Abstract
Purpose
The aim of this study was to investigate the relationship between the expressions of microRNA-9 (miR-9) and microRNA-200c (miR-200c) in human breast cancers and clinicopathological features.
Methods
We investigated the expressions of miR-9 and miR-200c in 68 patients with breast cancers using the quantitative reverse transcription–polymerase chain reaction method, and assessed the E-cadherin status using the immunohistochemistry method.
Results
The relative expression levels of miR-9 and miR-200c in breast cancer patients with lymph node metastasis were higher than that of patients without lymph node metastasis. The expression of miR-9 correlated inversely with E-cadherin expression.
Conclusions
The results showed that higher expressions of miR-9 and miR-200c in human breast cancers were associated with lymph node metastasis. This study indicated that the elevation of miR-9 and miR-200c in human breast cancers can induce an invasive phenotype and may serve as a molecular diagnostic marker for patients with breast cancer.
Key words: breast cancer, E-cadherin, metastasis, microRNA-9, microRNA-200c
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer deaths in women worldwide, accounting for 23% (1.38 million) of the total new cancer cases and 14% (458,400) of the total cancer deaths in 2008.1 Despite significantly improved diagnosis and clinical therapy methods, up to 90% deaths from breast cancer are due to metastasis. Even though much progress has been made in the mechanisms underlying the oncogenic transformation that leads to breast cancer initiation and progression, its molecular process regulating metastasis is still obscure. Therefore, novel predictive markers for metastasis are needed.
Recent researches show that epithelial-to-mesenchymal transition (EMT) is an essential process during embryogenesis, and its pathological activation during tumor development can contribute to cancer progression and metastasis.2 EMT is a multistep process characterized by the loss of cell-to-cell junctions and reorganization of the cytoskeleton, which together result in the loss of apical–basal cell polarity and the acquisition of spindle-shaped morphology.3 Increasing lines of evidences indicate that the early stages of the metastatic process are similar to EMT. E-cadherin, which plays an important role in intercellular epithelial adhesion, is a calcium-dependent transmembrane glycoprotein.4 Loss of E-cadherin is a key characteristic of EMT and is associated with tumor progression, metastasis, and poorer prognosis in breast cancer.4
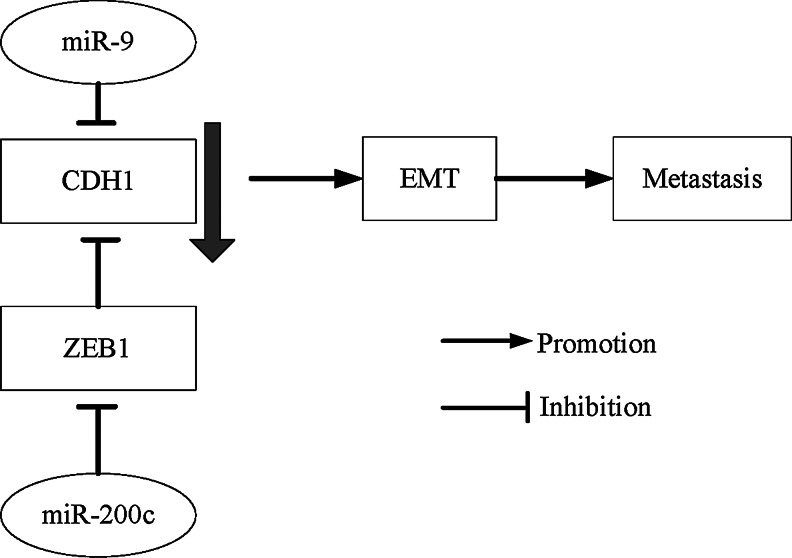
One of the emerging fields in cancer metastasis research is microRNAs (miRs). miRs are a new class of multifunctional small molecules that regulate the stability or translational efficiency of targeted messenger RNAs. Accumulating evidence indicates that the deregulation of miRs is associated with human malignancies and suggests a casual role of miRs in tumor initiation and progression.5 Several lines of evidence indicate that miRs play an important role in breast cancer metastasis.6 The microRNA-9 (miR-9), which directly targets the E-cadherin-encoding messenger RNA (CDH1) in breast cancer cells, leads to increased cell motility and invasiveness.7 Ma et al. investigated that miR-9 was significantly elevated in primary breast tumors from patients with diagnosed metastasis in comparison with patients who are metastasis free, in a small sample7; therefore, it was inferred that miR-9 was a putative metastasis promoter in breast cancer. The miR-9 has been added to a growing list of microRNAs that influence cancer metastasis. MicroRNA-200c (miR-200c) belongs to the microRNA-200 family and is located on chromosome 12p12.31. It has been shown that miR-200c is the only one to regulate E-cadherin transcriptional repressors zinc-finger E-box-binding homeobox 1 (ZEB1) and to restore E-cadherin expression in breast cancer cells.8 miR-200c indirectly increases E-cadherin expression by directly repressing ZEB1. The relationship among E-cadherin, ZEB1, miR-9, and miR-200c can be seen in Figure 1.
