Abstract
There is little evidence comparing treatment outcomes between adolescents and other age groups, particularly in resource-limited settings. A retrospective analysis of data from seven HIV clinics across urban Gauteng (n=5) and rural Mpumalanga (n=2), South Africa was conducted. The analysis compared HIV-positive antiretroviral treatment (ART)-naive young adolescents (10–14 years), older adolescents (15–19), and young adults (20–24 years) to adults (≥25 years) initiated onto standard first-line ART between April 2004 and August 2010. Log-binomial regression was used to estimate relative risk (RR) of failure to suppress viral load (≥400 copies/ml) or failure to achieve an adequate CD4 response at 6 or 12 months. The effect of age group on virological failure, mortality, and loss to follow-up (LTFU; ≥90 days since scheduled visit date) was estimated using Cox proportional hazards models. Of 42,427 patients initiating ART, 310 (0.7%) were young adolescents, 342 (0.8%) were older adolescents, and 1599 (3.8%) were young adults. Adolescents were similar to adults in terms of proportion male, baseline CD4 count, hemoglobin, and TB. Compared to adults, both older adolescents (6 months RR 1.75 95% CI 1.25–2.47) and young adults (6 months RR 1.33 95% CI 1.10–1.60 and 12 months RR 1.64 95% CI 1.23–2.19) were more likely to have an unsuppressed viral load and were more likely to fail virologically (HR 2.90 95% CI 1.74–4.86; HR 2.94 95% CI 1.63–5.31). Among those that died or were LTFU, the median time from ART initiation until death or LTFU was 4.7 months (IQR 1.5–13.2) and 10.9 months (IQR 5.0–22.7), respectively. There was no difference in risk of mortality by age category, compared to adults. Young adolescents were less likely to be LTFU at any time period after ART initiation (HR 0.43 95% CI 0.26–0.69) whereas older adolescents and young adults were more likely to be LTFU after ART initiation (HR 1.78 95% CI 1.34–2.36; HR 1.63 95% CI 1.41–1.89) compared to adults. HIV-infected adolescents and young adults between 15 and 24 years have poorer ART treatment outcomes in terms of virological response, LTFU, and virological failure than adults receiving ART. Interventions are needed to help improve outcomes and retention in care in this unique population.
Introduction
According to the World Health Organization (WHO), the number of adolescents on ART continues to increase.1 This reflects major improvements in access to antiretroviral therapy (ART) and successful treatment of perinatally infected children but also newly acquired HIV infections through high-risk behavior during early adolescence. A growing number of adolescents are entering care in adult-oriented HIV clinics, but there are few criteria in place to assist them or to guide clinic staff in how best to treat adolescents and young adults within these settings. The adult-oriented HIV care model may not meet the specific needs of adolescents and young adults as they face unique challenges in the management of HIV.2
Studies from the United States have suggested that HIV-positive adolescents and young adults in adult-oriented HIV clinics are less likely to start treatment, achieve viral suppression, and stay on treatment compared to HIV-infected adults.2,3 Two recent studies from southern Africa reported that adolescents have worse outcomes in terms of virological suppression, rates of virological failure, and adherence but similar rates of mortality and loss to follow-up compared to their adult counterparts.4,5 There have been limited studies assessing the clinical outcomes of HIV-infected adolescents and young adults receiving care in HIV clinics, particularly in resource-limited settings where the burden of HIV is the greatest.4–6
The majority of the previous studies on adolescents have been carried out in the United States and it is possible that these findings cannot be extrapolated to the African setting. Historically, evaluation of HIV/AIDS treatment programs in resource-limited settings has focused on adults and/or children and adolescents have been overlooked. In addition, other studies have not compared adolescent data directly with adult data. As HIV-positive children mature, it is important that appropriate services are available to counsel them on sexual safety, adherence to ART, and reproductive choices.6 The aim of the current study was to compare outcomes [mortality, loss to follow-up, failure to achieve an adequate CD4 response, failure to suppress viral load (≥400 copies/ml), virological failure, and time to first ART switch] between HIV-infected adolescents and adults attending clinics across urban Gauteng and rural Mpumalanga, South Africa (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid).
Materials and Methods
Study site and subjects
We analyzed prospectively collected data from multiple public-sector HIV Comprehensive Care Management and Treatment (CCMT) sites across Gauteng and Mpumalanga, South Africa. HIV-positive patients are eligible for ART initiation and are initiated onto standard public-sector first-line regimens according to the South African National Department of Health (DoH) ART treatment guidelines.7–9
Age categories were defined according to the WHO: young (early) adolescents from 10 to 14 years and older (middle and late) adolescents from 15 to 19 years.10 We defined young adults as 20–24 years and adults as 25 years and older. Individuals were categorized based on their age at ART initiation and were not switched between age categories during follow-up.
Eligible patients included HIV-positive patients initiated on ART at one of the five sites in Gauteng or one of the two sites in Mpumalanga. They were ART naive, 10 years of age and older, and initiated onto a standard first-line regimen of stavudine (d4T), zidovudine (AZT), or tenofovir (TDF) with lamivudine (3TC) or emtricitabine (FTC) and either efavirenz (EFV) or nevirapine (NVP) between April 2004 and August 2010. Young children or adolescents may be initiated on d4T or AZT with 3TC and either lopinavir/ritonavir (LPV/r) or EFV. Other first-line regimens may include 3TC with abacavir (ABC) and either EFV or NVP. The method of acquisition in this study population is mixed since we could not accurately determine whether the adolescents and young adults were infected via perinatal transmission or via high-risk behaviors.
Longitudinal clinical and demographic data are collected and stored on the electronic patient management system, TherapyEdge-HIV (Associated Biological Systems, South Africa). Use of data was approved by the Human Research Ethics Committee of the University of the Witwatersrand (HREC-Medical M060626/M110140).
