and receptor-operated Ca2+ channels (VOCC and ROCC respectively) or by release from intracellular stores, such as the endoplasmic reticulum, through endomembrane Ca2+ channels classified as inositol-1,4,5-trisphosphate receptors (InsP3Rs) and ryanodine receptors (RyRs). The variety of functions executed by Ca2+ depends on the speed, amplitude, and spatiotemporal pattern of Ca2+ signals and by interactions between Ca2+ and other signaling pathways [9]. For example, changes in [Ca2+]i following the activation of purinoceptors (P2X3, P2X4, P2Y1, and P2Y2) promote cell proliferation in murine ESCs [13]. On the other hand, glial excitability depends on Ca2+ waves that often occur as a result of adenosine-5′-triphosphate (ATP)-mediated signaling through P2Y receptors [14]. The entry of Ca2+ through VOCC and the release of Ca2+ from internal stores modulate neuronal excitability [15]. A transient increase in [Ca2+] regulates cellular secretion and cellular motility during neuronal development [16]. Both γ-aminobutyric acid (GABA) and glutamate have been shown to influence NP cell proliferation during development [17,18]. Hence, a detailed characterization of the Ca2+ signaling cascades that are activated by various stimuli is useful in determining the functional state of the cell and may even predict its fate.
In the previous work we have described a novel protocol for the efficient generation of NPs from hESCs [19]. Our results showed that (1) hESC NPs are able to differentiate into a neuronal phenotype and to develop into functionally active neurons; (2) P8 hESC NPs are the appropriate candidate for transplantation compared to undifferentiated hESCs and to P1, P5, and P10; (3) long-term maintenance in vitro decreases tumorogenisity, although simultaneously attenuates proliferative activity and differentiation potential. Our study further revealed that the profile of NPs changes with the length of maintenance in culture.
To date, the role of Ca2+, its homeostasis and signaling potential in ESCs differentiated to a neuronal phenotype have not been studied. To our knowledge there are only a few reports available on mouse ESCs [20,21] and on hESCs differentiated toward neurones [22–25]. Therefore, we undertook the present study to determine whether the functional properties of NPs change during long-term maintenance in culture. We also asked at what stage of differentiation are these cells in ideal physiological condition? We identified and analyzed molecular cascades of [Ca2+]i homeostasis and Ca2+ signaling in correlation with hESC differentiation into a neuronal phenotype.
Materials and Methods
Drugs and solutions
Chemicals obtained from Sigma-Aldrich: accutase, laminin, cadmium chloride, nickel chloride, nicardipine hydrochloride, ATP, α,β-Methyleneadenosine 5′-triphosphate lithium salt (α,β-MeATP), suramin sodium salt, pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS), 2′(3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP), l-Glutamic acid potassium salt monohydrate, N-Methyl-D-aspartic acid (NMDA), kainic acid, GABA, adenosine, β-mercaptoethanol, bovine serum albumin, and Triton-X 100; from GIBCO: Dulbecco's modified Eagle's medium (DMEM)/F12, l-glutamine, penicillin/streptomycin, fetal bovine serum, collagenase type IV, and B27; from Invitrogen: human recombinant fibroblast growth factor (hrFGF), Fura-2 AM 1 mM solution in anhydrous dimethyl sulfoxide (DMSO) cell permeant, and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI); from R&D Systems: human recombinant epidermal growth factor (hrEGF); from Molecular Probes (Eugene, OR): goat anti-mouse IgG conjugated with Alexa-Fluor 594, goat anti-rabbit IgG conjugated with Alexa-Fluor 488, and Pluronic F-127; from Tocris Bioscience: NF279; from Polysciences, Inc. (Warrington, PA): Aqua Poly/Mount mounting medium; from Alomone Labs Ltd.: caffeine, ryanodine, cyclopiazonic acid (CPA), ω-conotoxin MVIIC (MVIIC), and ω-conotoxin GVIA (GVIA).
Concentrated stock solutions of nicardipine, glutamate, and ryanodine were prepared in DMSO, while the remaining stock solutions of agonists/antagonists were dissolved in dH2O. For each experiment, caffeine was freshly dissolved in Normal Locke's buffer (NL) at 37°C and vortexed until the caffeine crystals dissolved. All concentrated stock solutions were stored at −20°C. Test solutions were daily prepared using aliquots from frozen stocks to obtain the working concentrations. All buffers and solutions in this study were made using ion-free dH2O from Merck, Germany.
hESC and hESC NP culture
hESCs line CCTL14 (complete information on the derivation and characterization of CCTL14 line of hESCs is available at www.isscr.org/science/sclines.htm) were cultured on a feeder layer of mitomycin-C-treated mouse embryonic fibroblasts in gelatin-coated tissue culture dishes. The culture medium (CM) was (D-MEM/F-12 without l-glutamine) supplemented with 15% fetal bovine serum, 1% nonessential amino acids, 2 mM l-glutamine, penicillin, and streptomycin at 50 U/mL, 0.1 mM β-mercaptoethanol, and 4 ng/mL hrFGF. Colonies of hESCs were passaged every 4–7 days using either mechanical scraping with a glass pipette to provide low-density cultures of undifferentiated cells or enzymatic dissociation with collagenase type IV to provide high-density cultures of undifferentiated cells [19].
NPs were derived from the CCTL14 line of human ESCs as described previously [19]. Briefly, to induce neural differentiation, clumps of undifferentiated hESCs were plated in agarose-coated tissue culture dishes with CM without hrFGF for 4 days, then with CM supplemented with Noggin for the next 4 days. At this time, 70%–90% of the colonies formed embryoid bodies (EBs). Aggregates whose diameter exceeded 0.5 mm were dissected into smaller clumps with 20 G surgical blades, then replaced in serum-free medium or NP medium (NPM) and cultured for 6 days. At this stage, cells in the EBs were defined as passage 1. NPM consisted of DMEM/F12 medium (1:1), B27 supplement (1:50), 2 mM l-glutamine and penicillin, and streptomycin at 50 U/mL, supplemented with 20 ng/mL hrEGF and 20 ng/mL hrFGF. For the long-term propagation of hESC NPs, the EBs were dissociated by accutase and the cells were plated onto laminin-coated culture dishes. NPs were cultured in NPM and passaged by accutase each 5–7 days.
Antibodies and immunocytochemistry
Cells plated onto laminin-coated coverslips were washed in phosphate-buffered saline (0.1 M PBS, pH 7.2) and fixed with 4% paraformaldehyde in PBS for 15 min. The fixed cells were washed twice in PBS prior to immunostaining. Permeabilization and blocking were carried out in a blocking buffer consisting of 0.4% Triton-X 100 and 10% bovine serum albumin in 0.1 M PBS for 45 min at 22°C. Primary antibodies (See Table 1) were diluted in buffer consisting of 0.1% Triton-X 100 and 2% bovine serum albumin in PBS overnight at 4°C. After 2 washes with PBS, appropriate secondary antibodies (Alexa Fluor 488-conjugated goat anti-rabbit IgG [1:200] or Alexa Fluor 594-conjugated goat anti-mouse IgG [1:200]), were applied for 30 min at 22°C. To visualize the cell nuclei, the coverslips were incubated with 300 nM DAPI in PBS for 5 min at 22°C. Finally, the coverslips with cells were mounted using Aqua Poly/Mount mounting medium and examined using a ZEISS LSM 510 DUO confocal microscope (Carl Zeiss).
Table 1.
Antibodies Used for Immunocytochemistry
| Antibody | Dilution | Host/Antibody type | Company | Secondary antibody used |
|---|---|---|---|---|
| Anti-NeuN | 1:100 | Mouse monoclonal | Merck Millipore | Alexa Fluor 594 goat anti-mouse IgG |
| Anti-β-tubulin isotype III | 1:1000 | Mouse monoclonal | Sigma-Aldrich | Alexa Fluor 594 goat anti-mouse IgG |
| Anti-MAP-2 | 1:1000 | Mouse monoclonal | Merck Millipore Chemicon | Alexa Fluor 594 goat anti-mouse IgG |
| Anti-nestin | 1:2000 | Mouse monoclonal | Merck Millipore | Alexa Fluor 594 goat anti-mouse IgG |
| Anti-myelin/oligodendrocyte specific protein | 1:250 | Mouse monoclonal | Merck Millipore Chemicon | Alexa Fluor 594 goat anti-mouse IgG |
| Anti-S100 | 1:400 | Rabbit polyclonal | DAKO Denmark | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-GFAP | 1:80 | Rabbit | Sigma-Aldrich | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-glutamine synthetase | 1:1000 | Mouse monoclonal | Merck Millipore | Alexa Fluor 594 goat anti-mouse IgG |
| Anti-glutamate | 1:1000 | Mouse monoclonal | Sigma-Aldrich | Alexa Fluor 594 goat anti-mouse IgG |
| Anti-GABA | 1:500 | Mouse monoclonal | Sigma-Aldrich | Alexa Fluor 594 goat anti-mouse IgG |
| Anti-Iba1 | 1:1000 | Rabbit polyclonal | Wako Chemicals GmbH | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-NG2 | 1:400 | Rabbit polyclonal | Merck Millipore | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-L-type Ca2+ CP α1C | 1:100 | Rabbit polyclonal | Santa Cruz Biotechnology | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-N type Ca2+ CP α1B | 1:100 | Rabbit polyclonal | Santa Cruz Biotechnology | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-P/Q-type Ca2+ CP α1A | 1:100 | Rabbit polyclonal | Santa Cruz Biotechnology | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-P2X2 | 1:100 | Rabbit polyclonal | Alomone Labs | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-P2X3 | 1:100 | Rabbit polyclonal | Alomone Labs | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-P2X4 | 1:100 | Rabbit polyclonal | Alomone Labs | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-P2X6 | 1:100 | Rabbit polyclonal | Alomone Labs | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-P2X7 | 1:100 | Rabbit polyclonal | Alomone Labs | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-RyR 3 | 1:100 | Rabbit polyclonal | Alomone Labs | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-RyR 2 | 1:200 | Rabbit polyclonal | Alomone Labs | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-RyR 1 | 1:200 | Rabbit polyclonal | Alomone Labs | Alexa Fluor 488 goat anti-rabbit IgG |
| Anti-NMDAɛ4 | 1:100 | Rabbit polyclonal | Santa Cruz Biotechnology | Alexa Fluor 594 goat anti-rabbit IgG |
GFAP, glial fibrillary acidic protein; GABA, γ-aminobutyric acid; RyR, ryanodine receptor; NMDA, N-Methyl-D-aspartic acid.