FIG. 1.
E-cadherin, ZEB1, miR-9, and miR-200c involve in the metastasis-regulating signaling network [miR-9 directly targets the E-cadherin-encoding messenger RNA (CDH1), and miR-200c regulates E-cadherin transcriptional repressors zinc-finger E-box-binding homeobox 1 (ZEB1)].
Recent studies showed that miR-9 upregulated in the metastasis of breast cancer.7 Dysregulation of miR-200c had been found in cancer of the ovary,9 cervix,10 and melanocyte lineage,11 while there was no reported study demonstrating the relationship between the expressions of miR-9 and miR-200c in human breast cancer tissue and clinicopathological features. Thus, we took advantage of quantitative reverse transcription (qRT)–polymerase chain reaction (PCR) to determine the expressions of miR-9 and miR-200c in human breast cancers and explored the relationship between their expressions and clinicopathological features of patients. We report here that miR-9 and miR-200c are frequently overexpressed in primary breast cancers. More importantly, the patients with elevated expressions of miR-9 and miR-200c are found to be associated with lymph node metastasis.
Materials and Methods
Patients and tissues
Fresh breast cancer tissues and matched adjacent noncancerous tissues of 68 consecutive patients were derived from patients undergoing breast surgery at the Affiliated Tumor Hospital of Harbin Medical University (Harbin, China) from January 2012 to March 2012. All the patients were women, and most of them (85.3%) had infiltrating ductal cancers. The usage of the tissue samples for the experiments was approved by all the patients and by the Ethics Committee of the Affiliated Tumor Hospital of Harbin Medical University. None of the patients had received chemotherapy or radiotherapy before the surgery. All the samples were divided into two parts. One was immediately snap-frozen in liquid nitrogen for 30 minutes and then stored at −80°C until RNA extraction, and the remaining tissues were routinely fixed in 10% formalin and embedded in paraffin wax for pathologic analysis. All cases were reviewed by two pathologists, and the histopathologic diagnosis was confirmed according to the AJCC criteria (the sixth edition, 2002). The clinical data were obtained from medical records within the hospital.
miRNA extraction from fresh tissues
Total RNA from fresh frozen breast samples was isolated in triplicate by using Trizol reagent (Invitrogen) according to the instructions of the manufacturer. Tissue miRNAs were extracted from 30 mg of frozen tissue sample using the MicroRNA Extraction and Purification Kit (Spin Column; Cat. No. N0114A; Novland) according to the manufacturer's protocol. The RNA concentration was quantified using NanoDrop ND-2000 Spectrophotometer (Thermo Fisher Scientific, Inc.). Extracted RNA samples were stored at −80°C until they were used.
qRT-PCR detection of miRNA
miR-9-, miR-200c-, and RNU6- (internal control) specific cDNAs were synthesized with the miScript Reverse Transcription Kit (Qiagen) according to the manufacture's protocols. A quantitative PCR was performed using the miScript SYBR-Green PCR Kit (Qiagen). The reaction was carried out with the manufacturer's protocols. Expression analysis was performed in triplicate for each sample. Both melting curve analysis and agarose gel run were used to confirm the specificity of the amplification reactions. The relative levels of miR-9 and miR-200c expression were calculated from the relevant signals by normalization with the signal for small-nuclear RNA U6 expression. The miRNA expression level was quantified using the ABI PRISM 7900 Sequence Detection System (Applied Biosystems). The relative expression of each miRNA was calculated using the 2−ΔΔCt method, with the ratio of the median expression sample among all cancers with adjacent noncancerous tissues samples being used as the calibrator.
