Abstract
The AMP-activated protein kinase (AMPK) mediates rapid, stress-induced loss of the inhibitor of differentiation (Id)2 in blastocysts and trophoblast stem cells (TSC), and a lasting differentiation in TSC. However, it is not known if AMPK regulates other potency factors or regulates them before the blastocyst stage. The caudal-related homeodomain protein (Cdx)2 is a regulatory gene for determining TSC, the earliest placental lineage in the preimplantation mouse embryo, but is expressed in the oocyte and in early cleavage stage embryos before TSC arise. We assayed the expression of putative potency-maintaining phosphorylated Cdx2 ser60 in the oocyte, two-cell stage embryo, blastocyst, and in TSC. We studied the loss of Cdx2 phospho ser60 expression induced by hyperosmolar stress and its underlying mechanisms. Hyperosmolar stress caused rapid loss of nuclear Cdx2 phospho ser60 and Id2 in the two-cell stage embryo by 0.5 h. Stress-induced Cdx2 phospho ser60 and Id2 loss is reversed by the AMPK inhibitor compound C and is induced by the AMPK agonist 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide in the absence of stress. In the two-cell stage embryo and TSC hyperosmolar, stress caused AMPK-mediated loss of Cdx2 phospho ser60 as detected by immunofluorescence and immunoblot. We propose that AMPK may be the master regulatory enzyme for mediating stress-induced loss of potency as AMPK is also required for stress-induced loss of Id2 in blastocysts and TSC. Since AMPK mediates potency loss in embryos and stem cells it will be important to measure, test mechanisms for, and manage the AMPK function to optimize the stem cell and embryo quality in vitro and in vivo.
Introduction
Maintenance of potency is important in several medical protocols, including culturing and cryopreserving oocytes and embryos, and in isolating and maintaining stem cells. Early oocyte and embryo quality in vivo is also important in producing high-quality pre- and postnatal life. Yet, stress arises during all these processes and it is important to understand how oocytes, embryos, and stem cells sense and respond to stress and how this affects potency. Measurement and management of stress and stress responses are key to producing highly quality stem cells and embryos.
It was previously shown that the AMP-activated protein kinase (AMPK) mediates stress-induced and normal, hormone-induced oocyte maturation [1,2]. Within 4 days of fertilization of oocytes, stress leads to differentiation in blastocysts and trophoblast stem cells (TSC) derived from blastocysts [3,4]. We have previously shown that both hyperosmolar stress and genotoxic stress induce loss of the potency factor inhibitor of differentiation (Id)2 in TSC and blastocysts in an AMPK-dependent manner [5–9]. These data suggest that this mechanism may be shared by a broad spectrum of stresses. Id2 loss occurs normally and is required for normal differentiation of placental TSC. Thus, stress induces a normal mechanism of differentiation, but knockouts suggest that AMPK is not essential in a normal vivarium for this process [3,10]. Thus, stress enzymes like AMPK become important for the adaptation to higher levels of stress than those in a normal vivarium. Stress-induced Id2 protein loss is rapid in TSC and blastocysts, but persists for hours to days and results in differentiation of TSC [4,5,11]. Stress activates AMPK with similar kinetics in mouse blastocysts, TSC, and embryonic stem cells (ESC), suggesting stress initiates similar mechanisms in different stem cell types. This also suggests that isolated TSC and TSC in blastocysts respond similarly. In contrast to AMPK, the unrelated stress-activated protein kinase (SAPK) is activated slowly, persists for hours, and is necessary to mediate upregulation of the transcription factors required for differentiation of TSC [4,12–15]. However, SAPK does not regulate potency factors in TSC [15] or ESC in normal culture [16] or in TSC during stressed culture [5]. Thus, emerging data suggest that AMPK mediates stress-induced potency factor loss and SAPK mediates differentiation factor gain, but not potency factor loss. Together, the enzymes mediate stress-induced TSC differentiation.
Cdx2 is an essential transcription factor determining the placental lineage [17] whose zygotic expression at the eight-cell embryo stage (E2.5) is dependent on the transcription factor Transcriptional enhancer factor TEF-3 [encoded by the transcriptional enhancer factor domain family member (TEAD4) gene] [18]. In turn, the transcription factor Eomes is dependent on Cdx2 [17], transcription factor heart and neural crest derivatives (Hand1) are dependent on Eomes [17], and the first, postimplantation placental hormone placental lactogen-1 (PL1) is predominantly dependent on Hand1 [19]. Cdx2 acts to negatively regulate Oct4 in outside cells of the E3.5 blastocyst [20,21]. Cdx2 expression in the totipotent stages of development, oocytes and cleavage division embryos before the eight-cell stage is controversial. Cdx2 mRNA was not detected in the two-cell stage embryo in two reports [22,23]. More recently, maternal Cdx2 has been detected in the oocyte and before the eight-cell stage [24–26]. However, one of these reports that also detected the Cdx2 protein in oocytes and two-cell stage embryos [26] was retracted [27]. Thus, the expression and function of the Cdx2 mRNA and protein in the totipotent stages of mouse development has been controversial with no studies of the phosphorylated state of the Cdx2 protein.
After the eight-cell embryo stage, transcription factors become more pleiotropic. As stem cells restrict potency from totipotent to pluripotent (ESC) or multipotent (TSC), transcription factors protect potency and also prepare for differentiation. By early postimplantation, the E5.5 embryo expresses Cdx2 only in the TSC adjacent to the inner cell mass [17], the source of the fibroblast growth factor (FGF)4 necessary to maintain the cells in a proliferative, undifferentiated state [9,28]. This expression pattern is similar to the Id2 protein. In preparation for implantation, blastocysts express high levels of mRNA for Id2 and after implantation, greatly upregulate Hand1 [29] and downregulate Id2 and Cdx2 in differentiating cells. After implantation, Hand1 in mouse placental cells [19,30] activates the PL1 promoter resulting in detectable PL1 in maternal blood by E6.0 of gestation [31]. For Hand1 to induce PL1 in differentiated cells, the dominant negative transcription factor Id2 must be lost in placental cells [30]. A similar loss of Id2 is required to produce differentiation to an invasive phenotype in human early first trimester cytotrophoblasts [32]. Cdx2 is required to determine the trophoblast lineage, but its loss may be required for further differentiation of TSC to form the trophoblast giant cells that synthesize PL1. In support of this hypothesis, Hand1 is upregulated abnormally in blastocyst stage embryos after maternal and zygotic Cdx2 is knocked down at the zygote stage (24).
Cdx2 mediates potency in multipotent small intestine stem cells [33,34]. Whether phosphorylated at ser60 or not, Cdx2 enters the nucleus in intestine epithelial cells. A gene of terminal differentiation in small intestines is silenced in stem cells by Cdx2 phospho ser60 in the intestinal crypts. The same gene is activated by Cdx2 nonphospho ser60 in the villous epithelium. It is likely that nuclear Cdx2 phospho ser60 binds and silences promoters of genes of the differentiated state and this is a possible Cdx2 function in the preimplantation embryo.
Cdx2 mRNA is expressed at low levels in the meiotic (M)II oocyte through the eight-cell embryo stage, but by the blastocyst stage, Cdx2 mRNA increases 100-fold in outside polarized cells after compaction [24], blocks Oct4 transcription, upregulates itself, and thus establishes the trophoblast lineage [20,21]. Although intestinal epithelial stem cells and TSC are both considered to be multipotent, Cdx2 is only transiently needed to establish TSC lineage, but in intestinal stem cells remains as the stem cell differentiates.
There are two AMPK catalytic subunit genes (AMPKα1/α2) and knockout of either single gene has no gestational phenotype [35]. However, the double null has not been done, single AMPK null mutants have no peri-implantation defect in normal vivaria, and testing single null mutants for effects of gestational stress has not been done.
Aside from the AMPK dependence of stress-induced loss of Id2 in TSC and blastocysts [5], there are no other reports of stress-induced enzymatic control of transcription factors in stem cells in the preimplantation embryo. Is Id2 unique or does AMPK mediate stress-induced loss of other potency factors and are other pluripotent or multipotent stages of development? What are the roles of Cdx2 and its protein phosphorylation states in totipotent two-cell stage embryos and in TSC in the blastocyst? In this report, we perform analyses of stress-induced, AMPK-mediated regulation of loss of phosphorylated and nonphosphorylated Cdx2 ser60 and regulation of Id2 protein loss in totipotent two-cell stage embryos and multipotent TSC.
