Abstract
Previous studies have shown that miR-137 functions as a tumor suppressor in various cancers, but its role in the initiation and development of gliomas is still unknown. Currently, we found that miR-137 exhibited the most significant increase in normal brain tissues compared with glioma specimens, and the miR-137 expression was greatly decreased with the ascending of tumor pathological grades. Furthermore, overexpression of miR-137 in vitro by chemically synthesized miR-137 mimics suppressed the proliferation, inhibited cell cycle arrest in the G1/G0 phase, and induced cell apoptosis. The tumor-suppressive effects of miR-137 were indeed induced by Rac1, which was verified as a direct target of miR-137. These findings indicate that miR-137 inhibits the growth of gliomas cells by directly targeting Rac1, suggesting that miR-137 could be a new important therapeutic strategy for glioma treatment and warrants further investigation.
Key words: glioblastoma, miR-137, Rac1
Introduction
MicroRNAs, as small highly conserved noncoding RNA molecules of ∼18–25 nucleotides in length, regulate gene expression at the post-transcriptional level by binding complimentary sequences in the 3′-untranslated regions (UTRs) of target mRNAs. It has been extensively considered as a critical role in biological processes and is closely correlated with tumorigenesis.1,2 By negatively regulating their mRNA targets, they can function as tumor suppressors or oncogenes.3
As a well-known oncomir, miR-21 has been proved to be overexpressed in a wide variety of human cancer tissues, impacting cell proliferation, survival, and invasiveness. Plenty of evidence indicated that several genes such as PDCD4, Bcl-2, and PTEN had been confirmed to be the targets of miR-21.4–7 MiR-221/222 was frequently found to be decreased in gliomas, targeting p53 up-regulated modulator of apoptosis (PUMA) to regulate glioma cells apoptosis.8 Most recently, we reported that miR-10b was elevated in glioma samples and tightly associated with the pathological grade and malignancy. In addition, miR-10b induced glioma cell invasion by modulating tumor invasion factors MMP-14 and uPAR expression via HOXD10.9 However, the concrete mechanism of dysregulated miRNAs underlying gliomas remains murky. MiR-137 is located at the region on chromosome 1p21.3 and lies across a large CpG island.10 In this study, we aimed at miR-137, which was reported to be down-regulated in several cancers, such as gastric cancer, colorectal cancer, and oral cancer.
Earlier, we profiled miRNA expression in 5 glioblastoma cell lines (U251, TJ866, TJ905, TJ899, and A172) and human astrocytoma cell line (H4) through microarray analysis, and found that miR-137 decreased significantly in tumor cells. Here, we further examined the miR-137 expression in glioblastoma cell lines and glioma specimens through real-time polymerase chain reaction (PCR) assay, and characterized the biological functions of miR-137 on glioma cells. Our data showed that miR-137 was a potential tumor-suppressor factor in human glioma.
Materials and Methods
Cells and cell culture
The human U87, LN229, U251, TJ905, and U373 glioblastoma cell lines were purchased from the Chinese Academy of Sciences Cell Bank. All glioma cell lines were maintained in a 37 C, 5% CO2 incubator in Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (Invitrogen).
Glioma samples and normal brain tissues
All the glioma samples were obtained from patients who underwent surgical resection at the first affiliated hospital of Nanjing Medical University, and their consent was also obtained. Overall, 18 specimens with clinical data were collected from May 2009 to October 2010, including 7 grade I-II tumors, 6 grade III tumors, and 5 grade IV tumors (glioblastomas). Normal brain tissues were obtained from patients with traumatic brain injury for internal decompression. This study was approved by the institutional review boards of the hospitals, and written informed consent was obtained from all patients. After resection, all samples were immediately frozen into liquid nitrogen until RNA extraction.
Oligonucleotides and cell transfection
The 2′-O-methy1 (2′-OMe-) miR-137 mimics were chemically synthesized by GenePharma, and the sequences were as follows: 5′-UUA UUG CUU AAG AAU ACG CGU AG-3′, 5′-ACG CGU AUU CUU AAG CAA UAA UU-3′, and scramble sequence 5′-UUC UCC GAA CGU GUC ACG UTT-3′, 5′-ACG UGA CAC GUU CGG AGA ATT-3′. For transfection, oligonucleotides were allowed to form transfection complexes with Lipofectamine 2000 (Invitrogen), subsequently added to glioma cells at a final concentration of 50 nmol/L, and left to incubate for 8 hours before medium change. We also designed a double-stranded siRNA oligonucleotide against Rac1 (sense: 5′-GGA GAT TGG TGC TGT AAA A-3′ and anti-sense: 5′UUU UAC AGC ACC AAU CUC C-3′).