Outcomes
Immunological and virological responses
We assessed failure to achieve an adequate CD4 count response (defined as failure to increase CD4 count by ≥50 cells/mm3 at 6 months or by ≥100 cells/mm3 at 12 months after ART initiation).11–14 Furthermore, the change in CD4 count during the course of therapy was calculated by subtracting the CD4 count at 6 or 12 months from the baseline CD4 count and presenting the absolute change [median and interquartile (IQR) range] at each time point.15
Virological failure (late failure) was defined as two or more consecutive HIV-RNA viral loads ≥400 copies/ml following suppression below this level (<400 copies/ml).16–18 Never achieving an RNA PCR viral load (<400 copies/ml) or a detectable HIV viral load (≥400 copies/ml) at 6 or 12 months after ART initiation was defined as failure to achieve virological suppression.19
Mortality and loss to follow-up
All-cause mortality was established from patient records. Data from Themba Lethu Clinic (TLC), Johannesburg were verified against the South African National Vital Registration System using patient national identity document numbers.12,20,21 Loss to follow-up (LTFU) was defined as having missed a clinic appointment (clinical assessment, antiretroviral drug pickup or counselor visit) by ≥3 months after the scheduled visit date.14 LTFU and mortality were assessed at three points: (1) ever—any time during follow-up, (2) during the first 12 months after ART initiation, or (3) after the first 12 months. For death or LTFU, person-time accrued from ART initiation until the earliest of death, LTFU, completed 12 months of follow-up (where applicable), or close of the dataset on July 31, 2011. Patients who transferred to another facility were censored at their last clinic visit. Time on ART (in months) was calculated from ART initiation until the earliest of LTFU, death, transfer out, or close of dataset.
First ART switch
Time to ART switch was defined as any change from the initiating ART regimen including single drug substitutions or change to a second-line regimen.
Statistical analysis
Patient characteristics at initiation of ART were stratified into the following groups: (1) young adolescents (10–14 years), (2) older adolescents (15–19 years), (3) young adults (20–24 years), and (4) adults (≥25 years). Groups were described and compared using Student's t test or Kruskal–Wallis for continuous variables and Chi-square (χ2) test for proportions (p value<0.05 was considered significant).
We used log-binomial regression models to estimate the relative risk (RR) and 95% confidence interval (CI) of age category on failure to achieve virological suppression or adequate CD4 count response at 6 or 12 months after ART initiation. Models were adjusted for gender, CD4 count, hemoglobin, body mass index (BMI), TB, site, year of initiation, and WHO stage at ART initiation. For the median change in CD4 count, p values were obtained by comparing each age category to adults and calculated using Kruskal–Wallis for continuous variables.
Kaplan–Meier curves and the log-rank test were used to describe and compare time-to-event distributions for ART outcomes (mortality, LTFU, virological failure, and ART switching) between the groups. We estimated crude and adjusted hazard ratios (HR) of the ART outcomes between the groups using Cox proportional hazard models. Proportional hazard assumptions were checked by including time-dependent covariates in the Cox model using interactions with log (time) and by using tests and graphs based on the Schoenfeld residuals. Analyses were performed using the SAS 9.1 statistical software package (SAS Institute, Inc., Cary, NC) and STATA 10.1 (StataCorp, Collage Station, TX).
Results
Baseline characteristics
Of a total of 75,900 HIV-positive individuals initiated on ART at multiple sites across Gauteng and Mpumalanga, we excluded those who initiated ART outside the study period April 2004–August 2010 (n=12,117), were ART experienced at presentation to the clinic (n=12,782), were less than 10 years of age (n=970), were not initiated onto a standard first-line regimen according to the South African Department of Health treatment guidelines (n=1,133), or who were initiated outside of the five HIV clinics in Gauteng or the two HIV clinics in Mpumalanga (n=6,471). The remaining 42,427 individuals were included in the analysis.
Of the 42,427 individuals included in the analysis, a total of 94.7% (40,176) were adults, 3.8% (1,599) young adults, 0.8% (342) older adolescents, and 0.7% (310) young adolescents (Table 1). The proportion attending HIV clinics in Gauteng ranged from 67% (1,074/1,599) for young adults, 64% (218/342) for older adolescents, and 52% (161/310) for young adolescents, compared to 33% (525/1,599), 36% (124/342), and 48% (149/310) from the Mpumalanga clinics, respectively. Immunodeficiency was advanced in all age groups, as reflected by baseline CD4 counts and WHO stage III/IV classification. As expected, the majority of young adolescents (167/185; 90.3%) had a BMI <18.5 kg/m2; however, since BMI is not commonly used in the pediatric population, weight-for-age (WAZ) was used instead.22 The WAZ score showed that 58.6% (102/174) of young adolescents and 31.0% (78/252) of older adolescents were undernourished, defined as a WAZ score<–2.23 This was similar to 34.2% (88/257) of older adolescents, 23.3% (274/1,178) of young adults, or 18.6% (5,536/29,812) of adults with a BMI <18.5 kg/m2. The majority of patients were initiated on an efavirenz-based regimen. The prevalence of pregnancy was lower in adult women compared to older adolescent and young adult women (8.1% vs. 21.6% and 22.9%; p<0.0001). The median time on treatment ranged from 15.6 months (IQR 7.3–29.4) for older adolescents to 23.9 months (IQR 12.3–36.7) for young adolescents.
Table 1.