Materials and Methods
Reagents
Sorbitol, FGF4, and heparin were from Sigma Chemical Co. Dulbecco's minimum essential media/F-12, fetal bovine serum, and RPMI1640 were from Gibco. The potassium simplex optimization medium (KSOM) and KSOM+ amino acids (KSOMaa) were from Specialty Media. The primary antibodies for total Cdx2 were from Biogenex, Abgent, and Orbigen. Cdx2 total protein (C terminus), Cdx2 phospho ser60, and Cdx2 nonphospho ser60 were used as previously [34,36]. The AMPK inhibitors compound C and adenosine arabinoside agonist were from Calbiochem, respectively. The AMPK agonist 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide (AICAR) was from Cell Signaling.
Collection and culture conditions for mouse embryos
Standard techniques were used for obtaining mouse embryos [37]. Female MF-1 mice (4–5 weeks old; Harlan Sprague Dawley) were super-ovulated, and their embryos were obtained as described previously [38,39] or their unfertilized oocytes were collected 6 h after human chorionic gonadotropin (hCG) (germinal vesicle breakdown-GVDB-stage) or 15 h after hCG (meiosis or MII stage). Animal use protocols were approved by the Wayne State University Animal Investigation Committee. In all studies, embryos were equilibrated for at least 1 h in lowest stress KSOMaa media [38] and stressed with the reagent dose for the time period indicated. KSOMaa was 239 mOsmol, increasing 2.8-fold to 674 mOsmol with the addition of 400 mM sorbitol. For inhibitor studies (except where indicated), the inhibitors were preincubated with embryos 3 h before the stress and continued during the stress.
Cell lines and culture conditions
The mouse TSC line was from Dr. Rossant (Lunenfeld Research Institute). TSC were cultured as described previously [39–41]. TSC media is 298 mOsmol (data not shown), increasing 2.4-fold to 719 mOsmol at 400 mM sorbitol. Sorbitol at 200 mM added to TSC media increases osmolarity 1.7-fold to 498 mM. In the text, the level of sorbitol (w/v) added is used to produce the given molarity of sorbitol. For inhibitor studies, the inhibitors were preincubated with cells for 2 h before stress was added and during stress. The doses of the AMPK inhibitor compound C used in this study are 10 and 20 μM. TSC were preincubated with compound C for 2 h, and then cells were treated with 200 mM sorbitol in the continuing presence of compound C for 4 h. For the AICAR study, TSC were incubated with 0.5 mM AICAR for 0.5 h without sorbitol presence.
Western blot
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis were performed as previously described [39,42]. For oocytes and embryos, 150–200 were used per lane during SDS-PAGE. TSC were grown to 70%–80% confluency, stimulated with 50 ng/mL FGF4 for the times indicated, washed twice with ice-cold phosphate-buffered saline, and lysed with a cold cell lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM ethylene glycol tetraacetic acid, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycero-phosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, and 1 mM phenyl methylsulfonyl fluoride; Cell Signaling) plus Phosphatase Inhibitor Cocktail (PIC1; Sigma) and PIC2 (Sigma) for 20 min. Twenty microgram of whole-cell extracts was separated by electrophoresis on a 10% SDS-PAGE gel using a Hoefer Mighty Small II SE 250 apparatus, and then transferred to ECL Hybond nitrocellulose membranes (Amersham) at 15 V for 30 min using a Bio-Rad Semi-dry Transfer Cell. The membranes were blocked overnight with 5% nonfat milk in Tris-buffered saline-Tween 20 (TTBS) and blotted with the specified primary antibodies for 1 h, incubated in the horseradish peroxidase-conjugated secondary antibody for 1 h followed by extensive washing with TTBS. Primary and secondary antibodies were diluted in 1% nonfat milk/TTBS. The protein bands were visualized using the enhanced chemiluminescence assay system (Amersham).
Real-time quantitative polymerase chain reaction
Primer sequences for were used from PrimerBank Harvard [43]. Real-time polymerase chain reaction (PCR) was performed using SmartCycler Thermal Cycler (Cepheid) and carried out with QuantiTect SYBR Green PCR Master Mix (Qiagen), which contained the HotStarTaq DNA Polymerase, QuantiTect SyBR Green PCR Buffer, and SYBR Green I. The real-time PCR reaction mixture contained 1× QuantiTect SYBR Green PCR Master Mix, 0.3 μM primer pairs, and 1 μL cDNA in a total volume of 25 μL. Melting curves were done by incremental temperature increases of 1.0°C from 60°C to 90°C. These were repeated to ensure that primer-dimers and other nonspecific products had been eliminated.
Sequencing real-time quantitative polymerase chain reaction products
Quantitative polymerase chain reaction (qPCR) products were submitted to the DNA Sequencing Core at the Wayne State University, and were sequenced with the Applied Biosystems BigDye Terminator v3.1 Cycle Sequencing Kit. All sequencing reactions were done on either an Applied Biosystems 2720 Thermal Cycler or GeneAmp PCR System 9700 and purified with a Sephadex G-50 plate. The purified sequencing reactions were analyzed on an Applied Biosystems 3730 DNA Analyzer with Sequencing Analysis Software Version5.2. The DNA sequence data were compared with reference sequences of mouse Cdx2 from the National Center for Biotechnology Information Entrez Nucleotide database and were confirmed as mouse Cdx2.
Statistical analysis
Statistical significance was determined by using one-way analysis of variance (ANOVA). If ANOVA showed significant differences, least significant difference post hoc tests were used to analyze differences between significantly different paired groups that were normally distributed. Values are presented as means±standard error. Differences between treatments and/or groups were considered insignificant if P≥0.05 and significant if P<0.05. All experiments were repeated as least twice with similar results. Single representative experiments were shown. Calculations of IC50s of signal transduction enzyme inhibitors for biological function in TSC were done using Probit analysis to generate regression lines using SPSS software V10.0 (SPSS, Inc.).
Results
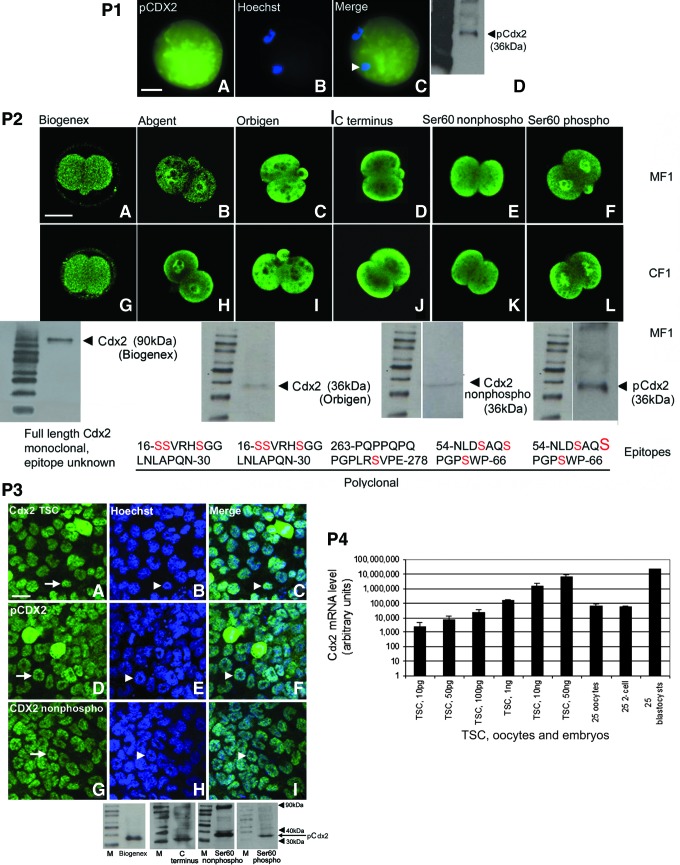
Since the Cdx2 protein was reported to be expressed in oocytes, we first tested oocytes for expression of Cdx2 phospho ser60 (Cdx2 phospho). Cdx2 phospho ser60 colocalized with Hoechst-stained DNA and was detected in the cytoplasm in the unfertilized germinal vesicle breakdown, and MII oocyte 6 and 15 h after induction of ovulation by hCG, respectively (Fig. 1, Part 1). An immunoblot of this stage oocyte produced a single band at 36 kDa as expected. We next tested for the presence of total Cdx2 protein and Cdx2 phospho and nonphospho ser60 in two-cell stage embryos. Ex vivo late two-cell stage embryos (E1.5) from outbred mice from a cross of Laboratory Animal Centre strain A and CS1 from Scientific Products farm (MF1) and Carworth farms mice (not Swiss-derived) (CF1) females mice expressed nuclear Cdx2 phospho ser60 in both nuclei, but Cdx2 nonphospho ser60 and total Cdx2 protein were cytoplasmic (Fig. 1, Part 2). Coincubation of an antibody with a 100-fold excess of the immunizing peptide completely ablated fluorescence, suggesting that Cdx2 epitopes are detected by immunofluorescence in embryos (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). Also, Cdx2 phospho and nonphospho ser60 were detected at 36 kDa by immunoblot of blastocysts using antibodies, but not with no-antibody controls. Since the Biogenex antibody produced only a 90-kDa band in two-cell stage embryos, its use at this stage, or at any stage without validation by immunoblot, is questionable.
