Abstract
Mild traumatic brain injury (mTBI) results in an estimated 75–90% of the 1.7 million TBI-related emergency room visits each year. Post-concussion symptoms, which can include impaired memory problems, may persist for prolonged periods of time in a fraction of these cases. The purpose of this study was to determine if an erythropoietin-mimetic peptide, pyroglutamate helix B surface peptide (pHBSP), would improve neurological outcomes following mTBI. Sixty-four rats were randomly assigned to pHBSP or control (inactive peptide) 30 μg/kg IP every 12 h for 3 days, starting at either 1 hour (early treatment) or 24 h (delayed treatment), after mTBI (cortical impact injury 3 m/sec, 2.5 mm deformation). Treatment with pHBSP resulted in significantly improved performance on the Morris water maze task. Rats that received pHBSP required 22.3±1.3 sec to find the platform, compared to 26.3±1.3 sec in control rats (p=0.022). The rats that received pHBSP also traveled a significantly shorter distance to get to the platform, 5.0±0.3 meters, compared to 6.1±0.3 meters in control rats (p=0.019). Motor tasks were only transiently impaired in this mTBI model, and no treatment effect on motor performance was observed with pHBSP. Despite the minimal tissue injury with this mTBI model, there was significant activation of inflammatory cells identified by labeling with CD68, which was reduced in the pHBSP-treated animals. The results suggest that pHBSP may improve cognitive function following mTBI.
Key words: adult brain injury, traumatic brain injury, treatment strategies for traumatic brain injury
Introduction
Mild traumatic brain injury (mTBI) is a syndrome of physical, cognitive, emotional, and sleep-related symptoms induced by a blow or jolt to the head.1 The clinical spectrum of mTBI ranges from brief confusion with rapid recovery to loss of consciousness for up to 30 min with prolonged disabling symptoms. An estimated 75–90% of the 1.7 million TBI-related emergency room visits each year are a result of mTBI.
Treatment of mTBI is primarily symptomatic. Several neuroprotective agents have been investigated in the laboratory in mTBI models, including tumor necrosis factor-α antagonists such as 3,6′-dithiothalidomide,2 sulfonylurea inhibitors such as glibenclamide,3 the PKC activator bryostatin 1,4 insulin-like growth factor,5 adenosine A1 receptor activation to inhibit microglia proliferation,6 glutamate antagonists like N-methyl-D-aspartate,7 free radical scavengers such as vitamin E,8 exercise,9 Nogo-A inhibitory peptide (NEP1-40),10 the nicotinic acetylcholine-receptor activator donepezil,11 dehydroepiandrosterone sulfate,12 and mexiletine,13 but none so far has been successful in clinical studies. Most of these agents target a specific pathophysiological injury mechanism. Given the complex cascade of injury mechanisms induced by TBI, a drug that has multiple neuroprotective activities might be more advantageous.
One such agent is erythropoietin (EPO). It has neuroprotective activities that include suppression of cellular inflammatory conditions, inhibiting apoptosis, and improving cerebrovascular function, and it may enhance recovery by stimulating neurogenesis and angiogenesis.14–21 However, the erythropoietic activities of EPO are problematic in some clinical settings. Stimulating erythropoiesis may be an advantage in severe TBI, for which anemia is a common complication. However, in mTBI, for which the hematocrit is usually normal, an increase in hematocrit is more likely to predispose to thrombotic complications, some of which can be serious and life-threatening.
Using derivatives of EPO, Leist and associates demonstrated that the erythropoietic and cytoprotective effects of EPO can be separated.22 A novel cell surface receptor, comprised of the classical EPO receptor (EPOR) disulfide, linked to the beta common receptor CD131, may mediate the non-hematopoietic effects of EPO.23 An 11-amino acid synthetic peptide was subsequently developed to mimic the 3D structure of EPO. This peptide retains EPO's neuroprotective activities, but not the erythropoietic actions that are responsible for the thrombogenic adverse effects of EPO.24 The peptide, called pyroglutamate helix B surface peptide (pHBSP), is a linear peptide containing the amino acids from the aqueous face of helix B (amino acids 58–85) of erythropoietin (QEQLERALNSS). Modification of the N-terminal Q to pyroglutamate (U) for stabilization results in a peptide (UEQLERALKSS) that can be stored for 2 years at 4°C, or up to 12 months at 25°C. Therefore pHBSP does not require refrigeration and may have lower risk of adverse effects, which are important advantages over EPO as a therapeutic agent in the treatment of mTBI.
In a model of mTBI followed by hemorrhagic shock, pHBSP had neuroprotective activities equivalent to EPO.25 The purpose of this study was to examine the neuroprotective effects of pHBSP in a model of uncomplicated mTBI.
Methods
Sixty-four Long Evans rats weighing 300–400 grams were used for this study. This protocol was approved by the institutional animal protocol review committee at Baylor College of Medicine, using guidelines for the humane care and use of animals that were developed for the National Institutes of Health.
Treatment groups
Rats were randomly assigned to receive either pHBSP or control treatment starting at 1 h (early treatment), or 24 h (delayed treatment) after injury. Sixteen animals were assigned to each of the four groups. The control group received an inactive peptide consisting of the same amino acid components as pHBSP, but in a scrambled sequence (ULSEARNQSEL). In a previous study this control was similar to a saline control.25 The dose of both the pHBSP and the control was 30 μg/kg IP starting at the assigned time, and then every 12 h for 3 days. The investigators conducting the injury experiment and the outcome measures were blinded to the treatment assignment.
Anesthesia
General anesthesia was induced using 5% isoflurane in 100% oxygen. The rats were placed in a vented anesthesia gas chamber for approximately 3–5 min. Immediately after anesthesia induction, the rats were intubated using a 14-gauge catheter. The rats were then mechanically ventilated with 2% isoflurane in 100% oxygen, and the ventilator was adjusted to maintain arterial partial carbon dioxide pressure between 35 and 40 mm Hg, and partial oxygen pressure > 100 mm Hg.
Craniectomy and controlled cortical impact
The rat's head was fixed in a stereotaxic frame for the injury. The surgical procedure was performed using aseptic technique. The rat's scalp was shaved and then cleaned using an iodine-based antiseptic solution. Placement of sterile linens over the rat's head was done to minimize the risk of contamination. A median skin incision was performed and the scalp (including the periosteum) and the temporalis muscle were reflected. To expose the brain for the impact injury, a 10-mm-diameter craniectomy was made using a dental drill over the right parietal cortex between the bregma and the lambda. Care was taken to not injure the dura. A small amount of saline solution was directed at the site of drilling to prevent thermal injury to the brain tissue.
To position the impactor device, the impactor tip was centered in the craniectomy site perpendicular to the exposed surface of the brain at an angle of approximately 45 degrees from the vertical. Then the tip was lowered until it just touched the dural surface. The impactor rod was then retracted, and the tip advanced an additional distance to produce a brain deformation of 2.5 mm at the time of the impact. To induce a mild injury, the controlled cortical impact device was adjusted to 35 psi, giving an impact velocity of 3 m/sec.
With the help of a heating lamp aimed at the head of the rat, the brain temperature was kept between 36 and 37°C. Body temperature was continuously monitored and maintained between 36 and 37°C with a heating pad controlled by a rectal probe.
