Abstract
There are currently few detailed studies describing HIV-1 recombination events or the potential impact of recombination on drug resistance. We describe here the viral recombination dynamics in a drug-naive patient initially infected with a circulating recombinant form 19 (CRF19) virus containing transmitted drug resistance mutations followed by superinfection with “wild-type” subtype B virus. Single genome analysis showed replacement of the primary CRF19 virus by recombinants of the CRF19 virus and the superinfecting subtype B virus. The CRF19/B recombinant virus dominating after superinfection had lost drug resistance mutations and at no time was the superinfecting subtype B variant found to be dominant in blood plasma. Furthermore, the detection of recombinant viruses in seminal plasma indicates the potential for onward transmission of these strains.
Transmitted drug resistance and HIV-1 superinfection are issues of concern with implications for HIV-1 disease progression and treatment. Transmitted drug resistance and persistence of resistant variants reduce treatment options in treatment-naive patients, while superinfection can potentially enhance viral fitness and evolutionary adaptation within the patient. Here, we examine the dynamics of recombination between a CRF19 virus containing multiple transmitted drug resistance mutations and a superinfecting subtype B virus without drug resistance mutations.
HIV-1 superinfection occurs despite the preexisting host immune response to the initial virus.1 Rates of superinfection have been estimated to be close to rates of initial infection, indicating a lack of protective immunity against newly acquired HIV-1 infection by preexisting infection. However, superinfection may be difficult to detect when the superinfecting virus is of the same subtype as the initial virus, and the detection of possible consequent recombinants is restricted to a handful of reports.2–6
It has previously been reported that superinfection with a virus of different subtype can result in replacement of the original virus.2,7–9 Fang et al.4 showed replacement of subtype A virus with AC, but did not show the presence of “pure” subtype C virus, so the superinfection could have been with an AC recombinant. Others have reported dual infection10 or superinfection3,5,6 with the same subtype followed by recombination. Koelsch et al.11 described complete replacement of drug-resistant virus with superinfecting wild-type virus, while Pingen et al.12 described complete replacement of wild-type virus with drug-resistant virus, but recombination was not detected in these reports.
Here, we report an examination of HIV-1 superinfection using single genome analysis. Our data show almost complete replacement in blood plasma of the initial transmitted drug-resistant strain by recombinants of the initial and secondary infecting HIV-1 variants. To our knowledge, this is the first study to examine HIV-1 recombination over time after superinfection in such detail using single genome analysis.
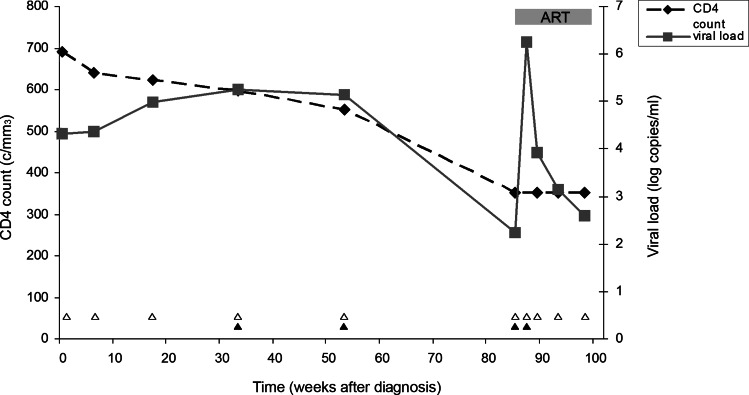
As part of a study examining the persistence of transmitted drug resistance-associated mutations, we performed population sequencing of HIV-1 protease (PR) and reverse transcriptase (RT) regions from longitudinal blood plasma samples and a seminal sample from a 28-year-old male who has sex with men (MSM). The patient was determined to have been recently infected with HIV-1 (<6 months) by serological incidence testing.13 The viral load and CD4 counts over the period of study are shown in Fig. 1. Antiretroviral treatment (darunavir, ritonavir, and emtricitabine) was started at 87 weeks postdiagnosis. Sequencing was performed in both directions using overlapping internal primers.14 Sequences (bases 2269–3509 in HXB2) were analyzed using Sequencher software (Gene Codes) with a minority population cut-off of 25%. Drug resistance mutations were defined using the Stanford University HIV drug resistance database.
FIG. 1.
