Abstract
Immune response modifiers are being studied as therapeutic agents for viral infections and cancer. These molecules include agonists for the Toll-like receptors (TLR), a family of innate immune receptors. TLR7 and 8, located in cellular endosomes, bind single-stranded RNA characteristic of viral genomes, and trigger intracellular signaling pathways that induce inflammatory cytokines and antiviral innate immune factors. We studied the anti-HIV-1 effects of gardiquimod, a specific TLR7 agonist when used at concentrations below 10 μM, in macrophages and activated peripheral blood mononuclear cells (PBMCs). Gardiquimod, added prior to or within 2 days after infection with X4, R5, or dual-tropic (R5/X4) strains of HIV-1, significantly reduced infection in these cells. Cocultures of activated PBMCs added to gardiquimod-treated and HIV-1-exposed macrophages demonstrated minimal HIV-1 replication for up to 10 days, suggesting that gardiquimod inhibited activated PBMCs viral amplification from HIV-1-exposed macrophages. Gardiquimod treatment of both activated PBMCs and macrophages induced interferon-alpha (IFN-α) transcription within hours of addition, and sustained IFN-α protein secretion for several days. Treatment of cells with a peptide inhibitor to the MyD88 adaptor protein blocked the induction of IFN-α by gardiquimod, and partially reversed the anti-HIV effects in activated PBMCs. Blocking the IFN-α receptor with a neutralizing antibody also reduced the anti-HIV effect of gardiquimod. Gardiquimod inhibited HIV-1 reverse transcriptase, an early step in the life cycle of HIV-1. These findings suggest that gardiquimod, functioning as both an immune system modifier and a reverse transcriptase inhibitor, could be developed as a novel therapeutic agent to block systemic and mucosal transmission of HIV-1.
Introduction
Toll-like receptors (TLR) are a family of highly conserved pattern recognition receptors involved in innate immune responses to pathogen infection. Some of the more than two dozen members of this class of receptors that include TLR3, TLR7, TLR8, and TLR9 are localized within intracellular vesicles including the endoplasmic reticulum, endosomes, lysosomes, and endolysosomes. These intracellular TLR recognize microbial nucleic acids,1 and once activated, induce rapid antiviral responses characterized by the production of innate immune factors including inflammatory cytokines and antiviral factors. TLR7 and TLR8 were originally identified by the ability to recognize imidazoquinoline derivatives such as imiquimod and resiquimod, and guanine analogs such as loxoribine that possess antiviral and antitumor properties. TLR7 and TLR8 recognize single-stranded RNA from viruses including vesicular stomatitis virus, influenza A virus, and the human immunodeficiency virus (HIV),2,3 and also recognize synthetic RNA molecules including small interfering RNA (siRNA).4
TLR7 is highly expressed by plasmacytoid dendritic cells,2,3 and is also found on other leukocyte subpopulations including macrophages,5 B cells,6 CD4-T cells,7 as well as CD8-T cells.8 Binding of ligand to TLR7 results in the activation of this receptor, and the induction of an intracellular signaling cascade promoted by the adaptor protein termed myeloid differentiation primary response gene 88 (MyD88). MyD88 then activates the transcription factors nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interferon regulatory factor 7 (IRF7), leading to inflammatory cytokine and type I interferon production. In dendritic cells, the cytokine storm in response to viral infection is totally dependent on TLR7, suggesting that TLR7 serves as a sensor to infection by single-stranded RNA viruses.
Although the natural ligands for TLR7 derive from viral pathogens, a number of molecules have been identified that act either as agonists or antagonists for these receptors, and are reported to induce immune responses that lead to control of viral replication or cancer cell killing. Isatoribine, a TLR7 agonist, was reported to reduce plasma viral levels of hepatitis C in otherwise untreated patients with chronic hepatitis C infection.9 In patients with cutaneous T cell lymphoma (CTCL), the combination of cytokine therapy using interferon-gamma (IFN-γ) plus interleukin-15 (IL-15), together with 3M-007, a synthetic imidazoquinoline that functions as a TLR7 and TLR8 agonist, significantly increased the natural killer (NK) cytolytic activity against CTCL tumor cell lines, compared to treatment with either cytokines or 3M-007 alone.10
Imiquimod and other compounds in this class were initially developed as antiviral agents, although recently they have been found to have potent effects on the immune system.11 Because these molecules stimulate antigen-presenting cells (APC) via TLR activation, they act as immunologic adjuvants. By triggering cytokine production, TLR agonists enhance the ability of APC to present foreign antigens to T cells, and amplify T helper cell responses by the increased production of cytokines including type I and type II interferons.12–15
The use of TLR7, TLR8, or TLR9 agonists to block infection by retroviruses has been studied by several groups. Brichacek et al. reported that the TLR9 agonist CpG oligonucleotide (CpG ODN) markedly inhibited infection by both X4 as well as R5 tropic strains of HIV-1 in primary explants prepared from tonsil tissue. 16 In contrast, Wang et al.17 reported that the TLR7 agonist imiquimod and the TLR9 agonist CpG-ODN applied vaginally to rhesus macaques resulted in an inflammatory response that led to an influx of immune cells and an enhancement of simian immunodeficiency virus (SIV) infection compared to the untreated control animals. This heightened inflammatory response following sustained TLR 7/8 stimulation was confirmed in a separate study in which mice administered resiquimod (R-848) or uridine-rich oligonucleotides demonstrated a progressive lymphopenia, splenomegaly, elevated levels of proinflammatory cytokines, and other abnormalities associated with chronic HIV-1 disease.18
Our current studies sought to determine whether gardiquimod, an imidazoquinoline compound reported to act as a TLR7/8 agonist, could inhibit HIV-1 infection of macrophages and activated peripheral blood mononuclear cells (PBMCs). Our findings demonstrate that gardiquimod, added to macrophages or to activated PBMCs prior to or shortly after infection with HIV-1, significantly inhibited infection by X4 (T-tropic), R5 (macrophage-tropic), or dual-tropic strains of HIV-1. Moreover, in cocultures of activated PBMCs added to gardiquimod-treated and HIV-1-exposed macrophages, we observed a significant inhibition of viral amplification by the activated PBMCs, suggesting that gardiquimod not only protected the macrophages from infection, but prevented transmission of HIV-1 from the macrophages to the activated PBMCs. Activated PBMCs and macrophages treated with gardiquimod expressed interferon-alpha (IFN-α) mRNA within 2 h of treatment, and demonstrated a sustained release of IFN-α protein for up to 48 h after treatment. Blocking the action of the adaptor protein, MyD88, with an inhibitory peptide prevented the induction of IFN-α in gardiquimod-treated activated PBMCs and also partially reversed the anti-HIV effects of gardiquimod. Although not previously reported, we show that gardiquimod significantly inhibited HIV-1 reverse transcriptase activity in a modified cDNA synthesis assay demonstrating its utility as a reverse transcriptase inhibitor. In sum, gardiquimod has potent anti-HIV-1 activity with two apparent modes of action, suggesting that this molecule could be developed as an antiviral therapeutic agent to block HIV-1 infection.
Materials and Methods
Reagents
Gardiquimod (Invivogen, San Diego, CA), an imidazoquinoline analog, is a TLR 7/8 agonist and is reported to specifically activate TLR7 when used at concentrations below 10 μM (manufacturer's documentation). In our study, gardiquimod was used at concentrations between 0.03 μM and 10 μM. We tested the ability of gardiquimod to block HIV-1 infection of primary cultures of activated PBMCs and purified populations of macrophages, and to inhibit production of HIV-1 from cocultures of HIV-1-infected macrophages and activated T cells [the primary target of HIV-1 infection in phytohemagglutinin (PHA)-activated PBMCs]. Gardiquimod was reconstituted in sterile, endotoxin-free water, and stored frozen at −20°C in 20-μl aliquots. Each aliquot was thawed once prior to use.