Real-time quantification of miRNAs by stem-loop reverse transcription-polymerase chain reaction
RNA was extracted using Trizol reagent (Invitrogen). For the TaqMan-based real-time reverse transcription-polymerase chain reaction (RT-PCR) assays, the ABI 7300 HT Sequence Detection system (Applied Biosystems) was used. Primers and probes of the miR-137 for Taqman miRNA assays were purchased from Applied Biosystems. Relative gene expression was calculated via a 2−ΔΔCt method.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay for cell proliferation
The effect of miR-137 on cell viability was determined by using the cell proliferation reagent MTT (KeyGEN). U251 and LN229 cells were seeded into 96-well plates at 2×103 cells per well. Subsequently, 50 μL of MTT dilution was added into each well at each day of consecutive 3 days after transfection, and the cells were incubated for additional 4 hours. Finally, the supernatant was discarded, and 150 μL of dimethyl sulfoxide was added to each well to dissolve the precipitate. Optical density was measured at the wavelength of 570 nm. These data are presented as the mean±SD, which are derived from five samples of at least three independent experiments.
Cell cycle analysis
U251 and LN229 cells were treated with miR-137 oligonucleotide, and harvested in the log phase of growth in 48 hours post-transfection. After extensive washing, the cells were suspended in Hank's Balanced Salt Solution containing 50 μg/mL PI (Propidium Iodide) (Sigma) and 50 μg/mL RNase A (Boehringer Mannheim), incubated for 1 hour at room temperature, and analyzed by FACScan (Becton Dickinson).
Apoptosis assays by annexin staining
U251 and LN229 cells were plated in six-well plates and transfected with oligonucleotide. After 48 hours post-transfection, the parental and transfected cells in the log phase of growth were harvested and collected. AnnexinV FITC Apoptosis Detection Kit (Invitrogen) was used, and the apoptotic cells were detected and quantified by FACScan (Becton Dickinson).
Transwell invasion assay
The transwell filters (Costar) were coated on the upper surface of the polycarbonic membrane (diameter 6.5 mm, pore size 8 μm) with matrigel (3.9 μg/μL, 60–80 μL, Becton Dickinson). Harvested cells of transfected and control groups (1×105) suspended in 100 μL of serum-free DMEM were added into the upper compartment of the chamber. A total of 200 μL conditioned medium from U251 and LN229 was used as a source of chemoattractant and placed in the bottom compartment of the chamber. After 24 hours of incubation at 37°C with 5% CO2, the medium was removed from the upper chamber. The noninvaded cells on the upper side of the chamber were scraped off with a cotton swab. The cells that had migrated from matrigel and into the pores of the inserted filter were fixed with 100% methanol, stained with hematoxylin, mounted, and dried at 80°C for 30 minutes. The number of cells invading through the matrigel was counted by three randomly selected visual fields, each from the central and peripheral portion of the filter using an inverted microscope at 200× magnification. Each test was performed in triplicate.
Western blot assay
Total proteins were extracted, and the protein concentration was determined by BSA method (KeyGEN). From each sample, 40 μg of protein lysates were subjected to sodium dodecyl sulfate -polyacrylamide gel electrophoresis in 10% acrylamidegel and transferred to polyvinylidene fluoride membranes (Millipore Corporation). The membrane was blocked in 5% nonfat milk and incubated with diluted antibodies against Cyclin D1, MMP-2, Bcl-2, Bax, and Rac1 (1:500, Cell Signal Technology) overnight at 4°C, followed by incubation with HRP-conjugated secondary antibody (1:2000, Santa Cruz Biotechnology). After stripping, the membrane was reprobed with GAPDH (1:2000, Kangchen Biotechnology) using ultra-enhanced chemoluminescence Western blot detection reagents (Multi Science Biotech). All Western bands were quantified by densitometry and are presented in the form of a bar graph.
Luciferase reporter assay
pGL3-WT-Rac1-promoter reporters were created by ligation of miR-137-binding sites in the Rac1 promoter into the BglII site of the pGL3 control vector (Promega). pGL3-MUT-Rac1-promoter reporters were constructed from pGL3-WT-Rac1-promoter reporters by deleting the binding sites. Cells of 60%–70% confluence in 24-well plates were cotransfected with luciferase reporter vector and miR-137 expressing vector, and 1 ng pRLSV40 Renilla luciferase construct was used for normalization. After 48 hours, luciferase activity was measured with the Dual-luciferase reporter assay system.