Longitudinal analysis of CD4 T cell count and HIV-1 viral load of patient under study. CD4 T cell count (cells/mm3) is indicated with black diamonds (left y-axis) and viral load (log10 copies/ml) is indicated with gray squares (right y-axis). The patient started antiretroviral therapy after the sample at 87 weeks postdiagnosis was taken as indicated with a gray bar. Time points for which population-based sequencing has been performed are indicated with open triangles and time points used for single genome analysis are indicated with closed triangles.
At diagnosis, the blood plasma sample of this patient showed the presence of numerous drug resistance mutations in RT, namely D67N, K70R, K101E, Y181C, G190A, T215L, and K219E, indicating high level transmitted drug resistance to nucleoside and nonnucleoside reverse transcriptase inhibitors (Table 1). Three further blood plasma samples taken 6, 18, and 34 weeks postdiagnosis showed a similar pattern of resistance mutations. In a blood plasma sample taken 54 weeks after diagnosis the same resistance mutations could be detected, but many mixed peaks were also observed in the sequence chromatograms. Further samples, taken 85 to 98 weeks after diagnosis, showed no major resistance mutations except for one sample showing Y181Y/C 93 weeks postdiagnosis (see Table 1).
Table 1.
Drug Resistance Profiles and Subtypes Observed in Longitudinal Samples by Population-Based Sequencing
| Sample type | Time postdiagnosis (weeks)a | RT drug resistance mutations |
|---|---|---|
| Blood plasma | 1 | D67N, K70R, K101E, Y181C, G190A, T215L, K219E |
| 6 | D67N, K70R, K101E, Y181C, G190A, T215L, K219E | |
| 18 | D67N, K70R, K101E, Y181C, G190A, T215L, K219E | |
| 34 | D67N, K101E, Y181C, G190A, T215L, K219E | |
| 54 | Mix at multiple codons | |
| 85 | None | |
| 87 | None | |
| 89 | None | |
| 94 | Y181Y/C | |
| 98 | None | |
| Seminal plasma | 87 | None |
Antiretroviral treatment was started after the samples at 87 weeks postdiagnosis were taken. Population-based sequencing results of HIV-1 pol from blood plasma RNA and from one seminal RNA sample are shown.
Subtype analysis using the SCUEAL15 algorithm and phylogenetic analysis using the neighbor-joining method in MEGA5 Software16 that included HIV-1 reference sequences from the Los Alamos HIV Sequence Database (www.hiv.lanl.gov/) identified the population sequences prior to 54 weeks postdiagnosis as the circulating recombinant form 19 (CRF19). In CRF19, the region of the HIV-1 pol gene we sequenced with our population sequencing protocol (bases 2269–3509 in HXB2 numbering) is derived from subtype D.17 However, at later time points (from 85 weeks postdiagnosis onward) subtype analysis identified the plasma-derived population sequences as either subtype B or recombinant variants of B and D subtypes. The seminal plasma sample from this patient taken at 87 weeks postdiagnosis showed subtype B virus without drug resistance mutations by population sequencing.
The heterogeneous PR and RT population sequence detected in blood plasma at 54 weeks postdiagnosis indicates the presence of evolutionary distant HIV-1 variants in the patient's plasma. Moreover, the detection of differing HIV-1 subtypes in plasma before and after this “mix” was detected points to HIV-1 superinfection with a different subtype. To investigate this further and examine the evolutionary dynamics of the disappearance of transmitted drug resistance mutations detected in this patient, we performed single genome sequencing on blood plasma samples taken at 34, 54, 85, and 87 weeks postdiagnosis. Viral RNA was reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen) with primer 3560- (5′-TGGCTCTTGATAAATTTGATATGTCC). Before polymerase chain reaction (PCR) amplification with Platinum PCR SuperMix (Invitrogen), the cDNA was serially diluted in 5 mM Tris–HCl, pH 8.0, and only PCR products from the dilution yielding 30% or less of positive PCR reactions were sequenced. First round PCR was performed using primers 1849+ (5′-GATGACAGCATGTCAGGGAG) and 3560-; nested PCR was performed using primers 1870+ (5′-GAGTTTTGGCTGAGGCAATGAG) and 3501- (5′-GCTATTAAGTCTTTTGATGGGTCATA). Sequencing of PCR products was performed in forward and reverse directions and sequences (bases 1893–3408 in HXB2) were analyzed using Sequencher software (Gene Codes). Any sequences containing double peaks in the chromatographs were excluded. As the last two available samples before the patient started antiretroviral therapy were less than 2 weeks apart at 85 and 87 weeks postdiagnosis, we have combined the results of these two samples and, for the benefit of simplicity, refer to these data as “85–87 weeks postdiagnosis.”