After the cortical injury the skull defect was closed using an artificial bone flap made of dental acrylic to avoid extrusion of brain tissue. The incision was then closed using 5-0 Vicryl™ sutures.
Postoperative management and assessment
Anesthesia was discontinued to allow the rats to recover. Escape, righting, head support, corneal, pinna, paw, and tail reflexes were assessed and recorded every minute for a maximum of 30 min until the animal was awake and breathing spontaneously. The animals were then returned to their cages and allowed free access to food and water. The animals were given buprenorphine 0.05 mg/kg subcutaneously daily for analgesia, and fluoroquinolone 0.05 mL/kg subcutaneously daily to prevent postoperative infections for 3 days post-injury.
The weight of each rat was recorded on the day before surgery, on the day of surgery, on days 1–5 post-surgery, and on days 17–21 post-surgery, using a manual scale. On days 1–5 post-injury, the animals performed beam-walking and beam-balancing tasks. On days 17–21 post-injury, the animals were tested on the Morris water maze task. Following the last behavioral assessment, the animals were euthanized and the brains removed for histological examination.
Behavioral testing
Beam-walking task
Each rat was pre-trained 2 days before surgery to walk down a beam that was 1 m long, 2.5 cm wide, and 1 m above the ground, into a darkened goal box to escape white noise of 90 db. At the beginning of each training and test trial, the rat sat in the goal box for 30 sec. During training trials, the animal was placed at successively longer distances from the goal box (fifths of the way) until it learned to walk down the entire beam. Any distance from which the rat did not walk down the beam into the goal box was repeated until it did. The rat was given a 30-sec rest period in the goal box between trials. After it had traversed the beam in 5 sec or less on three successive trials, four plastic pegs (7.5 cm high) were placed in holes in the beam at approximately 20-cm intervals alternating from side to side, 5 mm in from the edge of the beam. The rat was then trained to another criterion of three consecutive trials completed in 10 sec or less. If both of these criteria were not met by 30 trials, the rat was disqualified. The final criterion for inclusion in the study was beam walking times on the day of surgery with the pegs present that were 5 sec or less on three consecutive trials within 15 trials. Beam walking with the pegs present was assessed on days 1–5 post-surgery.
Beam-balancing task
Each rat was placed lengthwise along the center of a beam 1.5 cm wide, 1 m long, and 1 m above the ground. The rat was required to balance on the beam for 60 sec for three trials on the day of surgery, and was attempted again on days 1–5 post-surgery. The rat was taken off the beam and placed in the goal box for 30 sec between trials.
Morris water maze test
The Morris water maze consisted of a 5-foot-diameter galvanized steel pool that contained a 10×10-cm acrylic glass platform 26 cm high and hidden 2 cm below the water's surface. Four sequences that corresponded to both the position of the platform (1, 2, 3, and 4), and the starting position (north, south, east, and west quadrants), were developed using a Latin square. An equal number of rats from each treatment group was randomly assigned to each trial sequence. Each rat in each group was given a specific sequence to follow throughout the water maze trials. The maze was filled with water until it was 2 cm above the acrylic glass platform. For each trial, the rat was placed in the water facing the wall in the indicated position. If the rat did not find the platform within 120 sec on any trial, it was placed on the platform for 30 sec. If the rat found the platform within 120 sec, it was allowed to remain on the platform for 30 sec. Four trials were performed each day with a 4-min rest period between trials; the rat was kept warm with a heating lamp between trials. The rat was tracked in the water maze with a video tracking system. Pre-injury performance was not tested. Instead, maze performance was assessed on each of days 17–21 post-injury. A probe trial followed the fourth trial on day 21, in which the platform was removed from the quadrant and the time spent by the animal in the platform quadrant was determined. Finally, a visible platform was placed in the quadrant, and the time taken to find the platform was determined.
Histopathological assessment
On day 21, all animals were deeply anesthetized with 5% isoflurane in 100% oxygen and perfused transcardially with normal saline solution followed by phosphate-buffered 4% paraformaldehyde. The entire brain was removed and fixed in 4% paraformaldehyde for 24 h, and then transferred to a 30% sucrose solution. Following sucrose infiltration and immediately prior to subsequent processing, an 18-gauge needle was inserted ventrally on the left side of the brain from a rostral to caudal position. The brains were frozen and sectioned in the coronal plane on a cryostat at a thickness of 30 μm. The sections were sequentially placed in 48-well plates in a Tris-buffered/polyethylene glycol solution to maintain tissue integrity when stored at −20°C. Every ninth section was collected and immediately mounted, in sequential order, onto gelatin-coated slides and dried on a slide warmer.
The mounted sections were stained with hematoxylin and eosin (H&E), dehydrated, and cover-slipped. The slides were then scanned on an Epson Expression 1680 scanner driven by Adobe Photoshop CS3 at a resolution of 1200 dpi. Lesion volumes were determined using ImageJ software. Briefly, after establishing scale, the lesion cavity was outlined and filled, generating the lesion area per section. The lesion volume per animal was subsequently determined as the sum of the area measurement of each section×the thickness of the section (30 μm)×the sampling interval.
Quantification of CA1 and CA3 neurons
Neuronal counts were performed on brain sections immunolabeled with a monoclonal antibody raised against the pan-neuronal marker NeuN (Millipore, Billerica, MA). Briefly, sections were pre-incubated in PBS containing 0.06% hydrogen peroxide to quench endogenous peroxidase activity. The sections were then blocked in PBS containing Triton-X100 (Sigma-Aldrich, St. Louis, MO) with 5% horse serum for 1 h. The sections were then incubated in anti-NeuN antibody (mouse monoclonal; Millipore) overnight at 4°C at a concentration of 0.75 μg/mL. The sections were then incubated in horse anti-mouse biotinylated secondary antibody, followed by avidin-biotin-complex (ABC Elite Kit; Vector Laboratories, Burlingame, CA). NeuN immunoreactivity was subsequently visualized using diaminobenzidine (DAB) as a substrate, with nickel chloride (8%) to enhance, and hydrogen peroxidase as a catalyst. The sections were rinsed, mounted, dried, and dehydrated through increasing concentrations of ethanol. The slides were then cover-slipped using a non-aqueous mounting medium. Counts of NeuN-immunoreactive (-IR) neurons were performed in the ipsilateral hippocampal CA1 and CA3 regions as follows. Six sections per brain were examined in the coronal plane in a rostral-to-caudal direction. The range of sections was standardized by comparing H&E-labeled sections with the images in the rat brain atlas.26 The rostro-caudal series of NeuN-immunolabeled sections began at the following approximate coordinates: interaural 6.44 mm, bregma −2.56 mm to interaural 4.84 mm, bregma −4.16 mm. Images were captured using an Olympus BX61 microscope attached to a SPOT-RT digital camera under brightfield conditions using a 40× air objective, focusing on the CA1 and CA3 regions of the ipsilateral hippocampus. The captured images were then processed/quantified using Nikon NIS Elements image analysis software. Individual neurons within the CA1 and CA3 regions were autodetected in images thresholded to permit distinct resolution of NeuN-positive cell bodies. Cells were counted within an image field of 382.35×382.35 μm and stored. Data consisted of the cell count means of the six NeuN-immunolabeled sections.