Statistical analysis
Data were analyzed with SPSS 12.0. All experiments were done thrice, and statistical evaluation for data analysis was determined by t-test. Differences with p<0.05 were considered statistically significant.
Result
MiR-137 was epigenetically inactivated in gliomas
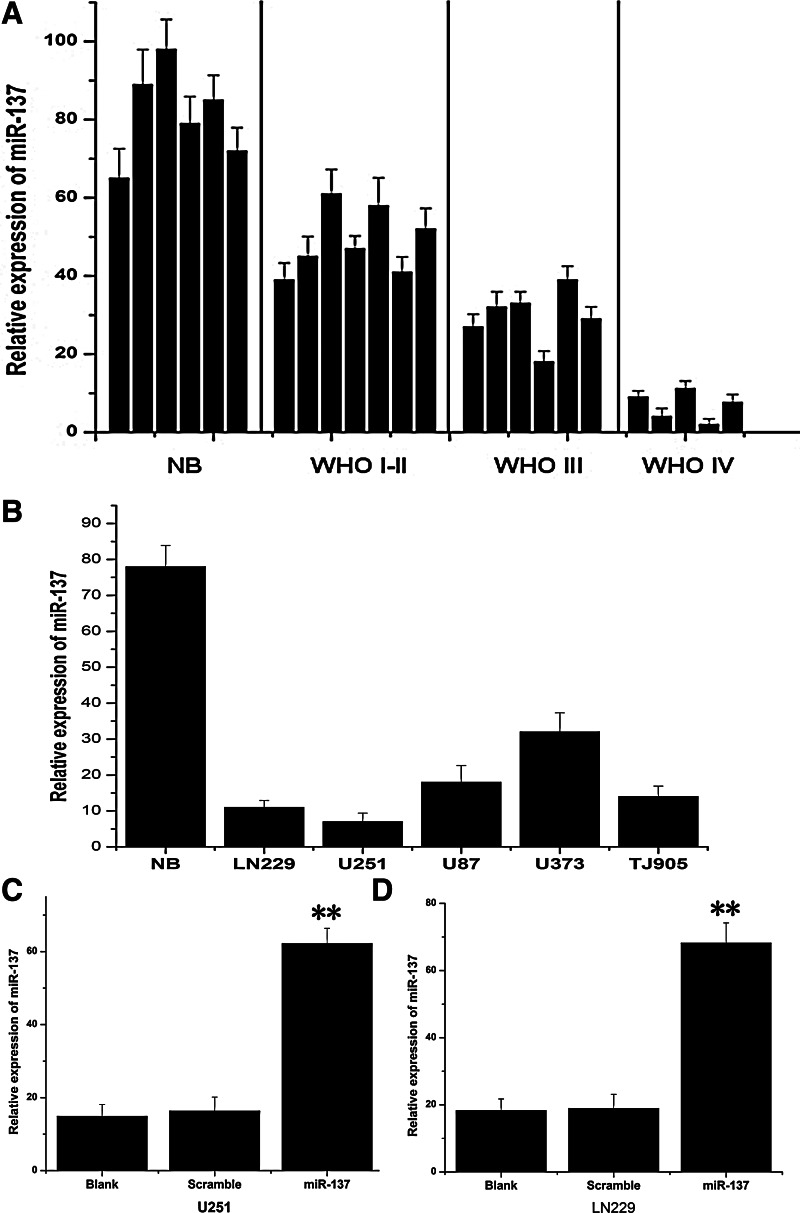
Real-time PCR assay was used to detect the miR-137 expressing level in glioma tissue specimens that were divided according to WHO classification, including 7 I-II, 6 III, and 5 IV grades. Six normal brain tissues were used as a control. Just as shown in Figure 1A, miR-137 expressing level in normal brain tissues was indeed higher than glioma specimens. Moreover, it was an obvious trend that miR-137 expression significantly decreased with the ascending of tumor pathological grades. In addition, miR-137 expression also statistically significantly reduced in glioma cell lines, especially in U251 and LN229 cells in comparison to normal brain tissues. Therefore, we chose U251 and LN229 cells for the following studies.
FIG. 1.
MiR-137 was down-expressed in glioma tissues and glioma cell lines. (A) The expression of miR-137 in glioma tissues was measured by real-time polymerase chain reaction assay, and six normal brain tissues were counted as a control. MiR-137 expressing level in normal brain tissues were indeed higher than in glioma specimens, and significantly decreased with the ascending of tumor pathological grades. (B) The expression of miR-137 was showed in glioma cell lines LN229, U251, U87, U373, and TJ905. (C, D) MiR-137 mimics could effectively activate the expression of miR-137 in LN229 and U251glioma cells. **p<0.01, compared with the control.