Table 2 summarizes the single genome sequencing results. As expected, at 34 weeks postdiagnosis, the closest time point available before the detection of the mixed population in plasma, a relatively homogeneous population of virus variants was detected. All 25 single genome sequences were CRF19 and all contained the following NRTI and NNRTI drug resistance mutations: D67N, K101E, Y181C, G190A, T215L, and K219E, with the exception of a single genome that additionally contained K70R.
Table 2.
Drug Resistance Profiles and Subtypes of Longitudinal Single Genome Sequences
| Time postdiagnosis (weeks) | No. of sequences/total no. of sequences at time point (%) | Subtypea | RT resistance mutations |
|---|---|---|---|
| 34 | 24/25 (96%) | CRF19 | D67N, K101E, Y181C, G190A, T215L, K219E |
| 1/25 (4%) | CRF19 | D67N, K70R, K101E, Y181C, G190A, T215L, K219E | |
| 54 | 18/25 (72%) | CRF19 | D67N, K101E, Y181C, G190A, T215L, K219E |
| 1/25 (4%) | B | None | |
| 3/25 (12%) | CRF19/B | None | |
| 1/25 (4%) | CRF19/B/CRF19 | None | |
| 1/25 (4%) | B/CRF19 | D67N, K101E, Y181C, G190A, T215L, K219E | |
| 1/25 (4%) | CRF19/B | D67N, K101E | |
| 85 and 87b | 34/43 (79%) | CRF19/B | None |
| 2/43 (5%) | B | None | |
| 3/43 (7%) | B/CRF19/B | None | |
| 1/43 (2%) | CRF19/B/CRF19/B | None | |
| 1/43 (2%) | CRF19/B | D67N, K101E, Y181C, G190A | |
| 2/43 (5%) | CRF19 | D67N, K101E, Y181C, G190A, T215L, K219E | |
| 87 seminal plasma | 18/21 (86%) | B | None |
| 3/21 (14%) | CRF19/B | None |
HIV-1 subtypes were defined using SimPlot.
The results from blood plasma samples from weeks 85 and 87 postdiagnosis were combined, as the time points were less than 2 weeks apart.
In contrast, the plasma RNA sample at 54 weeks postdiagnosis showed a considerable mix of viruses. Most (18/25, 72%) were CRF19 variants similar to those found at the preceding time point. However, one subtype B variant (1/25, 4%) and six recombinants of the two subtypes (6/25, 24%) were also detected. The majority of genomes (20/25, 80%) still contained some drug resistance mutations at this time point. Eighteen of these 20 genomes with drug resistance mutations were CRF19, one was a CRF19/B recombinant with RT being CRF19, and the last was a CRF19/B recombinant showing drug resistance mutations only in the 5′ region of RT where the amplicon was CRF19 (D67N and K101E). Five of the 25 (20%) variants at 54 weeks showed no drug resistance mutations. One of these was subtype B, while the four remaining genomes without drug resistance mutations were recombinants between CRF19 and B with the N-terminal region of RT being subtype B.
By 85–87 weeks postdiagnosis, the majority of genomes detected (40/43, 93%) did not contain any drug resistance mutations. Of these, two were subtype B and 38 were CRF19/B recombinants with most of the RT region derived from subtype B. Only 3/43 (7%) sequences had resistance mutations. One was a CRF19/B recombinant with drug resistance mutations only up to G190A in RT where the amplicon was CRF19, and the remaining two were CRF19 variants with several drug resistance mutations (Table 2).
To examine a compartment relevant for sexual transmission of HIV-1, we also performed single genome sequencing on a seminal plasma sample collected at 87 weeks postdiagnosis. This showed that the majority of the sequences (18/21) in semen were subtype B with the remaining three sequences being CRF19/B recombinants. None of the seminal plasma single genome sequences contained any drug resistance mutations.