Demographic and Clinical Characteristics of 42,427 HIV-Positive Patients Initiating Antiretroviral Therapy in Gauteng or Mpumalanga, South Africa Stratified by Age Category
| Young adolescents 10–14 years N=310 | Older adolescents 15–19 years N=342 | Young adults 20–24 years N=1599 | Adults ≥25 years N=40176 | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Gender, male, n (%) | 158 (51.0%) | 60 (17.5%) | 205 (12.8%) | 14,799 (36.8%) |
| Unemployed, n (%) | N/A | 216 (63.2%) | 1,194 (74.7%) | 22,983 (57.2%) |
| Education (primary or secondary), n (%) | 163/305 (53.4%) | 205/335 (61.1%) | 885/1,553 (60.4%) | 19,807/39,237 (50.5%) |
| Ethnic group—African, n (%) | 306 (98.7%) | 333 (97.4%) | 1560 (97.6%) | 38,937 (97.0%) |
| CD4, median (IQR) | 109 (24–195) | 133 (54–198) | 130 (57–189) | 105 (43–169) |
| ≤50 cells/mm3, n (%) | 95/266 (35.7%) | 78/318 (24.5%) | 357/1,497 (23.8%) | 10,531/37,477 (28.1%) |
| 51–100, n (%) | 32/266 (12.0%) | 49/318 (15.4%) | 239/1,497 (16.0%) | 7,640/37,477 (20.4%) |
| 101–200, n (%) | 78/266 (29.3%) | 114/318 (35.9%) | 602/1,497 (40.2%) | 14,571/37,477 (30.9%) |
| >201 cells/mm3, n (%) | 61/266 (22.9%) | 77/318 (24.2%) | 299/1,497 (20.0%) | 4,735/37,477 (12.6%) |
| Aspartate transaminase (AST; IU/liter), median (IQR) | 40.5 (31.0–57.0) | 29.0 (22.0–39.0) | 30.0 (23.0–44.0) | 35.0 (27.0–49.0) |
| Alanine aminotransferase (ALT; IU/liter), median (IQR) | 24.0 (16.0–38.0) | 19.0 (13.0–28.0) | 20.0 (14.0–30.0) | 23.0 (17.0–35.0) |
| Hemoglobin (Hb; g/dl), Median (IQR) | 10.7 (9.6–11.7) | 10.5 (9.0–11.9) | 10.7 (9.4–12.0) | 11.2 (9.7–12.7) |
| <8g/dl, n (%) | 10/206 (4.9%) | 34/259 (13.1%) | 128/1210 (10.6%) | 2,400/32,054 (7.5%) |
| Body mass index (BMI; kg/m2), Median (IQR) | 14.5 (13.0–16.4) | 20.4 (17.2–23.6) | 21.5 (18.7–24.5) | 21.8 (19.3–25.1) |
| <18.5kg/m2, n (%) | 167/185 (90.3%) | 88/257 (34.2%) | 274/1178 (23.3%) | 5,536/29,812 (18.6%) |
| HIV viral load (copies/ml) | ||||
| ≤100,000, n (%) | 86 (27.8%) | 62 (18.1%) | 326 (20.4%) | 7,953 (19.8%) |
| >100,000, n (%) | 37 (11.9%) | 43 (12.6%) | 206 (12.9%) | 6,302 (15.7%) |
| Missing, n (%) | 187 (60.3%) | 237 (69.3%) | 1067 (66.7%) | 25,921 (64.5%) |
| TB at initiation, n (%) | 39 (12.6%) | 29 (8.5%) | 146 (9.1%) | 4,571 (11.4%) |
| WHO stage III/IV, n (%) | 114/160 (71.3%) | 120/226 (53.1%) | 529/1075 (49.2%) | 14,753/27,845 (53.0%) |
| ART regimen | ||||
| EFV based, n (%) | 301 (97.1%) | 207 (60.5%) | 941 (58.8%) | 34,186 (85.1%) |
| NVP based, n (%) | 6 (1.9%) | 100 (29.2%) | 451 (28.2%) | 4,083 (10.2%) |
| PI based, n (%) | 2 (0.7%) | 34 (1.0%) | 207 (12.9%) | 1,854 (4.6%) |
| Other, n (%) | 1 (0.3%) | 1 (0.3%) | 0 (0.0%) | 53 (0.1%) |
| Follow-up time, months, Median (IQR) | 23.9 (12.3–36.7) | 15.6 (7.3–29.4) | 17.0 (7.3–32.0) | 20.5 (10.2–36.8) |
| 12 month outcomes | ||||
| Alive and in care, n (%) | 246 (79.4%) | 228 (66.7%) | 1077 (67.4%) | 29,631 (73.8%) |
| Loss to follow-up, n (%) | 19 (6.1%) | 75 (21.9%) | 295 (18.4%) | 4,324 (10.8%) |
| Died, n (%) | 21 (6.8%) | 15 (4.4%) | 110 (6.9%) | 3,795 (9.4%) |
| Transferred out, n (%) | 24 (7.7%) | 24 (7.0%) | 117 (7.3%) | 2,426 (6.0%) |
| Change in regimen, n (%) | 109 (35.2%) | 111 (32.5%) | 555 (34.7%) | 15,177 (37.8%) |
| Reason for regimen change, | ||||
| Virological failure, n (%) | 2 (1.8%) | 4 (3.6%) | 15 (2.7%) | 460 (3.0%) |
| Abnormal fat redistribution, n (%) | 5 (4.6%) | 9 (8.1%) | 30 (5.4%) | 1,344 (8.9%) |
| Lactic acidosis/hyperlactatemia, n (%) | 1 (0.9%) | 4 (3.6%) | 16 (2.9%) | 1,066 (7.0%) |
| Toxicity, n (%) | 2 (1.8%) | 3 (2.7%) | 15 (2.7%) | 534 (3.5%) |
| Noncompliance, n (%) | 3 (2.8%) | 10 (9.0%) | 23 (4.1%) | 492 (3.2%) |
| Pregnancy, n (%) | 0 (0.0%) | 13 (11.7%) | 117 (21.1%) | 715 (4.7%) |
IQR, interquartile range; EFV, efavirenz; NVP, nevirapine; PI, protease inhibitor.
Immunological and virological responses
Adjusted log-binomial regression models showed that young adolescents were less likely to fail immunologically (RR 0.48 95% CI 0.33–0.69); in other words, compared to adults, they were more likely to increase their CD4 count by ≥50 cells/mm3 by 6 months on treatment (Table 2). Compared to adults, young adults were more likely to have a detectable viral load at 6 (RR 1.33 95% CI 1.10–1.60) and 12 months (RR 1.64 95% CI 1.23–2.19). Older adolescents were more likely to have an unsuppressed viral load at 6 months (RR 1.75 95% CI 1.25–2.47), while young adolescents were more likely to have an unsuppressed viral load at 12 months (RR 2.30 95% CI 1.38–3.82). Compared to adults, all the age categories showed an increase in the median change in CD4 count from baseline to both 6 and 12 months.