FIG. 1.
The caudal-related homeobox protein 2 (Cdx2) protein is detected in oocytes, two-cell stage embryos, blastocysts, and trophoblast stem cells (TSC), and Cdx2 phospho ser60 is detected in the nuclei of both blastomeres in the two-cell stage embryo and all trophoblast nuclei in blastocysts and TSC. Cdx2 mRNA is detected throughout these developmental stages, but at similar levels in oocytes and two-cell stage embryos and >100-fold higher in blastocysts. Part 1: The Cdx2 phospho ser60 protein (pCdx2 in the figure) is expressed in the cytoplasm and nuclear DNA (arrowhead) of an oocyte. Oocytes were flushed from the oviduct 15 h after human chorionic gonadotropin (hCG) (A–C) fixed and stained for Cdx2 phospho ser60 protein, Hoechst stain (B), or (C) merge, or oocytes were lysed and tested using western blot (D). Bar in (A) shows 25 μM. Part 2: Late two-cell stage embryos from MF1 (outbred mice from a cross of Laboratory Animal Centre strain A and CS1 from Scientific Products farm) and CF1 [Carworth farms mice (not Swiss-derived)] mouse strains were collected at E1.5, fixed, and stained with six antibodies for Cdx2. At top is immunofluorescence adjusted to the same intensity to show location of Cdx2. In A–F six antibodies to Cdx2 were tested in 2-cell stage embryos from MF1 mice and in G–L the same antibodies were tested in 2-cell stage embryos from CF1 mice. Four antibodies were also tested using western blots, producing single bands at 36 kDa for three and a single band at 90 kDa for the fourth. The epitope of each antibody is also shown at bottom. Note there are no tyrosine or threonine residues that are possible alternate phosphorylation sites, but that there are other possible serine residues besides the one at position 60. The only overt phospho-specific antibody is indicated by a large S at the far right. Part 3: TSC were cultured, fixed, and stained with three antibodies for Cdx2. At top is immunofluorescence adjusted to the same intensity to show location of Cdx2. Four antibodies were also tested using immunoblots, and all detected bands at 36 kDa. Three antibodies were tested by using immunofluorescence, and all detected nuclear localization of all forms of Cdx2. Arrows in (A, D, G) show nuclear Cdx2 and arrowheads in (B, C, E, F, H, I) show nuclei. Bars in micrograph (A) in Parts 1, 2 and Part 3, show 25, 50, and 10 μm, respectively. Part 4: Cdx2 mRNA was tested using real-time quantitative polymerase chain reaction for a dilution series of TSC from 10 pg–50 ng, and 25 oocytes, 25 two-cell stage embryos, and 25 blastocysts assayed ex vivo. All biological experiments were repeated at least three times. Color images available online at www.liebertpub.com/scd
When micrographs were exposed to produce the same level of fluorescence intensity in two-cell stage embryos, the Biogenex antibody detected only the cytoplasmic protein, as did the commercial antibodies from Orbigen and two antibodies produced by us as reported previously [34,36]. Cdx2 phospho ser60 was detected in the nucleus of both blastomeres in the two-cell stage embryo and in all nuclei in the four-cell embryo (Fig. 1, Part 2, data not shown). Total Cdx2 (C terminus) antibody did not detect protein in the nucleus although Cdx2 phospho ser60 antibody did, suggesting that the C terminus antibody had less affinity and/or there was very little antigen in the nucleus. The Cdx2 nonphospho ser60 was detected in the cytoplasm in a pattern complementary pattern to Cdx2 phospho ser60 in the nucleus.
Commercial antibodies from Orbigen and Abgent were synthesized in rabbits in response to the same oligopeptide immunogen at amino acid resides from 16 to 30 using the carrier protein (same keyhole limpet hemocyanin), but produced different patterns of Cdx2 localization. The Abgent antibody detected nuclear Cdx2 stain similar to Cdx2 phospho ser60 and the Orbigen antibody produced a cytoplasmic Cdx2 stain similar to total Cdx2 protein detected by the C terminus antibody. The immunogen at amino acid residues from 16 to 30 had three serine, which might be phosphorylated, so it is possible that a dominant plasma cell clone in the Abgent (but not Orbigen) antiserum detected a serine phosphorylated at the same time as Ser60. However, the Abgent antibody is not characterized as a specific phospho-specific antibody, so we used the characterized Cdx2 phospho ser60-specific antibody here. By immunoblot, two-cell stage embryos expressed Cdx2 total protein (C terminus), Cdx2 phospho, and nonphospho ser60. All were detected at 36 kDa, validating the use of these antibodies. There were additional bands at 90 kDa for the Cdx2 C Terminus antibody and the nonphospho Ser60. We chose to use the Cdx2 nonphospho Ser60 as it gives definitive information on the absence of phosphorylation and the 90 kDa band was often quite minor. Whenever we used the C terminus antibody it was corroborated with the Orbigen antibody, which produced only a 36-kDa band. The six antibodies were studied using equivalent fluorescence intensity (Fig. 1, Part 2). The expression and function of Cdx2 is well established in TSC. In TSC, the three forms of Cdx2 phospho and nonphospho ser60, and total Cdx2 (C terminus) are all detected in the nucleus (Fig. 1, Part 3) and produce 36 kDa bands on immunoblots. This nuclear localization of total Cdx2 also occurs in the blastocyst, unlike the two-cell stage embryo where only Cdx2 phospho ser60 is nuclear.
Next, we assayed for Cdx2 mRNA using real-time PCR. On a logarithmic scale, 10 pg–50 ng of total RNA from TSC produced a reproducible standard curve with the relative amounts of Cdx2 from 25 oocytes or 25 twenty-cell embryos interpolated between 100 pg and 1 ng TSC RNA (Fig. 1, Part 4). Cdx2 mRNA from 25 blastocysts was expressed at about 400-fold greater amounts per embryo than two-cell stage embryos or oocytes. This high upregulation of Cdx2 mRNA from pre- to postcompaction embryo has been reported by others [24]. Interestingly, a large upregulation of Oct4 between oogenesis and pre-compaction to postcompaction embryo stages has also been reported [44]. The amplimers from the qPCR for two-cell stage embryos were sequenced and were mouse Cdx2 (see “Materials and Methods”).
Whether phosphorylated or not, Cdx2 enters the nucleus in the intestine and binds gene promoters [34]. A gene of terminal small intestinal differentiation is silenced in stem cells in the crypts by Cdx2 phospho ser60 and activated in the villous epithelium by Cdx2 nonphospho ser60. Thus, we tested whether stress induces the Cdx2 phospho ser60 loss that would be associated with differentiation.
We first tested for the regulation of loss of nuclear Cdx2 phospho ser60 in two-cell stage embryos. Cdx2 phospho ser60 was lost from the nucleus after 0.5 h of 400 mM sorbitol in an AMPK-dependent manner as the AMPK inhibitor compound C largely reversed the loss of nuclear Cdx2 (Fig. 2, Part 1). In addition, Cdx2 phospho ser60 loss from the nucleus was also caused by the AMPK agonist AICAR, suggesting that AMPK is necessary for stress-induced loss of Cdx2 phospho ser60 and sufficient for Cdx2 phospho ser60 loss without stress. Stress also induces the loss of Cdx2 phospho ser60 from trophectoderm in the E3.5 blastocysts (Fig. 2, Part 2).
FIG. 2.