These data suggest that the patient was superinfected with a subtype B variant between 34 and 54 weeks postdiagnosis. Coincidentally, the patient was diagnosed with rectal Chlamydia infection at 36 weeks and urethral Gonococcus and Chlamydia infections at 46 weeks postdiagnosis, indicating risk behavior during this period. HIV-1 superinfection has previously been associated with a peak in viral load,1 but this has not always been reported and, indeed, in this case we do not detect a change in the viral load between 34 and 54 weeks (see Fig. 1). After superinfection, recombinants between the original multidrug-resistant CRF19 virus and the superinfecting subtype B virus gradually became the predominant species in the patient. To analyze this in detail, we determined the breakpoints of the mosaic viruses by SimPlot18 using a randomly selected CRF19 single genome sequence from time point 34 weeks and the subtype B sequence from time point 54 as parental sequences, HXB2 (accession no. K03455) as an outgroup sequence, and a 200 bp window and a 20 bp step size. The breakpoint areas were estimated from the crossover points in the similarity plots by maximizing the χ2 value using informative site array positions (Supplementary Table S1 and Fig. 1; Supplementary Data are available online at www.liebertpub.com/aid). As expected, control HIV-1 sequences from subtype B, CRF19, and subtype D variants showed no breakpoints using this method (data not shown).
The analysis showed that the six recombinant viruses present at 54 weeks postdiagnosis are potentially the result of five different recombination events (Fig. 2). Of the sequences at 85–87 weeks postdiagnosis, 35/43 (81%) were CRF19/B recombinants. The majority of these had a breakpoint upstream of the RT start codon: either between nucleotides 2363 and 2442, equivalent to codons 38–64 of PR (18/43, 42%), or between nucleotides 2487 and 2540, equivalent to codons 79–96 of PR (3/43, 7%). A total of 13/43 (30%) had a breakpoint immediately downstream of the RT start codon, similar to one of the recombinants detected at 54 weeks postdiagnosis. The last CRF19/B recombinant had a breakpoint further into RT between nucleotides 3119–3137 (equivalent to codons 190–196 of RT) and retained drug resistance mutations where the RT was derived from the parental CRF19 virus.
FIG. 2.
Breakpoint analyses of mosaic viruses. For each single genome sequence the region of the amplicon that is CRF19 (white), the region that is subtype B (dark gray), and the region within which the putative breakpoint lies (light gray) are depicted. Sequences with overlapping breakpoint regions are grouped together and the results of time points 85 and 87 weeks postdiagnosis are combined. The number of times each sequence variant is detected at that time point is indicated on the right of each bar representing the sequence variant. The RT start codon is indicated with a filled triangle and the black lines indicate drug resistance mutations defined using the Stanford University HIV drug resistance database. The dotted line indicates that not all grouped sequences contain the specified drug resistance mutation. The numbers above and below the bars indicate HXB2 nucleotide numbering.
Four of the sequences detected at 85 and 87 weeks postdiagnosis seemed to represent more complex recombinants: three were determined to be CRF19/B/CRF19 recombinants and one was a putative CRF19/B/CRF19/B recombinant, none of which contained drug resistance mutations. Our analysis also showed that all 25 single genomes detected at 34 weeks postdiagnosis were pure CRF19 in the sequenced region, as were 18/25 (72%) of the sequences at 54 weeks and 2/43 (5%) at 85–87 weeks postdiagnosis. However, 1/25 (4%) sequences at 54 weeks and 2/43 (5%) sequences at 85–87 weeks postdiagnosis were pure subtype B. Thus, these data show the selection of a limited number of CRF19/B recombinants without drug resistance mutations over time, predominantly with a breakpoint near the start codon of RT despite the detection of “pure” subtype viruses and the other rare complex recombinants.
Analysis of the single genome sequences from the seminal plasma sample taken at week 87 postdiagnosis showed three CRF19/B recombinant viruses (3/21, 14%) with breakpoints between nucleotides 2397 and 2408 (codons 48–52 of PR), 2436–2442 (codons 61–63 of PR), and 2553–2588 (codons 1–13 of RT), respectively. The first two CRF19/B recombinants could be grouped with the most common recombinant sequence found in blood plasma (breakpoint region 2363–2442), whereas the third had the second-most common breakpoint region (2553–2588) found in blood plasma. However, the majority of the single genome sequences detected in seminal plasma were pure subtype B (18/21, 86%), while no “pure” CRF19 sequences were detected.