Table 2.
Immunological and Virological Responses at 6 and 12 Months After Antiretroviral Therapy Initiation, Stratified by Age Category
| |
6 months |
12 months |
||||
|---|---|---|---|---|---|---|
| n (%) | Crude RR (95% CI) | Adjusted RR (95% CI)a | n (%) | Crude RR (95% CI) | Adjusted RR (95% CI)a | |
| Failure to increase CD4 countb | ||||||
| Adults | 6,291 (24.3%) | 1.0 | 1.0 | 6,518 (30.7%) | 1.0 | 1.0 |
| Young adults | 212 (22.6%) | 0.93 (0.82–1.05) | 0.95 (0.84–1.07) | 190 (26.3%) | 0.86 (0.76–0.97) | 0.94 (0.80–1.12) |
| Older adolescents | 43 (23.6%) | 0.97 (0.75–1.26) | 0.95 (0.73–1.23) | 42 (31.8%) | 1.04 (0.81–1.33) | 1.06 (0.76–1.48) |
| Young adolescents | 24 (12.6%) | 0.52 (0.36–0.76) | 0.48 (0.33–0.69) | 36 (24.7%) | 0.80 (0.61–1.07) | 0.80 (0.48–1.32) |
| Failure to suppress viral load—detectable HIV viral load (≥400 copies/ml)c | ||||||
| Adults | 2,314 (9.3%) | 1.0 | 1.0 | 1,147 (9.7%) | 1.0 | 1.0 |
| Young adults | 100 (11.6%) | 1.25 (1.04–1.51) | 1.33 (1.10–1.60) | 57 (14.7%) | 1.51 (1.18–1.93) | 1.64 (1.23–2.19) |
| Older adolescents | 28 (16.2%) | 1.74 (1.24–2.45) | 1.75 (1.25–2.47) | 10 (16.7%) | 1.71 (0.97–3.02) | 1.65 (0.87–3.11) |
| Young adolescents | 24 (14.0%) | 1.50 (1.03–2.18) | 1.31 (0.90–1.90) | 24 (29.6%) | 3.04 (2.17–4.28) | 2.30 (1.38–3.82) |
| |
6 months |
12 months |
||||
|---|---|---|---|---|---|---|
| n (%) | Median (IQR) | p value | n (%) | Median (IQR) | p value | |
| Median (IQR) change in CD4 from baseline | ||||||
| Adults | 25,871 (64%) | 120 (52–210) | 21,265 (53%) | 163 (79–264) | ||
| Young adults | 937 (59%) | 150 (62–268) | <0.0001d | 722 (45%) | 209 (94–338) | <0.0001d |
| Older adolescents | 182 (53%) | 170 (56–288) | 0.0004d | 132 (39%) | 214 (55–368) | 0.0097d |
| Young adolescents | 190 (61%) | 243 (111–377) | <0.0001d | 146 (47%) | 315 (113–479) | <0.0001d |
Relative risk (RR) estimated from log-binomial regression models.
Failure to increase CD4 count by ≥50 cells/mm3 at 6 months or ≥100 cells/mm3 at 12 months after ART initiation.
Failure to suppress viral load below 400 copies/ml at 6 months or 12 months after ART initiation.
p values were compared to adults using the Kruskal–Wallis test for continuous variables.
RR, relative risk; CI, confidence interval; IQR, interquartile range.
Young adolescents had the highest rate of virological failure [6.3/100 person years (pys)] compared to older adolescents (3.8/100 pys), young adults (2.6/100 pys), and adults (2.1/100 pys). Cox proportional hazard models showed that young adolescents (HR 2.94 95% CI 1.63–5.31), older adolescents (HR 2.90 95% CI 1.74–4.86), and young adults (HR 1.53 95% CI 1.13–2.08) were all at increased risk of virological failure compared to adults. Crude Kaplan–Meier curves and log-rank test (p<0.001) confirmed this finding. In adjusted models, those with a lower CD4 count (≤50 vs. >200 cells/mm3; HR 1.40 95% CI 1.11–1.77 or 50–100 vs. >200 cells/mm3; HR 1.31 95% CI 1.03–1.68) were at increased risk of virological failure.
Mortality and loss to follow-up
By 12 months on ART, 31,182 (73.4%) patients were still alive and in care, 4,713 (11.1%) were LTFU, 2,591 (6.2%) transferred to another facility, and a further 3,941 (9.3%) had died. Among those who died or were LTFU, the median time from ART initiation until death or LTFU was 4.7 months (IQR 1.5–13.2) and 10.9 months (IQR 5.0–22.7), respectively.
Rates of mortality (at any time during follow-up) were highest among adults (6.1/100 pys) followed by young adults (5.4/100 pys), older adolescents (4.4/100 pys), and then young adolescents (4.1/100 pys). Crude Kaplan–Meier curves, log-rank test (p=0.07), and multivariate Cox proportional hazard models showed that there was no difference in mortality by age category (Table 3).
Table 3.