Dye loading and measurements of intracellular [Ca2+]i
[Ca2+]i measurements on single cells were performed as previously reported [26–31]. Briefly, cultures of hESC NPs, plated on 24 mm glass-bottom dishes (WillCo-dish, WillCo Wells B.V, Amsterdam, Netherlands) coated with laminin, were incubated with 2.5 μM Fura-2 AM with 0.02% Pluronic F-127 in CM at 37°C and 5% CO2 for 40 min. Loaded cells were then washed and the CM replaced with NL buffer containing (in mM): NaCl, 140; KCl, 5; MgCl2, 1.2; CaCl2, 2.2; glucose, 10; and HEPES-Tris, 10; pH 7.25 and kept at 37°C throughout the time course of the experiment. The osmolarity of the buffer was 298–300 mosmol/l−1. Fluorescence measurements of [Ca2+]i were performed with a fast fluorescence microspectrofluorimetry system based on an inverted microscope (Axiovert, Zeiss) equipped for epifluorescence (Plan-Neofluar 100x/1.30 oil immersion objective). To achieve fast switching between different excitation wavelengths, a rotating filter wheel was mounted in the excitation light path. The cells were alternately illuminated (200 Hz) at 340±10 nm and 380±10 nm. To minimize the background noise of the Fura-2 signal, successive values were averaged to a final time resolution of 320 ms. A measuring amplifier was synchronized to the filter wheel to measure the fluorescence intensities resulting from different wavelengths. The FFP software controlled the acquisition of the intensity data and provided functions for adjusting the signal values and the display and storage of the measured data. A CCD camera was used to visualize the cells. The [Ca2+]i measurement values are expressed as the ratio units (RU) between the fluorescence obtained with 2 excitation wavelengths, 340 nm (A) and 380 nm (B). Fura-2 calibration was performed in these cells in vitro following the procedure described by Lambert et al. [32] and Jamen et al. [33], which yielded Rmin=0.08, Rmax=2.02, β=1.757, and Kd=224 at 37°C.
Drug application
As described previously [28,34,35], the control and test solutions were applied using a temperature controlled multichannel polypropylene capillary perfusion system (Warner Instruments, Inc.). A single outlet capillary tubing (100 μm inner diameter) with a flow rate of 250 μL/min was positioned close to the tested cell (0.5 mm). The selected cell was subjected to a constant flow of control buffer or test solutions. Each capillary was fed by a reservoir 45 cm above the bath and connected to a temperature control device (Harvard). The temperature of all solutions was maintained at 37°C. In this approach, switching the flow from one capillary to the next resulted in complete solution exchange within 1–3 s. After each application of the tested drug, the cells were washed with control buffer. This method allowed for the fast and reliable exchange of the solution surrounding the selected cell under observation without exposing the neighboring cells.
Data analysis and statistical methods
Origin 8.5.1 was employed for plotting and statistical procedures. The results are expressed as mean±SEM. The number of the sample size (n) given is the number of cells tested according to the same protocol (control, test drug, and recovery) for each group. The figures (traces) show on-line single cell measurements of the [Ca2+]i levels before and after the application of test substances, while bar diagrams and numerical data are given as mean±S.E.M. and present the peak amplitude of the [Ca2+]i increase as a ratio between the fluorescence values of 340/380 nm excitation wavelengths. The results were analyzed using one-way ANOVA. Differences were considered statistically significant if P≤0.05.
Results
[Ca2+]i responses in undifferentiated hESC
Undifferentiated hESCs (n=34) from 2 independent cultures were subjected to the application of K+ (50 mM), ATP (100 μM), glutamate (50 μM), GABA (10 μM), and CPA (10 μM). These cells were only partially responsive to ATP. To identify the specific involvement of the type(s) of active purinergic receptors in undifferentiated hESC, we tested the effect of adenosine diphosphate (ADP; 100 μM) and adenosine (2 μM). ADP had no effect, whereas adenosine caused rise in [Ca2+]i in only 18% (n=11). P2 receptor antagonist suramin (300 μM) inhibited ATP-induced [Ca2+]i by 33.3%±23% (n=4), PPADS (10 μM) had no effect (n=3).
[Ca2+]i responses in hESC NPs change during long term propagation in culture
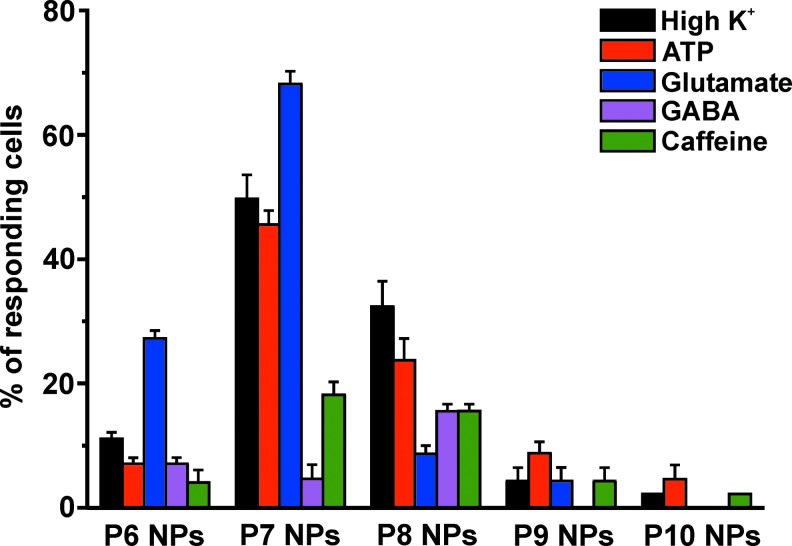
The functional properties of hESC NPs were studied during long-term propagation in vitro starting from P6 NPs to P10 NPs. All data within each passage were collected and analyzed from a minimum of 3 independent experiments. Cells were chosen randomly and subjected to the same experimental protocol. The cells from each passage were subjected to various stimuli such as K+ (50 mM), ATP (100 μM), glutamate (50 μM), GABA (10 μM), and caffeine (20 mM; Table 2). The results indicate that P6 NPs were sensitive to the application of glutamate and K+ (27%±1.3% and 11%±1%, respectively). Only a few cells were sensitive to other stimuli such as GABA or ATP (7%±1% each) and to caffeine (4%±2%). The percentages of P7 cells responding to K+, ATP, glutamate, GABA, and caffeine were 50%±3.9%, 46%±2.2%, 57%±0.3%, 5%±2.3%, and 18%±2.1%, respectively. Similarly, the percentages of P8 NPs responding to K+, ATP, glutamate, GABA, and caffeine were 32%±4%, 24%±3.5%, 9%±1.3%, 16%±1.1%, and 16%±1%, respectively. The cells from P9 were responsive to ATP (9%±1.9%) and nonresponsive to GABA. Less than 5% of cells were sensitive to other stimuli. P10 NPs were not sensitive to glutamate or GABA, while 5%±2.3% of cells were sensitive to ATP and 2.3% (1 out of 44 cells tested) to K+ or caffeine. Immunocytochemical staining for Ca2+ channels and RyRs revealed the expression of L-type and P/Q-type VOCC, ryanodine receptors RyR1 and RyR3, and the weak expression of RyR2 in all passages (Table 3). Since the number of cells responding to physiological stimuli was significantly higher in passage P7 NPs (59%±2.5%) than in other passages (P6 NPs: 41%±1.6%; P8 NPs: 49%±1.1%; P9 NPs: 15%±1.7%; and P10 NPs: 7%±0.2%), these cells were chosen for further experimentation in this study.
Table 2.
The Number of Cells Responding to Various Physiological Stimuli in Different Passages of Human Embryonic Stem Cell-Derived Neural Precursors (P6–P10 NPs)
| Number of cells tested | P6 NPs n=44 | P7 NPs n=44 | P8 NPs n=45 | P9 NPs n=45 | P10 NPs n=44 |
|---|---|---|---|---|---|
| Cells sensitive to 1 or more of applied stimuli | 41%±1.6% | 59%±2.5% | 49%±1.1% | 15%±1.7% | 7%±0.2% |
| K+ 50 mM | 11%±1% | 50%±3.9% | 32%±4% | 4%±2.2% | 2.3% |
| ATP 100 μM | 7%±1% | 46%±2.2% | 24%±3.5% | 9%±1.9% | 5%±2.3% |
| Glutamate 50 μM | 27%±1.3% | 57%±0.3% | 9%±1.3% | 4%±2.2% | 0 |
| GABA 10 μM | 7%±1% | 5%±2.3% | 16%±1.1% | 0 | 0 |
| Caffeine 20 mM | 4%±2% | 18%±2.1% | 16%±1.1% | 4%±2.1% | 2.3% |
ATP, adenosine-5′-triphosphate.
Table 3.
Expression of Various Neural Markers and Ca2+ Sensitive Channels in P6–P10 hESC NPs
| P6 NPs | P7 NPs | P8 NPs | P9 NPs | P10 NPs | |
|---|---|---|---|---|---|
| Neural markers | |||||
| Nestin | ++ | ++ | ++ | ++ | ++ |
| NeuN | ++ | ++ | ++ | + | + |
| βIII tubulin | +++ | +++ | +++ | ++ | ++ |
| MAP2 | − | − | − | − | − |
| Glutamate | + | +++ | − | − | − |
| GABA | + | − | + | − | − |
| GFAP | ++ | +++ | ++ | + | − |
| S100 | + | ++ | ++ | ++ | ++ |
| OLIG | − | ++ | − | − | − |
| GS | − | ++ | − | − | − |
| NG2 | − | − | − | − | + |
| Ca2+-sensitive channels | |||||
| L-type VOCC | ++ | +++ | ++ | ++ | ++ |
| N-type VOCC | − | − | − | − | − |
| P/Q-type VOCC | ++ | +++ | ++ | ++ | ++ |
| RyR 1 | +++ | +++ | +++ | +++ | +++ |
| RyR 2 | + | + | + | + | + |
| RyR 3 | ++ | +++ | ++ | ++ | ++ |
“−”-negative immunostaining.
“+”-faint positive immunostaining/few cells express the marker.
“++”-positive immunostaining/many cells express the marker.