CD68 visualization and quantification
Cellular immune response was chosen as an indication of overall inflammation following mTBI. CD68-immunolabeling was performed on a standardized rostro-caudal range of tissue sections determined using the rat brain atlas.26 The rostral range of sections began at the region indicated at interaural position 5.86 mm, bregma −3.14 mm, and included every eighth section, for a total of four sections assayed per animal. The sections were incubated in primary antibody (CD68 1 μg/mL) overnight at 4°C. CD68 was visualized following subsequent incubation in goat anti-mouse secondary antibody conjugated to Alexa-Fluor 488 (Invitrogen, Valencia, CA) for a period of 3 h. The sections were rinsed and mounted onto glass slides, allowed to dry, then cover-slipped using Fluoromount-G (Fisher Scientific, Pittsburgh, PA), an aqueous mounting medium. The sections were imaged on the same imaging platform described above, using widefield fluorescence parameters at 10× magnification. CD68-positive cellular profiles were automatically counted with Image Pro+ software (version 7.01; Media Cybernetics, Rockville, MD), using a user-generated area of interest of 1.48×0.97 mm applied to the ipsilateral hippocampus.
Statistical analysis
Summary data in text, tables, and figures are expressed as mean±standard error. Data were analyzed with IBM SPSS Statistics Version 19. Histology parameters were compared using a two-factor between-groups analysis of variance (ANOVA), with drug treatment (pHBSP versus control), and time of administration (early versus delayed), as the factors. The behavioral data over time were analyzed using a four-factor mixed ANOVA design, involving between-group effects of drug treatment and time of administration, and repeated-measures on day post-injury and trial within each day, was conducted. Effect size was evaluated with a partial η2, which is appropriate for ANOVAs having more than one factor.27,28 Interpretation of partial η2 is usually that 0.04 represents small, 0.25 medium, and 0.64 large effect size.
Results
Characteristics of the mild TBI model
Contusion volume and neuronal loss in the hippocampus was minimal with this mild cortical impact injury (Fig. 1). The median contusion volume from the impact was 0.3 mm3 (interquartile range 0.07–1.3 mm3). The average neuron counts in the CA1 and CA3 regions of the hippocampus were 98±3 and 69±1, respectively.
FIG. 1.
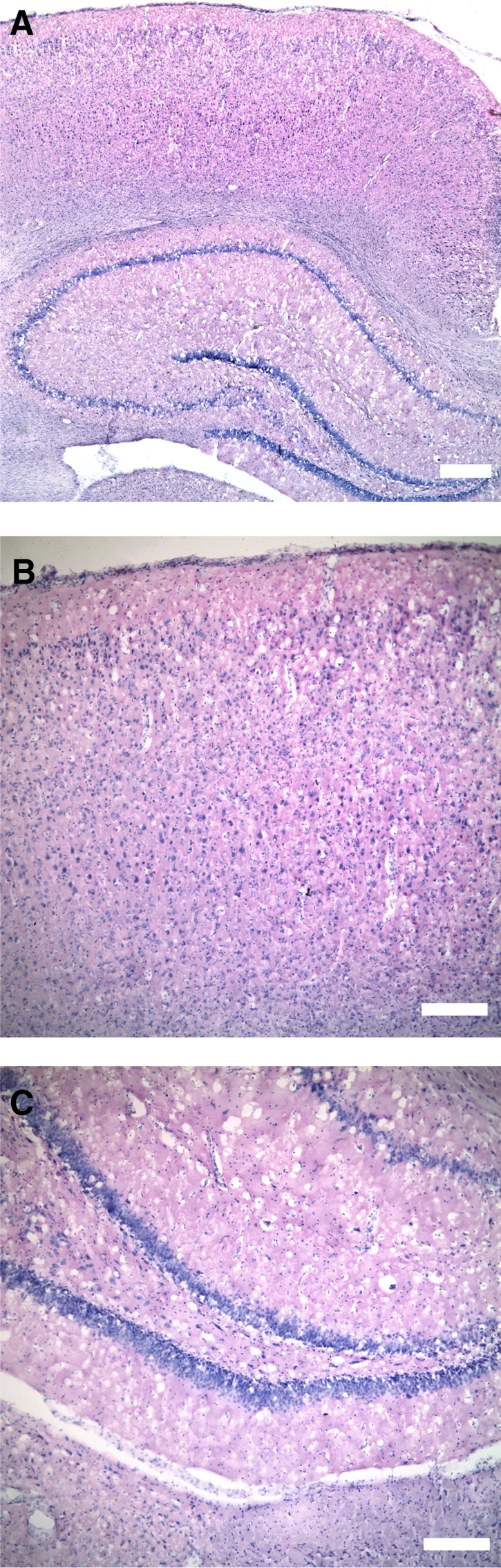
Typical appearance of the mild cortical impact injury. The images are taken from a coronal slice of the brain at the coordinates 5.86 mm from the interaural line and −3.14 mm from the bregma.26 No overt tissue damage (cavitation or cell loss) was observed grossly in either the cortex or underlying hippocampus under low (A; scale bar = 400 μm) or high magnification (B and C; scale bar = 200 μm).
Changes in the behavioral characteristics (Fig. 2) were likewise slight. The average time to recovery of the righting reflex was slightly increased, from 5.5±1.1 min with the mTBI compared to 4.8±0.8 min in sham-injured animals. Poorer performance on the Morris water maze task was the most consistent abnormality seen with mTBI (Fig. 2, left). The average time to find the platform was 26.3±1.3 sec in the mTBI animals, compared to 18.8±1.6 sec in the sham-injured animals. Beam-balance performance was mildly impaired on the first post-injury day compared to sham-injured animals (Fig. 2, center). Beam-walking performance was similar to that of sham-injured animals (Fig. 2, right).
FIG. 2.
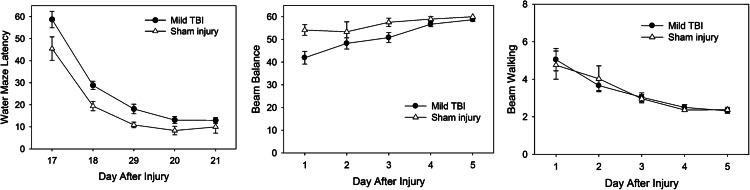
Behavioral characteristics of the mild traumatic brain injury (TBI) model, showing transient beam balancing deficits and mild impairment on the Morris water maze test compared to sham-injured animals.
Effects of pHBSP in the mild TBI model
Recovery of reflexes
As a measure of initial injury severity (prior to drug treatment), recovery of reflexes was recorded after the injury.29–31 The recovery time in minutes for escape, head support, righting, corneal, pinna, paw, and tail reflexes, were analyzed separately for each reflex, and for a measure combining all reflexes using two-factor between-groups ANOVA having treatment type and time of administration as the factors. No significant differences were seen among the treatment groups for any of the reflexes or for an average reflex measure (Table 1), suggesting that the injury severity was similar in all groups.
Table 1.