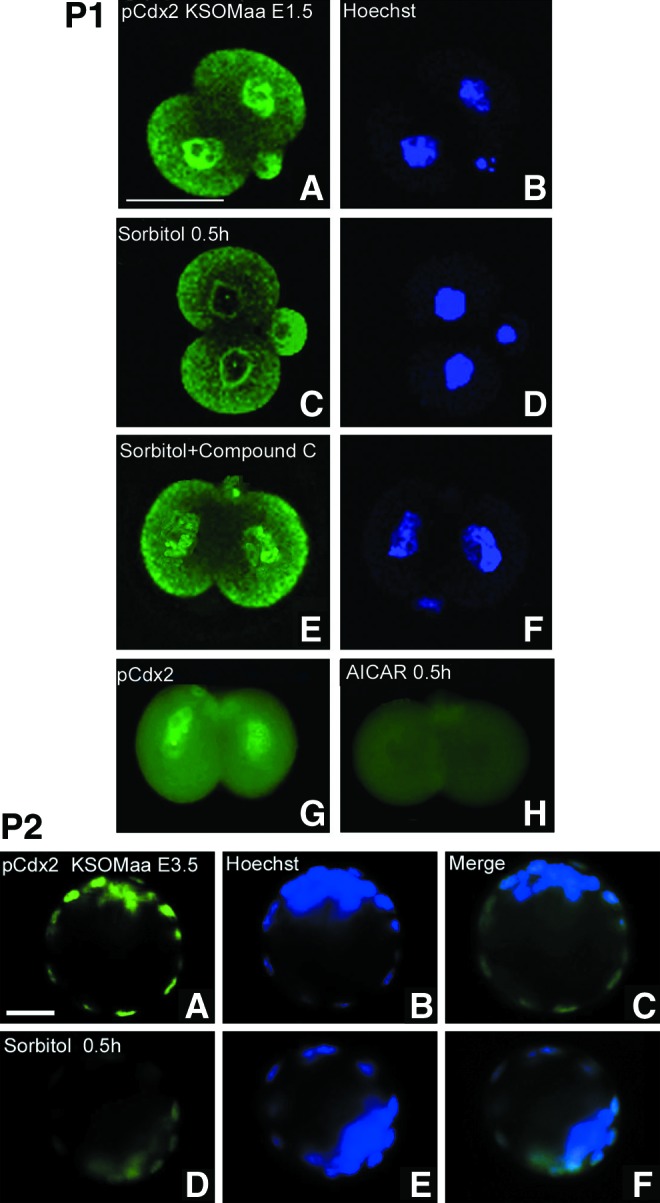
Hyperosmolar stress induced Cdx2 phospho ser60 protein loss from the nucleus after 0.5 h in E1.5 and E3.5 embryos and this was AMP-activated protein kinase (AMPK)-dependent (inhibitor sensitive) and AMPK sufficient [5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide (AICAR), AMPK agonist, without stress] in E1.5 embryos. Embryos were incubated for 0.5 h with/without 400 mM sorbitol, fixed, and stained for Cdx2 phospho ser60. Bar in (A) is 50 μM. Part 1: E1.5 embryos were cultured without stress (A, B), stressed for 0.5 h without (C, D) or with (E, F) compound C (an AMPK inhibitor) or without stress and with AICAR for 0.5 h (an AMPK agonist) (G, H). Part 2: E3.5 embryos were cultured without (A–C) or with stress (D–F) and stained for the Cdx2 phospho ser60 protein. Bar in (A) is 25 μM. Color images available online at www.liebertpub.com/scd
In preparation for implantation, blastocysts express mRNA for Id2 and greatly upregulate Hand1 [29]. For Hand1 to act, the dominant negative transcription factor Id2 must be lost [30] in mouse. Since benzopyrene and hyperosmolar stress cause Id2 loss in TSC [5,6], we tested for effects of stress on Id2 nuclear expression in two-cell stage embryos and blastocysts. Like Cdx2 phospho ser60, the Id2 protein was detected in both nuclei of the two-cell stage embryo and stress-induced loss was largely reversed by the AMPK inhibitor compound C (Fig. 3, Part 1). Also, like Cdx2 phospho ser60, Id2 protein loss is induced by the AMPK activator AICAR in the absence of stress. For both Cdx2 phospho ser60 and ID2 rapid loss occurs at 0.5 h whether by AMPK-mediated stress-induced loss or AICAR-induced loss in the absence of stress. We previously used immunoblots to show that AMPK activation kinetics, Id2 loss kinetics, and stress-induced Id2 loss is AMPK dependent in E3.5 blastocysts and TSC [5]. Here we show that stress-induced Id2 loss in TSC nuclei in blastocysts is rapid (Fig. 3, Part 2) as in two-cell stage embryos.
FIG. 3.
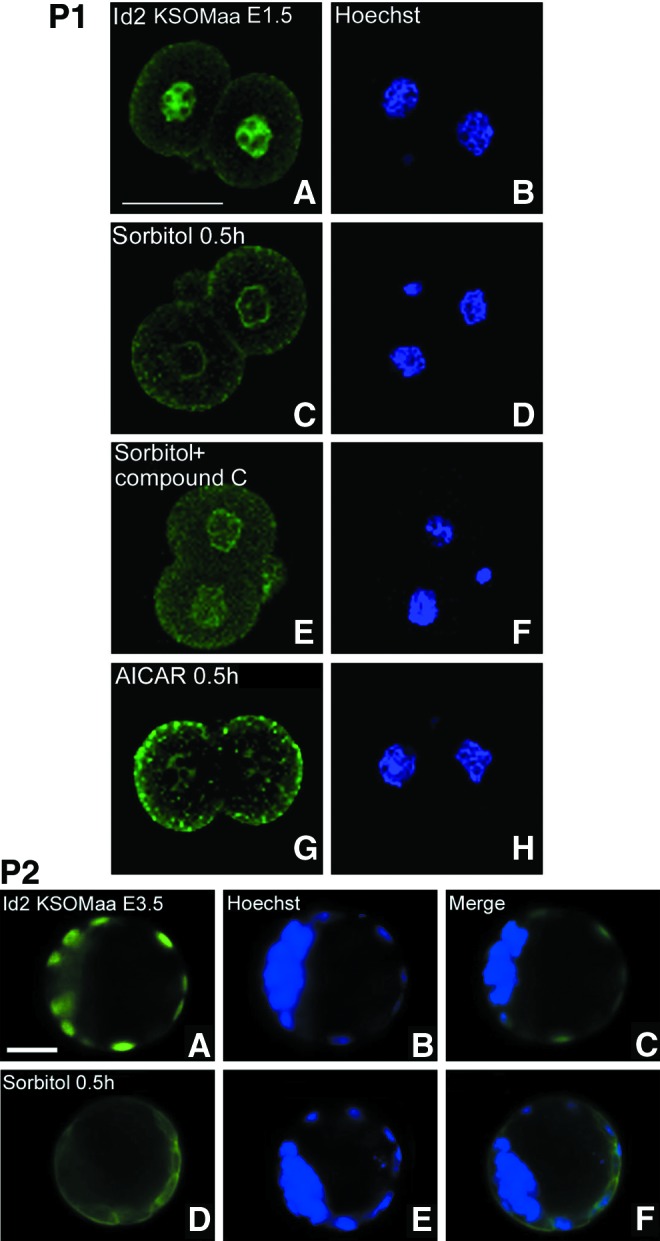
Hyperosmolar stress-induced inhibitor of differentiation 2 (Id2) protein loss from the nucleus after 0.5 h in E1.5 and. E3.5 embryos and this was AMPK-dependent (AMPK inhibitor sensitive) and AMPK-sufficient (AICAR, AMPK agonist, without stress) in E1.5 embryos. Embryos were incubated for 0.5 h with/without 400 mM sorbitol, fixed, and stained for phosphorylated Id2. Part 1: E1.5 embryos were cultured without stress (A, B), stressed for 0.5 h without (C, D) or with (E, F) compound C (an AMPK inhibitor) or without stress and with AICAR (an AMPK agonist) for 0.5 h (G, H). Bar in (A) is 50 μM. Part 2: E3.5 embryos were cultured without (A–C) or with stress (D–F) and stained for the Id2 protein. Bar in (A) is 25 μM. Color images available online at www.liebertpub.com/scd
To corroborate the AMPK-dependent and sufficient loss of Cdx2 phospho ser60 shown by immunofluorescence in two-cell stage embryos (Fig. 2, Part 1), we repeated these studies using immunoblots to confirm AMPK effects in TSC (Fig. 4, Part 1). As at the two-cell stage, stress-induced Cdx2 phospho ser60 loss is AMPK-dependent and in the absence of stress, AMPK-sufficient (AICAR sensitive). Significantly, stress-induced and AICAR-induced losses of the same magnitude and inhibition of AMPK by compound C completely prevents stress-induced Cdx2 phospho ser60 loss. As with blastocysts and two-cell stage embryos, nuclear Cdx2 phospho ser60 loss induced by hyperosmolar stress is rapid and complete by 0.5 h (Fig. 4, Part 2). Hyperosmolar stress induces loss of Cdx2 total protein (Fig. 4, Part 3) not just Cdx2 phospho ser60. Thus, in TSC, all forms of Cdx2 are in the nucleus and stress-induced loss of all forms is dependent on AMPK.