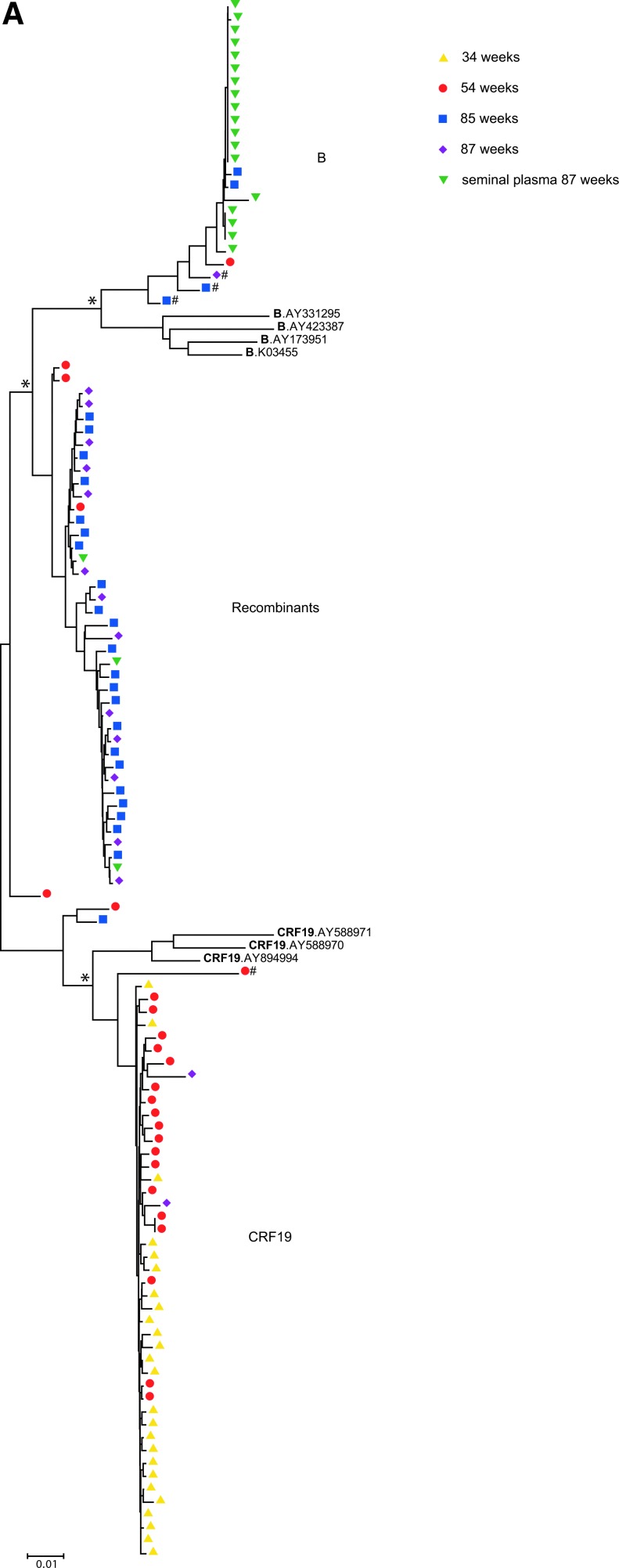
Next, we examined the relatedness of the viral sequences derived by single genome sequencing using phylogenetic analyses. Figure 3A shows a phylogenetic tree constructed using the neighbor-joining method and 1,000 bootstrap tests in MEGA5 Software16 including all the single genome sequences and subtype B and CRF19 reference sequences. In general, the analysis showed that the sequences segregated into three main clusters: the top and bottom clusters contained the “pure” subtype B and CRF19 sequences, respectively, whereas the middle cluster is composed of CRF19/B recombinant virus sequences. The CRF19 cluster contains all 25 sequences from the first time point tested (34 weeks postdiagnosis) as well as 19/25 sequences from the 54 week time point and 2/16 sequences from the 87 week time point. One of the sequences from 54 weeks in this cluster was identified as a CRF19/B recombinant by SimPlot with a breakpoint further upstream of the RT coding region (between nucleotides 2320 and 2374, equivalent to codons 22–41 of PR) and therefore contains only a small portion of subtype B and a large CRF19 region including the drug resistance mutations. Unsurprisingly, this sequence (indicated by “#” in Fig. 3A) grouped separately from the other SGS in the cluster exemplifying its marked divergence from the other CRF19 sequences.