“+++”-very positive/majority of cells express the marker.
VOCC, voltage-operated Ca2+ channels.
Characterization of [Ca2+]i transients mediated by Ca2+ channels in P7 hESC NPs
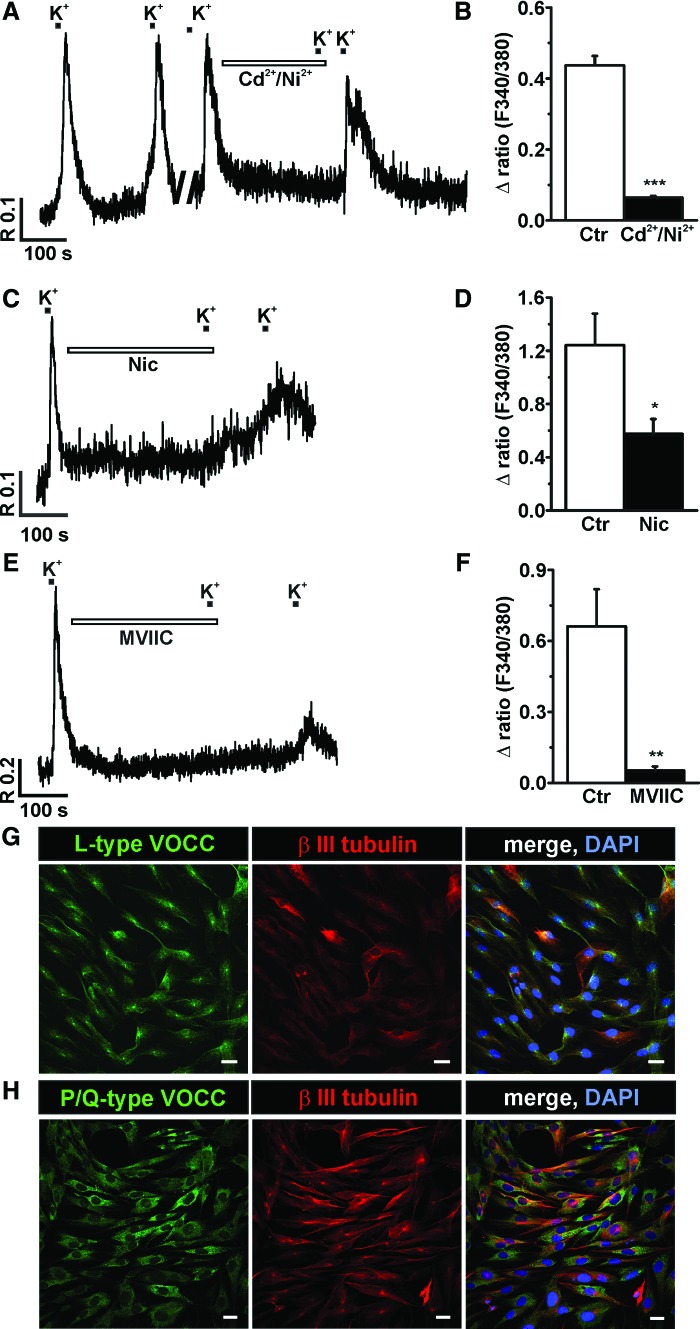
We monitored Ca2+ entry through VOCC as [Ca2+]i transients evoked by depolarization with 50 mM K+. The application of high K+ solution evoked a rapid increase in [Ca2+]i in 50% (n=145) of the tested cells. Preincubation with Cd2+ (100 μM), a nonspecific blocker of high-voltage activated (HVA) Ca2+ channels (L- N- P- Q- and R-types), together with Ni2+ (50 μM), a more specific blocker of low-voltage activated Ca2+ channels (T-type), for 5 min significantly reduced the [Ca2+]i responses induced by K+ in all cells tested by 85.3%±7.5% (n=7) indicating the involvement of voltage-activated Ca2+ channels in depolarization-induced Ca2+ entry (Fig. 1A, B). To further characterize the involvement of specific subtypes of HVA Ca2+ channels, we used specific Ca2+ channel blockers such as nicardipine (for L-type), MVIIC (for P/Q type), and GVIA (for N-type). Application of 10 μM nicardipine reduced [Ca2+]i responses in 67% of tested P7 NPs (n=12). In 2 out of 8 cells nicardipine totally abolished the [Ca2+]i responses (Fig. 1C, D). In the other 6 cells, it significantly reduced the amplitude of the responses by 54%±19.5% (P=0.03; n=6), suggesting the contribution of L-type Ca2+ channels in P7 NPs. The application of 300 nM MVIIC (Fig. 1E, F), which is known to block the P/Q-type of VOCC [36], reduced the [Ca2+]i responses by 92%±32% (P=0.004; n=5) in all cells. The application of MVIIC at a higher concentration (1 μM), which was reported to also block N-type Ca2+ channels, completely inhibited the K+-induced responses in the tested cells (n=3). Further, we used another specific N-type VOCC blocker, GVIA, at 2 different concentrations (500 nM and 800 nM), which only partially and reversibly reduced the K+-induced responses by 20%±14.6% (P=0.47; n=11) and 48%±31.8% (P=0.27; n=9), respectively. To confirm the above [Ca2+]i measurement results, we performed a series of immunocytochemical analyses. No positive immunostaining for the α1B subunit of the N-type of VOCC was observed, but positive immunostaining for the α1C subunit of the L-type of VOCC (Fig. 1G) and the α1A subunit of the P/Q-type of VOCC (Fig. 1H) was observed, suggesting the presence of L- and P/Q-type Ca2+ channels in P7 NPs.
FIG. 1.
Voltage-operated Ca2+ channels in P7 human embryonic stem cell-derived neural precursors (hESC NPs). Example of traces (A, C, E) from individual cells for each experimental design show the block of K+ responses by specific Ca2+ channel antagonists. The cells were first exposed to control K+ depolarization and the [Ca2+]i responses were monitored. Note that the amplitude of the control responses to high K+ is identical without any run down. Subsequently, the same cell was preincubated for 5 min with Ca2+ channel blockers [Cd2+/Ni2+; Nic: nicardipine and ω-conotoxin MVIIC (MVIIC)] as indicated on the trace and then again challenged with high K+. (B) Preincubation of cells with 100 μM Cd2+ together with 50 μM Ni2+ significantly reduced [Ca2+]i responses in all tested cells (n=7). The trace (C) and bar diagram (D) show the reduction of the high K+-induced [Ca2+]i responses by the L-type Ca2+ channel blocker 10 μM nicardipine (n=6). The trace (E) shows the K+-induced [Ca2+]i responses in the presence of and after preincubation with the P/Q-type Ca2+ channel blocker, MVIIC (300 nM). (F) Bar diagram shows the MVIIC inhibition of the high-K+ responses (n=5). G, H are confocal images of P7 hESC NPs, co-stained for βIII tubulin (G, H) (middle panel) and for L-type Ca2+ CP α1C (H-280) (G), (left panel) or P/Q-type Ca2+ CP α1A (H-90) (H), (left panel). Merged images are presented in the right panels (G, H). Scale bars=20 μm. *P=0.05; **P=0.005; ***P=0.0005. Color images available online at www.liebertpub.com/scd
[Ca2+]i signaling through purinergic receptors in P7 hESC NPs
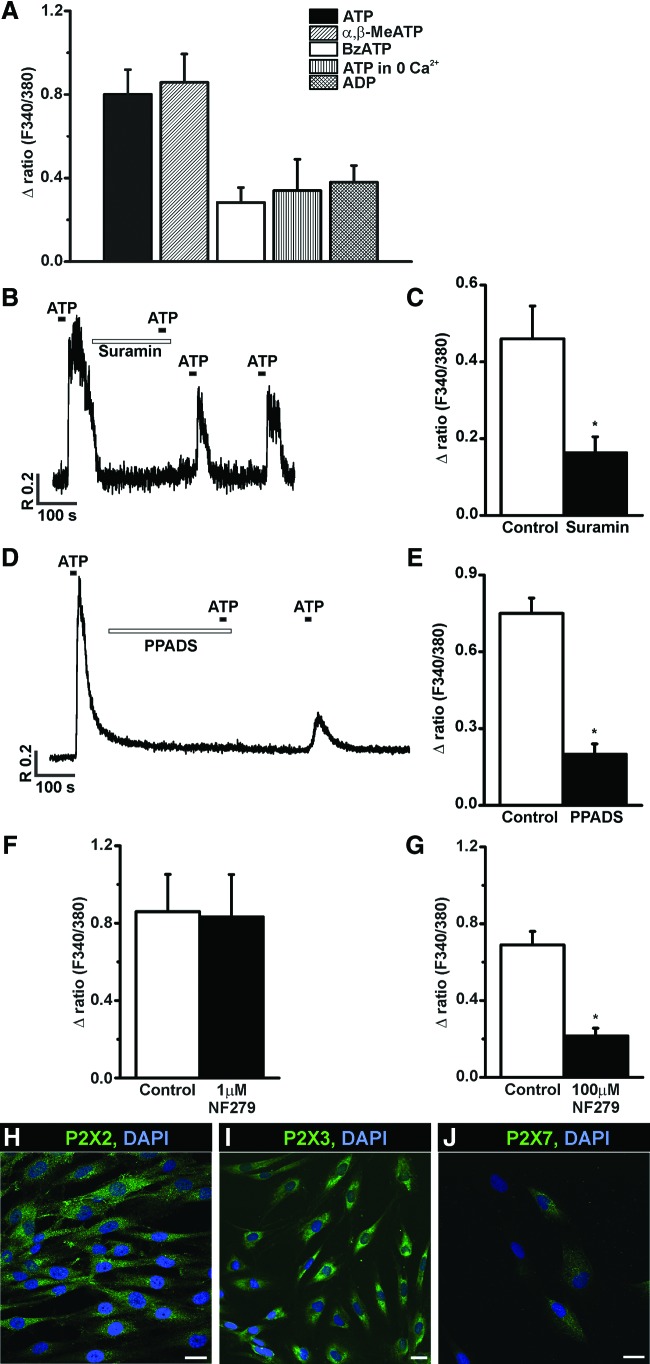
The application of ATP (100 μM) induced a rapid [Ca2+]i increase in 33 out of 71 (47%) NPs tested with the mean amplitude of 0.8±0.12 (RU) (Fig. 2A). The rise in [Ca2+]i in response to ATP was significantly inhibited (by 65%±25%) by the broad-spectrum P2 receptor antagonist suramin (300 μM) (P=0.01; n=8) (Fig. 2B, C). Another P2 receptor antagonist, PPADS, had a similar effect at a concentration of 10 μM, decreasing the [Ca2+]i response to ATP by 73%±20% (P=0.02; n=8), (Fig. 2D, E). These results suggest that functional P2 purinoceptors are present in P7 NPs.