FIG. 4.
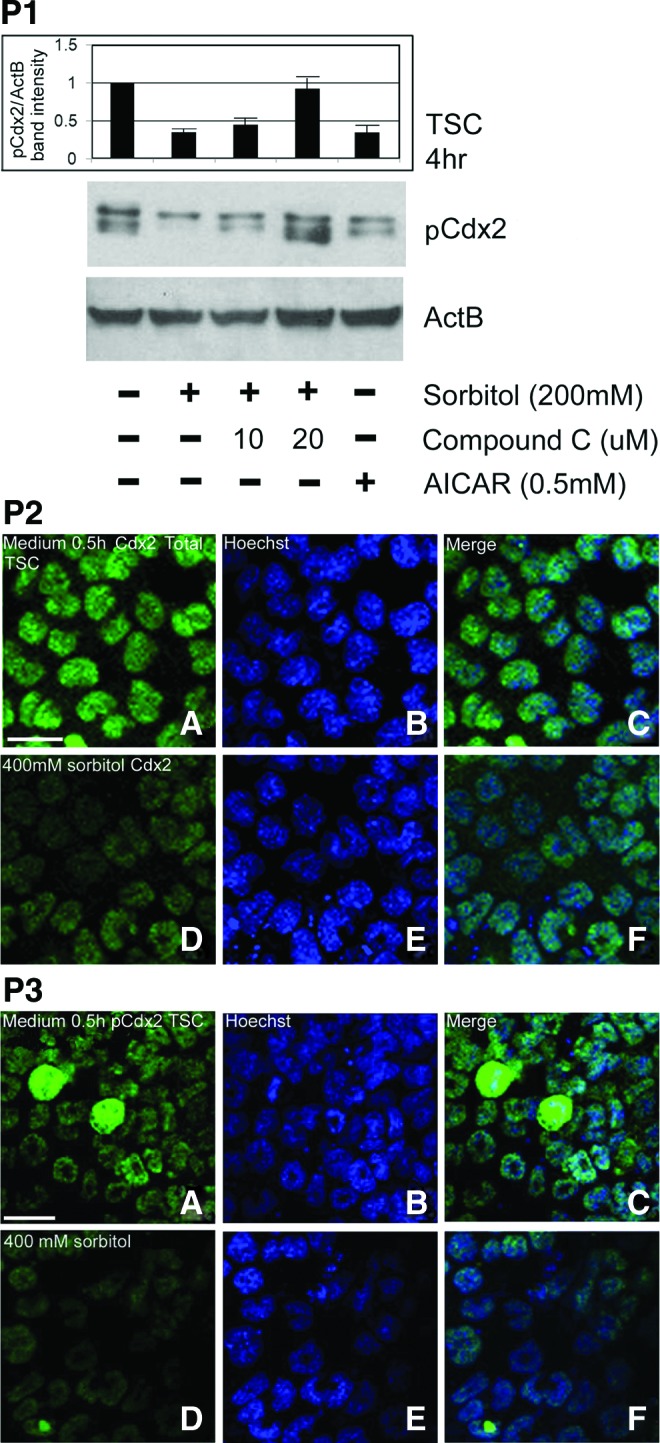
Cdx2 phospho ser60 in TSC undergoes hyperosmolar stress-induced, AMPK-dependent (AMPK antagonist sensitive) loss that is AMPK sufficient without stress (AMPK agonist sensitive). Part 1: TSC were stimulated with 200 mM sorbitol for 4 h with or without compound C, or with AICAR, lysed and the immunoblot stained for Cdx2 phospho ser60. Part 2: TSC were cultured without stress (A–C) or with 400 mM sorbitol for 0.5 h (D–F) stained using Cdx2 total (C terminus) antibody and immunofluorescence was detected. All experiments were repeated three times. Part 3: TSC were cultured without stress (A–C) or with 400 mM sorbitol for 0.5 h (D–F) stained using the Cdx2 phospho ser60 antibody and immunofluorescence was detected. All experiments were repeated three times. Color images available online at www.liebertpub.com/scd
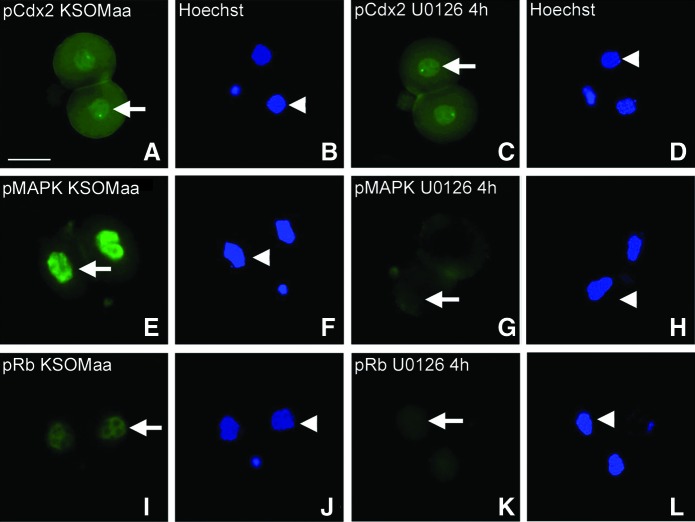
In small intestine Cdx2, phospho Ser60 is U0126 sensitive, indicating mitogen-activated protein kinase kinase (MEK) 1/2 phosphorylates Cdx2 in the nucleus of stem cells. We next tested whether nuclear phosphorylated Cdx2 phospho ser60 was U0126 sensitive. However, U0126 did not diminish nuclear Cdx2 phospho ser60 after 4 h (Fig. 5) or for other durations tested through 24 h (data not shown). It is likely that the Cdx2 protein turnover occurs within 24 h, so the lack of sensitivity to the inhibitor is not simply due to longevity of Cdx2 phospho ser60. To assure that U0126 inhibited a known function, we did find that it blocked phospho mitogen-activated protein kinase (MAPK) 1/3 Thr/Tyr 202/204 and retinoblastoma Ser795, which are known to be U0126 sensitive [45,46]. Thus, it appears that regulation of Cdx2 phosphorylation in two-cell stage embryos is not by MEK/MAPK signaling as it is in intestinal epithelial stem cells.
FIG. 5.
Unlike small intestine, phosphorylation of nuclear CDX2 phospho ser60 (pCDX2) is not mitogen-activated protein kinase kinase (MEK)1/2-dependent in two-cell stage embryos, although phosphorylated (p) mitogen-activated protein kinase (MAPK)1/3 and (p) retinoblastoma (Rb) are MEK1/2 dependent. Two-cell stage embryos were cultured with or without MEK1/2 inhibitor U0126 (1 μM) for 4 h, fixed, permeabilized, and stained for CDX2 phospho ser60 (A, C), phosphorylated (p)MAPK 1/3 Thr/Tyr 202/204 (E, G), and phosphorylated (p)Rb Ser795 (I, K). Hoechst-stained nuclei (B, D, F, H, J, L) complement antigen staining (A, C, E, G, I, K), respectively. Arrows show antigen staining and arrowheads show nuclear position. Color images available online at www.liebertpub.com/scd
Discussion
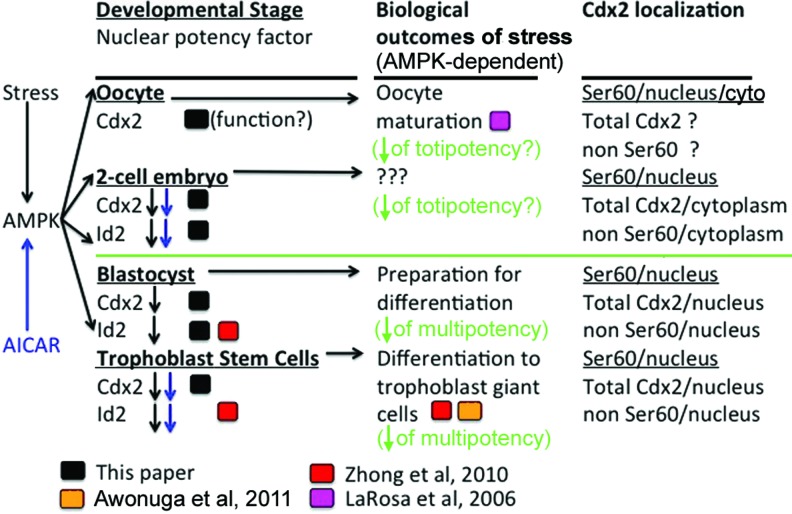
We have extended our past findings on stress-induced loss of transcription factors that can act as potency factors. AMPK is necessary for stress-induced loss of Cdx2 phospho ser60 and Id2 in totipotent two-cell stage embryos as well as multipotent TSC and is sufficient for their loss without stress (Fig. 6). Peak induction of stress-activated AMPK is rapid as it occurs by 15 min in TSC, ESC, and blastocysts [5]. This agrees with the rapid stress-induced loss of Cdx2 phospho ser60 and Id2 at all stem cell and embryo stages by 30 min observed here. Rapid stress-induced Id2 protein loss continues for hours to days and results in the differentiation of TSC [4,5], but this was not tested here. AMPK rapidly mediates potency factor loss and in contrast, SAPK mediates a slower, longer activation of transcription factors that mediate differentiation in response to hyperosmolar stress [4], hypoxic stress [14], and the genotoxic stress of benzopyrene [6]. The two stress enzymes are distinct as SAPK does not regulate potency factors in cultured ESC [16] or TSC [15] or in TSC in response to hyperosmolar or hypoxic stress [5,14]. Thus, AMPK is an important enzyme to diagnose stress and AMPK regulation may be used to prevent loss of potency in embryos that are stressed during in vitro fertilization (IVF)/assisted reproductive technology and during stress in vivo.