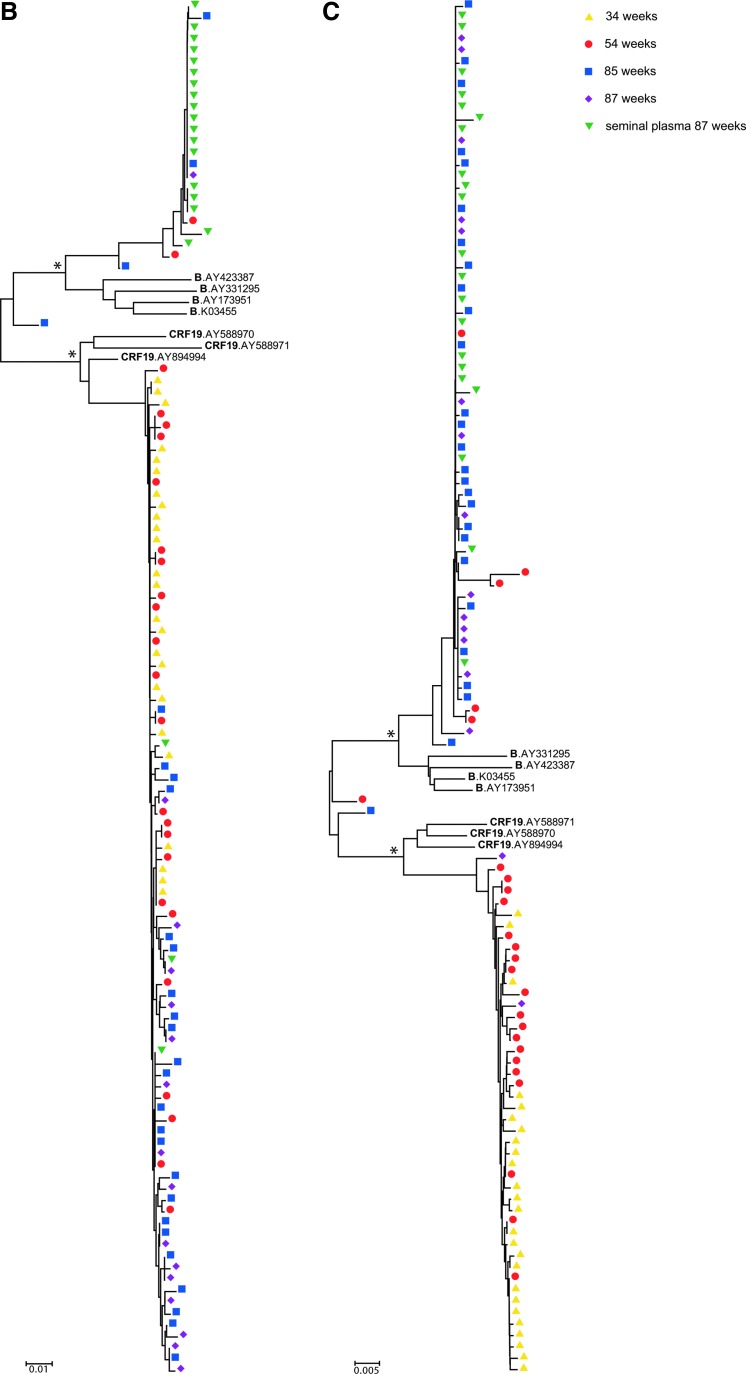
FIG. 3.
Neighbor-joining phylogenetic trees of pol single genome sequences. Neighbor-joining trees are shown of (A) all full-length single genome sequences—nucleotides 1893–3408, HXB2 numbering, (B) the 5′ end of all single genome sequences—nucleotides 1893–2390, and (C) the 3′ end of all single genome sequences—nucleotides 2590–3408. Depicted are single genome sequences of blood plasma RNA from 34 weeks (yellow triangles), 54 weeks (red circles), 85 weeks (blue squares), and 87 weeks (purple diamonds) postdiagnosis, and of seminal plasma RNA 87 weeks postdiagnosis (green upside down triangles). Hash tags (#) indicate sequences in CRF19 and subtype B clusters that were identified as CRF19/B recombinants by SimPlot (see the main text). Scale bars indicate genetic distance (amino acid substitutions per site). Asterisks (*) indicate that clustering of CRF19, subtype B, and recombinants is supported by bootstrap values of >75%. Color images available online at www.liebertpub.com/aid
The subtype B cluster is composed of the majority of sequences generated from the seminal plasma (18/21), the single subtype B blood plasma sequence from week 54 (1/25), four sequences from week 85 (4/27), and one sequence from week 87 (1/16). Two of the sequences from 85 weeks and the sequence of 87 weeks in this cluster (indicated by “#” in Fig. 3A) were identified as complex B/CRF19/B recombinants using SimPlot (Fig. 2 and Supplementary Table S1), illustrating that these recombination events are not supported by phylogenetic analysis. However, we cannot formally exclude that these three sequences are recombinant sequences as identified by SimPlot, but group in the subtype B cluster because the sequences contain only very small portions of CRF19.