FIG. 2.
Purinergic responses in P7 hESC NPs. P7 NPs express functional P2X and P2Y purinergic receptors. (A) Bar diagram showing the mean amplitude of the [Ca2+]i increase in response to various purinergic receptor agonists in P7 hESC NPs. The cells were exposed to100 μM adenosine-5′-triphosphate (ATP), 100 μM α,β-Methyleneadenosine 5′-triphosphate lithium salt (α,β-meATP), 20 μM 2′(3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP), and 100 μM adenosine diphosphate (ADP). The application of ATP also resulted in a rise in [Ca2+]i in the absence of external Ca2+. (B, D) Representative traces show the ATP-induced [Ca2+]i responses obtained in the presence of and after the washout of the purinergic receptor antagonists suramin and pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS). Incubation with 300 μM suramin or 10 μM PPADS after control ATP application significantly inhibited the ATP responses. (C, E) Bar diagrams showing the amplitude of the [Ca2+]i response to ATP before (control) and after incubation with the antagonists (C) 300 μM suramin (*P=0.01, n=8) and (E) 10 μM PPADS (*P=0.02, n=8). (F, G) Bar diagrams showing the effect of NF279 in P7 NPs. At 1 μM concentration NF279 had no effect (F), while at 100 μM concentration (G) it significantly inhibited ATP-induced [Ca2+]i responses (*P=0.04; n=3). Confocal images (H, I, J) showing the staining for P2X2 (H), P2X3 (I), and P2X7 (J) receptors. Cell nuclei are visualized with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) staining. Scale bars=20 μm. Color images available online at www.liebertpub.com/scd
To further identify which P2 receptors (ionotropic P2X or metabotropic P2Y) operate in hESC NPs, we studied the effect of the P2X agonist α,β-meATP. In 64% of the cells, the application of α,β-meATP (100 μM) transiently increased [Ca2+]i, by a mean amplitude of 0.86±0.14, n=16 (Fig. 2A). The application of 20 μM BzATP, another P2X receptor agonist, evoked a [Ca2+]i increase in 18% of the tested cells with the mean amplitude of 0.28±0.07, (n=22; Fig. 2A). The P2X receptors antagonist, NF279, was ineffective at concentrations from 20 nM to 1 μM at which it is specific agonist for P2X1 receptors (Fig. 2F), whereas at a higher concentration (100 μM), it inhibited the ATP-induced [Ca2+]i increase by 69%±20.6%, P=0.04; n=3 (Fig. 2G). Immunocytochemical staining with antibodies directed against P2X2, P2X3, P2X4, P2X6, and P2X7 receptors revealed expression of P2X2 (Fig. 2H), P2X3 (Fig. 2I) and P2X7 R (Fig. 2J). Finally, to investigate P2Y-mediated Ca2+ signaling, the effects of ATP were examined in Ca2+-free medium. In the absence of extracellular Ca2+, the application of ATP induced an increase in [Ca2+]i in 86% of the tested cells with a mean amplitude of 0.34±0.15, n=7 (Fig. 2A). The application of 100 μM ADP, a P2Y1 and P2Y12 agonist, elevated [Ca2+]i by 0.38±0.08 (n=5) in 56% of the tested cells (Fig. 2A). These data suggest the involvement of both the P2X (P2X2, P2X3, and P2X7) and P2Y purinoceptors in Ca2+ signaling in P7 NPs.
Ca2+ release from intracellular Ca2+ stores and spontaneous [Ca2+]i transients in P7 hESC NPs
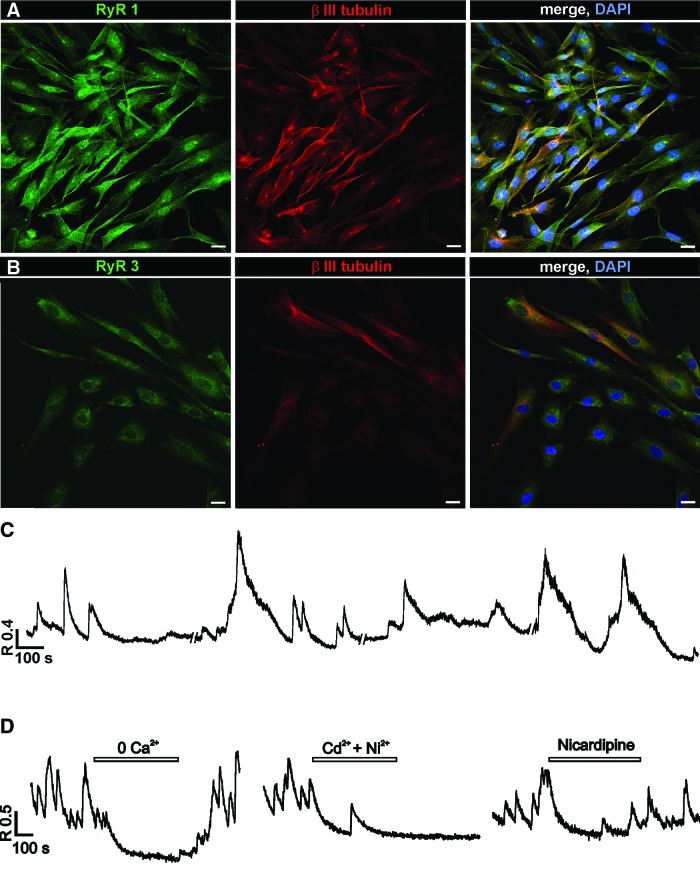
To check for functional intracellular Ca2+ stores in P7 NPs, we used caffeine (20 mM), which in millimolar concentrations acts as an inhibitor of intracellular receptors for IP3 while being a potent activator of RyR. We also applied ryanodine at 1 μM, the concentration that opens the RyR. A brief application (10 s) of either caffeine or ryanodine induced a [Ca2+]i rise in 18% (n=44) and 22% (n=9) of P7 hESC NPs, respectively. Immunostainings for RyR1 (Fig. 3A) and RyR3 (Fig. 3B) were positive in all tested cells. The application of 10 μM CPA, a potent, selective, and reversible inhibitor of the sarco-endoplasmic reticulum Ca2+-ATPase pump, caused an intracellular [Ca2+]i increase in 12 out of 13 tested cells (92%) with a mean amplitude of 0.38±0.05.
FIG. 3.
Ca2+ release from intracellular Ca2+ stores and spontaneous [Ca2+]i transients in P7 hESC NPs. (A, B) Confocal images of P7 hESC NPs. (A) Ryanodine receptor (RyR) 1 and (B) RyR 3 receptors are present in all cells and are co-localized with immunostaining for βIII tubulin. Scale bars=20 μm. (C) A representative trace showing spontaneous [Ca2+]i oscillations observed in the same cell. These types of oscillations were observed in about 31% of tested P7 hESCs. (D) Traces showing the inhibition of spontaneous [Ca2+]i activity after removal of extracellular Ca2+, application of voltage-operated Ca2+ channels (VOCC) blockers Cd2+ (100 μM) and Ni2+ (50 μM) and L-type VOCC blocker nicardipine (10 μM). Color images available online at www.liebertpub.com/scd
Spontaneous [Ca2+]i transients were observed in 51 out 164 of tested cells (31%) in the absence of any stimuli (Fig. 3C). The amplitude of the spontaneous [Ca2+]i transients was 0.74±0.05 (n=91), and they appeared with a mean frequency of 1 per 3.2 min. These transients were partially inhibited by the application of VOCC blockers (100 μM Cd2+, 50 μM Ni2+, and 10 μM nicardipine) or completely by the removal of extracellular Ca2+ (Fig. 3D).
[Ca2+]i responses to glutamate in P7 hESC NPs
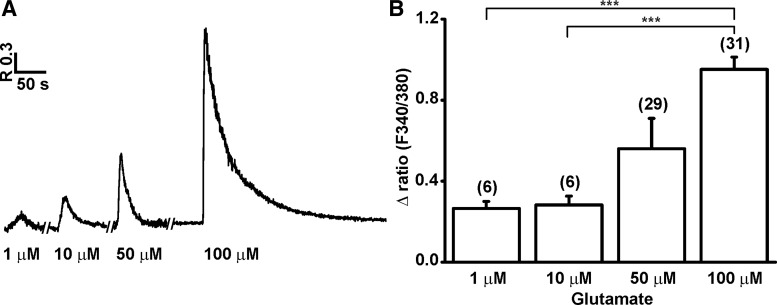
We tested the [Ca2+]i responses of P7 NPs to various concentrations of glutamate (1 μM–1 mM; n=118). Out of 118, the glutamate caused an elevation of [Ca2+]i in 80 cells (68%). Glutamate at 1 mM caused cell death, which was determined by the loss of Ca2+ signals (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). Therefore, the concentration-dependent elevation of [Ca2+]i was determined for the doses ranging between 1 and 100 μM. The amplitude of the glutamate-induced [Ca2+]i responses at various concentrations ranged, respectively at 1 μM=0.27±0.04, n=6; at 10 μM=0.29±0.04, n=6; at 50 μM=0.56±0.15, n=29; and at 100 μM=0.95±0.06, n=31 (Fig. 4A, B). When the cells were exposed briefly, 10 s, to 1 mM glutamate, there was a robust increase in [Ca2+]i, the Ca2+ level was high, sustained without any decay, and did not return to resting level even after wash of glutamate for a longer duration (Supplementary Fig. S1.) This phenomenon was observed in all 8 cells tested. Glutamatergic signals in the nervous system are mediated by ionotropic [NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate] and metabotropic (mGlu) receptors. Two out of 9 tested cells were sensitive to the application of NMDA (100 μM), while only 1 out of 6 cells was sensitive to kainic acid (100 μM). To determine the contribution of metabotropic glutamate receptors, we applied glutamate in the absence of extracellular Ca2+; this caused a [Ca2+]i increase in only 1 out of 9 cells suggesting that the sensitivity of P7 NPs to glutamate is mediated mainly by ionotropic glutamate receptors.