Cox Proportional Hazard Ratios for Death and Lost to Follow-up, Stratified by Age Category Among Antiretroviral Therapy Patients Attending Clinics Across Gauteng and Mpumalanga, South Africa (n=42,427)
| |
Death (ever)a |
LTFU (ever)c |
||||
|---|---|---|---|---|---|---|
| Deaths, n (%) n=5,591 | Crude HR(95% CI) | Adjusted HR(95% CI)b | Loss, n (%) n=9,374 | Crude HR (95% CI) | Adjusted HR(95% CI)b | |
| Age category | ||||||
| Adults | 5,366 (13.5%) | 1.0 | 1.0 | 8,642 (21.7%) | 1.0 | 1.0 |
| Young adults | 170 (10.8%) | 0.86 (0.71–1.05) | 0.92 (0.69–1.22) | 551 (34.9%) | 1.76 (1.61–1.92) | 1.63 (1.41–1.89) |
| Older adolescents | 26 (7.7%) | 0.54 (0.32–0.94) | 0.58 (0.29–1.15) | 138 (40.8%) | 2.25 (1.91–2.67) | 1.78 (1.34–2.36) |
| Young adolecents | 29 (9.4%) | 1.06 (0.72–1.56) | 0.65 (0.29–1.46) | 43 (13.9%) | 0.61 (0.45–0.82) | 0.43 (0.26–0.69) |
| Sex | ||||||
| Female | 2,459 (44.0%) | 1.0 | 1.0 | 3,477 (27.1%) | 1.0 | 1.0 |
| Male | 3,132 (56.0%) | 1.47 (1.37–1.57) | 1.25 (1.13–1.38) | 5,897 (62.9%) | 1.17 (1.13–1.21) | 1.20 (1.14–1.27) |
| Baseline CD4 count, cell/mm3 | ||||||
| 201–350 | 320 (6.1%) | 1.0 | 1.0 | 1,206 (13.9%) | 1.0 | 1.0 |
| 101–200 | 1,488 (28.3%) | 1.09 (0.96–1.24) | 0.97 (0.79–1.19) | 3,452 (39.6%) | 0.87 (0.83–0.91) | 1.01 (0.92–1.09) |
| 51–100 | 1,179 (22.5%) | 1.85 (1.62–2.11) | 1.47 (1.19–1.81) | 1,716 (19.7%) | 0.90 (0.85–0.95) | 0.99 (0.90–1.09) |
| 0–50 | 2,263 (43.1%) | 3.04 (2.70–3.42) | 1.98 (1.63–2.40) | 2,335 (26.8%) | 0.94 (0.89–0.99) | 1.04 (0.95–1.14) |
| Baseline hemoglobin, g/dl | ||||||
| ≥8g/dl | 3,802 (86.1%) | 1.0 | 1.0 | 6,639 (91.5%) | 1.0 | 1.0 |
| <8g/dl | 615 (13.9%) | 2.38 (2.14–2.65) | 1.86 (1.61–2.15) | 614 (8.5%) | 1.49 (1.39–1.60) | 1.39 (1.26–1.53) |
| Baseline BMI | ||||||
| ≥18.5 kg/m2 | 2,644 (64.8%) | 1.0 | 1.0 | 5,336 (79.4%) | 1.0 | 1.0 |
| <18.5 kg/m2 | 1,438 (35.2%) | 2.75 (2.53–2.99) | 1.94 (1.75–2.16) | 1,384 (20.6%) | 1.40 (1.34–1.47) | 1.42 (1.33–1.51) |
| Baseline WHO stage | ||||||
| I/II | 1,061 (31.0%) | 1.0 | 1.0 | 2,755 (47.4%) | 1.0 | 1.0 |
| III/IV | 2,366 (69.0%) | 1.89 (1.74–2.07) | 1.38 (1.24–1.54) | 3,054 (52.6%) | 1.24 (1.19–1.30) | 1.06 (1.00–1.13) |
Mortality obtained from the National Vital Registration system at the South African Department of Home Affairs.
Models adjusted for gender, CD4 count, hemoglobin, BMI, TB, site, year of initiation, and WHO stage at ART initiation.
Lost to follow-up defined as ≥3 months since the last scheduled visit.
LTFU, lost to follow-up; HR, hazard ratio; CI, confidence interval; pys, person years; BMI, body mass index.
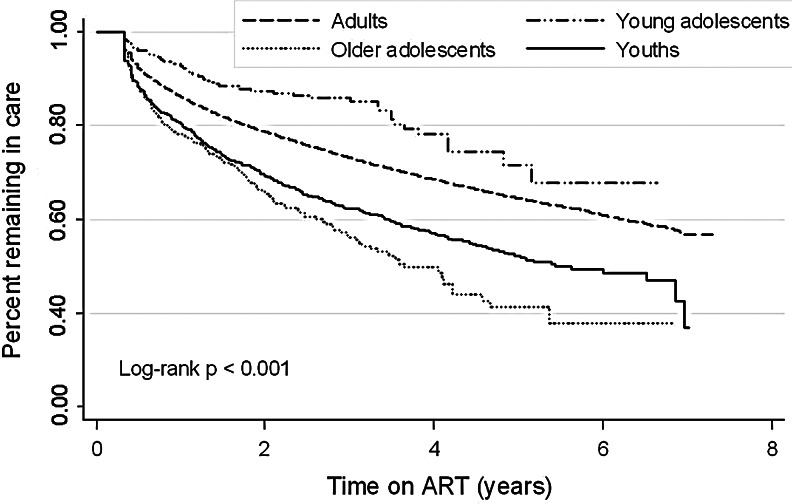
Older adolescents had the highest rate of LTFU (at any time during follow-up) (23.3/100 pys) followed by young adults (17.6/100 pys) and then adults (9.8/100 pys) while young adolescents had the lowest rate of LTFU (6.1/100 pys). Crude Kaplan–Meier curves showed that older adolescents had the highest risk of LTFU when compared to the other age groups (log-rank test p<0.001; Fig. 1). Compared to adults, young adolescents were less likely to be LTFU (HR 0.43 95% CI 0.26–0.69) while young adults (HR 1.63 95% CI 1.41–1.89) and older adolescents (HR 1.78 95% CI 1.34–2.36) were more likely to be LTFU (Table 3).
FIG. 1.
Crude Kaplan–Meier survival curve showing percentage remaining in care any time after ART initiation, stratified by age category [young adolescents (n=310), older adolescents (n=342), young adults (n=1,599) and adults (n=40,176)]. The log-rank test for the percentage remaining in care was p<0.001.