The recombinant cluster contained the majority of sequences from weeks 85 and 87 postdiagnosis (33/43) as well as 4/25 sequences from week 54 and 3/21 of the sequences from seminal plasma.
Finally, since the majority of recombination events were estimated to have occurred between nucleotides 2390 and 2590 (HXB2 numbering), we divided the sequences into two regions, a 5′ region consisting of nucleotides 1890–2390 and a 3′ region consisting of nucleotides 2590–3408. Neighbor-joining phylogenetic trees were then generated for each region (Figs. 3B and 3C). As expected, the sequences in both phylogenetic trees formed two main clusters composed of CRF19 and subtype B sequences, respectively. This is in agreement with the data showing that the mosaic viruses are CRF19 and subtype B recombinants and that most of the plasma viruses at later time points consist of CRF19 in the 5′ region and subtype B in the 3′ region of PR/RT.
To conclude, we report here the detailed examination of a case of HIV-1 superinfection with a subtype B virus after initial infection with a CRF19 virus containing multiple drug resistance mutations. Single genome amplification from longitudinal blood plasma samples enabled us to track the appearance of superinfection and recombinant virus variants over time. The primers used for single genome amplification were designed to detect all different HIV-1 subtypes and, though it cannot be completely excluded, we have no indication of preferential amplification of one subtype over another. We performed single genome amplification instead of molecular cloning of PCR products generated from “bulk” PCR to exclude the possibility of experimentally introduced recombination.
Taken together, our data indicate that the patient was first infected with a drug-resistant CRF19 virus and then superinfected with a drug-sensitive subtype B virus between weeks 34 and 54 postdiagnosis. Initially following superinfection, many different recombinants were observed with varying levels of drug resistance mutations, along with CRF19 and subtype B variants. Eventually, the virus population was almost completely replaced by drug-sensitive CRF19/B recombinants, with the recombination occurring around the reverse transcriptase start codon so that the CRF19 RT region containing drug resistance mutations is replaced by drug-sensitive subtype B RT. While the superinfecting subtype B virus never became the dominant species in blood plasma it was the dominant species in the seminal sample we tested. In addition, recombinant drug-sensitive viruses were detected in the seminal plasma, indicating the possibility for further transmission of these recombinant viruses.
We speculate that the presence of multiple drug resistance mutations in RT of the original CRF19 virus negatively influenced its viral fitness in the absence of therapy. But the low number of “pure” subtype B virus sequences detected in blood plasma and the selection of CRF19/B recombinant variants suggest that some factor separate from the drug resistance in pol of the original virus conferred replicative advantage in the patient over the superinfecting subtype B virus, possibly immune escape mutations. On the other hand, no CRF19 RT was detected that had lost drug resistance mutations, indicating reversion of these collective drug resistance mutations was not a preferred pathway.
A recent study in Kenya found that ∼30% of HIV-1-infected patients carried unique recombinant forms,19 indicating the potential for the emergence of new recombinants. Within the United Kingdom, subtype is still largely associated with risk group, in that most MSM are infected with subtype B and heterosexuals with non-B.20,21 This study gives an example of how these associations are starting to decline with the possible consequent emergence of new recombinant forms.
Sequence Data
Sequences are deposited in GenBank under the following accession numbers: JX628919–JX629043.