Immunohistochemistry for E-cadherin
The E-cadherin status of the breast cancer tissues was assessed by immunohistochemistry (IHC). Immunostaining was performed as follows. Four-μm-thick tissue sections were cut from the paraffin-embedded blocks. The IHC protocol was in accordance with the secondary antibody kits' manufacturer's instructions. The primary antibody, rabbit monoclonal anti-E-cadherin antibody (BA0475; Boster), was diluted by 1: 100. The sections were incubated overnight at 4°C in the appropriate dilution of the antibody. The secondary antibody kit, Goat anti-rabbit SABC Kit (SA1022; Boster), was purchased from Boster. Positive expression was shown by brown DAB (AR1022; Boster) precipitate, and the nuclei were stained with hematoxylin. The primary antibody was replaced by the phosphate-buffered saline in negative controls, and three replicates were carried out in the experiment. Immunoreactivity was classified as follows: E-cadherin antibody stained the membrane intensely and the cytoplasm of cancer cells weakly. E-cadherin expression was semiquantitatively analyzed according to the percentage of tumor cells showing membrane positivity: 0, 0%–10%; 1+, 10%–25%; 2+, 25%–50%; 3+, 50%–75%; 4+, 75%–100%. E-cadherin expression was considered positive when scores were ≥3 (see Fig. 2), and negative when scores were 0–2 (see Fig. 3). A case with cytoplasmic staining only was determined as E-cadherin negative.
FIG. 2.
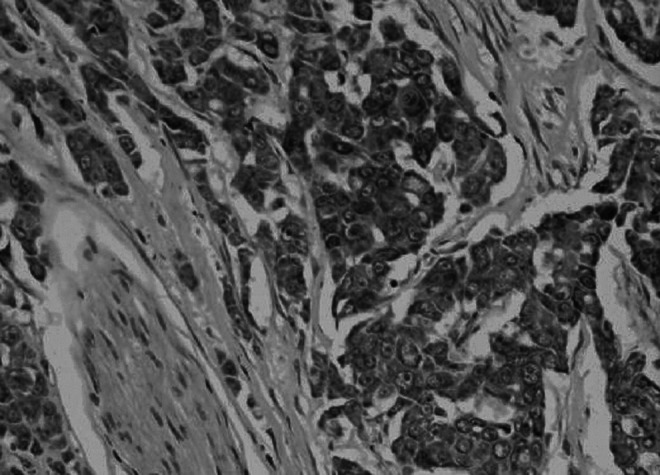
Positive expression of E-cadherin in human breast cancer (amplification,×200, strong E-cadherin expression was observed in the membrane of the breast cancer cells).
FIG. 3.
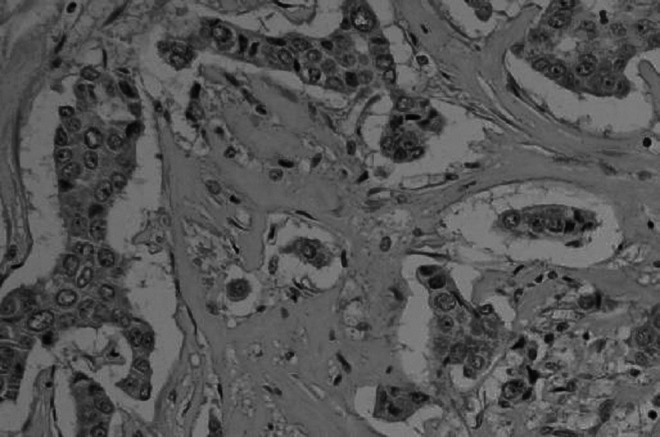
Negative expression of E-cadherin in human breast cancer (amplification,×200, there was no immunoreactivity occurred among the membrane and the cytoplasm of the beast cancer).
Statistical analysis
SPSS 16.0 software (SPSS) was used for statistical analysis. Differences of E-cadherin expression between groups were calculated by chi-squared test (χ2 test). All the results of miRs are expressed as the means±standard deviation (SD). The correlation between the expression levels of microRNAs and clinicopathologic features of the patients was evaluated by analysis of variance. In the experiment, p<0.05 was defined as statistically significant.
Results
The relationship between the E-cadherin expression and the clinicopathological features
The results of immunohistochemical analysis of E-cadherin in breast cancers are summarized in Table 1. Strong membranous E-cadherin immunostaining was observed in normal breast tissues. Thirty-eight of 68 (55.9%) cases of breast cancer had indicated positive expression of E-cadherin. The E-cadherin expression rate was much higher (76.2%) in patients with nodal-negative carcinoma than in patients with nodal-positive carcinoma (46.8%), and p value <0.05. However, no significant association was found between the E-cadherin expression with cell differentiation, age, tumor size, estrogen receptor, and progesterone receptor.