First ART switch
Multivariate Cox proportional hazard models showed that there was no difference in time to first ART switch by age category. In young adolescents, the most common reason for change from initiating an ART regimen was abnormal fat redistribution (lipodystrophy/lipoatrophy) (4.6%), while pregnancy was the most common reason among the older adolescents (11.7%) and young adults (21.1%). Abnormal fat redistribution (8.9%) and lactic acidosis/hyperlactatemia (7.0%) were common reasons for change from initiating an ART regimen among the adult group (Table 1).
Discussion
We aimed to compare ART treatment outcomes between HIV-infected adolescents and adults attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. First, we demonstrate no difference in mortality or time to first ART switch by age category. Second, compared to adults, young adolescents are more likely to achieve a favorable immunological response at 6 months after ART initiation but are more likely to have a detectable viral load at 12 months or fail virologically. Third, compared to adults, older adolescents and young adults are more likely to have a detectable viral load at 6 months after ART initiation, to be LTFU, and to fail virologically. Results support other reports that recommend that adolescents be evaluated separately from the adult population.5
Literature on mortality and LTFU in adolescents is sparse.5 In this study, we demonstrate no difference in adolescent mortality by age category, compared to adults. This is consistent with several reports from HIV clinics in Africa.5,6 Mortality rates (per 100 person years) for adolescents and young adults were similar to those recently reported from a community-based ART clinic in Cape Town, South Africa.5 We found that male gender was an independent predictor of mortality. This finding was consistent with reports from a recent study by Bakanda and co-workers6 conducted in a public health sector ART cohort from Uganda and suggests that male patients typically initiate ART late, have more advanced illness, and have worse clinical outcomes.6,24,25 In addition to male gender, we demonstrate that low CD4 count (<100 cells/mm3), low hemoglobin levels, low BMI, and WHO stage III or IV at ART initiation were associated with mortality, suggesting advanced HIV disease.
We demonstrate that young adolescents are less likely to be LTFU, which may be due to longer follow-up in this group. Compared to adults, older adolescents and young adults are more likely to be LTFU at any time during follow-up. Rates of LTFU are similar to those reported by Nglazi and co-workers5 and those from a recent multisite study in the private health sector of southern Africa.4 Poor rates of clinic retention among adolescents suggest that barriers exist to full commitment to HIV care.2 Transition to adolescence leads to many changes such as growing independence, increased risk-taking behavior, psychiatric problems, increased peer pressure, fear of stigmatization, and separation from parental involvement. Unlike younger children, many adolescents may not have caregivers who actively participate in HIV care and treatment and this may contribute to the increased LTFU rates observed among older adolescents and young adults.26
We report a greater immunological response and higher median change in CD4 count following ART in young adolescents compared to adults. This is consistent with other reports that suggest a younger and more robust immune system in adolescents.5,27 Immunological parameters improved from entry to the end of follow-up in young adolescents, older adolescents, and young adults—with the greatest increases in CD4 count during the early weeks. Results are similar to those reported from the PACTG 381 study.28,29 Results suggest that adolescents and young adults may have a greater capacity for immune reconstitution compared to adults. Since the capacity for CD4 recovery may be related to the baseline CD4 count, these findings support early identification and initiation of ART in HIV-positive adolescents.27,29
We report that young adolescents are more likely to achieve a favorable immunological response at 6 months after ART initiation but are also more likely to have a detectable viral load at 12 months or fail virologically. Lower rates of long-term suppression among adolescents can be explained by more rapid viral rebound.4 The PACTG 381 study showed that short- and long-term virological outcomes of HIV-infected adolescents starting on ART are poorer than outcomes observed in adults, with only 59% and 24% of the subjects meeting the study criteria of virological success at 24 weeks and 3 years, respectively.28,29 Flynn and co-workers28 reported that adherence to medication during the first 16 weeks of therapy was the most important factor predicting controlled viral replication. Although young adolescents show a good short-term response to ART, poor adherence or poor clinic attendance may be associated with poor virological outcomes and virological failure.2,4 Adolescents have been identified as a high-risk group for poor adherence to, and defaulting from, antiretroviral therapy.30–34 Some of the reasons for nonadherence include reckless or high-risk behavior, lack of social support, cost, poverty, or medication-related issues such as adverse effects, pill burden, or complexity of drug regimen.4,6,30 Just as the lack of parental involvement may contribute to increased LTFU rates, so it may contribute to poor ART adherence and poorer outcomes in young patients with HIV.35 Adherence in children is also influenced by age and maturity-related tolerance. Sustained suppression of HIV replication is dependent on the correct dose of appropriate ART being taken consistently and correctly and it is, therefore, important to review ART dosing as children move from pediatric to adult doses.
Many pediatric and adolescent systems (mainly in the developed settings) of HIV care provide coordinated comprehensive support that includes nursing, psychosocial, mental health, case management, and nutritional services delivered holistically in one setting. Fragmented services may disrupt HIV care and treatment in this group. Provision of adult health services may be available only in a location that lacks the infrastructure to provide the comprehensive services the youth have been accustomed to. Adult health services may see more patients (clinics are busier) and staff may spend less time with patients and may not always be sympathetic to the needs of adolescents. Although young persons may be considered adults, their life experience and the pressures of coping with a stigmatized, serious medical condition may leave them without the skill to manage their illness independently and successfully. Chronically ill youngsters may be less mature because their caregivers are more protective of them, ensuring that they take their medication, attend their clinic visits, and collect their medication regularly. Some may also come from poor socioeconomic backgrounds and may be AIDS orphans running child-headed households.23 More specialized adolescent health care clinics providing counseling, testing, and treatment have been developed in some countries, such as South Africa, to meet the needs of HIV-positive adolescents and young adults. Strategies such as directly observed therapy, education, and counseling interventions have been suggested to improve ART adherence. Adolescents have unique needs that require tailored services and targeted research. In this study, pregnancy was the most common reason for changing the ART regimen among the older adolescents and young adults, which also highlights the need for contraceptive counseling and improved access to birth control for this population.