FIG. 4.
Effect of glutamate in P7 hESC NPs. A representative trace (A) showing the [Ca2+]i responses after the application of different concentrations of glutamate (1–100 μM). A bar diagram (B) showing the amplitude (mean±SEM) of the [Ca2+]i responses to the application of increased concentrations of glutamate. The number of cells tested is given in the parentheses. ***P=0.0005.
Expression of neural markers in NPs derived from hESC
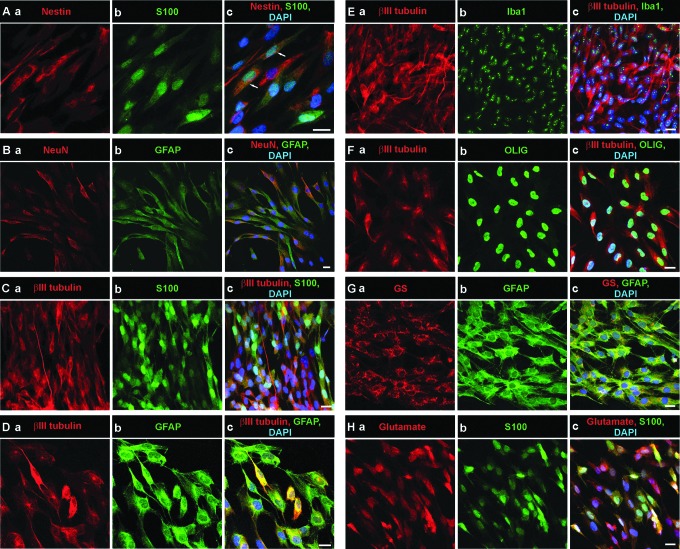
The expression of various neural markers was analyzed in passages 6–10 (P6–P10) NPs. Table 1 shows the list of primary and secondary antibodies used. All immunocytochemical results were obtained from 2 independent stainings with each antibody. The results from P6–P10 are summarized in Table 3. The expression of various neural markers in P7 NPs is shown in Fig. 5. The neuronal markers NeuN (Fig. 5 Ba) and βIII tubulin (Fig. 5 Ca, Da, Ea, Fa) were expressed in all passages, with the highest expression in P6–P8. Immunostaining for neuronal marker MAP-2 was negative in all passages. Staining for nestin (Fig. 5 Aa), a marker of neural progenitor cells, revealed the presence of numerous nestin-positive cells in all passages of hESC. Glial cells markers such as glial fibrillary acidic protein (GFAP) (Fig. 5 Db, Gb) and astrocytic marker S100 (Fig. 5 Ab, Bb, Cb, Hb) were also expressed throughout all passages except GFAP in P10. However, another astrocytic marker glutamine synthetase (Fig. 5 Gb) and oligodendrocyte marker OLIG (Fig. 5 Fb) were detected only in P7 NPs. P7 NPs also expressed microglial marker Iba1 (Fig. 5 Eb). In contrast, P10 NPs, but not other passages showed positive immunostaining with anti-NG2 chondroitin sulphate proteoglycan. Immunostaining with anti-glutamate was positive in P6 NPs, intensely positive in P7 NPs (Fig. 5 Ha), and absent in passages 8–10 (Table 3). The low expression of GABA was observed only in P6 and P8 NPs.
FIG. 5.
Confocal microscopy analysis of the expression of neural cell markers in P7 NPs. P7 NPs express a number of neuron- and glia-specific markers. (A) The proliferative marker nestin (Aa) is expressed in a majority of P7 NPs and in some cells (indicated by arrows) is co-localized (Ac) with the glial marker S100 (Ab, Ac). (B) P7 NPs express neuronal markers: NeuN, a marker of postmitotic neurons (Ba) and βIII tubulin (Ca, Da, Ea, Fa). The glial markers S100 (Ab, Cb, Hb), glial fibrillary acidic protein (GFAP) (Bb, Db, Gb), and glutamine synthetase (Ga), the oligodendrocyte marker OLIG (Fb) and the microglial marker Iba1 (Eb) are also expressed in P7 NPs. The majority of cells from P7 NPs show positive staining for glutamate (Ha). Nuclei were labeled with DAPI. Scale bars=20 μm. Color images available online at www.liebertpub.com/scd
Discussion
Remodeling of the [Ca2+]i signaling toolkit in hESC NPs
Here, we demonstrated the functional remodeling of hESC NPs during propagation in vitro. While undifferentiated hESC were partially responsive only to ATP, predifferentiated cells expressed more sophisticated Ca2+ signaling mechanisms, characteristic of a neural phenotype. P7 and P8 hESC NPs express functional Ca2+ channels, ATP receptors, glutamate receptors, RyRs, and also demonstrate spontaneous Ca2+ oscillations. We found that the highest activity of Ca2+ signaling systems was observed at P7 and P8. This reflected data obtained in vivo, which showed that P8 NPs yielded the best results in terms of functional improvement after transplantation [19]. Thus, we may suggest that the behavior and fate of P7 and P8 hESC NPs after transplantation in vivo correlate with their elevated functional state as revealed by Ca2+ signaling. This rapid and transient remodeling of the Ca2+ the signaling toolkit represents, in our view, the most important finding of the present study. This shows that physiologically, hESCs cells may acquire a neuron-specific pattern of Ca2+ signaling but only for a short period limited to only 2 passages (Fig. 6). We have also determined the expression of a number of neuronal markers in undifferentiated and various passages of differentiated hESCs (Fig. 7; see also [19]) with the differential expression of various markers in hESC-NPs. We may further contemplate that the evaluation of Ca2+ signals in stem cells can be used to predict the fate of the cells during differentiation and can serve as an important criterion for assessing the quality of stem cells before their use in cell replacement therapy.
FIG. 6.
The number of cells responding to various physiological stimuli in different passages of hESC-derived neural precursors [passage 6–10 (P6–P10 NPs)]. The number of cells from different passages of hESC NPs responding to various physiological stimuli [eg, 50 mM K+; 100 μM ATP, 50 μM glutamate; 10 μM γ-aminobutyric acid (GABA); 20 mM caffeine] by a rise in [Ca2+]i. Cells responding to 1 or more of the applied stimuli were considered as physiologically active. Color images available online at www.liebertpub.com/scd
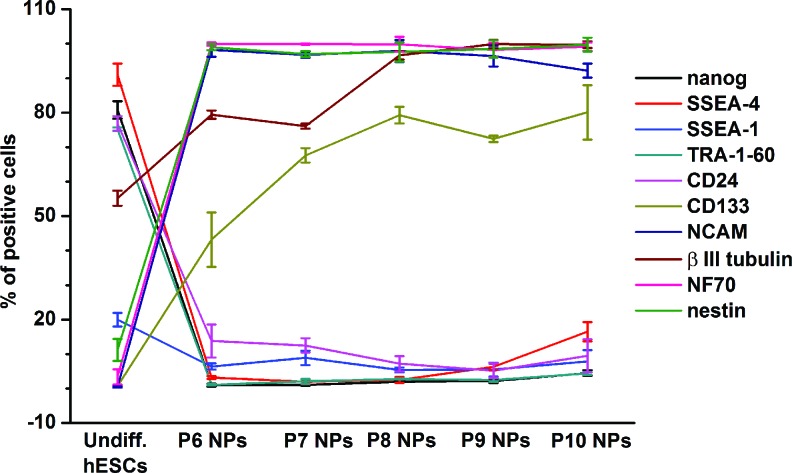
FIG. 7.
Fluorescence-activated cell sorting profiles of pluripotent and neural markers in undifferentiated hESCs and hESC-derived NPs (P6–P10) during long-term propagation in vitro. Predifferentiation of hESC led to the downregulation of pluripotent markers (nanog, SSEA-4, SSEA-1, TRA-1-60, and CD24) and upregulation of neural markers (CD133, NCAM, βIII tubulin, NF70, and nestin). Color images available online at www.liebertpub.com/scd
NPs derived from the CCTL14 line of hESCs at various passages show differences in their expression of genes and cell markers, and differences in the behavior of the cells after transplantation into the brain following middle cerebral artery occlusion in rats [19]. Therefore, our main attention was focused on studying the functional properties of NPs at different passages during maintenance in vitro that would be appropriate for transplantation.
Our results obtained from [Ca2+]i measurements in single cells indicated that P6 NPs were sensitive mostly to glutamate (27%), while only a small number of P6 NPs responded by an increase in [Ca2+]i to other agonists, such as 50 mM K+ (11%), ATP (7%), GABA (7%), or caffeine (4%), suggesting an insignificant role for VOCCs and the absence of ATP-, GABA-, and RyR-generated Ca2+ signals in P6 NPs. Likewise, only a small population of P9 NPs was sensitive to K+ (4%), ATP (9%), or caffeine (4%). The number of cells sensitive to glutamate significantly decreased to 9% in P9 NPs when compared with P6 NPs (27%), and GABA was without any effect on [Ca2+]i. Unlike all other passages, P10 NPs were not sensitive to any of the applied stimuli (ATP, 5%; K+, 2%; caffeine, 2%; or GABA, 0%). These results suggest that P10 NPs are generally devoid of Ca2+ signaling machinery. The highest number of cells responsive to at least one of the applied stimuli was found in NPs from passages 7 and 8 (59% and 49%, respectively). P7 NPs contained a significantly higher number of cells sensitive to glutamate (57% in P7 NPs vs. 9% in P8 NPs), K+ (50% in P7 NPs vs. 32% in P8 NPs), and ATP (46% in P7 NPs vs. 24% in P8 NPs). Almost equal populations of P7 NPs and P8 NPs were sensitive to caffeine (18% and 16%, respectively). The sensitivity to GABA increased to 16% in P8 NPs in comparison with P6 NPs (7%); however, it should be noted that P9 NPs and P10 NPs were totally insensitive to GABA. These findings are in agreement with immunocytochemical results showing the expression of glutamate only in passages 6 and 7, faint expression of GABA in passages 8 and 6, but not in other passages (Table 3). The reasons for such peculiar signaling patterns remain unclear. Interestingly, the number of cells sensitive to various stimuli was consistently the highest in P7 NPs when compared with the other passages (Table 2, Fig. 6). Therefore, P7 NPs were chosen for the further detailed characterization of Ca2+ signaling mechanisms.