Recovery of Reflexes after Injury for the Treatment Groups
| |
Early treatment (min) |
Delayed treatment (min) |
Main effects p values (partial η2) |
|||
|---|---|---|---|---|---|---|
| Recovery of reflex (minutes after injury) | pHBSP | Control | pHBSP | Control | Drug | Time of administration |
| Escape reflex | 7.4±0.5 | 6.9±0.4 | 6.7±0.4 | 6.9±0.2 | 0.478 (0.01) | 0.387 (0.01) |
| Head support reflex | 6.6±0.4 | 6.2±0.4 | 6.1±0.3 | 6.2±0.3 | 0.484 (0.01) | 0.484 (0.01) |
| Righting reflex | 6.0±0.4 | 5.6±0.4 | 5.4±0.3 | 5.6±0.2 | 0.487 (0.01) | 0.372 (0.01) |
| Corneal reflex | 4.8±0.3 | 4.4±0.3 | 4.3±0.2 | 4.5±0.2 | 0.442 (0.01) | 0.442 (0.01) |
| Pinna reflex | 4.6±0.3 | 4.1±0.3 | 3.9±0.2 | 4.2±0.2 | 0.501 (0.01) | 0.347 (0.02) |
| Paw reflex | 3.5±0.3 | 3.0±0.3 | 2.6±0.2 | 3.1±0.2 | 0.813 (0.01) | 0.160 (0.03) |
| Tail reflex | 4.0±0.3 | 3.6±0.2 | 3.5±0.2 | 3.7±0.2 | 0.477 (0.01) | 0.320 (0.03) |
pHBSP, pyroglutamate helix B surface peptide.
Weight
Weight was recorded at baseline and daily and on days 1–5 and on days 17–21, as a measure of the general health of the animals post-injury. In all groups there was a small drop in weight on day 1 post-injury, with recovery and a gradual increase in weight thereafter (Table 2). Weight was analyzed separately for baseline prior to surgery, for days 1–5 post-injury, and for days 17–21 post-injury. Baseline weight was analyzed by a two-factor ANOVA with treatment and time of administration as the between-groups factors. No differences in baseline weight were observed among the treatment groups, indicating that the groups were similar in weight before surgery. Three-factor mixed ANOVAs with treatment and time as the between-groups factors, and days 1–5 or days 17–21 as the repeated-measures factors, were performed. The main effects of drug treatment and time of administration, as well as the drug×time interaction, were not significantly different for either days 1–5 or days 17–21.
Table 2.
Weight of the Animals Over Time in the Treatment Groups
| |
Early treatment (g) |
Delayed treatment (g) |
Main effects p values (partial η2) |
|||
|---|---|---|---|---|---|---|
| pHBSP | Control | pHBSP | Control | Drug | Time of administration | |
| Baseline | 366±8 | 369±8 | 360±7 | 371±8 | 0.416 (0.01) | 0.840 (0.01) |
| Day 1 | 361±8 | 361±9 | 353±7 | 368±8 | 0.389 (0.01) | 0.913 (0.00) |
| Day 2 | 363±8 | 364±8 | 354±7 | 369±8 | ||
| Day 3 | 366±7 | 365±8 | 356±7 | 371±8 | ||
| Day 4 | 367±7 | 367±9 | 358±7 | 373±8 | ||
| Day 5 | 368±7 | 369±9 | 361±6 | 376±8 | ||
| Day 17 | 413±7 | 413±11 | 408±6 | 425±14 | 0.211 (0.03) | 0.573 (0.01) |
| Day 18 | 415±7 | 414±12 | 410±6 | 427±14 | ||
| Day 19 | 416±7 | 415±12 | 411±6 | 429±14 | ||
| Day 20 | 417±7 | 416±12 | 414±7 | 432±14 | ||
| Day 21 | 419±7 | 418±12 | 414±6 | 433±14 | ||
pHBSP, pyroglutamate helix B surface peptide.
Effect of pHBSP on behavioral outcome measures
Beam-walking and beam-balance tests
Motor strength and coordination were tested prior to injury and daily (3 trials/day) on post-injury days 1–5 using the beam-balance and beam-walking tests. For the beam-walking test, the length of time required to cross a narrow beam was recorded for each trial, with a shorter time representing better performance. For trials in which the rat fell off or could not complete the task within 30 sec, a value of 31 sec was used in the analysis. For the beam-balance test, the length of time that the animals could balance on a narrow beam was recorded for each trial, with a longer time indicating better performance. The details of the statistical analysis are summarized in Table 3.
Table 3.
Details of the Statistical Analysis of the Outcome Measures
| |
p Values (partial η2) for main effects |
p Values (partial η2) for interactions |
||||
|---|---|---|---|---|---|---|
| Outcome measure | Drug treatment (pHBSP or control) | Time of administration (early or delayed) | Day post-injury | Trial each day | Drug × time | Day × trial |
| Histology (two-factor ANOVA) | ||||||
| Contusion volume | 0.817 (0.06) | 0.252 (0.02) | 0.787 (0.06) | |||
| CA1 neuron count | 0.312 (0.02) | 0.768 (0.00) | 0.590 (0.01) | |||
| CA3 neuron count | 0.917 (0.00) | 0.558 (0.01) | 0.220 (0.03) | |||
| CD68-labeled cell count | 0.040 (0.07) | 0.723 (0.00) | 0.767 (0.00) | |||
| Morris water maze (four-factor mixed ANOVA) | ||||||
| Time to find platform | 0.022 (0.08) | 0.014 (0.10) | 0.000 (0.75) | 0.000 (0.34) | 0.901 (0.00) | 0.000 (0.16) |
| % Time spent in platform quadrant | 0.825 (0.00) | 0.148 (0.03) | 0.000 (0.19) | 0.000 (0.19) | 0.860 (0.00) | 0.000 (0.25) |
| Distance traveled to find platform | 0.019 (0.09) | 0.029 (0.08) | 0.000 (0.74) | 0.000 (0.34) | 0.599 (0.01) | 0.000 (0.16) |
| Swim speed | 0.448 (0.01) | 0.534 (0.01) | 0.000 (0.11) | 0.028 (0.05) | 0.027 (0.08) | 0.000 (0.17) |
| Probe trial: time spent in platform quadrant | 0.416 (0.01) | 0.954 (0.00) | 0.914 (0.00) | |||
| Visible platform: time to find platform | 0.263 (0.02) | 0.996 (0.00) | 0.177 (0.03) | |||
| Beam walking (three- or four-factor mixed ANOVA) | ||||||
| Pre-injury time to cross beam | 0.458 (0.01) | 0.895 (0.00) | 0.000 (0.13) | 0.411 (0.01) | ||
| Post-injury time to cross beam | 0.658 (0.00) | 0.294 (0.02) | 0.000 (0.34) | 0.000 (0.14) | 0.366 (0.01) | 0.001 (0.05) |
| Beam balance (four-factor mixed ANOVA) | ||||||
| Post-injury balance time | 0.815 (0.00) | 0.911(0.00) | 0.000 (0.38) | 0.178 (0.028) | 0.997 (0.00) | 0.012 (0.04) |
pHBSP, pyroglutamate helix B surface peptide; ANOVA, analysis of variance.