MiR-137 was up-regulated after transfection
To determine the biological functions of miR-137 on glioma cells, chemically synthesized modified oligonucleotides were transfected into U251 and LN229 cell lines. Then, real-time PCR assay was initially used to measure miR-137 expression at 48 hours post-transfection of miR-137 mimics in glioma cells. The results showed that miR-137 expression increased about 4.19- and 3.72-fold, respectively, in U251 and LN229 cells compared with their blank control groups (Fig. 1C and D), suggesting that miR-137 was activated effectively after transfection in glioma cell lines.
MiR-137 suppressed the proliferation of glioma cells in vitro through leading to cell cycle arrest at G0/G1 phase
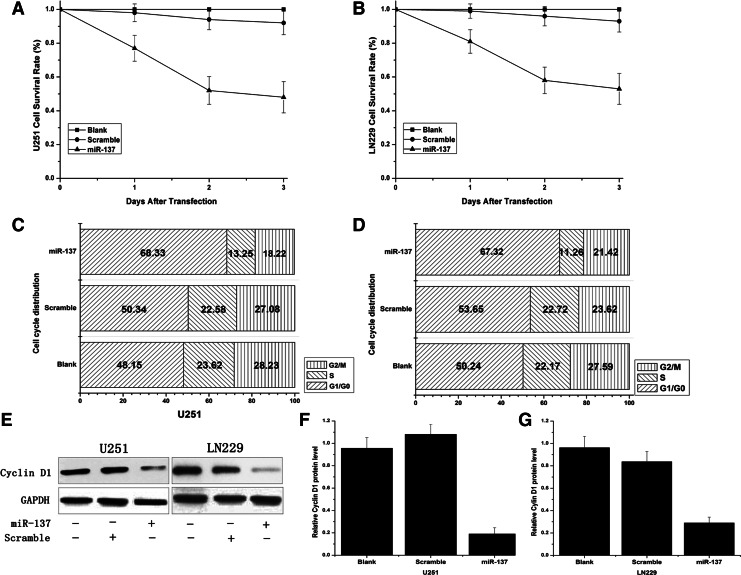
MTT assay showed a significant reduction in proliferation after transfection of miR-137 mimics in glioma cells. About 0.77±0.076, 0.52±0.082, and 0.48±0.091 survival rate at 1, 2, and 3 days post transfection of miR-137 mimics in U251, while about 0.81±0.71, 0.58±0.078, and 0.53±0.089 in LN229 cells were shown (Fig. 2A and B). For further studies as to whether decreased growth viability was a result of cell cycle arrest, the cell cycle distribution was analyzed by flow cytometry (FCM). In U251 cells, as shown in Figure 2C and D, the G0/G1 phase fraction in the control and scramble groups was 48.15% and 50.34%, respectively, but increased significantly in the miR-137 mimics group to 68.33%. In LN229 cells, the G1/G0 phase fraction of the control and scramble groups was 50.24% and 53.65%, respectively, while the miR-451 mimics group increased to 67.32%. In addition, miR-137 mimics had no effect on the G2/M phase fraction of glioma cells. The above results revealed that miR-137 could inhibit the cells growth by leading to an arrest in the cells at G0/G1 phases. Western blot assay showed that Cyclin D1 protein level in miR-137 mimics group was significantly reduced than in the untreated group.
FIG. 2.
MiR-137 mimics inhibited growth of glioma cells. (A, B) Cell proliferation was suppressed after the cells had been transfected with MiR-137 mimics by MTT assay. (C, D) MiR-137 could induce the glioma cells arrest at G0/G1 phases and delay the progression of the cell cycle. (E–G) Cyclin D1 protein expression was examined by Western blot assay, and GAPDH was regarded as an endogenous normalizer.