FIG. 6.
Summary diagram for stress-induced AMPK-dependent and -sufficient loss of Cdx2 phospho ser60 and Id2 in two-cell stage embryos, blastocysts, and TSC are shown in the two columns to the left. Cellular localization of Cdx2 phospho ser60, Ser60 nonphospho ser60, and total Cdx2 are shown in the column on the right. Putative functions mediated by stress-induced AMPK are shown in the column next to one on the right. Black boxes show data from this report, red boxes show data from Zhong et al. [5], orange box shows data from Awonuga et al. [4], and purple box shows data from LaRosa and Downs [2]. Blue arrows indicate outcomes induced by AICAR and black arrows indicate outcomes induced by stress in an AMPK-dependent manner. The green line separates the earlier oocyte and two-cell embryo stages of development that are totipotent from the cultured TSC and the TSC in the blastocysts that are multipotent. Color images available online at www.liebertpub.com/scd
Although potency loss is rapid in studies in TSC, continuing stress maintains low levels of the Id2 protein for 48 h and differentiation events blocked by Id2 are activated to high levels [4,11]. These results suggest that care be taken when using AMPK activators such as the drugs Metformin [47], or aspirin [48], or the nutritional source resveratrol [49], to treat women during pregnancy. Since benzopyrene also induces AMPK-dependent Id2 loss in TSC [6], an environmental exposure to cigarette smoke or diesel exhausts might additively increase Id2 loss induced by nutritional or medicinal sources. Finally, the common function of AMPK in regulating potency in diverse totipotent two-cell blastomeres and multipotent TSC in the blastocyst suggests this may be a common reporter of stress and predicts potency loss in many stem cell types.
This article is the first to use immunoblots to confirm expression of Cdx2 phospho ser60 in oocytes and Cdx2 nonphospho and phospho ser60 in two-cell stage embryos (Fig. 6) as well as in blastocyst-derived TSC. Cdx2 phospho ser60 could bind and putatively inactivate differentiation-mediating genes, thus mediating potency in all developmental stages tested. It is also the first demonstration that stress induces the rapid, AMPK-dependent loss of two different nuclear potency factors (Cdx2 phospho ser60 and Id2) from the nucleus at the two-cell stage embryo stage and in TSC. Finally, it is the first to identify epitopes of six different Cdx2 antibodies that have different cellular localization in two cell blastomeres.
We report that two-cell stage embryos (E1.5) from MF1 and CF1 female mice expressed nuclear Cdx2 phospho ser60, but Cdx2 nonphospho ser60 and total protein for Cdx2 are cytoplasmic. However, in blastocysts and TSC, all Cdx2 forms are detected in the nucleus. The significance of regulation of nuclear localization in embryos before compaction may be important in explaining Cdx2 function. In intestinal stem cells and their differentiated progeny, Cdx2 is always transported into the nucleus, but only in crypt stem cells is active MAPK transported to the nucleus where it phosphorylates and activates the silencing function of Cdx2 [33,34]. Transcriptionally inactive nuclear Cdx2 phospho ser60 was detected in the potent crypt intestinal stem cells, whereas Cdx2 nonphospho ser60 was detected in differentiated cells of the apical epithelium [34]. Thus, Cdx2 acts to silence differentiation-mediating genes when it is phosphorylated on ser60 and to activate these genes when it is not phosphorylated on ser60.
In contrast, Cdx2 phospho ser60 was detected in nuclei of potent cells from all developmental stages from oocytes to two-cell stage embryos, blastocysts, and TSC. However, only Cdx2 phospho ser60 was in totipotent blastomeres of the two-cell stage embryos, whereas total Cdx2 protein and Cdx2 nonphospho ser60 is detected in TSC and trophectodermal cells in the blastocyst. This suggests that unlike all intestinal stages, blastocysts and TSC, nuclear import is regulated at the two-cell stage. However, this regulation is not by MEK/MAPK and phosphorylation in the nucleus is also not MEK/MAPK-dependent as in crypt stem cells of the intestine. The biological significance of nuclear localization of all forms of Cdx2 in less potent blastocysts and TSC is not clear. However, phosphomimetic and nonphosphorylatable point mutant ser60 Cdx2 expression vectors are available to test for function [33,34].
In human and mouse ESC, polycomb and oct4 are colocalized in hundreds of promoters that would mediate differentiation to many lineages [50,51] if these promoters were active. We hypothesize that Cdx2 phospho ser60 mediates potency by suppressing promoters of differentiation-mediating genes. The gene-silencing role of Cdx2 phospho ser60 is established in intestinal stem cells and a similar role in two-cell blastomeres is consistent with the data reported here.
Although one report suggests maternal Cdx2 is required for compaction at the eight-cell stage [25], three other reports, using more effective knockout and knockdown techniques show that neither maternal not zygotic Cdx2 is required for compaction [17,24,52]. However, Cdx2 knockdown does severely limit the development of mitochondrial function in the eight-cell stage embryo [24], suggesting some functions for Cdx2 before its function in TSC lineage maintenance after the eight-cell stage.
Our ability to characterize the different locations of Cdx2 was due to the use of different antibodies. We found that the Biogenex antibody detected only the cytoplasmic protein in two-cell stage embryos, as did the commercial antibodies from Orbigen and two antibodies produced by us as reported previously [34,36]. However, the Biogenex antibody detected only a band of incorrect 90 kDa size in immunoblots of two-cell stage embryos. These data make use of this antibody problematic before the blastocyst stage. The Biogenex antibody was used in the oocyte and two-cell embryos, but only by immunofluorescence in an article that was later retracted [26]. However, the Biogenex antibody was also used more recently at the 8–12-cell stage when it is unclear if it detects a 90-kDa band or a 36-kDa band [24]. The Abgent antibody detected nuclear Cdx2 similar to the antibody for Cdx2 phospho ser60. The Orbigen antibody detected cytoplasmic Cdx2 similar to total Cdx2 protein detected by the C terminus antibody. By immunoblot, two-cell stage embryos expressed a Cdx2 at 36 kDa as previously reported. Interestingly, the Biogenex monoclonal antibody routinely used in several reports [17,21,26] produced a very weak immunofluorescence in two-cell stage embryos, even though the exposures were five to tenfold longer than used for the other five antibodies. Of the six antibodies, the Biogenex monoclonal is the only one with an unknown epitope. Also, one commercial antibody detects the nuclear Cdx2 phospho ser60, which silences differentiation-mediated genes (Abgent). Another commercial antibody detects the Cdx2 nonphospho ser60 (Orbigen), which activates differentiation-mediating genes. These associated functions should be considered when planning experiments and interpreting results.