Expression of VOCC in P7 hESC NPs
Ca2+ entering the cytosol via VOCC regulates enzyme activity, gene expression, and other biochemical processes in cells. In neurons, VOCC also initiate synaptic transmission [37]. While L- and T-type currents are found in a wide range of cells, N-, P, Q-, and R-type Ca2+ currents are most prominent in neurones. Depending on their localization in the cell, VOCC carry out different functions. For example, L-type VOCC located in the cell bodies and proximal dendrites induce gene activation, while N- and P/Q-type VOCC trigger the release of neurotransmitters at synaptic endings [9].
Previous studies have demonstrated the strong enhancement of neurogenesis by Ca2+ influx through L-type VOCC [38,39]. In neuronal cells derived from ESCs, Yu et al. [40] showed that cooperation between L-type VOCC and RyR2 is crucial for the activity-dependent neurogenesis induced by GABA signaling. In neural progenitors derived from hESCs after 30 days in culture, Malmersjo et al. observed an increase in [Ca2+]i evoked by 50 mM K+, but they did not specify which type of VOCC was activated [22]. Using reverse transcript (RT)-polymerase chain reaction (PCR) studies, others have shown the upregulation of VOCC expression in differentiated hESCs [23]. L-type channels are also expressed in NPs derived from human [25] and mouse ESCs [24].
Here, we identified the functional expression of L- and P/Q-type VOCC in P7 NPs. Our findings are in accordance with the reports mentioned above; however, to the best of our knowledge, our study is the first to document the functional expression of P/Q Ca2+ channels in NPs derived from hESCs. Another interesting observation in this study was the lack of an effect of GVIA on K+-induced [Ca2+]i responses and the absence of immunocytochemical staining for the α1B subunit of the N-type of Ca2+ channels.
Our data show that P/Q-type channels were functional in 50% of the cells tested (responsive to 50 mM K+) from P7 NPs. The application of 300 nM up to 2 μM MVIIC was reported to block several P/Q- and N-type Ca2+ channel-regulated physiological functions [36,41,42]. In our case, high K+ induced a [Ca2+]i increase that was blocked by 1 μM MVIIC in all tested cells, although the application of GVIA (500 and 800 nM), a selective N-type VOCC blocker, was not effective in blocking the K+-induced [Ca2+]i. This could be explained by the presence of P/Q channels, which are sensitive to MVIIC but not to GVIA [30,36,43]. Indeed, the application of MVIIC at a 300 nM concentration, effective in blocking the P/Q-type of VOCC, reduced the Ca2+ increase in all tested cells. In addition, immunocytochemical staining for the α1B subunit of the N-type of VOCC was negative, while immunostaining for the α1A subunit of the P/Q-type of VOCC was positive. Therefore, we conclude that P7 NPs express functional P/Q-, but not N-type, Ca2+ channels. An interesting finding was that there was no correlation between the immunocytochemical expression of VOCC and their functional activity. L- and P/Q-types of VOCC could be identified immunocytochemically in all passages without any significant differences, while functionally only P7 and P8 showed the expression of VOCC, while in other passages (P6, P9, and P10) VOCC were inactive.
It was previously shown that mouse ESC-derived neurones in the early stages of differentiation possess a complex pattern of VOCC, with a shift in channel contribution from N- and L-types in apolar cells to P/Q- and R-type channels in bi- and multipolar cells [21]. In our study, the [Ca2+]i increase in response to depolarization by K+ was partially reduced by nicardipine, suggesting a possible role for L-type VOCC.
Purinergic receptor activation in P7 hESC NPs
Purinergic receptors are widely distributed in the body and participate in the regulation of virtually all physiological processes. ATP acts as a fast excitatory neurotransmitter and has a potent long-term role in cell proliferation, growth, and development and also in disease and cytotoxicity [44,45]. Nervous system development, including progenitor cell proliferation, cell migration, neuronal and glial interactions, and differentiation and synaptic network formation are also controlled by purines [13,46].
In our study, we used a series of agonists and antagonists to determine which subtypes of purinergic receptors are functional in P7 NPs. Suramin is generally selective as an antagonist for P2 receptors versus other types of receptors, but it does not discriminate between P2X and P2Y receptors [47]. In the present study, suramin reversibly inhibited the ATP-induced [Ca2+]i increase by 65% in all tested cells, suggesting that P2 receptors are functional in P7 NPs. To discriminate between different types of P2X receptors, the selective P2X1,3,5,7 agonist α,β-me ATP was used; it displayed a similar high potency as ATP, which is typical for P2X1 and P2X3 receptors. The P2X1,2,3,5 selective antagonist PPADS effectively blocked the ATP-induced [Ca2+]i increase by 75% in all tested cells. Another antagonist NF279, was not effective in low concentrations (<100 μM), suggesting the absence of functional P2X1 and P2X2 receptors. BzATP was effective in 18% of the cells tested. These data suggest the presence of functionally active P2X3 and P2X7 receptors, which were also confirmed by immunocytochemistry. While we were unable to characterize the function of P2X2 receptors pharmacologically, immunocytochemical staining showed the presence of P2X2 receptors in P7 NPs.
To the best of our knowledge there is only 1 study by Young et al. [23] that has demonstrated the presence of purinergic receptors in hESC-derived neural progenitors. Though the authors did not perform functional studies on P2 receptors, they showed by RT-PCR that in the hESC line WA09, the P2X4 subunits are upregulated in hNPs but downregulated in differentiated hNPs, while P2X7 was not expressed at any stage. In contrast, in our study CCTL14 hESC NPs expressed P2X7, but not P2X4 receptors. This might be explained by the difference in the cell lines used and by differences between the differentiation protocols themselves, since it is now well established that the effect of various neurotrophic factors such as GDNF, BDNF, NGF, and NT-3varies in dependence with a targeted cell type [48]. Others have shown that GABAergic neurones derived from mouse ESCs elevate [Ca2+]i predominantly via the activation of P2X2, P2X4, and P2Y1 receptors [49]. In our study, we observed a [Ca2+]i increase in response to ATP also in the absence of extracellular Ca2+, suggesting the involvement of metabotropic P2Y receptors in the functioning of P7 NPs. We hypothesize that the functional properties of NPs are highly dependent on the origin of the cells and the differentiation conditions they are exposed to.
Ca2+ stores in P7 hESC NPs
In the nervous system Ca2+ release from internal stores plays an important role in regulating synaptic plasticity, neurite outgrowth, neurodegeneration, and secretion [50–52] and is also essential for triggering Ca2+ waves and oscillations in astrocytes [53]. It is regulated by 2 types of receptors, InsP3Rs and RyRs, which are localized on the endoplasmic reticulum and in mitochondria [54,55]. Millimolar concentrations (5–20 mM) of caffeine modulate intracellular Ca2+ signaling through the activation of RyR and at the same time the inhibition of InsP3R [56]. Therefore, depending on the relative densities of RyR and InsP3R in a particular cell, caffeine can either stimulate or block Ca2+ release from intracellular stores [57]. The release of Ca2+ from intracellular stores is well documented in ESC-derived cardiomyocytes [58,59]. In hESC-derived dopamine neurones, it was shown that dihydroxyphenylglycine-induced [Ca2+]i increase was observed in the absence of extracellular Ca2+ suggesting the involvement of intracellular stores [22]. Other reports have shown that the RyR2 receptor, acting through GABAA receptors and L-type Ca2+ channels, induces neurogenesis in ESCs [40]. In our experiments, the application of caffeine caused an increase in [Ca2+]i, suggesting the activation of RyR; these findings were further confirmed by positive immunostaining for the RyR1 and RyR3 receptor. The application of CPA in P7 NPs resulted in a slow, long-lasting [Ca2+]i increase. These data suggest that P7 NPs possess mature and functional endoplasmic reticulum Ca2+ stores.
Spontaneous [Ca2+]i oscillations in P7 hESC NPs
Another interesting phenomenon that we observed is that hESC-NPs exhibited spontaneous oscillations in [Ca2+]i. Spontaneous [Ca2+]i activity is an essential feature of developing neurones [15,35,60]. The elevation of [Ca2+]i in developing neurones in the form of Ca2+ spikes or Ca2+ waves regulates neuronal differentiation, axonal outgrowth, the development of potassium currents, the expression of GABA, and so on. We observed spontaneous [Ca2+]i transients in 31% of tested cells from P7 NPs with a mean frequency of 3.2 min. These spontaneous transients were completely abolished by either the removal of extracellular Ca2+, or partially by the presence of VOCC blockers (N-, L-, and P/Q-type). Of interest, Spitzer et al. identified a few types of spontaneous [Ca2+]i transients in developing neurones, including Ca2+ spikes and Ca2+ waves [60,61]. Ca2+ spikes functionally have been found to regulate the development of potassium currents and the expression of GABA [62,63], while Ca2+ waves regulate neurite outgrowth [64]. We would classify the spontaneous Ca2+ elevations, based on their characteristics, observed in P7 NPs as Ca2+ waves. These data clearly indicate that P7 hESC NPs function in a similar manner as early neuronal cells. Similarly, it was reported that post mitotic neurons from hESCs exhibit spontaneous [Ca2+]i transients, similar to [Ca2+]i waves, and are mediated by Gd3+/La3+. Blocking these transients led to a significant reduction in progenitor cell proliferation [25].