MiR-137 induced apoptosis in glioma cell lines
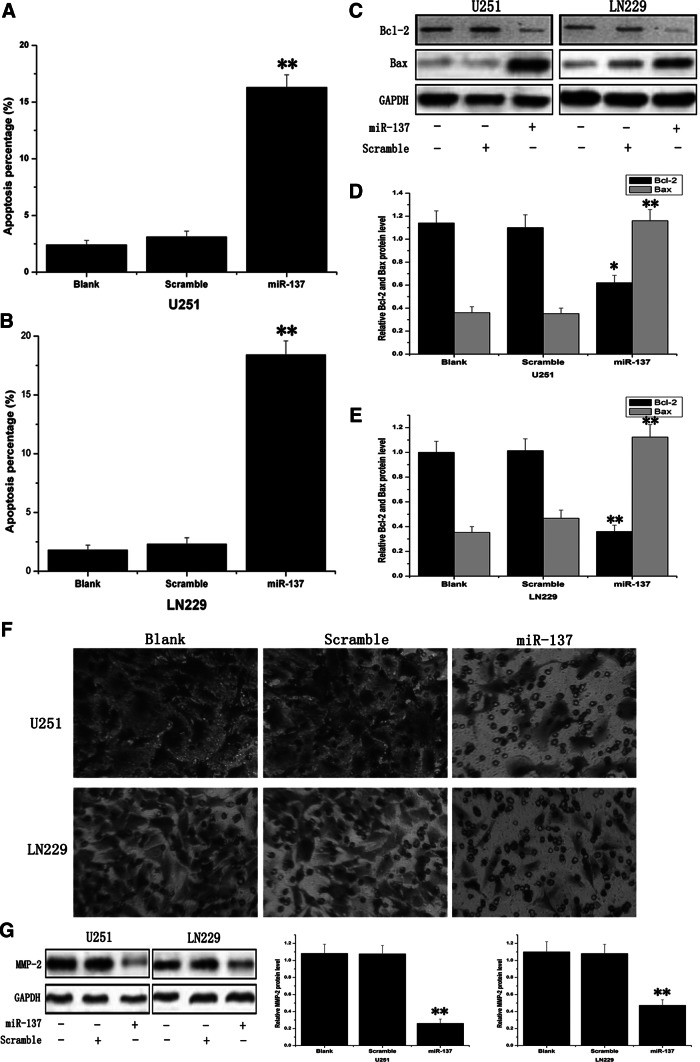
Annexin V and PI double staining assay was used to examine the effect of miR-137 on gliomas cell apoptosis. At 48 hours after transfection, the cell apoptosis was assessed by FCM. It was found that the apoptotic rate in miR-137 mimics-treated U251 cells was 16.3±1.32 and 18.4±1.15 in miR-137 mimics-treated LN229 cells, which showed statistically significant more apoptotic cells relative to their untreated cells and scramble-treated cells (Fig. 3A, B). Then, antiapoptotic protein Bcl-2 and pro-apoptotic protein Bax were examined by Western blot assay, showing that Bcl-2 expression was suppressed and Bax expression was activated (Fig. 3C).
FIG. 3.
MiR-137 induced apoptosis and inhibited the invasion ability in glioma cell lines. (A, B) Annexin V and Propidium Iodide double staining assay was utilized to appraise the cell apoptosis. (C–E) Anti-apoptotic protein Bcl-2 and pro-apoptotic protein Bax were measured by Western blot assay, and GAPDH was regarded as an endogenous normalizer. (F) Transwell matrigel invasion assay was employed to evaluate the cells' invasion ability, and miR-137 mimics effectively inhibited invasive activity. (G) MMP-2 was investigated by Western blot assay, and GAPDH was regarded as an endogenous normalizer. *p<0.05, **p<0.01 compared with the control.
MiR-137 inhibited the invasive ability of glioma cells
To appraise the impact of miR-137 on glioma cell invasive ability, we employed a transwell matrigel invasion assay. In U251 cells, miR-137 mimics inhibited invasive activity by ∼40%, as 62.8±7.28 cells field invaded the matrigel layer compared with 162.7±18.21 and 153.6±14.87 cells/field in the blank control and scramble-treated groups, respectively. Similarly, overexpression of miR-137 had greatly suppressed ability to invade matrigel in LN229 cells, exhibiting about 121.7±11.34, 103.9±10.38, and 52.8±6.65, respectively, in the blank group, scramble group, and mimics-treated group cells (Fig. 3F). Western blot assay showed that MMP-2 protein significantly decreased in miR-137 mimics-treated cells. These results strongly indicated that miR-137 was an important participant in the reduction of the invasive potential of glioma cells.