Two previous reports detected no Cdx2 mRNA at the two-cell stage [22,23]. However, two more recent reports have shown that Cdx2 mRNA is in precompaction embryos [24,25]. Like these more recent reports, we found that Cdx2 mRNA from blastocysts was expressed at about 400-fold greater amounts per embryo than the two-cell stage or oocyte. Our findings do not agree with two previous reports [22,23], which may be due to lack of expression in other mouse strains at the two-cell stage, or of a false-negative due to lack of characterization of the sensitivity of the reverse transcription-polymerase chain reaction used for Cdx2. It is well accepted that the function of Cdx2 after compaction is to maintain late blastocyst formation, support blastocyst hatching and implantation, but the function of Cdx2 before compaction is still unclear.
An earlier and more severe phenotype (than the Cdx2 knockout) was observed in embryos where maternal and zygotic Cdx2 was depleted before the compaction stage. Deletion of Cdx2 before compaction caused not only the failure of late blastocyst formation and maintenance, but also failure of cellular polarization, compaction, and trophectoderm specification [24,25]. These reports confirmed the presence of Cdx2 before the eight-cell stage of the embryo and suggested different functions of Cdx2 before compaction.
In the totipotent cells of the two-cell stage embryo, only the putative differentiation-silencing Cdx2 phospho ser60 form is detected in the nucleus (Fig. 6). However, in the multipotent TSC, Cdx2 phospho ser60, Cdx2 nonphospho ser60, and Cdx2 C terminus are all in the nucleus. If these different forms of the Cdx2 protein have the different functions in moving from stemness to differentiation that they have in intestinal cells (which are also multipotent), it suggests that the multipotent TSC are in a dynamic equilibrium of Cdx2 function where some tendency to differentiate is allowed that may not be allowed in the totipotent two-cell stage embryo. Stress would increase the tendency to differentiate if it increased Cdx2 phospho Ser60 loss more than Cdx2 nonphospho Ser60 loss, at least initially.
Unlike intestinal stem cells, Cdx2 does not remain in TSC after they differentiate, but is needed to induce Eomes, Eomes-dependent Hand1, and Hand1-dependent PL1, the first differentiated product of TSC after the blastocyst implants [3,4]. It will be important to test whether the stress-induced loss of Cdx2 phospho ser60 is more rapid than the loss of Cdx2 nonphospo ser60 in blastocysts. Hypothetically, a transient increase in Cdx2 nonphospho in the absence of Cdx2 phospho ser60 should increase Eomes before the loss of Cdx2 during terminal differentiation.
Future studies are needed to understand the role of Cdx2 phospho ser60 during normal and stressed development of the oocyte and two- to eight-cell stage embryos. The roles of AMPK in regulating in normal and stressed early development also need testing. Functions of Id2 and Cdx2 phospho ser60 before the eight-cell stage are not clear, but the results here imply that both factors block differentiation. It is important to test whether stress-induced reduction of Id2 and Cdx2 phospho ser60 leads to loss of these factors on promoters of differentiated lineage markers that may be suppressed by them. It is likely that experimental regulation of the AMPK activity will improve IVF techniques such as cryopreservation and culture and also isolation and maintenance of highly potent ESC and TSC derived from eight-cell stage embryo blastomeres.
Supplementary Material
Acknowledgments
We thank Mike Kruger for advice on statistical analysis, Dr. Todd Leff for comments on the manuscript, and Drs. Mike Diamond and Ghassan Saed for help with qPCR and comments on the manuscript. This research was supported by a grant from the National Institute of Child Health and Human Development, NIH (R03HD061431, R01HD40972).
Author Disclosure Statement
No author has commercial or other interests that create conflict with the data presented and analyzed here.
References
- 1.Chen J. Downs SM. AMP-activated protein kinase is involved in hormone-induced mouse oocyte meiotic maturation in vitro. Dev Biol. 2008;313:47–57. doi: 10.1016/j.ydbio.2007.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaRosa C. Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006;74:585–592. doi: 10.1095/biolreprod.105.046524. [DOI] [PubMed] [Google Scholar]
- 3.Xie Y. Awonuga AO. Zhou S. Puscheck EE. Rappolee DA. Interpreting the stress response of early mammalian embryos and their stem cells. Int Rev Cell Mol Biol. 2011;287:43–95. doi: 10.1016/B978-0-12-386043-9.00002-5. [DOI] [PubMed] [Google Scholar]
- 4.Awonuga AO. Zhong W. Abdallah ME. Slater JA. Zhou SC. Xie YF. Puscheck EE. Rappolee DA. Eomesodermin, HAND1, and CSH1 proteins are induced by cellular stress in a stress-activated protein kinase-dependent manner. Mol Reprod Dev. 2011;78:519–528. doi: 10.1002/mrd.21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong W. Xie Y. Abdallah M. Awonuga AO. Slater JA. Sipahi L. Puscheck EE. Rappolee DA. Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction. 2010;140:921–930. doi: 10.1530/REP-10-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y. Abdallah ME. Awonuga AO. Slater JA. Puscheck EE. Rappolee DA. Benzo(a)pyrene causes PRKAA1/2-dependent ID2 loss in trophoblast stem cells. Mol Reprod Dev. 2010;77:533–539. doi: 10.1002/mrd.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rappolee DA. Awonuga AO. Puscheck EE. Zhou S. Xie Y. Benzopyrene and experimental stressors cause compensatory differentiation in placental trophoblast stem cells. Syst Biol Reprod Med. 2010;56:168–183. doi: 10.3109/19396360903431638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rappolee DA. It's not just baby's babble/Babel: recent progress in understanding the language of early mammalian development: a minireview. Mol Reprod Dev. 1999;52:234–240. doi: 10.1002/(SICI)1098-2795(199902)52:2<234::AID-MRD15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 9.Chai N. Patel Y. Jacobson K. McMahon J. McMahon A. Rappolee DA. FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Dev Biol. 1998;198:105–115. doi: 10.1006/dbio.1997.8858. [DOI] [PubMed] [Google Scholar]
- 10.Rappolee D. Xie Y. Zhou S. Puscheck E. Stress responses at the endometrial-placental interface suppress labyrinthine placental differentiation from trophoblast stem cells. Reproduction (in press) 2012 doi: 10.1530/REP-12-0240. [DOI] [PubMed] [Google Scholar]
- 11.Liu J. Xu W. Sun T. Wang F. Puscheck E. Brigstock D. Wang QT. Davis R. Rappolee DA. Hyperosmolar stress induces global mRNA responses in placental trophoblast stem cells that emulate early post-implantation differentiation. Placenta. 2009;30:66–73. doi: 10.1016/j.placenta.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong W. Xie Y. Wang Y. Lewis J. Trostinskaia A. Wang F. Puscheck EE. Rappolee DA. Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci. 2007;14:534–547. doi: 10.1177/1933719107307182. [DOI] [PubMed] [Google Scholar]
- 13.Xie Y. Zhong W. Wang Y. Trostinskaia A. Wang F. Puscheck EE. Rappolee DA. Using hyperosmolar stress to measure biologic and stress-activated protein kinase responses in preimplantation embryos. Mol Hum Reprod. 2007;13:473–481. doi: 10.1093/molehr/gam027. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S. Xie Y. Puscheck EE. Rappolee DA. Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta. 2011;32:475–481. doi: 10.1016/j.placenta.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abell AN. Granger DA. Johnson NL. Vincent-Jordan N. Dibble CF. Johnson GL. Trophoblast stem cell maintenance by fibroblast growth factor 4 requires MEKK4 activation of Jun N-terminal kinase. Mol Cell Biol. 2009;29:2748–2761. doi: 10.1128/MCB.01391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu P. Davis RJ. c-Jun NH2-terminal kinase is required for lineage-specific differentiation but not stem cell self-renewal. Mol Cell Biol. 2010;30:1329–1340. doi: 10.1128/MCB.00795-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strumpf D. Mao CA. Yamanaka Y. Ralston A. Chawengsaksophak K. Beck F. Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 18.Yagi R. Kohn MJ. Karavanova I. Kaneko KJ. Vullhorst D. Depamphilis ML. Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134:3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- 19.Riley P. Anson-Cartwright L. Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- 20.Wang K. Sengupta S. Magnani L. Wilson CA. Henry RW. Knott JG. Brg1 is required for Cdx2-mediated repression of Oct4 expression in mouse blastocysts. PLoS One. 2010;5:e10622. doi: 10.1371/journal.pone.0010622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwa H. Toyooka Y. Shimosato D. Strumpf D. Takahashi K. Yagi R. Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Meissner A. Jaenisch R. Generation of nuclear transfer-derived pluripotent ES cells from cloned Cdx2-deficient blastocysts. Nature. 2006;439:212–215. doi: 10.1038/nature04257. [DOI] [PubMed] [Google Scholar]