Macrophage and peripheral blood mononuclear cell (PBMC) cultures
Peripheral blood cells were obtained from healthy donors over the age of 18 who were donating platelets at the Dartmouth-Hitchcock Medical Center. Leukocytes trapped in a filter during plateletpheresis and normally discarded were used as a source of PBMCs. All donors were screened for blood-borne pathogens prior to their platelet donation. No information about the donor other than their age and gender was obtained. The mononuclear cell fraction was isolated by Ficoll-Hypaque (Amersham, Piscataway, NJ) as previously described.19
To purify monocytes, PBMCs were resuspended at a concentration of 5×106 cells/ml in serum-free RPMI (Invitrogen, Grand Island, NY) and added to large (150-cm2) tissue culture flasks to permit monocyte attachment. After a 1 h incubation at 37°C, each flask of cells was washed with additional media to remove nonadherent leukocytes, leaving behind the adherent monocytes. After attachment, the monocytes were maintained in RPMI containing 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 50 units/ml penicillin, plus 50 μg/ml streptomycin, and further supplemented with 10 ng/ml granulocyte-monocyte colony-stimulating factor (GM-CSF; R & D Systems, Minneapolis, MN) to promote maturation to macrophages. The cells were maintained in a humidified incubator at 37°C with 5% CO2 for a total of 8 days. On the fifth day of culture, the medium was refreshed and one-half of the cells were treated with 0.6–3.0 μM gardiquimod for 3 days prior to infection with HIV-1 on day 8 of culture. Control macrophages were left untreated.
For PBMC isolation, mononuclear cells isolated by Ficoll-Hypaque were washed and resuspended to 2×106 cells/ml in RPMI supplemented with 10% FBS, 2 mM glutamine, and 50 units/ml penicillin plus 50 μg/ml streptomycin (complete RPMI). PBMCs were activated for 2 days in the presence of 2 μg/ml phytohemagglutinin (PHA-P, Invitrogen). After incubation with PHA, PBMCs were washed three times and resuspended in complete RPMI containing 10 units/ml IL-2 (Invitrogen). This population of cells contains primarily activated T cells. Gardiquimod was added to the activated PBMCs at various times prior to or after infection with HIV-1, from 1 h prior to up to 72 h after infection.
To assess the purity of the isolated cells, a portion of the attached and nonattached cells was incubated with fluorescein-conjugated anti-CD14 (for monocytes) and anti-CD3 (for T cells) and analyzed by flow cytometry. All isotype control and receptor-specific antibodies were obtained from R & D Systems (Minneapolis, MN).
Infection of macrophages and activated PBMCs with HIV-1
Human macrophages were infected on day 8 of culture with 200 TCID50 HIV-1Ba-L (R5-tropic strain of HIV-1) for 1 h, and then washed three times to remove unincorporated virus. Fresh complete RPMI without exogenous GM-CSF or additional gardiquimod was then added to the cells. Macrophages were harvested on day 4 postinfection, and viral DNA quantified as described below for activated PBMCs by real-time PCR.
PHA-activated PBMCs were washed to remove PHA after a 48-h culture, resuspended in complete RPMI at 2×106 cells/ml, and infected with 20 TCID50/ml of various strains of HIV-1, including HIV-1Ba-L or HIV-1CM235 (R5-tropic), HIV-1HC4 (X4-tropic), and HIV-1C7/86 (R5/X4 dual-tropic). All stocks of virus were pretreated for 30 min with 10 units/ml of DNase 1 to remove contaminating genomic DNA. Cells were exposed to HIV-1 for 1 h, followed by three washes to remove any unincorporated virus. The cells were resuspended after the final wash in complete RPMI containing 10 units/ml of IL-2. Gardiquimod, at a final concentration of 1 μM, was added at various time points before or after exposure to HIV-1. These times ranged from 1 h prior to infection to 72 h postinfection with HIV-1. In other experiments, gardiquimod at concentrations ranging from 0.01 μM to 10 μM was added to activated PBMCs or macrophage cultures 1 h after infection with HIV-1Ba-L. Activated PBMCs were then incubated for an additional 4 days before analysis of infection by measuring HIV-1 DNA by real-time PCR, or for up to 9 days before measuring p24 levels in culture supernatant by ELISA (Dupont, Wilmington, DE).
Inhibition of HIV-1 amplification in co-cultures of activated PBMCs and gardiquimod-treated macrophages
We assessed the ability of gardiquimod to inhibit activated PBMC amplification of virus from HIV-1-infected macrophages. Macrophages were either left untreated or were treated with gardiquimod as described above. All macrophages were then infected with 200 TCID50 HIV-1Ba-L for 1 h, washed extensively to remove unbound virus, and resuspended in complete media. Either immediately after infection, or 1 or 2 days later, 2×106 PHA-activated PBMCs (not treated with gardiquimod) were added to 1×106 macrophages. HIV-1 DNA was quantified on the cell mixture (coculture) by real-time PCR on day 10 after macrophage infection, and p24 levels in the culture supernatant were measured on day 4 of coculture by p24 ELISA. A sample of the macrophage culture supernatant was collected prior to the addition of PHA-activated PBMCs to confirm the absence of viral particles by p24 ELISA.
Quantification of viral DNA by real-time PCR
Cellular DNA was isolated from HIV-1-infected PBMCs or macrophage cultures using lysis buffer from the Gentra PureGene Tissue Kit (catalog 158667, Qiagen, Valencia, CA) and columns from the QIAamp DNA extraction kit (catalog 51306, Qiagen). DNA was analyzed by real-time PCR for HIV-1 DNA. Quantification of HIV-1 DNA was performed in triplicate by primers complementary to both the reverse transcription product and integrated virus as described.20 Genomic DNA (100–200 ng) isolated from HIV-1-infected cells was subjected to real-time PCR. HIV-1 reverse transcription levels were normalized to genomic human β-actin. The primer sequences used for these reactions were as follows: For HIV-1, the forward primer was 5′- GGAACCCACTGCTTAAGCCTCAA-3′ and the reverse primer was 5′- TGTTCGGGCGCCACTCTTCCAGCCTTCCTTCC -3′. For β-actin, the forward primer was 5′- CACTCTTCCCAGCCTTCCTTCC-3′ and the reverse primer was 5′- CTGTGTTGGCGTACAGGTCT-3′. The relative starting quantity was determined using 2–ΔΔCT analysis.
Measurement of interferon-alpha (IFN-α) by gardiquimod
We measured mRNA levels of IFN-α in PHA-activated PBMCs after gardiquimod treatment. For these studies, PHA-activated PBMCs (2×106 cells/well in triplicate) were incubated with media alone or with 1 μM gardiquimod. Cells were removed from wells at 0, 2, 24, and 48 h post-gardiquimod treatment and total cellular RNA was isolated using the RNeasy Plus Mini Kit (Qiagen). To remove contaminating genomic DNA, an on-column DNase digestion was performed according to the manufacturer's specifications (Qiagen protocol). Two micrograms of total RNA from each time point was reverse transcribed to cDNA using random hexamer primers in a 50-μl reaction volume as described.20 Duplicate samples without added reverse transcriptase were run to verify the absence of genomic DNA contamination. Eight microliters of the cDNA reaction was used for real-time PCR measurement of IFN-α mRNA levels at an annealing temperature of 57°C. Data from each condition were normalized to GAPDH mRNA. Subsequently, the level of each transcript was expressed relative to that measured in cells prior to the addition of gardiquimod (time 0). Data were analyzed using the ΔΔCT method. For IFN-α, the forward primer sequence was 5′- GCACCGAACTCTACCAGCAGC-3′ and the reverse primer sequence was 5′- TCTGACAACCTCCCAGGCACA-3′. For GAPDH, the forward primer sequence was 5′- GGACCTGACCTGCCGTCTA-3′ and the reverse primer sequence was 5′- TGCTGTAGCCAAATTCGTTG-3′.
Blockade of IFN-α gene expression using the myeloid differentiation primary response gene 88 (MyD88) inhibitor
To determine whether preventing the induction of IFN-α expression in gardiquimod-treated cells would diminish the protection against HIV-1 infection, we blocked the downstream signaling events triggered by TLR7 activation with an MyD88 inhibitory peptide. The MyD88 inhibitory peptide blocks the action of MyD88, the universal adaptor protein used by all TLR (except TLR3) to activate downstream gene expression. For studies to measure inhibition of IFN-α expression, PHA-activated PBMCs were treated for 4 h with 10 μg/ml of the MyD88 inhibitory peptide (tlr1-pimyd, Invivogen, San Diego, CA) or a control peptide. Following the 4-h incubation, the cells were treated with 1 μM gardiquimod. Cell pellets were collected at 2, 24, and 48 h after the addition of gardiquimod for extraction of total cellular RNA as described above. Transcription levels of the IFN-α mRNA were expressed relative to the baseline levels at time 0, prior to the addition of either the MyD88 inhibitory or control peptide. For studies to measure the inhibition of HIV-1, PHA-activated PBMCs were first infected with HIV-1Ba-L for 1 h, washed, and then treated with either the MyD88 inhibitory peptide or the control peptide as described above. Cellular DNA was isolated and assessed for levels of HIV-1 DNA.