Table 1.
Relationship Between E-Cadherin Expression and Clinicopathological Features of the Patients
| |
|
E-cadherin |
|
|
|---|---|---|---|---|
| Variable | Number | Positive (%) (n=38) | Negative (%) (n=30) | p-Value |
| Age (years) | >0.05 | |||
| <50 | 35 | 20 (57.1%) | 15 (42.9%) | |
| ≥50 | 33 | 18 (54.5%) | 15 (45.5%) | |
| Cell differentiation | >0.05 | |||
| Good–moderate | 40 | 26 (65.0%) | 14 (35.0%) | |
| Poor | 28 | 12 (42.9%) | 16 (57.1%) | |
| pT | >0.05 | |||
| T1 | 23 | 14 (60.9%) | 9 (39.1%) | |
| T2 | 24 | 13 (54.2%) | 11 (45.8%) | |
| T3 | 21 | 11 (52.4%) | 10 (47.6%) | |
| pN | <0.05 | |||
| Negative | 21 | 16 (76.2%) | 5 (23.8%) | |
| Positive | 47 | 22 (46.8%) | 25 (53.2%) | |
| Estrogen receptor | >0.05 | |||
| Negative | 29 | 16 (55.2%) | 13 (44.8%) | |
| Positive | 39 | 22 (56.4%) | 17 (43.6%) | |
| Progesterone receptor | >0.05 | |||
| Negative | 32 | 17 (53.1%) | 15 (46.9%) | |
| Positive | 36 | 21 (58.3%) | 15 (41.7%) | |
The correlation between miR-9 and clinicopathological features of human breast cancers
Table 2 shows the correlations between miR-9 expression and the clinicopathological features of the patients with breast cancer. We analyzed the correlation between miR-9 and clinicopathological features of patients with breast cancer to better understand the potential roles of miRs. The means of relative expression levels of miR-9 in tumors and adjacent noncancerous tissues were, respectively, 1.54±2.42 and 1.0±0.35, and the expression of the tumors was significantly higher than in adjacent noncancerous tissues (p<0.05). A significant association was found between higher miR-9 expression and lymph node metastasis of breast cancer. miR-9 was found associated inversely with the expression of E-cadherin. However, no significant association was found between miR-9 expression and other clinicopathological features.
Table 2.
Association Between miRNAs and Clinicopathological Features in 68 Patients with Breast Cancers
| |
|
miR-9 (2−⊿⊿Ct) in tumor |
MiR-200c (2−⊿⊿Ct) in tumor |
||
|---|---|---|---|---|---|
| n | Mean±SD | p-Value | Mean±SD | p-Value | |
| Age (years) | |||||
| <50 | 35 | 1.64±2.49 | >0.05 | 2.02±2.99 | >0.05 |
| ≥50 | 33 | 1.35±1.98 | 2.60±4.67 | ||
| Cell differentiation | |||||
| Good–moderate | 40 | 1.90±2.13 | >0.05 | 2.49±4.18 | >0.05 |
| Poor | 28 | 0.93±1.89 | 2.03±3.54 | ||
| pT | |||||
| T1 | 23 | 0.76±1.63 | >0.05 | 1.90±3.62 | >0.05 |
| T2 | 24 | 1.50±2.41 | 2.25±5.36 | ||
| T3 | 21 | 2.30±4.37 | 2.79±4.98 | ||
| pN | |||||
| Negative | 21 | 0.60±1.32 | <0.05 | 1.39±3.36 | <0.05 |
| Positive | 47 | 1.90±4.68 | 2.71±6.93 | ||
| Estrogen receptor | |||||
| Negative | 29 | 1.13±1.64 | >0.05 | 2.19±4.08 | >0.05 |
| Positive | 39 | 2.00±2.53 | 2.38±4.19 | ||
| Progesterone receptor | |||||
| Negative | 32 | 1.20±3.26 | >0.05 | 1.98±4.12 | >0.05 |
| Positive | 36 | 1.77±4.10 | 2.58±4.62 | ||
| E-cadherin | |||||
| Negative | 30 | 3.25±3.74 | <0.05 | 1.35±3.61 | >0.05 |
| Positive | 38 | 1.10±2.31 | 1.62±3.97 | ||
Bold values indicate that both of the expression levels of microRNA-9 and microRNA-200c in breast cancer patients with lymph node metastasis were higher than that of patients without lymph node metastasis. MicroRNA-9 affects lymph node metastasis of human breast cancer through the miR-9/E-cadherin axis-dependent mechanism.