Rac1 was a direct target of miR-137
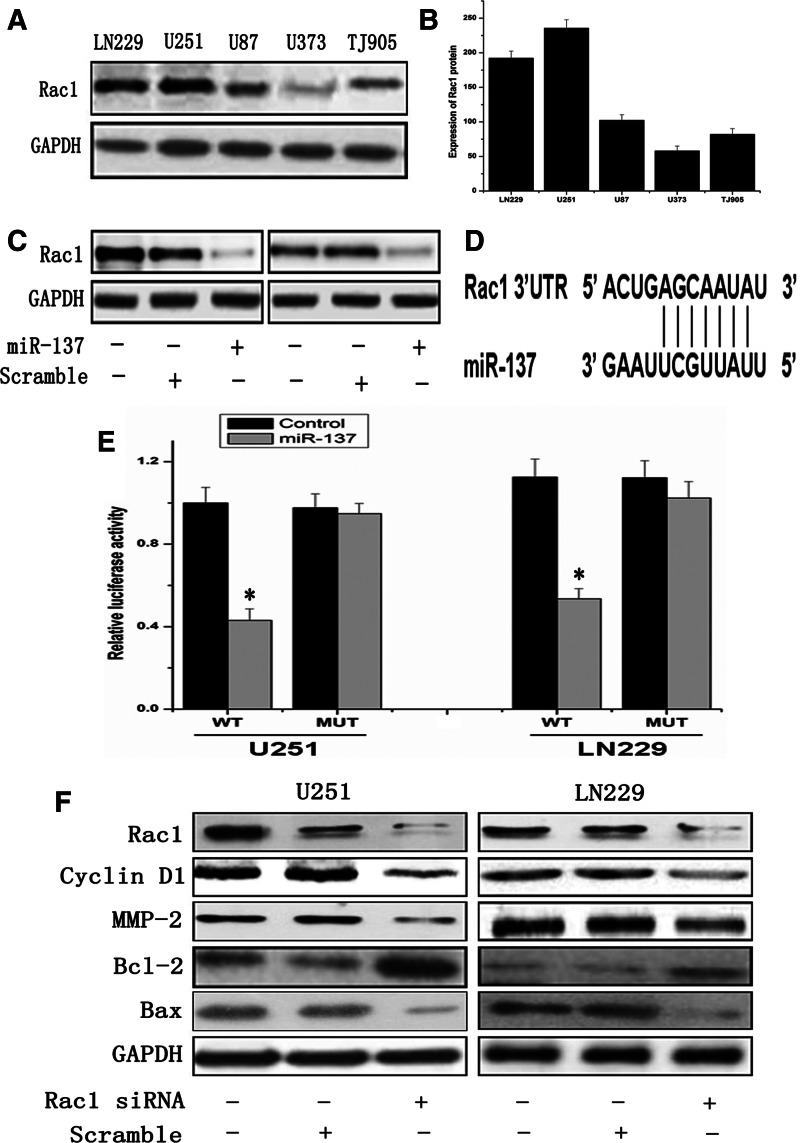
Rac1 that alternates between an inactive GDP-bound form and an active guanosine triphosphate (GTP)-bound form regulates a variety of signal pathways that are involved in malignant phenotypes.11 In the present study, we examined Rac1 expression in diverse glioma cell lines. It was interesting to note that both of U251 and LN229 cells which expressed a lower level of miR-137 were overexpressed Rac1 protein, and the inverse situation occurred in U373, U87, and TJ905 cell lines (Fig. 4A, B). In order to explore the association between miR-137 and Rac1, miR-137 mimics were transfected in U251 and LN229 glioma cells with lower expression of miR-137, and it was found that Rac1 expression in both cell lines was significantly diminished normalized to their untreated cells (Fig. 4C). The above results indicated that miR-137 suppressed Rac1 expression in glioma cells. Using bioinformatics, the 3′UTR of Rac1 was found to contain target sequences for miR-137 (Fig. 4D). To further confirm whether Rac1 was a direct target of miR-137 in glioma cells, a luciferase reporter assay was conducted. Then, the constructed pGL3-WT Rac1-promoter and pGL3-MUT Rac1-promoter within the miR-137 binding site were co-transfected in glioma cells. The relative luciferase activity of the WT construct of Rac1 3′UTR was significantly reduced in the presence of miR-137, and the mutation could abrogate the suppressive effect of miR-137 inhibition on luciferase activity in both U251 and LN229 cells (Fig. 4E). These data revealed that miR-137 directly regulates Rac1 gene by binging to 3′UTR region of Rac1.
FIG. 4.
MiR-137 directly targets the 3′UTR of RAC1 mRNA in glioma cell line. (A, B) Rac1 expression levels in glioma cell lines LN229, U251, U87, U373, and TJ905 were examined by Western blot assay, and GAPDH was regarded as an endogenous normalizer. (C) Rac1 expression was significantly diminished in miR-137 mimics-treated cells normalized to their untreated cells. (D) The predicted binding sites between miR-137 and Rac1 3′UTR in humans. (E) pGL3-WT-Rac1-promoter and pGL3-Rac1-Rac1-promoter reporters were transfected into cells, which were then treated with miR-137 mimics. Luciferase activity was determined 48 hours after transfection. Luciferase activity of control group was normalized as one in the WT group. Error bars represent standard deviation and were obtained from three independent experiments. (F) Rac1, CyclinD1, MMP-2, Bcl-2, and Bax protein levels were examined by Western blot assay, and GAPDH was regarded as an endogenous normalizer. *p<0.05 compared with the control.