IFN-α receptor blockade
To determine whether the binding of IFN-α to its receptor was required for the inhibition of HIV-1 replication in gardiquimod-treated cells, we blocked the IFN-α receptor using a blocking antibody (IFN-α R2, MAB1155, Millipore, Temecula, CA) prior to gardiquimod treatment. PHA-activated PBMCs (2×106 cells/well) were first infected with 20 TCID50 HIVBa-L for 1 h, washed extensively to remove unbound virus, and either left untreated, treated with 2 μg/ml of a control IgG2a isotype antibody, or 2 μg/ml of the anti-IFN-α R2 antibody. One hour after antibody treatment, cells were treated with 1 μM gardiquimod. Supernatants from all cell groups were collected on day 7 postinfection and HIV-1 p24 levels were measured by ELISA.
Reverse transcription inhibition assay
To determine whether gardiquimod could inhibit HIV-1 reverse transcriptase (RT) activity, a modified cDNA synthesis biochemical reaction was performed using HIV-1 RT (catalog #3555, NIH AIDS Research and Reference Program, Germantown, MD) as the source of reverse transcriptase, in the presence or absence of gardiquimod. Azidothymidine (AZT) served as a positive RT inhibitor at a range of concentrations from 40 μM to 400 μM. Total RNA was isolated from a monocytic cell line (U937 cells) and used as the source of mRNA. The final cDNA synthesis reaction contained 1 μg total RNA, 1 × first strand cDNA synthesis buffer (Invitrogen), 80 μM dNTP, 0.15 μg HIV-1 RT, 50 U Roche Protector RNase inhibitor, 5 mM DTT, and gardiquimod (6– 60 μM) or AZT (40–400 μM) in a 50 μl volume. Of the CD45 mRNA antisense primer (5′-GGTGCTTGCGGGTGAGAAT-3′) 2 μM was used for cDNA synthesis, and all reactions were performed in triplicate. The cDNA synthesis steps were as follows: 25°C for 5 min, 50°C for 45 min, 55°C for 15 min, and 65°C for 15 min. Ten microliters of the cDNA synthesis reaction was then used as a template in a real-time PCR amplification reaction using the CD45-specific sense primer (5′-CACGGCTGACTTCCAGATATGA-3′) and the CD45 antisense primer (5′- GGTGCTTGCGGGTGAGAAT-3′), with an annealing temperature of 56°C. Levels of cDNA product from reactions containing gardiquimod or AZT were compared to untreated controls.
Cell metabolic activity and apoptotic assays
The metabolic activity of gardiquimod-treated cells was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI). Macrophages or PHA-activated PBMCs (2×104 cells) were added to replicate wells of a 96-well plate, and 20 μl of CellTiter reagent was added to each well. Metabolic activity was determined by a colorimetric change of the substrate at 490 nm and was measured daily from 1 to 7 days posttreatment.
To determine whether gardiquimod induced apoptosis in cells, we measured the intensity of annexin V and propidium iodide staining in control and gardiquimod-treated activated PBMCs by flow cytometry using the FITC-Annexin-V Apoptosis kit (catalog #556547, BD Biosciences, Sparks, MD). Tumor necrosis factor-alpha (TNF-α) at 100 ng/ml was used as a positive control to induce apoptosis. Cells were analyzed in triplicate after 48 h of gardiquimod treatment by the addition of 5 μl of FITC-annexin V or 5 μl propidium iodide.
Statistical analysis
Analysis of datasets comparing two groups was performed by paired Student's t-test. Data were considered statistically significant at p≤0.05 and specific p values are noted in each figure legend. In all figures, data are represented as the mean±standard deviation of the mean (mean±SD) of triplicate samples from either two or three different blood cell donors.
Results
Gardiquimod inhibits HIV-1 replication in primary human macrophages
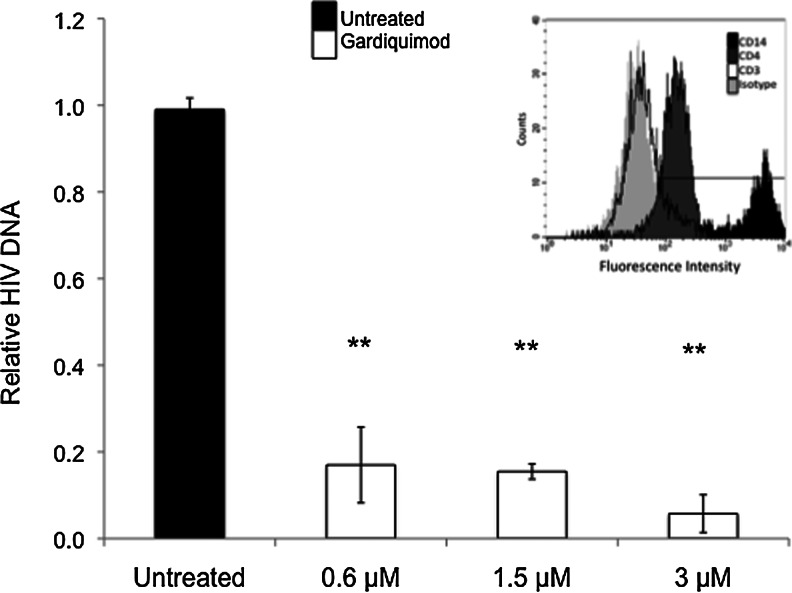
Human monocytes were matured to macrophages for 8 days in the presence of GM-CSF and then analyzed for purity and maturation status by expression of CD14 and CD4 using immunofluorescence and flow cytometry. As shown in Fig. 1 (insert), cell cultures contained greater than 95% macrophages on day 8 of culture as determined by CD14 and CD4 expression, and lack of CD3 expression. Treatment of macrophages with gardiquimod on day 5 of culture, followed by infection with HIV-1Ba-L on day 8, significantly reduced levels of HIV-1 DNA in the cells (p≤0.001) at all gardiquimod concentrations tested compared to untreated controls, as determined on day 4 postinfection (Fig. 1). This effect was consistently observed among all three donors tested.
FIG. 1.
Gardiquimod inhibits HIV-1Ba-L infection in primary human macrophages. Macrophages were incubated with FITC-conjugated anti-CD3, anti-CD4, or anti-CD14 antibodies to assess purity. Flow cytometric analysis showed 95% CD14 (black histogram), 80% CD4 (dark gray histogram), and 2% CD3 (white histogram) expression in the cell population (inset). Macrophages were exposed to HIV-1Ba-L (black bar) or treated with 0.6–3 μM gardiquimod (white bars) for 72 h prior to exposure to HIV-1. Cells were assessed for total HIV-1 DNA on day 4 postinfection. Data are displayed by setting the levels of HIV-1 DNA from the untreated controls to “1.” Data are a compilation from three donors, each of which was assayed in triplicate. All gardiquimod-treated cultures were at a statistically significant (**p<0.001) level compared to untreated controls.
Gardiquimod inhibits HIV-1 replication in PHA-activated PBMCs
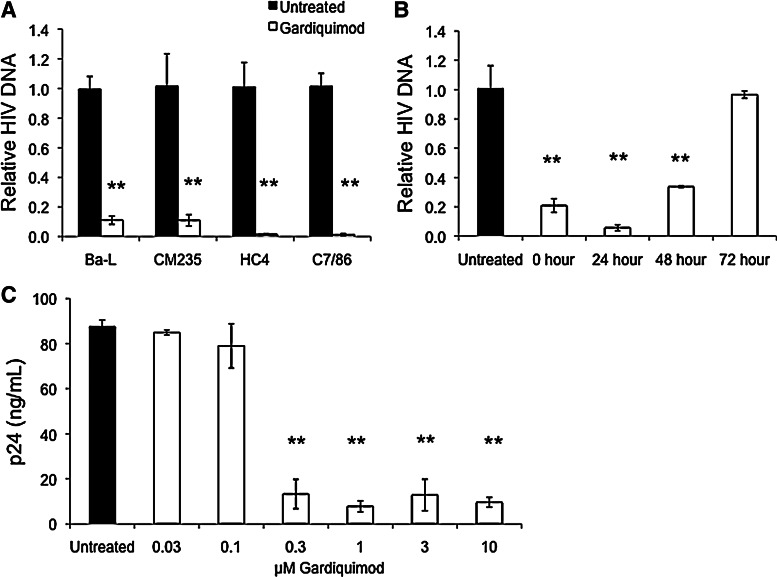
Freshly isolated PBMCs were activated with PHA, washed, and treated for 1 h with 0.6 μM gardiquimod prior to infection with various strains of HIV-1. As shown in Fig. 2A, levels of HIV-1 DNA measured by real-time PCR were significantly lower in gardiquimod-treated cells compared to untreated controls on day 9 postinfection. Viral inhibition was observed for R5 strains (Ba-L, CM235), an X4 strain (HC4), and a dual-tropic strain (C7/86).