The correlation between miR-200c and clinicopathological features of human breast cancers
The correlations between miR-200c and the clinicopathological features of 68 patients with breast cancer are also summarized in Table 2. The means of relative expression levels of miR-200c in tumors and adjacent noncancerous tissues were, respectively, 2.30±4.19 and 1.0±0.35, and the expression of the tumors was also significantly higher than in adjacent noncancerous tissues (p<0.05). Moreover, we found that the expression of miR-200c in the patients with lymph node metastasis was higher than that of the patients without lymph node (p<0.05). No associations were found between miR-200c expression and age, tumor size, differentiation, expression of E-cadherin, and the status of hormonal receptors. High expressions of miR-9 and miR-200c in tissues of patients with breast cancer were associated with lymph node metastasis.
Discussion
The metastasis is a complex multistep process, which is associated with not only growth factors, cytokines, chemokines, proangiogenic factors, and extracellular matrix-remodeling molecules, but also the regulators associated with the EMT.12 Low E-cadherin expression is a key characteristic of EMT and has been reported to have a high incidence of metastasis in a variety of in vitro and in vivo models.13 Although miR deregulation has been observed in a variety of human tumors and to our knowledge, there are no studies that specifically investigate the expressions of miR-9 and miR-200c in primary breast cancer tissues. The present study, for the first time, simultaneously investigated the expressions of miR-9 and miR-200c in human fresh frozen breast cancer tissues and analyzed the association of expressions of miRs with the status of E-cadherin and clinicopathologic features of the patients with breast cancer. Our results showed that overexpression of miR-9 and miR-200c in human breast cancers was associated with lymph node metastasis.
Recent studies have investigated that miR deregulation of human breast cancer tissues correlates with the initiation and progression of the disease.6 miR-9, which is selectively expressed in neural tissues,7 is induced by Myc in breast cancer cells where it targets the major epithelial adherent junction protein E-cadherin. In clinical breast cancer, miR-9 is upregulated in primary tumors relative to normal mammary tissues,14 and the upregulation of microRNA-9 was also involved in the metastases of many types of cancer. However, we do not know whether miR-9 plays a role in breast cancers, so we investigated the expression of microRNA-9 in human primary breast cancer tissues and compared the expression levels of patients with the clinicopathological features in 68 patients.
Several studies have implicated that the microRNA-200 family (including microRNA-200a, microRNA-200b, microRNA-200c, microRNA-141, and microRNA-429) plays an important role in the regulation of E-cadherin transcriptional repressors ZEB1 and ZEB2 and in the induction of EMT in cancer cells.15 It has been hypothesized that enforced expression of these miRs will limit metastasis. ZEB1, which has crucial effects on various processes of malignant tumor progression and promotes metastasis, has been identified as direct target of the microRNA-200c. Dysregulation of miR-200c also occurs in multiple cancer types. Overexpression of the hsa-miR-200c leads to increased expression of E-cadherin in breast cancer cells MDA-MB-231.16 Low or absent miR-200c expression results in aberrant expression of ZEB1 and consequent repression of E-cadherin.17 Some researchers found that the miR-200c level is associated with the differentiation of breast cancer cell lines, and it was high in well-differentiated, but extremely low in poorly differentiated, cancer cells. The effects of miR-200 on tumor cell invasion are significantly contextual, as reports on breast cancer cells have shown that the miR-200 family members increase with neoplastic transformation and promote tumor cell invasion and metastasis.18 Bockmeyer et al. determined the microRNA expression pattern of healthy mammary epithelial cells and breast cancer subtypes. The results indicated that microRNA-200c cells were predominantly healthy luminal cells and basal-like breast cancer.19 Baffa et al. performed a microRNA microarray analysis on 13 paired primary tumors of breast cancer and one of their related metastatic lymph nodes. They found that the miR-200c downregulates in breast samples.20
The results of the present study showed that both of miR-9 and miR-200c in patients with lymph node metastasis are higher than that of patients with the lymph node absent. It suggests that miR-9 and miR-200c are involved in the lymph node metastasis of breast cancers. To identify the mechanism of action of miR-9 and miR-200c, the IHC method was performed. A significant association was found between higher miR-9 expression and the lower expression of E-cadherin. Hence, we speculate that miR-9 affects lymph node metastasis of human breast cancer through an miR-9/E-cadherin axis-dependent mechanism. The results of miR-200c expression in breast cancer tissues significantly increased compared to normal tissues, and that of patients with lymph node metastasis were higher than that of patients without lymph node. It is controversial to the expression analyses of cultured cell lines and could not be solely explained by its ability to target ZEB1. It may be associated with the micromanagement of miR that each miR controls tens or hundreds of gene targets. The discrepancy of the results may attribute to the different extent of tumor growth in cancer cells. Therefore, the mechanisms that miR-200c regulating lymph node metastasis of patients with breast cancer are needed for further studies. However, in the present study, we did not find the relationship between expression of miR-200c and the differentiation of a tumor cell.