- 23.Wang H. Xie H. Guo Y. Zhang H. Takahashi T. Kingsley PJ. Marnett LJ. Das SK. Cravatt BF. Dey SK. Fatty acid amide hydrolase deficiency limits early pregnancy events. J Clin Invest. 2006;116:2122–2131. doi: 10.1172/JCI28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu G. Gentile L. Fuchikami T. Sutter J. Psathaki K. Esteves TC. Arauzo-Bravo MJ. Ortmeier C. Verberk G. Abe K. Scholer HR. Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2. Development. 2010;137:4159–4169. doi: 10.1242/dev.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jedrusik A. Bruce AW. Tan MH. Leong DE. Skamagki M. Yao M. Zernicka-Goetz M. Maternally and zygotically provided Cdx2 have novel and critical roles for early development of the mouse embryo. Dev Biol. 2010;344:66–78. doi: 10.1016/j.ydbio.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deb K. Sivaguru M. Yong HY. Roberts RM. Cdx2 gene expression and trophectoderm lineage specification in mouse embryos. Science. 2006;311:992–996. doi: 10.1126/science.1120925. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy D. Editorial expression of concern. Science. 2006;314:592. doi: 10.1126/science.314.5799.592b. [DOI] [PubMed] [Google Scholar]
- 28.Rappolee DA. Basilico C. Patel Y. Werb Z. Expression and function of FGF-4 in peri-implantation development in mouse embryos. Development. 1994;120:2259–2269. doi: 10.1242/dev.120.8.2259. [DOI] [PubMed] [Google Scholar]
- 29.Wang QT. Piotrowska K. Ciemerych MA. Milenkovic L. Scott MP. Davis RW. Zernicka-Goetz M. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell. 2004;6:133–144. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- 30.Cross JC. Flannery ML. Blanar MA. Steingrimsson E. Jenkins NA. Copeland NG. Rutter WJ. Werb Z. Hxt encodes a basic helix-loop-helix transcription factor that regulates trophoblast cell development. Development. 1995;121:2513–2523. doi: 10.1242/dev.121.8.2513. [DOI] [PubMed] [Google Scholar]
- 31.Ogren L. Southard JN. Colosi P. Linzer DI. Talamantes F. Mouse placental lactogen-I: RIA and gestational profile in maternal serum. Endocrinology. 1989;125:2253–2257. doi: 10.1210/endo-125-5-2253. [DOI] [PubMed] [Google Scholar]
- 32.Janatpour MJ. McMaster MT. Genbacev O. Zhou Y. Dong J. Cross JC. Israel MA. Fisher SJ. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development. 2000;127:549–558. doi: 10.1242/dev.127.3.549. [DOI] [PubMed] [Google Scholar]
- 33.Boudreau F. Rings EH. van Wering HM. Kim RK. Swain GP. Krasinski SD. Moffett J. Grand RJ. Suh ER. Traber PG. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem. 2002;277:31909–31917. doi: 10.1074/jbc.M204622200. [DOI] [PubMed] [Google Scholar]
- 34.Rings EH. Boudreau F. Taylor JK. Moffett J. Suh ER. Traber PG. Phosphorylation of the serine 60 residue within the Cdx2 activation domain mediates its transactivation capacity. Gastroenterology. 2001;121:1437–1450. doi: 10.1053/gast.2001.29618. [DOI] [PubMed] [Google Scholar]
- 35.Viollet B. Andreelli F. Jorgensen SB. Perrin C. Flamez D. Mu J. Wojtaszewski JF. Schuit FC. Birnbaum M, et al. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 36.Liu J. Puscheck EE. Wang F. Trostinskaia A. Barisic D. Maniere G. Wygle D. Zhong W. Rings EH. Rappolee DA. Serine-threonine kinases and transcription factors active in signal transduction are detected at high levels of phosphorylation during mitosis in preimplantation embryos and trophoblast stem cells. Reproduction. 2004;128:643–654. doi: 10.1530/rep.1.00264. [DOI] [PubMed] [Google Scholar]
- 37.Hogan B. Beddington R. Constantini F. Lacy B. Manipulating the Mouse Embryo, A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 2002. [Google Scholar]
- 38.Wang Y. Puscheck EE. Wygle DL. Lewis JJ. Trostinskaia AB. Wang F. Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril. 2005;83:1144–1154. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 39.Xie Y. Wang T. Sun T. Wang Y. Wang F. Puscheck EE. Rappolee DA. Acquisition of essential somatic cell cycle regulatory protein expression and implied activity occurs at the second to third cell division in mouse preimplantation embryos. FEBS Lett. 2005;579:398–408. doi: 10.1016/j.febslet.2004.10.109. [DOI] [PubMed] [Google Scholar]
- 40.Zhong W. Sun T. Wang Q. Wang Y. Xie Y. Johnson A. Leach R. Puscheck EE. Rappolee DA. SAPK/JNK1, 2, but not SAPK/JNK3 mRNA transcripts, are expressed in early gestation human placenta and mouse eggs, preimplantation embryos, and trophoblast stem cells. Fertil Steril. 2004;82:1140–1148. doi: 10.1016/j.fertnstert.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Xie Y. Sun T. Wang QT. Wang Y. Wang F. Puscheck E. Rappolee DA. Acquisition of essential somatic cell cycle regulatory protein expression and implied activity occurs at the second to third cell division in mouse preimplantation embryos. FEBS Lett. 2005;579:398–408. doi: 10.1016/j.febslet.2004.10.109. [DOI] [PubMed] [Google Scholar]
- 41.Xie Y. Wang Y. Sun T. Wang F. Trostinskaia A. Puscheck EE. Rappolee DA. Six post-implantation lethal knockouts of genes for lipophilic MAPK pathway proteins are expressed in preimplantation mouse embryos and trophoblast stem cells. Mol Reprod Dev. 2005;71:1–11. doi: 10.1002/mrd.20116. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y. Wang F. Sun T. Trostinskaia A. Wygle D. Puscheck E. Rappolee DA. Entire mitogen activated protein kinase (MAPK) pathway is present in preimplantation mouse embryos. Dev Dyn. 2004;231:72–87. doi: 10.1002/dvdy.20114. [DOI] [PubMed] [Google Scholar]
- 43.Wang X. Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurosaka S. Eckardt S. McLaughlin KJ. Pluripotent lineage definition in bovine embryos by Oct4 transcript localization. Biol Reprod. 2004;71:1578–1582. doi: 10.1095/biolreprod.104.029322. [DOI] [PubMed] [Google Scholar]
- 45.Iryo Y. Matsuoka M. Wispriyono B. Sugiura T. Igisu H. Involvement of the extracellular signal-regulated protein kinase (ERK) pathway in the induction of apoptosis by cadmium chloride in CCRF-CEM cells. Biochem Pharmacol. 2000;60:1875–1882. doi: 10.1016/s0006-2952(00)00510-4. [DOI] [PubMed] [Google Scholar]
- 46.Guo J. Sheng G. Warner BW. Epidermal growth factor-induced rapid phosphorylation at Ser780 and Ser795 is mediated by ERK1/2 in small intestine epithelial cells. J Biol Chem. 2005;280:35992–35998. doi: 10.1074/jbc.M504583200. [DOI] [PubMed] [Google Scholar]
- 47.Legro RS. Barnhart HX. Schlaff WD. Carr BR. Diamond MP. Carson SA. Steinkampf MP. Coutifaris C. McGovern PG, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 48.Hawley SA. Fullerton MD. Ross FA. Schertzer JD. Chevtzoff C. Walker KJ. Peggie MW. Zibrova D. Green KA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baur JA. Pearson KJ. Price NL. Jamieson HA. Lerin C. Kalra A. Prabhu VV. Allard JS. Lopez-Lluch G, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee TI. Jenner RG. Boyer LA. Guenther MG. Levine SS. Kumar RM. Chevalier B. Johnstone SE. Cole MF, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyer LA. Plath K. Zeitlinger J. Brambrink T. Medeiros LA. Lee TI. Levine SS. Wernig M. Tajonar A, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 52.Wang K. Chen Y. Chang EA. Knott JG. Cibelli JB. Dynamic epigenetic regulation of the Oct4 and Nanog regulatory regions during neural differentiation in rhesus nuclear transfer embryonic stem cells. Cloning Stem Cells. 2009;11:483–496. doi: 10.1089/clo.2009.0019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.