FIG. 2.
Gardiquimod inhibits HIV-1 infection in phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells (PBMCs). (A) PBMCs were treated with 0.6 μM gardiquimod for 1 h prior to infection with the following HIV-1 strains: Ba-L, CM235, HC4, or C7/86. Nine days postinfection, total HIV-1 DNA levels were measured as described in Fig. 1. Data are a compilation from three donors, each assayed in triplicate. **p<0.005. (B) PHA-activated PBMCs were treated with 0.6 μM gardiquimod either immediately after exposure to HIV-1C7/86 or 24, 48, or 72 h after infection. HIV-1 levels were determined on day 9 postinfection by real-time polymerase chain reaction (PCR) and normalized to HIV-1 DNA levels from the untreated controls, which were arbitrarily set to a value of “1.” **p<0.005. (C) Gardiquimod was tested for its ability to inhibit HIV-1 infection of PBMCs over a range of concentrations. PBMCs were infected with 20 TCID50 HIV-1Ba-L prior to treatment with gardiquimod from 0.03 μM to 10 μM. Supernatants were collected on day 9 postinfection and p24 levels were measured by enzyme-linked immunosorbent assay (ELISA). Shown is a representative dose–response from a single donor performed in triplicate. Differences in p24 levels from cultures treated with gardiquimod at concentrations of 0.3 μM or greater were statistically significant in all three donors tested compared to untreated controls. **p<0.005.
We next determined whether the addition of gardiquimod to activated PBMCs after exposure to HIV-1 could still inhibit HIV-1 replication. As shown in Fig. 2B, activated PBMCs were infected with the dual-tropic strain HIV-1C7/86, washed, and then immediately treated with 0.6 μM gardiquimod (0 h), or treated 24, 48, or 72 h after infection. Analysis of HIV-1 DNA from cells treated with gardiquimod immediately after infection with HIV-1, or 24 or 48 h after infection, showed significantly reduced levels as determined on day 9 postinfection. Treatment with gardiquimod at 72 h after exposure to HIV-1 had no effect on HIV-1 DNA levels compared to untreated controls.
We then determined the active dose range for gardiquimod using concentrations that ranged from 0.03 μM to 10 μM, and found significant inhibition of HIV-1Ba-L p24 production in activated PBMCs at gardiquimod concentrations equal to or greater than 0.3 μM (Fig. 2C). In this experiment, gardiquimod was added to the activated PBMCs immediately after the cells were washed following a 1-h exposure to HIV-1, and p24 values were measured on day 9 postinfection. Levels of p24 collected immediately after infection (day 0) were consistently below the limit of detection of the ELISA assay (data not shown).
The reduction in either HIV-1 DNA or p24 levels in gardiquimod-treated activated PBMC cultures was not due to the induction of apoptosis, as determined by measuring levels of annexin 5 and propidium iodide by flow cytometry following 48 h of exposure to gardiquimod (Table 1). We also confirmed that gardiquimod did not affect cell metabolism, viability, or cell numbers throughout the culture duration (data not shown).
Table 1.
Gardiquimod Treatment of Peripheral Blood Mononuclear Cells Does Not Induce Apoptosis
| PBMC treatmenta | % Annexin Vb | % Propidium iodideb |
|---|---|---|
| Untreated | 10.7±0.6 | 10.7±1.5 |
| TNF-α, 100 ng/ml | 16.3±0.6* | 12.3±0.6 |
| Gardiquimod, 1 μM | 10.3±0.6 | 11.5±0.7 |
Freshly isolated PBMCs were incubated with either medium alone (untreated), TNF-α, or gardiquimod at the indicated dosages for 48 h prior to staining with annexin V or propidium iodide.
The percentages of annexin V or propidium iodide staining cells were assessed by flow cytometry. Values represent the percentage of positive cells using unstained/untreated cells to set the gates on the flow cytometer. Values represent the mean±SD of triplicates.
p≤0.0002 compared to untreated cells stained with either annexin V or propidium iodide.
PBMC, peripheral blood mononuclear cells; TNG, tumor necrosis factor.
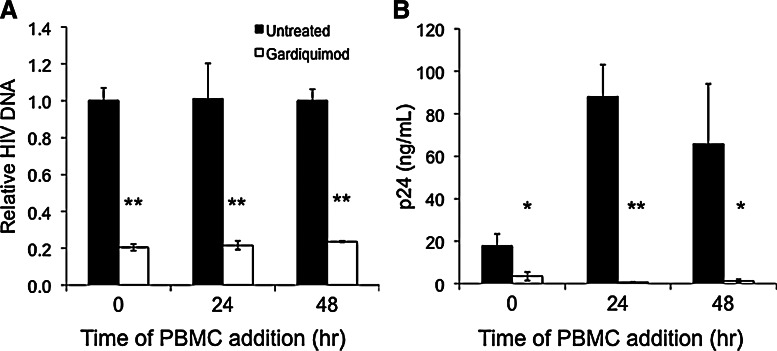
Gardiquimod treatment of macrophages prior to HIV-1 infection inhibits subsequent viral amplification by activated PBMCs in coculture. We treated macrophages with 3 μM gardiquimod for 3 days prior to infection with 200 TCID50 HIV-1Ba-L. In a dose–response analysis, we found that 3 μM gardiquimod was the lowest effective dose that consistently inhibited HIV-1 infection in macrophages (data not shown). After a thorough washing to remove unincorporated virus, PHA-activated PBMCs were added to the macrophage cultures either immediately (0 h), or 24 or 48 h later. Cells from the coculture were harvested and analyzed by real-time PCR for HIV-1 DNA after 10 days of coculture (Fig. 3A). We also measured p24 levels in the culture supernatant 4 days after the addition of activated PBMCs to the macrophage cultures (Fig. 3B). We observed significantly lower levels of HIV-1 DNA and HIV-1 p24 in gardiquimod-treated and HIV-1-exposed macrophages cocultured with activated PBMCs, regardless of the time of PBMC addition.
FIG. 3.
Gardiquimod treatment of macrophages inhibits HIV-1 amplification by PBMCs in coculture. Activated PBMCs were added to gardiquimod-treated and HIV-1-exposed macrophages either immediately after HIV-1 exposure (0 h) or at 24 h or 48 h after HIV-1 exposure. (A) HIV-1 levels were determined on day 10 postinfection by real-time PCR and normalized to HIV-1 DNA levels from the untreated controls, which were arbitrarily set to a value of “1.” Data are a compilation from three donors, each of which was assayed in triplicate. All cultures containing PBMCs and gardiquimod-treated macrophages had levels of HIV-1 DNA that were significantly lower compared to those in the control cultures. (B) p24 levels were quantified in supernatants from the cocultures by ELISA 4 days after PBMC addition. Data are a compilation from three donors, each of which was assayed in triplicate. *p<0.02, **p<0.005.