Our study may be affected by the number of the enrolled patients with breast cancer, so we require to enlarge our samples for further studies about the expressions of miR-9 and miR-200c of the patients with breast cancer. Due to the short time of the onset, we cannot get the association of the expressions of miR-9 and miR-200c with the overall survival. The axillary lymph node metastasis is the most commonly involved region. Once the relationship between the expressions of miR-9 and miR-200c and metastasis was identified, we may discriminate the patients at high risk. If we give them aggressive treatments after operation, the group patients may benefit from the early discovery. We hope that miRNA signatures may have diagnostic and therapeutic values in patients with breast cancer. The elucidation of the role of miRs in metastasis can lead to new treatments for patients with breast cancer. In conclusion, our findings showed that patients with human breast carcinoma with higher expressions of miR-9 and miR-200c may have a higher propensity to lymph node metastasis.
Acknowledgments
The study was supported by the Foundation of Heilongjiang Provincial Health Bureau (Grant No. 674) and the Foundation of the Third Affiliated Hospital of Harbin Medical University (Grant No. JJ2010-21).
Disclosure Statement
No competing financial interests exist.
References
- 1.Jemal A. Bray F. Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Vincent-Salomon A. Thiery JP. Host microenvironment in breast cancer development: Epithelial-mesenchymal transition in breast cancer development. Breast Cancer Res. 2003;5:101. doi: 10.1186/bcr578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huber MA. Kraut N. Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Kowalski PJ. Rubin MA. Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Q. Gumireddy K. Schrier M, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 7.Ma L. Young J. Prabhala H, et al. MiR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurteau GJ. Carlson JA. Roos E, et al. Stable expression of miR-200c alone is sufficient to regulate TCF8 (ZEB1) and restore E-cadherin expression. Cell Cycle. 2009;8:2064. doi: 10.4161/cc.8.13.8883. [DOI] [PubMed] [Google Scholar]
- 9.Bendoraite A. Knouf EC. Garg KS, et al. Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: Evidence supporting a mesothelial-to-epithelial transition. Gynecol Oncol. 2010;116:117. doi: 10.1016/j.ygyno.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JW. Choi CH. Choi JJ, et al. Altered MicroRNA expression in cervical carcinomas. Clin Cancer Res. 2008;14:2535. doi: 10.1158/1078-0432.CCR-07-1231. [DOI] [PubMed] [Google Scholar]
- 11.Mueller DW. Rehli M. Bosserhoff AK. miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J Invest Dermatol. 2009;129:1740. doi: 10.1038/jid.2008.452. [DOI] [PubMed] [Google Scholar]
- 12.Ma L. Weinberg RA. Micromanagers of malignancy: Role of microRNAs in regulating metastasis. Trends Genet. 2008;24:448. doi: 10.1016/j.tig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Onder TT. Gupta PB. Mani SA, et al. Loss of E-Cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 14.Iorio MV. Ferracin M. Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 15.Park SM. Gaur AB. Lengyel E, et al. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurteau GJ. Carlson JA. Spivack SD, et al. Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res. 2007;67:7972. doi: 10.1158/0008-5472.CAN-07-1058. [DOI] [PubMed] [Google Scholar]
- 17.Cochrane DR. Spoelstra NS. Howe EN, et al. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther. 2009;8:1055. doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dykxhoorn DM. Wu Y. Xie H, et al. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS One. 2009;4:e7181. doi: 10.1371/journal.pone.0007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bockmeyer CL. Christgen M. Muller M, et al. MicroRNA profiles of healthy basal and luminal mammary epithelial cells are distinct and reflected in different breast cancer subtypes. Breast Cancer Res Treat. 2011;130:35. doi: 10.1007/s10549-010-1303-3. [DOI] [PubMed] [Google Scholar]
- 20.Baffa R. Fassan M. Volinia S, et al. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol. 2009;219:214. doi: 10.1002/path.2586. [DOI] [PubMed] [Google Scholar]