Glutamate and GABA receptors in P7 hESC NPs
Since glutamate and GABA are important neurotransmitters and play a role in neuronal development [65], we next tested whether P7 NPs are sensitive to these 2 substances. Only 5% of tested cells (n=44) were sensitive to the application of GABA. According to some reports [66–68], the [Ca2+]i increase induced by GABA causes neuronal depolarization mainly in cells undergoing neuronal differentiation and only in a fraction of precursor and progenitor cells; also, the number of responding cells decreases with time. In addition, there are some concerns about using [Ca2+]i to monitor the depolarizing action of GABA. Glutamate regulates proliferation and neuronal differentiation and also acts as a positive regulator in neurogenesis [69]. Glutamate acts via ionotropic NMDA, AMPA, and kainate receptors in addition to metabotropic mGlu receptors. Due to such a diversity of pathways, glutamate plays various roles in neurogenesis starting from the early stages of development [69,70]. In our study, we observed a [Ca2+]i increase even in response to low concentrations of glutamate in a majority of cells tested (68%). Similar findings have been previously reported in a few studies, for example in NP cells derived from hESCs differentiated into dopaminergic neurones [22]. Young et al. described the presence of AMPA- and kainate- and the absence of NMDA-mediated Ca2+ responses in hESC-derived neural progenitors [23]. In our study, only a few P7 NPs were sensitive to NMDA and kainic acid, 22% and 17% respectively. We did not make further attempts to test the responses to AMPA suggesting that glutamate responses were most likely not mediated by NMDA or kainate glutamate receptors. Other authors have reported that the application of 500 μM glutamate caused a rise in [Ca2+]i in 34 out of 68 tested human NP cells, while only 3 out of 68 cells were depolarized by 50 mM K+ [71]. In contrast, our results show that NP cells are sensitive to the application of even low (1 μM) concentrations of glutamate, and 50% of cells were depolarized by 50 mM K+. Glutamate, when applied at 1 mM caused Ca2+ overload led and to cell death (Supplementary Fig. S1). In the absence of extracellular Ca2+, the application of glutamate had no effect, suggesting the absence of functional mGlu receptors. Additionally, we found positive immunorectivity for the NR2D subunit of NMDA receptors in P7 NPs (Supplementary Fig. S2). This subunit has been shown to play an important role in synaptic transmission in the early stages of brain development.
Further, immunocytochemical results revealed that P7 NPs consist of heterogeneous cell populations including neural progenitor cells (nestin-positive), which have the capacity for self-renewal, and more mature cells showing a neuronal or glial phenotype. This heterogeneity is also reflected in the variety of Ca2+ channels and receptors present in P7 NPs. Some overlap in expression of neuronal marker βIII tubulin and glial marker GFAP was observed in all immunostaining throughout all passages. Of interest, it was previously reported [72–74] that both neurons and glial cells at certain time points can co-express βIII tubulin and GFAP. Interestingly, there was no significant difference between the immunocytochemical expression of VOCC and RyRs among P6–10 NPs, although the number of cells sensitive to the agonists of these receptors varied from P6 to P10, with significantly higher activation in P7 and P8 NPs.
Conclusions
Remarkable progress has been made recently in differentiating ESCs and other pluripotent stem cells into a neuronal phenotype [2–5,75,76]. To date, various growth factors and morphogenes, and cell markers, necessary for neuronal differentiation and development have been identified [77]. Nevertheless, many questions remain unanswered, in particular concerning the physiological development and functional activity of transplantable NPs derived from ESCs. Here, we correlate the histochemical and morphological features of hESC NPs with their physiological properties. Our findings clearly indicate that the predifferentiation of ESCs leads to an activation of Ca2+ signaling cascades and enhances the functional activity of the cells. We also showed that the Ca2+ signaling mechanisms and the physiological properties of hESC-derived NPs change during maintenance in vitro (Fig. 6). The mechanisms and factors that underlie these processes need to be established. Studying the functional properties of stem cells in vitro may help to predict their behavior and the fate of their physiopathological status in vivo and may serve as criteria to evaluate the quality of such cells. The preliminary results of this work have appeared as abstracts [78,79]. We conclude that the criteria to establish the histochemical characteristics and to identify the markers of differentiating cells do not reflect their functional state in terms of their signaling mechanisms. The evaluation of homeostatic signaling mechanisms could be considered as a key element in determining the “quality of stem cells”. Therefore, understanding the physiology of stem cells may allow us to better control their regenerative potential, which in turn may help to improve strategies for their use in transplantation and the treatment of neurodegenerative diseases.
Supplementary Material
Acknowledgments
G. Dayanithi is supported by the “Centre National de la Recherche Scientifique,” France. This work was supported by the grants GACR P304/11/2373 and GACR P304/12/G069 from the Grant Agency of the Czech Republic, the FP7 project AXREGEN (PITN-GA-2008-214003), and the FP7 project Edu-GLIA (PITN-GA-2009-237956) Initial Training Network. We thank Silvia Bernascone for her participation in preliminary experiments and Hana Voriskova, IEM ASCR, for help with the immunocytochemical staining. We thank Carl Zeiss, s.r.o. Prague, Czech Republic for support and consultation on imaging and fluorescence photometry systems. We are very grateful to James Dutt, IEM ASCR, for helpful discussions and critical reading of the article.
Author Disclosure Statement
The authors declare they have no conflicts of interest.
References
- 1.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SC. Wernig M. Duncan ID. Brustle O. Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 3.Reubinoff BE. Itsykson P. Turetsky T. Pera MF. Reinhartz E. Itzik A. Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 4.Schulz TC. Noggle SA. Palmarini GM. Weiler DA. Lyons IG. Pensa KA. Meedeniya AC. Davidson BP. Lambert NA. Condie BG. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22:1218–1238. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- 5.Li XJ. Du ZW. Zarnowska ED. Pankratz M. Hansen LO. Pearce RA. Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 6.De Smedt H. Verkhratsky A. Muallem S. Ca(2+) signaling mechanisms of cell survival and cell death: an introduction. Cell Calcium. 2011;50:207–210. doi: 10.1016/j.ceca.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Dayanithi G. Forostyak O. Ueta Y. Verkhratsky A. Toescu EC. Segregation of calcium signalling mechanisms in magnocellular neurones and terminals. Cell Calcium. 2012;51:293–299. doi: 10.1016/j.ceca.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Toescu EC. Dayanithi G. Neuroendocrine signalling: natural variations on a Ca(2+) theme. Cell Calcium. 2012;51:207–211. doi: 10.1016/j.ceca.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Berridge MJ. Lipp P. Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 10.Carafoli E. Santella L. Branca D. Brini M. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001;36:107–260. doi: 10.1080/20014091074183. [DOI] [PubMed] [Google Scholar]
- 11.Munaron L. Antoniotti S. Lovisolo D. Intracellular calcium signals and control of cell proliferation: how many mechanisms? J Cell Mol Med. 2004;8:161–168. doi: 10.1111/j.1582-4934.2004.tb00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orrenius S. Zhivotovsky B. Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 13.Majumder P. Trujillo CA. Lopes CG. Resende RR. Gomes KN. Yuahasi KK. Britto LR. Ulrich H. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic Signal. 2007;3:317–331. doi: 10.1007/s11302-007-9074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North RA. Verkhratsky A. Purinergic transmission in the central nervous system. Pflugers Arch. 2006;452:479–485. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- 15.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 17.LoTurco JJ. Owens DF. Heath MJ. Davis MB. Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 18.Haydar TF. Wang F. Schwartz ML. Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozubenko N. Turnovcova K. Kapcalova M. Butenko O. Anderova M. Rusnakova V. Kubista M. Hampl A. Jendelova P. Sykova E. Analysis of in vitro and in vivo characteristics of human embryonic stem cell-derived neural precursors. Cell Transplant. 2010;19:471–486. doi: 10.3727/096368909X484707. [DOI] [PubMed] [Google Scholar]
- 20.Rowe EW. Jeftinija DM. Jeftinija K. Jeftinija S. Development of functional neurons from postnatal stem cells in vitro. Stem Cells. 2005;23:1044–1049. doi: 10.1634/stemcells.2005-0037. [DOI] [PubMed] [Google Scholar]
- 21.Arnhold S. Andressen C. Angelov DN. Vajna R. Volsen SG. Hescheler J. Addicks K. Embryonic stem-cell derived neurones express a maturation dependent pattern of voltage-gated calcium channels and calcium-binding proteins. Int J Dev Neurosci. 2000;18:201–212. doi: 10.1016/s0736-5748(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 22.Malmersjo S. Liste I. Dyachok O. Tengholm A. Arenas E. Uhlen P. Ca2+ and cAMP signaling in human embryonic stem cell-derived dopamine neurons. Stem Cells Dev. 2010;19:1355–1364. doi: 10.1089/scd.2009.0436. [DOI] [PubMed] [Google Scholar]
- 23.Young A. Machacek DW. Dhara SK. Macleish PR. Benveniste M. Dodla MC. Sturkie CD. Stice SL. Ion channels and ionotropic receptors in human embryonic stem cell derived neural progenitors. Neuroscience. 2011;192:793–805. doi: 10.1016/j.neuroscience.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L. Blackman BE. Schonemann MD. Zogovic-Kapsalis T. Pan X. Tagliaferri M. Harris HA. Cohen I. Pera RA, et al. Estrogen receptor beta-selective agonists stimulate calcium oscillations in human and mouse embryonic stem cell-derived neurons. PLoS One. 2010;5:e11791. doi: 10.1371/journal.pone.0011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weick JP. Austin Johnson M. Zhang SC. Developmental regulation of human embryonic stem cell-derived neurons by calcium entry via transient receptor potential channels. Stem Cells. 2009;27:2906–2916. doi: 10.1002/stem.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komori Y. Tanaka M. Kuba M. Ishii M. Abe M. Kitamura N. Verkhratsky A. Shibuya I. Dayanithi G. Ca(2+) homeostasis, Ca(2+) signalling and somatodendritic vasopressin release in adult rat supraoptic nucleus neurones. Cell Calcium. 2010;48:324–332. doi: 10.1016/j.ceca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Dayanithi G. Widmer H. Richard P. Vasopressin-induced intracellular Ca2+ increase in isolated rat supraoptic cells. J Physiol. 1996;490(Pt 3):713–727. doi: 10.1113/jphysiol.1996.sp021180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viero C. Mechaly I. Aptel H. Puech S. Valmier J. Bancel F. Dayanithi G. Rapid inhibition of Ca2+ influx by neurosteroids in murine embryonic sensory neurones. Cell Calcium. 2006;40:383–391. doi: 10.1016/j.ceca.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Dayanithi G. Chen-Kuo-Chang M. Viero C. Hamel C. Muller A. Lenaers G. Characterization of Ca2+ signalling in postnatal mouse retinal ganglion cells: involvement of OPA1 in Ca2+ clearance. Ophthalmic Genet. 2010;31:53–65. doi: 10.3109/13816811003698117. [DOI] [PubMed] [Google Scholar]