Gardiquimod induces IFN-α transcription and IFN-α protein in PHA-activated PBMCs
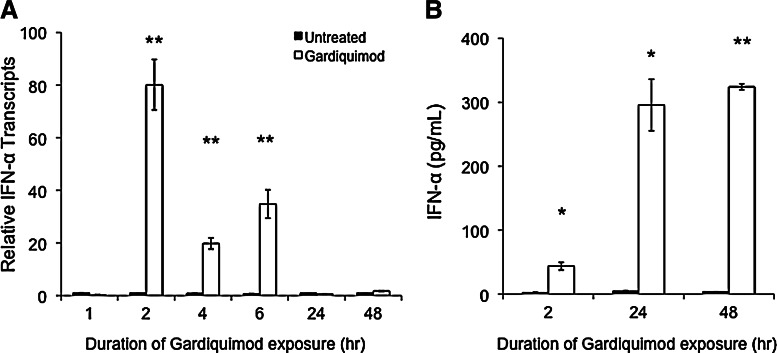
We determined whether gardiquimod induced IFN-α transcription in activated PBMCs. IFN-α is an innate immune cytokine that could contribute to the anti-HIV effects we observed in gardiquimod-treated cells. PHA-activated PBMCs were exposed to 1 μM gardiquimod to activate TLR7 for various time points from 1 h to 48 h. RNA isolated from untreated cells contained levels of IFN-α transcripts below the limit of detection and this level of expression was arbitrarily set to “1.” Gardiquimod significantly increased IFN-α mRNA levels 80-, 20-, and 35-fold above the level of detection at 2, 4, and 6 h posttreatment, respectively, as measured by real-time PCR (Fig. 4A). No detectable levels of IFN-α mRNA were measured in the untreated PBMCs or at the 1 h time point in gardiquimod-treated cells. Levels of IFN-α mRNA returned to baseline by 24 h posttreatment and were undetectable throughout the remainder of the culture period.
FIG. 4.
Gardiquimod induces interferon (IFN)-α mRNA transcription and IFN-α protein in PBMCs. (A) Levels of IFN-α transcripts were measured by real-time PCR in PBMCs exposed to gardiquimod for 1–48 h. Levels of IFN-α mRNA are displayed relative to those detected in untreated cells. **p<0.005. (B) Levels of IFN-α protein secreted into the culture supernatant were measured by ELISA at 2, 24, and 48 h after the addition of gardiquimod. Significant levels of IFN-α protein were detected at all time points. *p<0.05, **p<0.005.
Levels of IFN-α protein in these activated PBMC culture supernatants were measured at 2, 24, and 48 h by ELISA and were found to increase by the 2 h time point, with even greater levels detected at the 24 and 48 h time points (Fig. 4B). No significant IFN-α protein was detectable in the supernatant from untreated PHA-activated PBMCs.
Inhibition of TLR7 intracellular signaling pathways activated by gardiquimod blocks IFN-α transcription and reduces the inhibition of HIV-1 replication
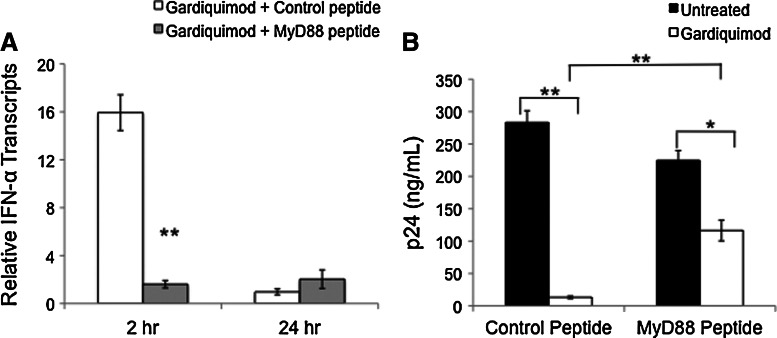
We determined whether blocking downstream signaling events induced by TLR7 activation would prevent IFN-α transcription and the subsequent loss of inhibition of HIV-1 replication in activated PBMCs. PHA-activated PBMCs were treated for 4 h with either 10 μg/ml of the MyD88 inhibitory peptide or a control peptide, prior to the addition of 1 μM gardiquimod. Cells were harvested for RNA isolation at 2 h and 24 h after gardiquimod treatment. The MyD88 inhibitory peptide blocked the increase in IFN-α transcription induced by gardiquimod at the 2 h time point (Fig. 5A).
FIG. 5.
Blocking TLR7 downstream signaling events induced by gardiquimod inhibits IFN-α transcription and reduces protection from HIV-1 replication. (A) PHA-activated PBMCs were treated either with 10 μg/ml of the MyD88 inhibitory peptide or a control peptide for 4 h prior to the addition of 1 μM gardiquimod. Cells were harvested at 2 h and 24 h after gardiquimod treatment and levels of IFN-α transcript were measured by RT-PCR. The MyD88 inhibitory peptide significantly reduced levels of IFN-α transcripts at 2 h. **p<0.005. (B) To determine the effects of blocking downstream signaling pathways using the MyD88 inhibitory peptide on HIV-1 replication, PHA-activated PBMCs were infected with HIV-1 for 1 h, washed, and treated with either the control or inhibitory peptide for 4 h, followed by the addition of gardiquimod. Supernatants were collected on day 6 after HIV-1 infection and levels of p24 were measured by ELISA. The addition of the MyD88 inhibitory peptide reduced the inhibitory effect of gardiquimod compared to cultures that received the control peptide and gardiquimod, although p24 levels were still significantly suppressed compared to the non-gardiquimod-treated cells. *p<0.05.
To determine whether the addition of the MyD88 inhibitory peptide also prevented the inhibition of HIV-1 replication by gardiquimod, we infected PHA-activated PBMCs with HIV-1Ba-L for 1 h, washed the cells to remove unbound virus, and then added 10 μg/ml MyD88 inhibitory peptide or the control peptide for an additional 4 h. Gardiquimod (1 μM) was then added to one-half of the cells in each treatment group, and supernatants were collected for p24 measurement 6 days after HIV-1 infection. In cells treated with the control peptide, gardiquimod significantly inhibited HIV-1 p24 compared to cells that did not receive gardiquimod. Treatment with the MyD88 inhibitory peptide largely, but not completely, abolished the protective effects of gardiquimod (Fig. 5B).
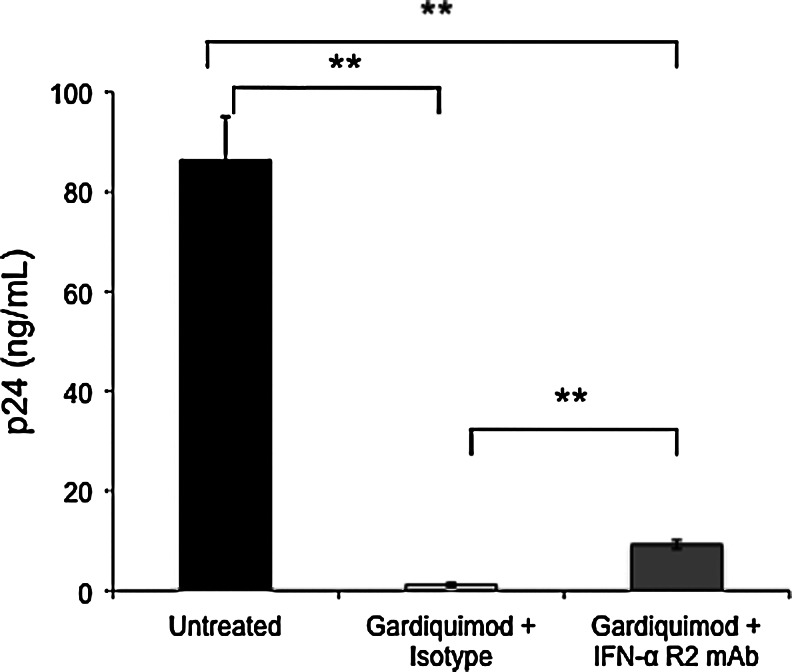
Blockade of the IFN-α receptor partially inhibits the anti-HIV effect afforded by gardiquimod
To determine whether the induction of IFN-α by gardiquimod was necessary and sufficient for protection against HIV-1 infection, we blocked the receptor prior to gardiquimod treatment using a neutralizing IFN-α receptor-blocking antibody. PHA-activated PBMCs were first infected with HIVBa-L, washed extensively to remove unbound virus, and then treated with either an IgG2a isotype control antibody or the anti-IFN-R2 neutralizing antibody to block the IFN-α receptor. One hour after antibody treatment, cells were divided into two groups, with one group remaining untreated and the other group treated with 1 μM gardiquimod. Supernatants from all treatment groups were collected on day 7 postinfection, and HIV-1 p24 levels were measured by ELISA. As shown in Fig. 6, p24 levels were significantly reduced in the gardiquimod-treated cells that were pretreated with the isotype control antibody. The addition of the IFN-α receptor blocking antibody partially, but not completely, reversed the protection afforded by gardiquimod.