The tumor-suppressive effect of miR-137 was induced by Rac1 activity and its down-stream effectors
To explore the molecular mechanism of miR-137 in glioma cells, we constructed an Rac1 siRNA plasmid vector to inhibit the expression of Rac1 in U251 and LN229 cells. It was found that CyclinD1, MMP-2, and Bcl-2 were down-regulated, while Bax was up-regulated in glioma cells. The results suggested that miR-137 suppressed glioma cells growth by regulating CyclinD1, MMP-2, Bcl-2, and Bax mediated via Rac1.
Discussion
MiR-137 has been postulated to function as a tumor suppressor in various cancers, such as colorectal, oral, and gastric.12–15 In the present study, we examined miR-137 expression in different grades of primary human glioma specimens and normal brain tissues as well as glioblastoma cell lines, suggesting that miR-137 was frequently inactivated in glioma specimens compared with normal brain tissues. Furthermore, the expression of miR-137 statistically decreased with the ascending of tumor grades. Accordingly, the expression of miR-137 in glioma cell lines was notably higher than in normal brain tissues. The aforementioned studies along with our results firmly support the notion that miR-137 expression is associated with advanced clinical stages and malignancy of gliomas. In order to further study the effects of miR-137 on glioma cells, we used synthesized mimics to elevate the expression of miR-137 in human gliomablastoma (GBM) cells (U251 and LN229). Real-time PCR assay was used to detect the expression of miR-137, suggesting that the mimics could effectively activate the miR-137 expression in U251 and LN229 GBM cells.
Furthermore, we kept studying its biology functions in human glioblastoma cells. By ectopic expression of miR-137 in U251 and LN229 GBM cells, the miR-137 mimics could suppress the proliferation, induce the cells' arrest at G0/G1 phase, and promote the apoptosis, suggesting a firm antitumor role of miR-137 in gliomas. To investigate the molecule mechanisms involved in the tumorigenesis of gliomas, we examined the expression of CyclinD1, MMP-2, Bcl-2, and Bax protein by using Western blotting methods.
Loss of normal cell cycle control is a hallmark of cancer. Cyclin D1 gene encodes the regulatory subunit of a holoenzyme that phosphorylates and inactivates the retinoblastoma protein and promotes progression through the G1-S phase of the cell cycle.15 Dysregulation of cyclin D1 expression plays key role during glioma tumorigenesis.16 Overexpression of cylin D1 is known to correlate with the early onset of cancer and the risk of tumor progression and proliferation. In the present study, the result showed that miR-137 could suppress cylcin D1 express, which is responsible for the cell cycle arrest and proliferation inhibition.
The degradation of extracellular matrix is an essential step in invasiveness and malignancy of glioma. MMP-2, one of the most plenty in glioma, is thought to be the key enzyme involved in the degradation progression. High levels of MMP-2 in tissues are associated with tumor cell growth and invasion.17 Pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins are known to regulate glioma cells apoptosis. Low levels of Bax and high levels of Bcl-2 protein could protect glioma cells from apoptosis induced by a wide variety of insults, including chemotherapeutic drugs and radiation.18 Transwell invasion assay and apoptosis analysis suggested that miR-137 could induce invasion and apoptosis in U251 and LN229 cells. Meanwhile, MMP-2 expression was significantly suppressed by miR-137, while Bcl-2 decreased and Bax expression was promoted. These data proved that miR-137 could induce the invasion and apoptosis of glioma cells.
Previous studies have shown that cdc42 was a direct target of miR-137 in colorectal cancer cells and gastric cancer cells, and miR-137 induced apoptosis and cell G1 arrest by negatively regulating cdc42 expression. Chen reported that both MITF and CDK6 genes have miR-137 complementary sites in their 3′UTR region, and miR-137 can act as a tumor suppressor in uveal melanoma cell proliferation through downregulation of targets MITF and CDK6.19
Rac1 is subject to the Rho family, which constitutes a subgroup of the Ras superfamily of small GTPases. It has been reported that Rac1 participated in multiple cellular events such as cell cycle progression, growth, cytoskeletal reorganization, and cell motility. We examined the Rac1 expression level in glioma cell lines, and found that Rac1 protein level was inversely correlated to miR-137 expression in glioma cells. Therefore, we assumed that Rac1 was a direct target of miR-137. Using bioinformatics analysis, we observed that the 3′UTR of Rac1 gene harbors a putative site which is recognized by miR-137. Results from a luciferase reporter assay indeed validated that Rac1 was a direct target of miR-137. Subsequently, the protein expression level of Rac1 was found to be decreased by exogenously expressed miR-137.