These findings should be considered in light of the study limitations. First, loss to follow-up may have led to a misclassification of mortality as LTFU. Despite active tracing, mortality is substantially underestimated among HIV-positive patients lost from HIV treatment programs. Where possible and with valid national identification numbers, mortality was obtained and verified against the South African National Vital Registration system, which provides a more accurate assessment of mortality.12 After the death linkage for patients in this cohort, mortality rates increased from 3.5/100 pys to 6.1/100 pys for adults, 3.3/100 pys to 5.4/100 pys for youths, 2.2/100 pys to 4.4/100 pys for older adolescents, and 3.7/100 pys to 4.1/100 pys for young adolescents, while the LTFU rates decreased (13.7/100 pys to 9.8/100 pys for adults, 21.7/100 pys to 17.6/100 pys for youths, 27.0/100 pys to 23.3/100 pys for older adolescents, and 8.2/100 pys to 6.1/100 pys for young adolescents). This may minimize the effect of misclassifying mortality as LTFU. Furthermore, we note that the risk factors for mortality and LTFU were similar and we considered the possibility that LTFU was merely a surrogate for mortality. However, the point estimates of the effects of mortality and LTFU are very different, even in an opposite direction when comparing older adolescents and adults, and thus we feel this is unlikely to completely explain this similarity. We note, in addition, that the proportion of those LTFU who were actually deceased was found to be 37% in a study among a sample from the Themba Lethu Clinical Cohort, Johannesburg, South Africa by Fox and co-workers,12 again strengthening our sense that the outcomes described are indeed different.
Second, we do not have any data on whether adolescent HIV infection is acquired perinatally or behaviorally. Using growth failure [defined by a height-for-age (HAZ) z score<–2 for stunted growth] as a proxy for perinatal HIV acquisition, approximately 36.5% (85/233) of young adolescents and 19.8% (60/303) of older adolescents had a HAZ score<–2 and may have acquired HIV perinatally. However, this is considerably lower than reported in other studies.5 We did not access other associated factors, such as social issues, that may have been responsible for some of the differences observed between the groups.
Third, data are from public-sector HIV clinics and may, therefore, affect the extrapolation of the findings to other clinics, which may differ by region and program level. Lastly, the analysis is limited by the lack of data on adherence, incomplete CD4 and HIV viral load data, and lack of antiretroviral resistance testing. Routine patient data on HIV viral load or antiretroviral resistance testing are not available in these settings. The measurement of adherence is incomplete as we rely solely on self-reporting. These problems are also common in other resource-constrained settings. Since this is an observational retrospective analysis, no conclusions about causality can be made.
Strengths include the large samples size and long-term follow-up, which provide insight into the overall program outcomes of adolescents receiving ART at public-sector clinics across Gauteng and Mpumalanga, South Africa.
Conclusions
We report no difference in mortality by age category; however, HIV-infected adolescents and young adults between 15 and 24 years receiving ART have poorer treatment outcomes in terms of virological response, rates of LTFU, and virological failure than adults. Poor adherence, factors influencing transition, and barriers to full participation in HIV care may be responsible for the poorer treatment outcomes and increased LTFU in this unique group. Despite lower long-term virological suppression rates and higher rates of virological failure, young adolescents are more likely to achieve a favorable short-term immune response and are less likely to be LTFU. Results suggest a younger and more robust immune system in young adolescents, accompanied by more rapid viral rebound. The presence of a caregiver actively participating in HIV care and treatment may contribute to the lower rates of LTFU or better adherence to ART. Studies to determine barriers to adherence in adolescents and to develop interventions to address low rates of virological suppression and high rates of LTFU among this unique group are sorely needed in this setting.
Supplementary Material
Acknowledgments
We would like to acknowledge the directors and staff of Themba Lethu Clinic (TLC), CHRU, and Right to Care (RTC)—a PEPFAR (US President's Emergency Plan for AIDS Relief) funded NGO. We would also like to acknowledge the Gauteng Provincial and National Department of Health for providing for the care of the patients as part of the National Comprehensive Care, Management and Treatment (CCMT) of HIV and AIDS program. Lastly, we would like to sincerely thank the patients for their continued trust in the treatment and care provided at the clinics.
This study is made possible by the generous support of the American people through the United States Agency for International Development (USAID) and the National Institutes of Health (USAID-674-A-12-00029). Denise Evans is supported by funding from the Claude Leon Foundation and NIH/CFAR/IAS Creative and Novel Ideas in HIV Research (CNIHR) program (subaward with the UAB Center for AIDS Research: P30AI027767). The opinions expressed herein are those of the authors and do not necessarily reflect the views of USAID, NIH, or the United States government.
Prior abstract publication: Evans D, Menezes C, Mahomed K, Macdonald P, Untiedt S, Levin L, Jaffray I, Bhana N, Firnhaber C, and Maskew M: Treatment outcomes of HIV-positive adolescents in adult-orientated clinics across Gauteng and Mpumalanga, South Africa. 19th Conference on Retroviruses and Opportunistic Infections, March 5–8, 2012, Seattle, WA. Abstract 978.
Author Disclosure Statement
Right to Care (RTC) provided some of the funding for the current research and also supports the provision of treatment for the patients in the study.