Baseline beam-walking and beam-balance performance were tested prior to the surgery to produce the impact injury. To analyze the pre-injury beam walking data, a three-factor mixed ANOVA with the between-group effects of drug treatment (pHBSP or control), time of administration (early or late), and repeated measures on trial (1–3). There were no significant differences in baseline beam-walking performance by drug treatment or time of administration, indicating that the groups had similar performance on these motor tasks prior to the injury. For the beam-balance task, all animals were able to balance for the full 60 sec prior to injury.
Beam-walking and beam-balance performance post-injury were analyzed with a mixed four-factor ANOVA design involving the between-group effects of drug treatment (pHBSP or control), time of administration (early or late), repeated measures on day (1–5) post-injury, and trial (1–3) within each day. Beam-walking time improved significantly over time in all animals, from 5.4±0.6 sec on day 1, to 2.4±0.1 sec on day 5. And on each day, beam walking improved with each subsequent trial. This improvement with repeated trials was greatest on day 1. Beam balance also improved significantly over time, from 42.5±2.2 sec on day 1, to 57.8±0.9 sec on day 5. There were no significant effects of drug treatment or time of administration, and the drug×time of administration interaction was not significant for either the beam-walking or beam-balance testing.
Morris water maze test
Spatial learning was tested daily (4 trials/day) using the Morris water maze on post-injury days 17–21. For trials for which the rat successfully found the platform, the length of time required was used in the analysis. For trials for which the rat was not able to find the platform within 120 sec, a value of 121 sec was assigned for the analysis. The path length traveled to find the platform and the swim speed were also recorded for each trial. To analyze these variables, a four-factor mixed ANOVA design involving between-group effects of drug treatment (pHBSP or control), time of administration (early or late), repeated measures on day (17–21) post-injury, and trial (1–4) within each day was conducted. The results of the statistical analyses are summarized in Table 3.
For all rats, the length of time required to find the platform decreased progressively over time, from 55.5±2.3 sec on day 17, to 11.6±0.8 sec on day 21 (p<0.001, partial η2=0.75), and decreased on each day with subsequent trials. This decrease with repeated trials was largest on the first day of testing (day 17 post-injury), and decreased progressively over the 5 days of testing.
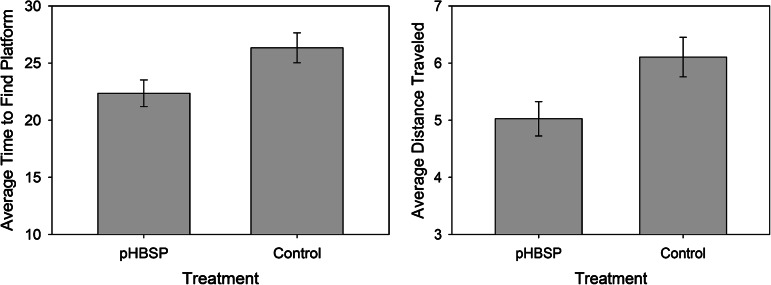
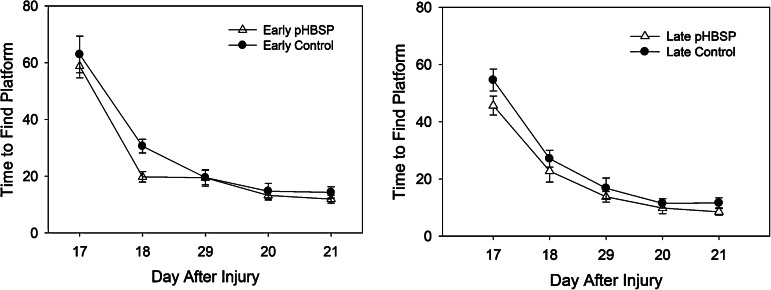
Rats that received pHBSP required a significantly shorter time to find the platform, 22.4±1.2 sec, compared to 26.3±1.2 sec in rats that received control treatment (p=0.022, partial η2=0.08). The rats that received pHBSP also traveled a significantly shorter distance to get to the platform, 5.0±0.3 meters, compared to 6.1±0.3 meters in rats that received control treatment (p=0.019, partial η2=0.09). There was no significant interaction between the drug treatment and the time of administration for either time to find the platform or for distance traveled to get to the platform, suggesting that the improvement in spatial learning with pHBSP was similar when given early after injury and when delayed until 24 h post-injury. The average time to find the platform over all 5 days of testing is shown in Figure 3 (lef). Plots of the average time to find the platform for each day of testing are shown in Figure 4 (animals treated early on the left, animals treated at 24 h on the right). The average distance traveled to find the platform over all 5 days of testing is shown in Figure 3 (right). For both the early and the late treatment times, the animals treated with pHBSP required a shorter time to find the platform compared to the rats that received control treatment.
FIG. 3.
The average time to find the platform was significantly shorter (left, p=0.022), and the distance traveled to find the platform was shorter (right, p=0.019), in the animals treated with pHBSP compared to the control animals (pHBSP, pyroglutamate helix B surface peptide).
FIG. 4.
Average time to find the platform by day after injury was slightly shorter in the animals that received pHBSP compared to those that received control treatment, both with early treatment (left) and with treatment at 24 h post-injury (right). These differences were greatest on the first 2 days of testing (pHBSP, pyroglutamate helix B surface peptide).
There was also a significant main effect of administration time (p=0.014, partial η2=0.10), with the animals that were treated early post-injury (both pHBSP- and control-treated animals) requiring longer to find the platform than animals having treatment that was delayed 24 h. This effect can be seen in Figure 4, where the average time to find the platform for both early treatment groups (pHBSP and control) was longer than the times for both late treatment groups on the first day of testing. Since this difference was not limited to a specific treatment (pHBSP or control), it was not clear what aspect of administering the drug early post-injury resulted in this finding.
Swim speed in meters per second decreased significantly over time (p=0.001, partial η2=0.75). There was a significant drug×time of administration interaction (p=0.027, partial η2=0.08), with the rats treated with pHBSP early having a slower swim speed than the control rats treated early, while the rats treated with pHBSP at 24 h post-injury had a faster swim speed than control rats treated at 24 h post-injury. Swim speed was not related to the group differences in time to find the platform.
The time required to find the platform on post-injury day 21 during the probe trial and the visible platform trial were analyzed using a two-factor ANOVA, with between-groups factors of drug treatment (pHBSP or control), and time of administration (early or late). There was no significant difference in performance during these tasks for drug treatment, time of administration, or an interaction.
Effect of pHBSP on histological outcome measures
As described under the characteristics of the mTBI model, contusion volume and neuronal loss in this mild injury was small in the majority of animals. Only 11 animals (17%, 2 or 3 animals per treatment group) had a contusion volume greater than 2 mm3. Contusion volume was not significantly different among the treatment groups. Likewise neuronal loss in the hippocampus was minimal, and there were no significant differences among treatment groups.