FIG. 6.
Blockade of the IFN-α receptor partially reverses the HIV-1 inhibitory effect of gardiquimod. PBMCs infected with HIV-1 and then treated either with an isotype control antibody or an antibody that blocks the IFN-α receptor were treated with gardiquimod and levels of HIV-1 p24 were measured on day 7 postinfection. PBMCs treated with the isotype control antibody plus gardiquimod showed significant inhibition of p24, whereas cells treated with the blocking antibody plus gardiquimod showed a partial inhibition of the protection, although levels of p24 were still significantly suppressed compared to the untreated PBMCs. **p<0.005.
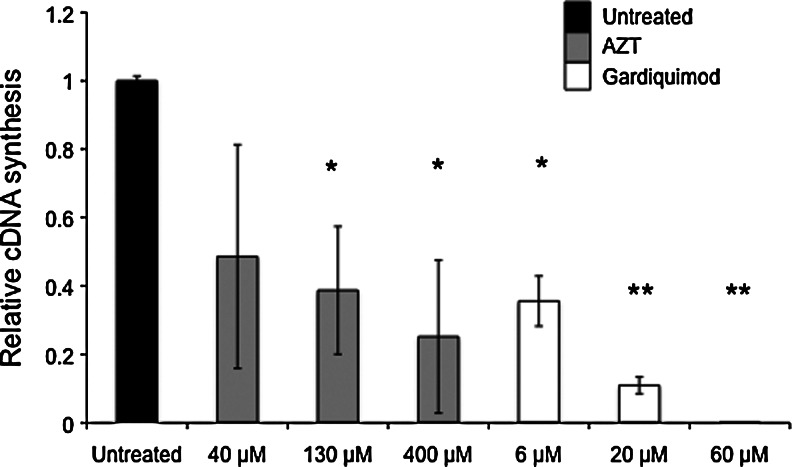
Gardiquimod inhibits HIV-1 reverse transcription
We determined the extent to which gardiquimod inhibits the HIV-1 reverse transcriptase (RT) reaction using a biochemical assay to synthesize cDNA. We found significant inhibition of cDNA synthesis by HIV-1 reverse transcriptase when gardiquimod was added to the reaction at concentrations between 6 μM and 60 μM (Fig. 7). Gardiquimod inhibited the RT reaction more effectively than AZT, which was used as a control nucleoside reverse transcriptase inhibitor. The concentrations of gardiquimod used in this biochemical assay are not directly analogous to those used in the in vitro assays in which we inhibited HIV-1 infection of cells. Furthermore, we found that the ability of gardiquimod to inhibit HIV-1 RT was not as effective when we used deoxynucleotide triphosphates (dNTP) at a higher concentration (320 μM rather than 80 μM dNTP), indicating a potential competitive inhibition of gardiquimod with the dNTP.
FIG. 7.
Gardiquimod inhibits HIV-1 reverse transcriptase. Gardiquimod was added to a modified cDNA synthesis reaction using HIV-1 RT to synthesize cDNA copies of total RNA isolated from uninfected lymphocytes. All conditions used identical template RNA, and total levels of cDNA were determined by CD45 specific real-time PCR to assess inhibition of the cDNA synthesis reaction. Values are expressed relative to untreated controls, and azidothymidine (AZT) was used at a range of concentrations as a positive control for RT inhibition. *p<0.02, **p<0.005.
Discussion
Infection of CD4+ target cells by HIV-1 leads to the induction of immune responses designed to protect the host and prevent viral transmission. These responses, however, heighten the activation state of responding immune cells,21 which increases surface chemokine receptor expression,22 induces chemokine secretion to attract other immune target cells to the site of infection,23–26 and results in the proliferation of responding cells. This provides a greater number of target cells for the newly synthesized viral particles to infect,27 and can lead to a more rapid dissemination of virus throughout the body.21 One approach to reduce viral transmission to the periphery would be to inhibit the local expansion of virus within and among cells at the initial portal of entry in mucosal tissues of the reproductive and gastrointestinal tracts.28–30 As macrophages and CD4+ T cells are the initial target cells of infection in mucosal tissues,31,32 targeting those cells with antiviral compounds would likely reduce infection and transmission while at the same time serve to enhance antiviral immune responses.33
Small molecule inhibitors that block HIV-1 binding,34–37 reverse transcription,38 integration,39,40 or the action of proteases41 are presently being developed as novel approaches to inhibit viral infection of target cells at mucosal sites.42 Some classes of small molecule inhibitors also activate innate immune responses by triggering cellular receptors involved in pathogen recognition. One class of innate immune receptors that plays an important role in responding to pathogen infection at sites of exposure includes the Toll-like receptors (TLR) expressed by myeloid, lymphoid, and epithelial cells. In mucosal tissues, TLR activation initiates a cascade of responses that form the first line of defense against pathogen invasion, including HIV-1. Ligands to some of the TLR are potent inducers of innate immunity against viral infections, including HIV-1, because they mimic the binding of pathogen to TLR. We sought to determine the effects of gardiquimod, a TLR 7/8 agonist, on HIV-1 infection of macrophages and T cells. Gardiquimod is an imidazolequinoline compound that is reported to induce NF-κB activation in cell lines. Our studies demonstrated that micromolar quantities of gardiquimod potently and significantly inhibited viral production from macrophages and activated T cells, and blocked the amplification of HIV-1 by activated PBMCs when cocultured with gardiquimod-treated and HIV-1-exposed macrophages. Our studies show that gardiquimod likely works in two ways: by inducing IFN-α expression, an antiviral cytokine, and perhaps other antiviral compounds, and by inhibiting reverse transcriptase activity. Thus, gardiquimod acts to block both a key step in the viral life cycle, and also serves to enhance innate immune responses to virus.
As freshly obtained peripheral blood-derived monocytes are difficult to infect with HIV-1 in vitro,43 we matured monocytes to macrophages in the presence of GM-CSF for 1 week. Macrophages are readily infected with HIV-1 strains that utilize the CCR5 coreceptor. Exposure to gardiquimod during the last 3 days of the maturation period was sufficient to block infection of macrophages with HIV-1. Because GM-CSF can itself protect macrophages from infection,44 we maintained macrophages in the absence of GM-CSF after exposure to HIV-1. Significant and sustained inhibition of viral infection was observed in macrophages throughout the culture period.
Similar findings were observed with PHA-activated PBMCs. In PBMC cultures, gardiquimod inhibited the replication of several strains of HIV-1, including those that utilize the CXCR4 coreceptor for infection. Moreover, infection was significantly inhibited throughout a prolonged 9-day culture period suggesting that virus transmission and amplification of infection were also prevented. Of note is the fact that activated PBMCs were treated for only 1 h prior to exposure to HIV-1 or immediately after infection with HIV-1, and cultures were maintained in the absence of additional gardiquimod. This suggests that the HIV-inhibitory effects of gardiquimod function at the time of the initial infection event and can protect cells for a sustained period of time. The finding that infection with both R5- and X4-tropic strains was inhibited indicates that gardiquimod is not blocking the binding of HIV-1 to a specific chemokine coreceptor. Furthermore, gardiquimod protected activated PBMCs from HIV-1 infection even when added up to 48 h after infection. This suggests that gardiquimod functions at a postbinding and preintegration step during the infectivity process, as measures of HIV-1 DNA were significantly lower in gardiquimod-treated cells. The addition of gardiquimod 72 h postinfection was not effective in protecting activated PBMCs from infection, however, suggesting that gardiquimod is likely ineffective at reducing infectivity after HIV-1 has undergone reverse transcription and integration.
We next determined whether gardiquimod could prevent the amplification of virus by activated PBMCs when PBMCs were added to gardiquimod-treated and HIV-1-exposed macrophages. Macrophages were treated with gardiquimod for 3 days and then infected with HIV-1. Immediately after infection, or up to 2 days later, activated PBMCs were added to the macrophages and the cell coculture was assessed for levels of HIV-1 DNA and for p24 4 days later. We observed that levels of HIV-1 were significantly suppressed in cultures containing gardiquimod-treated macrophages, regardless of the time of PBMC addition. Whether gardiquimod protects macrophages from infection, or prevented viral transmission from infected macrophages to activated PBMCs, is not known, but these findings indicate that viral release is effectively suppressed by gardiquimod-treated macrophages.