References
- 1.World Health Organization: Towards Universal Access: Scaling up priority HIV/AIDS interventions in the Health Sector. WHO Press; Geneva, Switzerland: 2007. Progress Report April 2007. [Google Scholar]
- 2.Ryscavage PA. Anderson EJ. Sutton SH, et al. Clinical outcomes of adolescents and young adults in adult HIV care. J Acquir Immune Defic Syndr. 2011;58:193–197. doi: 10.1097/QAI.0b013e31822d7564. [DOI] [PubMed] [Google Scholar]
- 3.Agwu A. Rutstein R. Gaur A, et al. and the HIV Research Network. Starting late, stopping early: Disparities in HAART utilization for behaviorally HIV-infected youth. Conference on Retroviruses and Opportunistic Infections; Boston. Abstract 692. [Google Scholar]
- 4.Nachega JB. Hislop M. Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nglazi MD. Kranzer K. Holele P, et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis. 2012;12:21. doi: 10.1186/1471-2334-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakanda C. Birungi J. Mwesigwa R, et al. Survival of HIV-infected adolescents on antiretroviral therapy in Uganda: Findings from a nationally representative cohort in Uganda. PLoS One. 2011;6(4):e19261. doi: 10.1371/journal.pone.0019261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Department of Health, Republic of South Africa. The South African national antiretroviral treatment guidelines. 2004. http://southafrica.usembassy.gov/media/2004-dohart-guidelines.pdf. [Jan 21;2013 ]. http://southafrica.usembassy.gov/media/2004-dohart-guidelines.pdf
- 8.National Department of Health, Republic of South Africa. The South African national antiretroviral treatment guidelines. 2010. www.fidssa.co.za/Guidelines/2010_Adult_ART_Guidelines.pdf. [Jan 21;2013 ]. www.fidssa.co.za/Guidelines/2010_Adult_ART_Guidelines.pdf
- 9.Fox M. Maskew M. MacPhail A. Long L. Brennan A. Westreich D. MacLeod W. Majuba P. Sanne I. Cohort profile: The Themba Lethu Clinical Cohort, Johannesburg, South Africa. Int J Epidemiol. 2012;10:1–10. doi: 10.1093/ije/dys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNICEF/WHO 1995: A picture of health—a review of and annotated bibliography of health of young people in developing countries. UNICEF New York. WHO; Geneva: [Google Scholar]
- 11.Maskew M. Mahlangeni G. Fox M, et al. Short-, long-term effect of Kaposi sarcoma on the response to HAART in the setting of the South African HIV epidemic. Conference on Retroviruses and Opportunistic Infections; Feb 16–19;2010 ; San Francisco, California. Abstract 763. [Google Scholar]
- 12.Fox MP. Brennan A. Maskew M, et al. Using vital registration data to update mortality among patients loss to follow-up from ART programmes: Evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15:405–413. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans DH. Maskew M. Sanne I. Increased risk of mortality and loss to follow-up among HIV positive patients with oropharyngeal candidiasis and malnutrition before antiretroviral therapy initiation: A retrospective analysis from a large urban cohort in Johannesburg, South Africa. Oral Med. 2012;113:362–372. doi: 10.1016/j.oooo.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan AT. Maskew M. Sanne I, et al. The importance of clinic attendance in the first six months on antiretroviral treatment: A retrospective analysis at a large public sector HIV clinic in South Africa. J Int AIDS Soc. 2010;13:49. doi: 10.1186/1758-2652-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maskew M. MacPhail P. Sanne I, et al. Prevalence and effect of KSHV seropositivity on immunologic and virologic outcomes among HIV-infected adults initiating HAART in Johannesburg, South Africa. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. Abstract 874. [Google Scholar]
- 16.Meya D. Spacek LA. Tibenderana H, et al. Development and evaluation of a clinical algorithm to monitor patients on antiretrovirals in resource-limited settings using adherence, clinical and CD4 cell count criteria. J Int AIDS Soc. 2009;12(3) doi: 10.1186/1758-2652-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins GK. Johnson KL. Chang Y, et al. Predicting virological failure in an HIV clinic. Clin Infect Dis. 2010;50:779–786. doi: 10.1086/650537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanne I. Orrell C. Fox MP, et al. and the CIPRA-SA Study Team. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): A randomised non-inferiority trial. Lancet. 2010;376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westreich D. Evans D. Firnhaber C, et al. Prevalent pregnancy, biological sex and virologic response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60:489–494. doi: 10.1097/QAI.0b013e318256b310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairall LR. Bachmann MO. Louwagie GM, et al. Effectiveness of antiretroviral treatment in a South African program: A cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 21.Boulle A. Van Cutsem G. Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization: WHO Child Growth Standards. WHO Press; Geneva, Switzerland: 2006. Methods, development: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height, body mass index-for-age. [Google Scholar]
- 23.Macdonald P. Maskew M. Evans D, et al. HAART alone is not enough to deal with pediatric HIV: Treatment outcomes from a resource-limited setting in South Africa. Vulnerable Child Youth Studies. 2011;6:208–221. [Google Scholar]
- 24.May M. Boulle A. Phiri S, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: A collaborative analysis of scale-up programs. Lancet. 2010;376:449–457. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kigozi IM. Dobkin LM. Martin JN, et al. Late disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009;52:280. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson CM. Wright PF. Safrit JT, et al. Epidemiology of HIV infection and risk in adolescents and youth. J Acquir Immune Defic Syndr. 2010;54:S5–S6. doi: 10.1097/QAI.0b013e3181e243a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt PW. Deeks SG. Rodriguez RJ, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–1915. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 28.Flynn PM. Rudy BJ. Douglas SD, et al. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190:271–279. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 29.Flynn PM. Rudy BJ. Lindsey JC, et al. Long-term observation of adolescents initiating HAART therapy: Three-year follow-up. AIDS Res Hum Retroviruses. 2007;23:1208–1214. doi: 10.1089/aid.2006.0290. [DOI] [PubMed] [Google Scholar]
- 30.Murphy DA. Belzer M. Durako SJ, et al. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159:764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 31.Khan M. Song X. Williams K, et al. Evaluating adherence to medication in children and adolescents with HIV. Arch Dis Child. 2009;94:970–973. doi: 10.1136/adc.2008.156232. [DOI] [PubMed] [Google Scholar]
- 32.Gross R. Zhang Y. Grossberg R. Medications refill logistics and refill adherence in HIV. Pharmacoepidemiol Drug Saf. 2005;14:789–793. doi: 10.1002/pds.1109. [DOI] [PubMed] [Google Scholar]
- 33.Van Rossum AM. Bergshoeff AS. Fraaij PL, et al. Therapeutic drug monitoring of indinavir and nelfinavir to assess adherence to therapy in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2002;21:473–747. doi: 10.1097/00006454-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Bangsberg DR. Hecht FM. Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 35.Van Dyke RB. Lee S. Johnson GM, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109:e61. doi: 10.1542/peds.109.4.e61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.