Supplementary Material
Acknowledgments
We are indebted to the patient included in this study, to Adriana Alvarez and Andros Gavriel for performing the population-based PR-RT genotypic drug resistance testing, Kerry Hobbs for specimen collection, and David Pao for contribution to the transmitted drug resistance study design. This study was funded by the Health Protection Agency and the National Institute for Health Research Centre for Health Protection Research (project number 107496). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Waters L. Smit E. HIV-1 superinfection. Curr Opin Infect Dis. 2012;25(1):42–50. doi: 10.1097/QCO.0b013e32834ef5af. [DOI] [PubMed] [Google Scholar]
- 2.Piantadosi A. Chohan B. Chohan V. McClelland RS. Overbaugh J. Chronic HIV-1 infection frequently fails to protect against superinfection. PLoS Pathog. 2007;3(11):e177. doi: 10.1371/journal.ppat.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Streeck H. Li B. Poon AF, et al. Immune-driven recombination and loss of control after HIV superinfection. J Exp Med. 2008;205(8):1789–1796. doi: 10.1084/jem.20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang G. Weiser B. Kuiken C, et al. Recombination following superinfection by HIV-1. AIDS. 2004;18(2):153–159. doi: 10.1097/00002030-200401230-00003. [DOI] [PubMed] [Google Scholar]
- 5.Pernas M. Casado C. Fuentes R. Perez-Elias MJ. Lopez-Galindez C. A dual superinfection and recombination within HIV-1 subtype B 12 years after primoinfection. J Acquir Immune Defic Syndr. 2006;42(1):12–18. doi: 10.1097/01.qai.0000214810.65292.73. [DOI] [PubMed] [Google Scholar]
- 6.Kozaczynska K. Cornelissen M. Reiss P. Zorgdrager F. van der Kuyl AC. HIV-1 sequence evolution in vivo after superinfection with three viral strains. Retrovirology. 2007;4:59. doi: 10.1186/1742-4690-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jost S. Bernard MC. Kaiser L, et al. A patient with HIV-1 superinfection. N Engl J Med. 2002;347(10):731–736. doi: 10.1056/NEJMoa020263. [DOI] [PubMed] [Google Scholar]
- 8.Ramos A. Hu DJ. Nguyen L, et al. Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J Virol. 2002;76(15):7444–7452. doi: 10.1128/JVI.76.15.7444-7452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chohan B. Lavreys L. Rainwater SM. Overbaugh J. Evidence for frequent reinfection with human immunodeficiency virus type 1 of a different subtype. J Virol. 2005;79(16):10701–10708. doi: 10.1128/JVI.79.16.10701-10708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jobes DV. Daoust M. Nguyen VT, et al. Longitudinal population analysis of dual infection with recombination in two strains of HIV type 1 subtype B in an individual from a Phase 3 HIV vaccine efficacy trial. AIDS Res Hum Retroviruses. 2006;22(10):968–978. doi: 10.1089/aid.2006.22.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koelsch KK. Smith DM. Little SJ, et al. Clade B HIV-1 superinfection with wild-type virus after primary infection with drug-resistant clade B virus. AIDS. 2003;17(7):F11–F16. doi: 10.1097/00002030-200305020-00001. [DOI] [PubMed] [Google Scholar]
- 12.Pingen M. Nouwen JL. Dinant S, et al. Therapy failure resulting from superinfection by a drug resistant HIV variant. Antivir Ther. 2012;17:1621–1625. doi: 10.3851/IMP2267. [DOI] [PubMed] [Google Scholar]
- 13.Kothe D. Byers RH. Caudill SP, et al. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. J Acquir Immune Defic Syndr. 2003;33(5):625–634. doi: 10.1097/00126334-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 14.Cane P. HIV drug resistance testing. Methods Mol Biol. 2011;665:123–132. doi: 10.1007/978-1-60761-817-1_8. [DOI] [PubMed] [Google Scholar]
- 15.Kosakovsky Pond SL. Posada D. Stawiski E, et al. An evolutionary model-based algorithm for accurate phylogenetic breakpoint mapping and subtype prediction in HIV-1. PLoS Comput Biol. 2009;5(11):e1000581. doi: 10.1371/journal.pcbi.1000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura K. Dudley J. Nei M. Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 17.Casado G. Thomson MM. Sierra M. Najera R. Identification of a novel HIV-1 circulating ADG intersubtype recombinant form (CRF19_cpx) in Cuba. J Acquir Immune Defic Syndr. 2005;40(5):532–537. doi: 10.1097/01.qai.0000186363.27587.c0. [DOI] [PubMed] [Google Scholar]
- 18.Lole KS. Bollinger RC. Paranjape RS, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73(1):152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hue S. Hassan AS. Nabwera H, et al. HIV type 1 in a rural coastal town in Kenya shows multiple introductions with many subtypes and much recombination. AIDS Res Hum Retroviruses. 2012;28(2):220–224. doi: 10.1089/aid.2011.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal I. Smith M. Tatt ID, et al. Evidence for onward transmission of HIV-1 non-B subtype strains in the United Kingdom. J Acquir Immune Defic Syndr. 2006;41(2):201–209. doi: 10.1097/01.qai.0000179430.34660.11. [DOI] [PubMed] [Google Scholar]
- 21.Fox J. Castro H. Kaye S, et al. Epidemiology of non-B clade forms of HIV-1 in men who have sex with men in the UK. AIDS. 2010;24(15):2397–2401. doi: 10.1097/QAD.0b013e32833cbb5b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.