As induction of inflammatory responses has been linked to enhanced susceptibility to HIV-1 infection45,46 we determined whether gardiquimod induces inflammatory responses in cells. Activated PBMCs treated with gardiquimod showed enhanced transcription of IFN-α, but mRNA transcripts were detectable only between 2 and 6 h posttreatment. IFN-α protein production, however, was first detected at the 2-h time point and cytokine levels increased in the culture supernatant throughout a 48-h period. It is likely that this short period of inflammatory cytokine production is insufficient to cause enhanced susceptibility to HIV-1 infection, but may contribute to the antiviral effect at the time of viral exposure. Additional time course and dosage experiments would need to be carried out to determine the appropriate level and duration of TLR activation that would result in an anti-HIV effect rather than to a generalized immune activation state.
To confirm that the IFN-α production was a direct result of TLR7 activation by gardiquimod, we blocked the downstream signaling events of TLR7 activation that are mediated by MyD88. MyD88 is the adaptor protein that links activation signals from TLR to intracellular signaling pathways that result in gene transcription. TLR7 activation by ligand, including HIV-1 single-stranded RNA, induces IFN-α production because of a signaling cascade mediated through MyD88.47 Using an inhibitory peptide to MyD88, we found that IFN-α transcripts were markedly suppressed in gardiquimod-treated cells exposed to the inhibitory peptide compared to cells exposed to the control peptide. We next assessed the contribution of IFN-α expression on the inhibition of HIV-1 replication. In HIV-1-infected PHA-activated PBMCs that were treated with the MyD88 inhibitory peptide and then exposed to gardiquimod, we saw a significant, although not complete, reversal of the inhibition of HIV-1 replication. This indicated that mechanisms other than type I interferon production were likely contributing to the anti-HIV-1 effect in these target cells. In a corollary set of experiments, we blocked the IFN-α receptor with an antibody that prevents the binding of IFN-α to its receptor. These studies also confirmed that IFN-α partially contributes to the anti-HIV effect in activated PBMCs, as cells infected with HIV-1 and then treated with the blocking antibody prior to gardiquimod exposure showed higher p24 levels compared to cells treated with the isotype control antibody.
To address potential anti-HIV-1 effects that are independent of IFN-α and other downstream antiviral mediators induced by gardiquimod and TLR7 activation, we measured the ability of gardiquimod to directly inhibit HIV-1 reverse transcriptase activity in a biochemical assay that measures cDNA synthesis. These studies were prompted by similarities in the molecular structure between gardiquimod and nucleosides, suggesting a potential direct effect of gardiquimod on HIV-1 reverse transcriptase. We designed a biochemical assay in which we could determine the ability of gardiquimod to interfere with HIV-1 reverse transcriptase and cDNA synthesis. We observed that gardiquimod was a potent inhibitor of HIV-1 reverse transcriptase, and suppressed enzymatic activity at levels that were equal to or better than azidothymidine (AZT). This effect could result from the ability of gardiquimod to mimic a nucleoside and block the enzymatic action of reverse transcriptase. As such, gardiquimod mimics the action of the class of antiretroviral compounds known as nucleoside reverse transcriptase inhibitors (NRTI), of which AZT belongs. Thus, it is likely that gardiquimod functions to block HIV-1 infection at an early postbinding step by interfering with reverse transcription as well as by inducing early antiviral responses including type I interferon production.
The potential for TLR7/8 activation to induce HIV-1 inhibitory cytokines has been reported in several studies. In one report,48 monocytes isolated from the peripheral blood of nine HIV-1-infected patients were incubated for 48 h in the presence of R-848 (resiquimod), a TLR 7/8 ligand, and levels of the inflammatory cytokines IL-12 and TNF-α as well as viral secretion were measured. A decrease in spontaneous HIV-1 secretion into culture supernatant in monocytes from five of the nine patients correlated with increased cytokine levels. Whether the anti-HIV activity also resulted from additional downstream effects following TLR7/8 activation, or from other mechanisms, was not determined. In fact, attempts to identify the relevant anti-HIV-1 cytokines induced by TLR7/8 activation have revealed a complex scenario in which multiple mechanisms likely contribute to the anti-HIV effects. Using lymphoid suspensions from tonsillar tissues and from PBMCs, activation of TLR7 or TLR8 resulted in the induction of several cytokines including IFN-α, TNF-α, IL-12, IL-15, and IL-18, and the subsequent inhibition of HIV-1 production.49 However, the anti-HIV effects were sustained, albeit reduced, even after IFN-α, IFN-γ, and TNF-α were neutralized with antibodies.49 These authors concluded that the anti-HIV effects resulted not only from the antiviral effects of the cytokines, but were critically dependent on the presence of dendritic cells, as these cells likely promoted the killing of virally infected targets via the cytolytic effects of natural killer (NK) cells and CD8+ T cells.49
The development of novel compounds to block HIV-1 infection at sites of initial infection is likely to both prevent viral transmission to the periphery and also to enhance antiviral responses in responding immune cells. Gardiquimod functions as both a reverse transcriptase inhibitor and also an immune system modifier, and reduces primary infection of immune cells as well as cell-to-cell transmission. Whether gardiquimod could serve as a microbicidal compound in mucosal tissues is at present unknown, but our preliminary studies using humanized mice that can be infected with HIV-1 intravaginally have indicated a significant protection against vaginal transmission if gardiquimod is applied intravaginally immediately prior to HIV-1 exposure (data not shown). Additional studies are needed to assess potential toxicity or inflammation, but it is likely that gardiquimod could be developed as a novel therapeutic agent that functions to inhibit viral transmission in HIV-1-exposed individuals. Studies to develop such innate immune modifiers must take into account the balance between anti-HIV effects and a generalized immune activation state that may, in some cases, promote HIV transmission. Such products, including immunosuppressive oligodeoxynucleotides, are being tested in humanized murine models and have shown significant promise as potential microbicides.50 In sum, gardiquimod shows promise as a potent and durable inhibitor of HIV-1 infection in macrophages and in activated lymphocytes, the earliest cells to acquire HIV-1 infection during sexual transmission.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kawai T. Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Akira S. Uematsu S. Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T. Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7(2):131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 4.Hornung V. Guenthner-Biller M. Bourquin C, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11(3):263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 5.Cros J. Cagnard N. Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33(3):375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douagi I. Gujer C. Sundling C, et al. Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. J Immunol. 2009;182(4):1991–2001. doi: 10.4049/jimmunol.0802257. [DOI] [PubMed] [Google Scholar]
- 7.Caramalho I. Lopes-Carvalho T. Ostler D. Zelenay S. Haury M. Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197(4):403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y. Zhuang Y. Zhai S, et al. Increased expression of TLR7 in CD8(+) T cells leads to TLR7-mediated activation and accessory cell-dependent IFN-gamma production in HIV type 1 infection. AIDS Res Hum Retroviruses. 2009;25(12):1287–1295. doi: 10.1089/aid.2008.0303. [DOI] [PubMed] [Google Scholar]
- 9.Horsmans Y. Berg T. Desager JP, et al. Isatoribine, an agonist of TLR7, reduces plasma virus concentration in chronic hepatitis C infection. Hepatology. 2005;42(3):724–731. doi: 10.1002/hep.20839. [DOI] [PubMed] [Google Scholar]