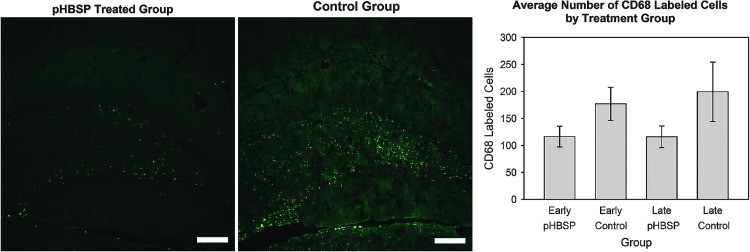
As a measure of the cellular inflammatory response to injury (Fig. 5), cells of monocyte/macrophage origin were labeled and counted using an antibody against CD68-IR cells in the hippocampus ipsilateral to the lesion site. Despite the minor nature of the contusion, there were numerous CD68-labeled cells in the majority of animals. The number of CD68-labeled cells was significantly less in the animals treated with pHBSP, 116±24 compared to 188±24, in the control animals (drug effect p=0.043, partial η2=0.07). As shown in the graph in Figure 5, the reduction in inflammation was similar regardless of the time of the start of treatment (drug×time of administration interaction p=0.744).
FIG. 5.
Despite an absence of grossly-detectable tissue damage, macrophages, either infiltrating or resident microglia, labeled with an antibody against CD68 were observed in the injured brain (left and middle images; scale bar=200 μm). There were fewer CD68-immunoreactive cellular profiles in the ipsilateral hippocampus of pHBSP-treated rats than in control-treated mTBI rats (right graph; main treatment effect p=0.043; pHBSP, pyroglutamate helix B surface peptide).
Summary of pHBSP effects
In summary, the major significant findings in this mTBI model are an improvement in Morris water maze performance and a reduction in the number of inflammatory cells in the injured cortex with pHBSP administration.
Discussion
pHBSP has been shown to have cytoprotective activity in models of stroke, myocardial and renal ischemia-reperfusion, renal and neuronal cisplatinum toxicity, diabetic neuropathy and retinopathy, sciatic nerve crush injury, status epilepticus, wound healing, and other models, when administered at doses in the range of 0.5–10 μg/kg.32–36
In a previous TBI study, pHBSP at a dose of 30 μg/kg every 12 h for 3 days reduced contusion volume and improved neurobehavioral recovery in a model of mTBI followed by hemorrhagic shock.25 Contusion volume was decreased by 70%, and neurobehavioral improvements were limited to motor tasks. The current study shows that pHBSP also improves some aspects of neurological outcome in an uncomplicated mTBI model. In addition, these improvements were no different when administration was delayed until 24 h post-injury.
Some aspects of these treatment effects with pHBSP are similar to those that have been described with EPO, and with the EPO derivative carbamylated EPO (CEPO) in more severe traumatic injuries. When EPO or CEPO were given within 6 h of severe TBI, major reductions in contusion volume and cellular inflammatory response,21 and improvements in hippocampal neuronal survival have been observed.14,15,37 Improvements in performance on behavioral tasks also occur.37 When treatment is delayed until 24 h post-injury, contusion volume is not reduced, but preservation of hippocampal neurons and improvement in performance on the Morris water maze task have been reported.37,38 These improvements with delayed treatment despite the lack of preservation of tissue at the impact site have been attributed to enhancement of recovery through neurogenesis. The lack of effect on contusion volume and hippocampal neuronal survival with early administration of pHBSP in the uncomplicated mTBI model is likely due to the very minor nature of the cellular loss in this mild TBI model.
Improvements in cognitive function with EPO have also been noted in patients treated with EPO for anemia,39 in normal volunteers who have been given single doses of EPO,40,41 and in schizophrenic patients treated for 3 months with weekly injections of EPO.40 Because the current study did not include a drug-treated and placebo-treated sham-injury group, it cannot be determined if the improvements on the Morris water maze task are due to neuroprotection/neurorestoration following mTBI, or to this enhancement of cognitive function that has been observed with EPO-like agents in other conditions.
The minor deficits due to the nature of the mild injury could be viewed as a limitation of the present study. The injury resulted in a very small contusion and only modest behavioral deficits compared to sham-injured animals. Therefore the improvements seen with any drug treatment would be necessarily small in this model. Performance on the motor tasks in the mTBI model was only transiently impaired (beam-balance test), or not impaired at all (beam-walking test), compared to sham-injured animals. No consistent improvements were seen in motor performance with pHBSP. The results in the Morris water maze showed a small but significant improvement in the time required to find the platform with pHBSP.
While this characteristic of the mTBI model could be considered a limitation of the study, this is also the nature of mTBI in humans. The major consequences of mTBI are cognitive problems and post-concussive symptoms. Motor symptoms are uncommon. The post-concussive symptoms that occur with mTBI are difficult to model in rodents, but cognitive tests are widely used in rodent studies. The Morris water maze is one of the more common rodent tests of cognition. It tests acquisition of spatial navigation, and is sensitive to hippocampal injury. In humans, wayfinding is commonly impaired after TBI, and spatial navigation has been tested and been found to be impaired using a virtual reality simulation of the Morris water maze.42 Therefore the findings of improved Morris water maze performance should be pertinent to mTBI patients. Other tests in future preclinical studies involving the mTBI model might include tasks of working memory and conflict active avoidance tasks.43,44
Another limitation of the study is the confounding finding of the time of administration. This finding did not seem to be related to the treatment, because both the pHBSP-treated and the control-treated animals had better performance in the Morris water maze with delayed treatment, compared to early treatment. It is possible that this was due to an imbalance in the randomization, with more severely injured animals being randomized to the two early treatment groups. However, the time to recovery of the righting reflex, which was used as a measure of the pre-treatment injury severity, was similar in all treatment groups. The only difference in the way that the early- and delayed-treatment animals were managed was in receiving the first dose of the drug at 1 h post-injury or at 24 h post-injury, respectively. Stress can alter physiological parameters in rodents, and could potentially alter the outcome from an mTBI injury. For example, an IP injection of saline can result in an increased glucocorticoid response to a second saline injection.45 Restraint stress can even alter aspects of brain metabolism, such as amyloid-beta and brain-derived neurotrophic factor production.46 A stronger study design that might have allowed better comparison of the early and late treatments would have been to give the delayed treatment groups placebo injections on the day of injury.
The neuroprotective/neurorestorative mechanism of action for pHBSP is thought to be similar to that of EPO, with actions that include anti-inflammatory effects and inhibition of apoptosis. Following experimental TBI, administration of EPO attenuated the injury-induced increase in interleukin-1β and tumor necrosis factor-α in the brain.47,48 Interleukin-6 levels and reduced numbers of activated glial cells have also been reported to be lower following EPO administration.21,49 The finding of a decrease in CD68-labeled cells is consistent with the reports for EPO,21 and suggests that inhibition of the inflammatory response to mTBI may play a role in the improved outcome seen with pHBSP in our mTBI model. Cytoprotective activities of pHBSP in other models have also included anti-inflammatory activities. In a hemorrhagic shock model, pHBSP significantly reduced the inflammatory response and subsequent injury to the lungs.50 The mechanism was thought to be due to activation of Akt, which enhances tissue survival by inhibiting GSK-3β, and it also inhibits the inflammatory response to hemorrhage by reducing the injury-induced activation of NF-κB. In a myocardial ischemia model induced by permanent ligation of a coronary artery, pHBSP decreased the infarct size when given in a dose of 1–60 μg/kg at 5 min after the ligation, but no change was seen when it was given 24 h after the ligation. pHBSP reduced the number of inflammatory cells (neutrophils and macrophages) by 34% in the area of myocardium at risk.32
Conclusions
The studies suggest that pHBSP may be a useful drug for mTBI. The findings of an improvement in spatial navigation acquisition are relevant to the cognitive impairments seen with TBI. Treatment with pHBSP can be delayed as long as 24 h post-injury, which is important with mTBI. Unlike more severe injuries, for which patients are brought immediately to the hospital after the accident, mTBI patients may not be as rapidly evaluated. Finally, since pHBSP does not stimulate erythropoiesis, it may be safer than EPO in a disorder like mTBI, for which the hemoglobin concentration is usually normal.