In addition, we knocked down the expression of Rac1 in glioma cells by transfecting Rac1 siRNA into U251 and LN229 glioma cells. Western blot assay showed that cylcin D1, MMP-2, and Bcl-2 were inactivated by Rac1 siRNA, and Bax was induced. The effects of Rac1 siRNA on glioma cells was mostly alike to miR-137 mimics, suggesting that miR-137 suppressed glioma cell growth by regulating cylcin D1, MMP-2, Bcl-2, and Bax mediated through Rac1.
Taken together, our data uncover the functions of miR-137 in glioma carcinogenesis, and enhance an understanding of the relevant molecular mechanism. The findings mentioned earlier strongly disclosed that miR-137 was a negative regulator of Rac1 expression, and it inhibited the growth of glioma cells through Rac1 gene and its downstream genes. Our data uncover that miR-137 may be a critical factor in glioma progression and could be used as a potential molecular marker for pathological diagnosis and prognosis evaluation for malignant gliomas.
Acknowledgments
This work was supported by the China Natural Science Foundation (81201976, 81000963), Jiangsu Province's Natural Science Foundation (BK2012670), Jiangsu Province's 333 Talent Program (BRA2011046), the Kunshan Social Development Foundation (Grant Number: KS1006, KS1009), and the Suzhou Social Development Foundation (SYS201063).
Disclosure Statement
No financial conflicts exist.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Calin GA. Sevignani CD. Dumitru , et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B. Pan X. Cobb GP, et al. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Frankel LB. Christoffersen NR. Jacobsen A, et al. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 5.Gaur AB. Holbeck SL. Colburn NH, et al. Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro Oncol. 2011;13:580. doi: 10.1093/neuonc/nor033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei K. Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papagiannakopoulos T. Shapiro A. Kosik KS, et al. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68:8164. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 8.Zhang CZ. Zhang JX. Zhang AL, et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Cancer. 2010;9:229. doi: 10.1186/1476-4598-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L. Yan W. Wang G, et al. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011;1389:9. doi: 10.1016/j.brainres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Liu M. Lang N. Qiu M, et al. miR-137 targets Cdc42 expression, induces cell cycle G1 arrest and inhibits invasion in colorectal cancer cells. Int J Cancer. 2011;128:1269. doi: 10.1002/ijc.25452. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q. Chen M. Zhang Q, et al. MiR-137 is frequently down-regulated in gastric cancer and is a negative regulator of Cdc42. Dig Dis Sci. 2011;56:2009. doi: 10.1007/s10620-010-1536-3. [DOI] [PubMed] [Google Scholar]
- 12.Kozaki K. Imoto I. Mogi S, et al. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res. 2008;68:2094. doi: 10.1158/0008-5472.CAN-07-5194. [DOI] [PubMed] [Google Scholar]
- 13.Raptis L. Arulanandam R. Geletu M, et al. The R(h)oads to Stat3: Stat3 activation by the Rho GTPases. Exp Cell Res. 2011;317:1787. doi: 10.1016/j.yexcr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Necela BM. Carr JM. Asmann YW, et al. Differential expression of microRNAs in tumors from chronically inflamed or genetic (APC(Min/+)) models of colon cancer. PLoS One. 2011;6:18501. doi: 10.1371/journal.pone.0018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu M. Wang C. Li Z, et al. Minireview: Cyclin D1: Normal and abnormal functions. Endocrinology. 2004;145:5439. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 16.Klein EA. Assoian RK. Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci. 2008;121:3853. doi: 10.1242/jcs.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Z. Shi W. Shao J, et al. Peroxisome proliferator-activated receptor gamma agonist pioglitazone inhibits beta-catenin-mediated glioma cell growth and invasion. Mol Cell Biochem. 2011;349:1. doi: 10.1007/s11010-010-0637-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang P. Zhen H. Jiang X, et al. Boron neutron capture therapy induces apoptosis of glioma cells through Bcl-2/Bax. BMC Cancer. 2010;10:661. doi: 10.1186/1471-2407-10-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X. Wang H. Shen J, et al. Epigenetics, microRNAs, and carcinogenesis: Functional role of microRNA-137 in uveal melanoma. Invest Ophthalmol Vis Sci. 2011;2:1193. doi: 10.1167/iovs.10-5272. [DOI] [PubMed] [Google Scholar]