- 30.Dayanithi G. Sabatier N. Widmer H. Intracellular calcium signalling in magnocellular neurones of the rat supraoptic nucleus: understanding the autoregulatory mechanisms. Exp Physiol. 2000;85 doi: 10.1111/j.1469-445x.2000.tb00010.x. Spec No:75S–84S. [DOI] [PubMed] [Google Scholar]
- 31.Viero C. Dayanithi G. Neurosteroids are excitatory in supraoptic neurons but inhibitory in the peripheral nervous system: it is all about oxytocin and progesterone receptors. Prog Brain Res. 2008;170:177–192. doi: 10.1016/S0079-6123(08)00416-0. [DOI] [PubMed] [Google Scholar]
- 32.Lambert RC. Dayanithi G. Moos FC. Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994;478(Pt 2):275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamen F. Alonso G. Shibuya I. Widmer H. Vacher CM. Calas A. Bockaert J. Brabet P. Dayanithi G. Impaired somatodendritic responses to pituitary adenylate cyclase-activating polypeptide (PACAP) of supraoptic neurones in PACAP type I-receptor deficient mice. J Neuroendocrinol. 2003;15:871–881. doi: 10.1046/j.1365-2826.2003.01075.x. [DOI] [PubMed] [Google Scholar]
- 34.Widmer H. Ludwig M. Bancel F. Leng G. Dayanithi G. Neurosteroid regulation of oxytocin and vasopressin release from the rat supraoptic nucleus. J Physiol. 2003;548:233–244. doi: 10.1113/jphysiol.2002.036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dayanithi G. Mechaly I. Viero C. Aptel H. Alphandery S. Puech S. Bancel F. Valmier J. Intracellular Ca2+ regulation in rat motoneurons during development. Cell Calcium. 2006;39:237–246. doi: 10.1016/j.ceca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Sabatier N. Richard P. Dayanithi G. L-, N- and T- but neither P- nor Q-type Ca2+ channels control vasopressin-induced Ca2+ influx in magnocellular vasopressin neurones isolated from the rat supraoptic nucleus. J Physiol. 1997;503(Pt 2):253–268. doi: 10.1111/j.1469-7793.1997.253bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsien RW. Lipscombe D. Madison DV. Bley KR. Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 38.Deisseroth K. Singla S. Toda H. Monje M. Palmer TD. Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 39.Webb SE. Moreau M. Leclerc C. Miller AL. Calcium transients and neural induction in vertebrates. Cell Calcium. 2005;37:375–385. doi: 10.1016/j.ceca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Yu HM. Wen J. Wang R. Shen WH. Duan S. Yang HT. Critical role of type 2 ryanodine receptor in mediating activity-dependent neurogenesis from embryonic stem cells. Cell Calcium. 2008;43:417–431. doi: 10.1016/j.ceca.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Pouille F. Cavelier P. Desplantez T. Beekenkamp H. Craig PJ. Beattie RE. Volsen SG. Bossu JL. Dendro-somatic distribution of calcium-mediated electrogenesis in purkinje cells from rat cerebellar slice cultures. J Physiol. 2000;527(Pt 2):265–282. doi: 10.1111/j.1469-7793.2000.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khosravani H. Zamponi GW. Voltage-gated calcium channels and idiopathic generalized epilepsies. Physiol Rev. 2006;86:941–966. doi: 10.1152/physrev.00002.2006. [DOI] [PubMed] [Google Scholar]
- 43.Lemos JR. Ortiz-Miranda SI. Cuadra AE. Velazquez-Marrero C. Custer EE. Dad T. Dayanithi G. Modulation/physiology of calcium channel sub-types in neurosecretory terminals. Cell Calcium. 2012;51:284–292. doi: 10.1016/j.ceca.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbracchio MP. Burnstock G. Verkhratsky A. Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Burnstock G. Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9. doi: 10.1038/cddis.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmermann H. Purinergic signaling in neural development. Semin Cell Dev Biol. 2011;22:194–204. doi: 10.1016/j.semcdb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Ralevic V. Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 48.Allodi I. Guzman-Lenis MS. Hernandez J. Navarro X. Udina E. In vitro comparison of motor and sensory neuron outgrowth in a 3D collagen matrix. J Neurosci Methods. 2011;198:53–61. doi: 10.1016/j.jneumeth.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Khaira SK. Pouton CW. Haynes JM. P2X2, P2X4 and P2Y1 receptors elevate intracellular Ca2+ in mouse embryonic stem cell-derived GABAergic neurons. Br J Pharmacol. 2009;158:1922–1931. doi: 10.1111/j.1476-5381.2009.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose CR. Konnerth A. Stores not just for storage. intracellular calcium release and synaptic plasticity. Neuron. 2001;31:519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- 51.Meldolesi J. Rapidly exchanging Ca2+ stores in neurons: molecular, structural and functional properties. Prog Neurobiol. 2001;65:309–338. doi: 10.1016/s0301-0082(01)00004-1. [DOI] [PubMed] [Google Scholar]
- 52.Rizzuto R. Intracellular Ca(2+) pools in neuronal signalling. Curr Opin Neurobiol. 2001;11:306–311. doi: 10.1016/s0959-4388(00)00212-9. [DOI] [PubMed] [Google Scholar]
- 53.Kettenmann H. Hanisch UK. Noda M. Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 54.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 55.Bootman MD. Berridge MJ. Roderick HL. Calcium signalling: more messengers, more channels, more complexity. Curr Biol. 2002;12:R563–R565. doi: 10.1016/s0960-9822(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 56.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 57.Vyleta NP. Smith SM. Fast inhibition of glutamate-activated currents by caffeine. PLoS One. 2008;3:e3155. doi: 10.1371/journal.pone.0003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sedan O. Dolnikov K. Zeevi-Levin N. Leibovich N. Amit M. Itskovitz-Eldor J. Binah O. 1,4,5-Inositol trisphosphate-operated intracellular Ca(2+) stores and angiotensin-II/endothelin-1 signaling pathway are functional in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:3130–3138. doi: 10.1634/stemcells.2008-0777. [DOI] [PubMed] [Google Scholar]
- 59.Satin J. Itzhaki I. Rapoport S. Schroder EA. Izu L. Arbel G. Beyar R. Balke CW. Schiller J. Gepstein L. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:1961–1972. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 60.Spitzer NC. Lautermilch NJ. Smith RD. Gomez TM. Coding of neuronal differentiation by calcium transients. Bioessays. 2000;22:811–817. doi: 10.1002/1521-1878(200009)22:9<811::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 61.Spitzer NC. Spontaneous Ca2+ spikes and waves in embryonic neurons: signaling systems for differentiation. Trends Neurosci. 1994;17:115–118. doi: 10.1016/0166-2236(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 62.Desarmenien MG. Spitzer NC. Role of calcium and protein kinase C in development of the delayed rectifier potassium current in Xenopus spinal neurons. Neuron. 1991;7:797–805. doi: 10.1016/0896-6273(91)90282-5. [DOI] [PubMed] [Google Scholar]
- 63.Spitzer NC. Debaca RC. Allen KA. Holliday J. Calcium dependence of differentiation of GABA immunoreactivity in spinal neurons. J Comp Neurol. 1993;337:168–175. doi: 10.1002/cne.903370111. [DOI] [PubMed] [Google Scholar]
- 64.Gu X. Olson EC. Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ben-Ari Y. Gaiarsa JL. Tyzio R. Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 66.Wang J. Reichling DB. Kyrozis A. MacDermott AB. Developmental loss of GABA- and glycine-induced depolarization and Ca2+ transients in embryonic rat dorsal horn neurons in culture. Eur J Neurosci. 1994;6:1275–1280. doi: 10.1111/j.1460-9568.1994.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 67.Obrietan K. van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maric D. Maric I. Wen X. Fritschy JM. Sieghart W. Barker JL. Serafini R. GABAA receptor subunit composition and functional properties of Cl- channels with differential sensitivity to zolpidem in embryonic rat hippocampal cells. J Neurosci. 1999;19:4921–4937. doi: 10.1523/JNEUROSCI.19-12-04921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schlett K. Glutamate as a modulator of embryonic and adult neurogenesis. Curr Top Med Chem. 2006;6:949–960. doi: 10.2174/156802606777323665. [DOI] [PubMed] [Google Scholar]
- 70.Melchiorri D. Cappuccio I. Ciceroni C. Spinsanti P. Mosillo P. Sarichelou I. Sale P. Nicoletti F. Metabotropic glutamate receptors in stem/progenitor cells. Neuropharmacology. 2007;53:473–480. doi: 10.1016/j.neuropharm.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 71.Piper DR. Mujtaba T. Rao MS. Lucero MT. Immunocytochemical and physiological characterization of a population of cultured human neural precursors. J Neurophysiol. 2000;84:534–548. doi: 10.1152/jn.2000.84.1.534. [DOI] [PubMed] [Google Scholar]
- 72.Draberova E. Del Valle L. Gordon J. Markova V. Smejkalova B. Bertrand L. de Chadarevian JP. Agamanolis DP. Legido A, et al. Class III beta-tubulin is constitutively coexpressed with glial fibrillary acidic protein and nestin in midgestational human fetal astrocytes: implications for phenotypic identity. J Neuropathol Exp Neurol. 2008;67:341–354. doi: 10.1097/NEN.0b013e31816a686d. [DOI] [PubMed] [Google Scholar]
- 73.Hol EM. Roelofs RF. Moraal E. Sonnemans MA. Sluijs JA. Proper EA. de Graan PN. Fischer DF. van Leeuwen FW. Neuronal expression of GFAP in patients with Alzheimer pathology and identification of novel GFAP splice forms. Mol Psychiatry. 2003;8:786–796. doi: 10.1038/sj.mp.4001379. [DOI] [PubMed] [Google Scholar]
- 74.Casper KB. McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Wernig M. Zhao JP. Pruszak J. Hedlund E. Fu D. Soldner F. Broccoli V. Constantine-Paton M. Isacson O. Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dimos JT. Rodolfa KT. Niakan KK. Weisenthal LM. Mitsumoto H. Chung W. Croft GF. Saphier G. Leibel R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 77.Yuan SH. Martin J. Elia J. Flippin J. Paramban RI. Hefferan MP. Vidal JG. Mu Y. Killian RL, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6:e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forostyak O. Kozubenko N. Bernascone S. Sykova E. Dayanithi G. Physiology of calcium signalling in human embryonic stem cell-derived neural precursors. 7th Federation of European Neuroscience Scocieties. Forum of European Neuroscience. 2010 FENS Abstract, 5: 041.8. [Google Scholar]
- 79.Forostyak O. Romanyuk N. Sykova E. Dayanithi G. Physiology of Ca2+ signalling in human embryonic stem cell-derived neural precursors. GLIA. 2011;59(Suppl:1):S119–S119. doi: 10.1089/scd.2012.0624. Pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.