Acknowledgment
This work was funded by U.S. Army Award numbers W81XWH-08-2-0132 (C.S.R.) and W81XWH-08-2-0150 (R.J.G.).
Author Disclosure Statement
The pHBSP was provided by Araim Pharmaceuticals, Inc. Dr. Hand is a shareholder of Warren Pharmaceuticals which shareholder of Araim Pharmaceuticals. Araim Pharmaceuticals is developing tissue-protective compounds including pHBSP.
References
- 1.Menon D.K. Schwab K. Wright D.W. Maas A.I. Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Baratz R. Tweedie D. Rubovitch V. Luo W. Yoon J.S. Hoffer B.J. Greig N.H. Pick C.G. Tumor necrosis factor-alpha synthesis inhibitor, 3, 6′-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J. Neurochem. 2011;118:1032–1042. doi: 10.1111/j.1471-4159.2011.07377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel A.D. Gerzanich V. Geng Z. Simard J.M. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J. Neuropathol. Exp. Neurol. 2010;69:1177–1190. doi: 10.1097/NEN.0b013e3181fbf6d6. [DOI] [PubMed] [Google Scholar]
- 4.Zohar O. Rubovitch V. Milman A. Schreiber S. Pick C.G. Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol. Exp. (Wars.) 2011;71:36–45. doi: 10.55782/ane-2011-1821. [DOI] [PubMed] [Google Scholar]
- 5.Rubovitch V. Edut S. Sarfstein R. Werner H. Pick C.G. The intricate involvement of the insulin-like growth factor receptor signaling in mild traumatic brain injury in mice. Neurobiol. Dis. 2010;38:299–303. doi: 10.1016/j.nbd.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Haselkorn M.L. Shellington D.K. Jackson E.K. Vagni V.A. Janesko-Feldman K. Dubey R.K. Gillespie D.G. Cheng D. Bell M.J. Jenkins L.W. Homanics G.E. Schnermann J. Kochanek P.M. Adenosine A1 receptor activation as a brake on the microglial response after experimental traumatic brain injury in mice. J. Neurotrauma. 2010;27:901–910. doi: 10.1089/neu.2009.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa T. Constantino L.C. Mendonca B.P. Pereira J.G. Herculano B. Tasca C.I. Boeck C.R. N-methyl-D-aspartate preconditioning improves short-term motor deficits outcome after mild traumatic brain injury in mice. J. Neurosci. Res. 2010;88:1329–1337. doi: 10.1002/jnr.22300. [DOI] [PubMed] [Google Scholar]
- 8.Aiguo W. Zhe Y. Gomez-Pinilla F. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil. Neural Repair. 2010;24:290–298. doi: 10.1177/1545968309348318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabo Z. Ying Z. Radak Z. Gomez-Pinilla F. Voluntary exercise may engage proteasome function to benefit the brain after trauma. Brain Res. 2010;1341:25–31. doi: 10.1016/j.brainres.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atalay B. Bavbek M. Ozen O. Nacar A. Gulsen S. Yigitkanli K. Caner H. Altinors N. Nogo-A inhibitory peptide (NEP1-40) increases pan-cadherin expression following mild cortical contusion injury in rats. Turk. Neurosurg. 2008;18:356–365. [PubMed] [Google Scholar]
- 11.Fujiki M. Kubo T. Kamida T. Sugita K. Hikawa T. Abe T. Ishii K. Kobayashi H. Neuroprotective and antiamnesic effect of donepezil, a nicotinic acetylcholine-receptor activator, on rats with concussive mild traumatic brain injury. J. Clin. Neurosci. 2008;15:791–796. doi: 10.1016/j.jocn.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Milman A. Zohar O. Maayan R. Weizman R. Pick C.G. DHEAS repeated treatment improves cognitive and behavioral deficits after mild traumatic brain injury. Eur. Neuropsychopharmacol. 2008;18:181–187. doi: 10.1016/j.euroneuro.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Atalay B. Caner H. Can A. Cekinmez M. Attenuation of microtubule associated protein-2 degradation after mild head injury by mexiletine and calpain-2 inhibitor. Br. J. Neurosurg. 2007;21:281–287. doi: 10.1080/02688690701364781. [DOI] [PubMed] [Google Scholar]
- 14.Brines M.L. Ghezzi P. Keenan S. Agnello D. de Lanerolle N.C. Cerami C. Itri L.M. Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherian L. Goodman C.J. Robertson C.S. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J. Pharmacol. Exp. Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- 16.Lu D. Mahmood A. Changsheng Q. Goussev A. Schallert T. Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J. Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 17.Marti H.H. Bernaudin M. Petit E. Bauer C. Neuroprotection and angiogenesis: Dual role of erythropoietin in brain ischemia. News Physiol. Sci. 2000;15:225–229. doi: 10.1152/physiologyonline.2000.15.5.225. [DOI] [PubMed] [Google Scholar]
- 18.Siren A.L. Fratelli M. Brines M. Goemans C. Casagrande S. Lewczuk P. Keenan S. Gleiter C. Pasquali C. Capobianco A. Mennini T. Heumann R. Cerami A. Ehrenreich H. Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl. Acad. Sci. USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villa P. Bigini P. Mennini T. Agnello D. Laragione T. Cagnotto A. Viviani B. Marinovich M. Cerami A. Coleman T.R. Brines M. Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L. Zhang Z. Wang Y. Zhang R. Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 21.Yatsiv I. Grigoriadis N. Simeonidou C. Stahel P.F. Schmidt O.I. Alexandrovitch A.G. Tsenter J. Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- 22.Leist M. Ghezzi P. Grasso G. Bianchi R. Villa P. Fratelli M. Savino C. Bianchi M. Nielsen J. Gerwien J. Kallunki P. Larsen A.K. Helboe L. Christensen S. Pedersen L.O. Nielsen M. Torup L. Sager T. Sfacteria A. Erbayraktar S. Erbayraktar Z. Gokmen N. Yilmaz O. Cerami-Hand C. Xie Q.W. Coleman T. Cerami A. Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 23.Brines M. Grasso G. Sfacteria A. Ghezzi P. Fratelli M. Latini R. Xie Q.W. Smart J. Su-Rick C. Pobre E. Diaz D. Gomez D. Hand C. Coleman T. Cerami A. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc. Natl. Acad. Sci. USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brines M. Patel N.S. Villa P. Brines C. Mennini T. De P.M. Erbayraktar Z. Erbayraktar S. Sepodes B. Thiemermann C. Ghezzi P. Yamin M. Hand C.C. Xie Q.W. Coleman T. Cerami A. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. USA. 2008;105:10925–10930. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson C.S. Cherian L. Shah M. Garcia R. Navarro J.C. Grill R.J. Hand C.C. Tian T.S. Hannay H.J. Neuroprotection with an erythropoietin mimetic peptide (pHBSP) in a model of mild traumatic brain injury complicated by hemorrhagic shock. J. Neurotrauma. 2012;29:1156–1166. doi: 10.1089/neu.2011.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. Academic Press; New York: 1998. [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 28.Ferguson C.J. An effect size primer: A guide for clinicians and researchers. Professional Psychol.y. 2009;40:532–538. [Google Scholar]