- 10.Wysocka M. Dawany N. Benoit B, et al. Synergistic enhancement of cellular immune responses by the novel Toll receptor 7/8 agonist 3M-007 and interferon-gamma: Implications for therapy of cutaneous T-cell lymphoma. Leuk Lymphoma. 2011;52(10):1970–1979. doi: 10.3109/10428194.2011.582202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaspari AA. Mechanism of action and other potential roles of an immune response modifier. Cutis. 2007;79(4 Suppl):36–45. [PubMed] [Google Scholar]
- 12.Hamilton MJ. Antignano F. von Rossum A. Boucher JL. Bennewith KL. Krystal G. TLR agonists that induce IFN-beta abrogate resident macrophage suppression of T cells. J Immunol. 2010;185(8):4545–4553. doi: 10.4049/jimmunol.1002045. [DOI] [PubMed] [Google Scholar]
- 13.Liu YC. Gray RC. Hardy GA, et al. CpG-B oligodeoxynucleotides inhibit TLR-dependent and -independent induction of type I IFN in dendritic cells. J Immunol. 2010;184(7):3367–3376. doi: 10.4049/jimmunol.0903079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopal D. Paturel C. Morel Y. Uematsu S. Akira S. Diebold SS. Plasmacytoid dendritic cell-derived type I interferon is crucial for the adjuvant activity of Toll-like receptor 7 agonists. Blood. 2010;115(10):1949–1957. doi: 10.1182/blood-2009-08-238543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lombardi V. Van Overtvelt L. Horiot S. Moingeon P. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-gamma, and IL-17A by naive CD4+ T cells. J Immunol. 2009;182(6):3372–3379. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 16.Brichacek B. Vanpouille C. Kiselyeva Y, et al. Contrasting roles for TLR ligands in HIV-1 pathogenesis. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y. Abel K. Lantz K. Krieg AM. McChesney MB. Miller CJ. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J Virol. 2005;79(22):14355–14370. doi: 10.1128/JVI.79.22.14355-14370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baenziger S. Heikenwalder M. Johansen P, et al. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2009;113(2):377–388. doi: 10.1182/blood-2008-04-151712. [DOI] [PubMed] [Google Scholar]
- 19.Asin SN. Heimberg AM. Eszterhas SK. Rollenhagen C. Howell AL. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses. 2008;24(5):701–716. doi: 10.1089/aid.2007.0108. [DOI] [PubMed] [Google Scholar]
- 20.Eszterhas S. Ilonzo N. Crozier J. Celaj S. Howell A. Nanoparticles containing siRNA to silence CD4 and CCR5 reduce expression of these receptors and inhibit HIV-1 infection in human female reproductive tract tissue explants. Infectious Dis Rep. 2011;3:52–61. doi: 10.4081/idr.2011.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira CF. Boven LA. Middel J. Verhoef J. Nottet HS. Induction of cyclooxygenase-2 expression during HIV-1-infected monocyte-derived macrophage and human brain microvascular endothelial cell interactions. J Leukoc Biol. 2000;68(3):423–428. [PubMed] [Google Scholar]
- 22.Kottilil S. Shin K. Planta M, et al. Expression of chemokine and inhibitory receptors on natural killer cells: Effect of immune activation and HIV viremia. J Infect Dis. 2004;189(7):1193–1198. doi: 10.1086/382090. [DOI] [PubMed] [Google Scholar]
- 23.Giagulli C. Magiera AK. Bugatti A, et al. HIV-1 matrix protein p17 binds to the IL-8 receptor CXCR1 and shows IL-8-like chemokine activity on monocytes through Rho/ROCK activation. Blood. 2012;119(10):2274–2283. doi: 10.1182/blood-2011-06-364083. [DOI] [PubMed] [Google Scholar]
- 24.Ross MJ. Fan C. Ross MD, et al. HIV-1 infection initiates an inflammatory cascade in human renal tubular epithelial cells. J Acquir Immune Defic Syndr. 2006;42(1):1–11. doi: 10.1097/01.qai.0000218353.60099.4f. [DOI] [PubMed] [Google Scholar]
- 25.Lee C. Liu QH. Tomkowicz B. Yi Y. Freedman BD. Collman RG. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J Leukoc Biol. 2003;74(5):676–682. doi: 10.1189/jlb.0503206. [DOI] [PubMed] [Google Scholar]
- 26.Peng H. Whitney N. Wu Y, et al. HIV-1-infected and/or immune-activated macrophage-secreted TNF-alpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia. 2008;56(8):903–916. doi: 10.1002/glia.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts L. Passmore JA. Mlisana K, et al. Genital tract inflammation during early HIV-1 infection predicts higher plasma viral load set point in women. J Infect Dis. 2012;205(2):194–203. doi: 10.1093/infdis/jir715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 29.Keele BF. Identifying and characterizing recently transmitted viruses. Curr Opin HIV AIDS. 2010;5(4):327–334. doi: 10.1097/COH.0b013e32833a0b9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenchley JM. Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1(1):23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehner T. Wang Y. Whittall T. Seidl T. Innate immunity and HIV-1 infection. Adv Dent Res. 2011;23(1):19–22. doi: 10.1177/0022034511399081. [DOI] [PubMed] [Google Scholar]
- 32.Shen R. Richter HE. Smith PD. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol. 2011;65(3):261–267. doi: 10.1111/j.1600-0897.2010.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen JS. Easterhoff D. Dewhurst S. Advances in HIV microbicide development. Future Med Chem. 2011;3(16):2101–2116. doi: 10.4155/fmc.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai WH. Huang L. Chen CH. HIV entry inhibitors: Progress in development and application. Yao Xue Xue Bao. 2010;45(2):131–140. [PubMed] [Google Scholar]
- 35.Singh IP. Chauthe SK. Small molecule HIV entry inhibitors: Part II. Attachment and fusion inhibitors: 2004–2010. Expert Opin Ther Pat. 2011;21(3):399–416. doi: 10.1517/13543776.2011.550876. [DOI] [PubMed] [Google Scholar]
- 36.Singh IP. Chauthe SK. Small molecule HIV entry inhibitors: Part I. Chemokine receptor antagonists: 2004–2010. Expert Opin Ther Pat. 2011;21(2):227–269. doi: 10.1517/13543776.2011.542412. [DOI] [PubMed] [Google Scholar]
- 37.Teissier E. Penin F. Pecheur EI. Targeting cell entry of enveloped viruses as an antiviral strategy. Molecules. 2011;16(1):221–250. doi: 10.3390/molecules16010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prajapati DG. Ramajayam R. Yadav MR. Giridhar R. The search for potent, small molecule NNRTIs: A review. Bioorg Med Chem. 2009;17(16):5744–5762. doi: 10.1016/j.bmc.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 39.Li X. Krishnan L. Cherepanov P. Engelman A. Structural biology of retroviral DNA integration. Virology. 2011;411(2):194–205. doi: 10.1016/j.virol.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Debyser Z. Christ F. On the cell biology of HIV integration from basic research to development of novel antiviral drugs. Verh K Acad Geneeskd Belg. 2010;72(5–6):219–237. [PubMed] [Google Scholar]
- 41.Steuber H. Hilgenfeld R. Recent advances in targeting viral proteases for the discovery of novel antivirals. Curr Top Med Chem. 2010;10(3):323–345. doi: 10.2174/156802610790725470. [DOI] [PubMed] [Google Scholar]
- 42.Jenwitheesuk E. Horst JA. Rivas KL. Van Voorhis WC. Samudrala R. Novel paradigms for drug discovery: Computational multitarget screening. Trends Pharmacol Sci. 2008;29(2):62–71. doi: 10.1016/j.tips.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergamaschi A. Pancino G. Host hindrance to HIV-1 replication in monocytes and macrophages. Retrovirology. 2010;7:31. doi: 10.1186/1742-4690-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kedzierska K. Maerz A. Warby T, et al. Granulocyte-macrophage colony-stimulating factor inhibits HIV-1 replication in monocyte-derived macrophages. AIDS. 2000;14(12):1739–1748. doi: 10.1097/00002030-200008180-00008. [DOI] [PubMed] [Google Scholar]
- 45.Thurman A. Doncel G. Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: Relationship to HIV acquisition. Am J Reprod Immunol. 2011;65(2):89–98. doi: 10.1111/j.1600-0897.2010.00902.x. [DOI] [PubMed] [Google Scholar]
- 46.Appay V. Sauce D. Immune activation and inflammation in HIV-1 infection: Causes and consequences. J Pathol. 2008;214(2):231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 47.Meier A. Alter G. Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81(15):8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nian H. Geng WQ. Cui HL, et al. R-848 triggers the expression of TLR7/8 and suppresses HIV replication in monocytes. BMC Infect Dis. 2012;12:5. doi: 10.1186/1471-2334-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlaepfer E. Speck RF. Anti-HIV activity mediated by natural killer and CD8+ cells after toll-like receptor 7/8 triggering. PLoS One. 2008;3(4):e1999. doi: 10.1371/journal.pone.0001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fraietta JA. Mueller YM. Do DH, et al. Phosphorothioate 2' deoxyribose oligomers as microbicides that inhibit human immunodeficiency virus type 1 (HIV-1) infection and block Toll-like receptor 7 (TLR7) and TLR9 triggering by HIV-1. Antimicrob Agents Chemother. 2010;54(10):4064–4073. doi: 10.1128/AAC.00367-10. [DOI] [PMC free article] [PubMed] [Google Scholar]