- 29.Hallam T.M. Floyd C.L. Folkerts M.M. Lee L.L. Gong Q.-Z. Lyeth B.G. Muizelaar J.P. Berman R.F. Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop models. J Neurotrauma. 2004;21:521–539. doi: 10.1089/089771504774129865. [DOI] [PubMed] [Google Scholar]
- 30.Hamm R.J. Neurobehavioral assessment of outcome following traumatic brain injury in rats: An evaluation of selected measures. J. Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt R.H. Scholten K.J. Maughan P.H. Cognitive impairment and synaptosomal choline uptake in rats following impact acceleration injury. J. Neurotrauma. 2000;17:1129–1139. doi: 10.1089/neu.2000.17.1129. [DOI] [PubMed] [Google Scholar]
- 32.Ahmet I. Tae H.J. Juhaszova M. Riordon D.R. Boheler K.R. Sollott S.J. Brines M. Cerami A. Lakatta E.G. Talan M.I. A small nonerythropoietic helix B surface peptide based upon erythropoietin structure is cardioprotective against ischemic myocardial damage. Mol. Med. 2011;17:194–200. doi: 10.2119/molmed.2010.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McVicar C.M. Hamilton R. Colhoun L.M. Gardiner T.A. Brines M. Cerami A. Stitt A.W. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes. 2011;60:2995–3005. doi: 10.2337/db11-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeger N. Zellinger C. Rode A. Roloff F. Bicker G. Russmann V. Fischborn S. Wendt H. Potschka H. The erythropoietin-derived peptide mimetic pHBSP affects cellular and cognitive consequences in a rat post-status epilepticus model. Epilepsia. 2011;52:2333–2343. doi: 10.1111/j.1528-1167.2011.03302.x. [DOI] [PubMed] [Google Scholar]
- 35.Ueba H. Brines M. Yamin M. Umemoto T. Ako J. Momomura S. Cerami A. Kawakami M. Cardioprotection by a nonerythropoietic, tissue-protective peptide mimicking the 3D structure of erythropoietin. Proc. Natl. Acad. Sci. USA. 2010;107:14357–14362. doi: 10.1073/pnas.1003019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zellinger C. Seeger N. Hadamitzky M. Fischborn S. Russmann V. Wendt H. Pankratova S. Bock E. Berezin V. Potschka H. Impact of the erythropoietin-derived peptide mimetic Epotris on the histopathological consequences of status epilepticus. Epilepsy Res. 2011;96:241–249. doi: 10.1016/j.eplepsyres.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Xiong Y. Mahmood A. Zhang Y. Meng Y. Zhang Z.G. Qu C. Sager T.N. Chopp M. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J. Neurosurg. 2011;114:549–559. doi: 10.3171/2010.10.JNS10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong Y. Mahmood A. Meng Y. Zhang Y. Qu C. Schallert T. Chopp M. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J. Neurosurg. 2010;113:598–608. doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickett J.L. Theberge D.C. Brown W.S. Schweitzer S.U. Nissenson A.R. Normalizing hematocrit in dialysis patients improves brain function. Am. J. Kidney Dis. 1999;33:1122–1130. doi: 10.1016/S0272-6386(99)70150-2. [DOI] [PubMed] [Google Scholar]
- 40.Miskowiak K. O'Sullivan U. Harmer C.J. Erythropoietin enhances hippocampal response during memory retrieval in humans. J. Neurosci. 2007;27:2788–2792. doi: 10.1523/JNEUROSCI.5013-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miskowiak K. Inkster B. O'Sullivan U. Selvaraj S. Goodwin G.M. Harmer C.J. Differential effects of erythropoietin on neural and cognitive measures of executive function 3 and 7 days post-administration. Exp. Brain Res. 2008;184:313–321. doi: 10.1007/s00221-007-1102-1. [DOI] [PubMed] [Google Scholar]
- 42.Livingstone S.A. Skelton R.W. Virtual environment navigation tasks and the assessment of cognitive deficits in individuals with brain injury. Behav. Brain Res. 2007;185:21–31. doi: 10.1016/j.bbr.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Abdel Baki S.G. Kao H.Y. Kelemen E. Fenton A.A. Bergold P.J. A hierarchy of neurobehavioral tasks discriminates between mild and moderate brain injury in rats. Brain Res. 2009;1280:98–106. doi: 10.1016/j.brainres.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 44.Kline A.E. Massucci J.L. Marion D.W. Dixon C.E. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 45.Drude S. Geissler A. Olfe J. Starke A. Domanska G. Schuett C. Kiank-Nussbaum C. Side effects of control treatment can conceal experimental data when studying stress responses to injection and psychological stress in mice. Lab. Anim. (NY) 2011;40:119–128. doi: 10.1038/laban0411-119. [DOI] [PubMed] [Google Scholar]
- 46.Ray B. Gaskins D.L. Sajdyk T.J. Spence J.P. Fitz S.D. Shekhar A. Lahiri D.K. Restraint stress and repeated corticotrophin-releasing factor receptor activation in the amygdala both increase amyloid-beta precursor protein and amyloid-beta peptide, but have divergent effects on brain-derived neurotrophic factor and pre-synaptic proteins in the prefrontal cortex of rats. Neuroscience. 2011;184:139–150. doi: 10.1016/j.neuroscience.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen G. Shi J.X. Hang C.H. Xie W. Liu J. Liu X. Inhibitory effect on cerebral inflammatory agents that accompany traumatic brain injury in a rat model: a potential neuroprotective mechanism of recombinant human erythropoietin (rhEPO) Neurosci. Lett. 2007;425:177–182. doi: 10.1016/j.neulet.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Lieutaud T. Andrews P.J. Rhodes J.K. Williamson R. Characterization of the pharmacokinetics of human recombinant erythropoietin in blood and brain when administered immediately after lateral fluid percussion brain injury and its pharmacodynamic effects on IL-1beta and MIP-2 in rats. J. Neurotrauma. 2008;25:1179–1185. doi: 10.1089/neu.2008.0591. [DOI] [PubMed] [Google Scholar]
- 49.Bian X.X. Yuan X.S. Qi C.P. Effect of recombinant human erythropoietin on serum S100B protein and interleukin-6 levels after traumatic brain injury in the rat. Neurol. Med. Chir. (Tokyo) 2010;50:361–366. doi: 10.2176/nmc.50.361. [DOI] [PubMed] [Google Scholar]
- 50.Patel N.S. Nandra K.K. Brines M. Collino M. Wong W.F. Kapoor A. Benetti E. Goh F.Y. Fantozzi R. Cerami A. Thiemermann C. A nonerythropoietic peptide that mimics the 3D structure of erythropoietin reduces organ injury/dysfunction and inflammation in experimental hemorrhagic shock. Mol. Med. 2011;17:883–892. doi: 10.2119/molmed.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]