Abstract
A recent national survey of HIV+ adults noted that nearly three-quarters of cognitively impaired individuals are categorized as having asymptomatic neurocognitive impairment (ANI), lacking documented compromise of everyday function. The clinical impact and long-term consequences of ANI are unknown and the importance of this asymptomatic diagnosis has raised concerns in clinical care settings where competing priorities often exist. In this study, we conducted structured tests of everyday functioning in a sample of HIV+ subjects over 60 years of age and asked subjects to rate their performance relative to peers. We demonstrate that individuals with neuropsychological testing impairment often lack self-awareness of functional performance deficits. Specifically, ANI subjects rated functional performance similar to that of HIV-negative control subjects, despite noted deficits in objective measures of function. These findings have important implications for use of self-report of function in the diagnosis of HIV-associated neurocognitive disorders (HAND), likely underestimating symptomatic impairment.
Introduction
The prevalence of neuropsychological testing deficits in the HIV+ population remains unchanged despite widespread use of combination antiretroviral therapy (cART). Current rates of HIV-associated neurocognitive disorder (HAND) approach 50% among community dwelling HIV+ adults,1,2 although overall severity of impairment is attenuated as compared to the pre-cART era, and the frequency of frank dementia is greatly diminished. Risk for HAND increases with age, and older adults tend to have higher rates of comorbid illnesses such as cardiovascular disease, which further increase risk for HAND.3,4 HAND diagnoses range from asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND) to HIV-associated dementia (HAD). Current diagnostic criteria rely on neuropsychological (NP) testing—with HAND patients demonstrating deficits on testing across two or more cognitive domains—and reporting of functional compromise by the HIV+ patient. Report of functional deficits differentiates between asymptomatic (ANI) and symptomatic (MND and HAD) impairment, although comparable cognitive deficits are present within both the ANI and MND categories.5 It is estimated that about 70% of cognitively impaired HIV+ individuals are categorized as ANI, owing to a lack of reported deficits in everyday functioning.5,6
The use of self-report to determine functional compromise, an approach long known to be fraught with limitations in literature related to non-HIV cognitive syndromes such as Alzheimer's disease (AD),7–9 may underestimate rates of symptomatic disease in the setting of HIV. The involvement of frontal-striatal circuits in HIV neuropathology may contribute to deficits in judgment and insight,10,11 leading to a lack of self-awareness of functional abilities that contributes to an asymptomatic diagnosis in patients who show evidence of cognitive impairment by NP testing. Researchers have previously noted a discordance between reporting of symptoms and evidence of impairment in the HIV+ population. Hinkin et al.12 reported that 26% of their subjects with memory impairment by NP testing denied deficits, and Rourke et al.13 found that those who evidenced learning deficits but who did not report memory complaints performed poorly on tasks of conceptual problem solving. Researchers have also noted inaccurate self-report of medication adherence in HIV.14 Use of self-report to establish functional compromise may be similarly unreliable.
The long-term clinical and functional outcomes of the ANI diagnosis remain unknown. Data that identify poor survival outcomes associated with cognitive impairment are based on symptomatically impaired patients.15 Most of the published literature defining functional compromise in HIV has been correlated with neuropsychological testing abnormalities without distinction between those with and without symptomatic impairment, with few exceptions among smaller studies.16–21 Moreover, neuropsychological testing impairment alone does not universally inform critical issues such as workforce reentry among disabled patients.22 Given the challenge in determining functional integrity in patients diagnosed as ANI, clinicians often have insufficient information to guide screening and treatment approaches. This has led to considerable tension in HIV clinical care, where physicians juggle competing priorities in busy primary care clinics and may not have time to assess function accurately.23
We undertook this study as a pilot project to determine whether HIV+ individuals with cognitive impairment by NP testing evidenced any deficits in insight by measuring their ability to objectively assess their own performance on tests of functional abilities. We evaluated 24 HIV+ individuals over the age of 60, using formal functional assessment techniques. Participants were stratified by cognitive diagnosis based on the revised 2007 criteria and both self-reporting and proxy reporting of everyday function.5 We compared data to 10 HIV-negative controls to determine the degree of functional impairment as well as insight into functional limitations. We hypothesize that all cognitively impaired individuals (ANI+MND) will demonstrate both deficits in functional performance and impaired insight.
Materials and Methods
Participant selection
We recruited a sample of subjects (n=24) enrolled in a larger cohort study of cognition in HIV+ individuals over age 60 (UCSF HIV Over 60 Cohort). Subjects were contacted based on stated willingness to participate in additional substudies and included HIV+ participants with normal cognition (HIV-NL), ANI, and MND. Among subjects contacted (n=34), eight HIV-NL, eight ANI, and eight MND completed this study; those who did not participate declined citing aversion to more testing, scheduling conflicts, or were unreachable. One subject did not complete all testing due to experimenter error and was not included in the analyses. For comparison, we used functional performance data captured from 10 healthy HIV-negative controls aged 60 and older, as previously reported.8 Controls were enrolled in the University of California San Francisco (UCSF) Alzheimer's Disease Research Center (ADRC) and had normal performance on NP testing, no report of functional or cognitive decline, and a normal neurological examination, or were members of the UCSF Memory and Aging Center staff (n=3). All participants signed IRB-approved consents.
Individuals recruited into the parent UCSF HIV Over 60 Cohort were residents of the San Francisco Bay area, over 60 years of age, and identified English as their primary language. Major exclusion criteria were a history of stroke, opportunistic brain infection, loss of consciousness greater than 30 min, and active illicit drug use within the past 6 months. Subjects who were not on antiretroviral therapy, as well as those who were hepatitis C antibody positive, diabetic, or had metal in their bodies, were initially excluded from enrollment; however, these exclusions were modified after the first year to better represent the general aging HIV population. Participants were recruited through broad community-based techniques including recruitment from AIDS service organizations, advertisement in local newspapers, physician referrals from community clinics, as well as peer referrals.
Participant cognitive assessments
All subjects in the UCSF HIV Over 60 Cohort were coenrolled into the ADRC at UCSF and underwent standardized comprehensive neurological and medical evaluations, including a complete neurological examination, medical history with HIV history, medications, family history, social history, laboratory tests, and a 1-h neuropsychological testing battery augmented with tests of psychomotor and motor speed (grooved pegboard task and finger tapping, respectively) for greater sensitivity in HIV. The NP battery tapped multiple domains including memory, executive function, psychomotor speed, visuospatial and motor abilities, and attention (Table 1). A proxy informant interview was completed by a certified technician using the Clinical Dementia Rating scale (CDR).24 Although not employed in HIV, the CDR is a widely validated formal interview assessing multiple domains of cognitive functioning and provides a global rating of functional compromise associated with dementia of the Alzheimer's type.25 Participants completed the Geriatric Depression Scale questionnaire (GDS, 30 items) to evaluate for depressive symptoms.26
Table 1.
Neuropsychological Testing Battery Grouped by Domain
| Cognitive domain | Neuropsychological tests |
|---|---|
| Memory | Delayed and immediate recall trials of the CVLT-II, Story Recall, and Benson Figure delayed recall |
| Executive function | Modified Trails, Trails B, STROOP Interference, Lexical Fluency (D words), Digits backward |
| Psychomotor speed | Trails A, WAIS Digit Symbol Modalities Test, STROOP Color Naming |
| Visuospatial | VOSP, Benson Figure Copy, pentagon copy |
| Motor | Grooved Pegboard, Finger Tapping |
| Attention | CVLT-II Trial 1, Digits forward |
| Other tests | GDS, Design Fluency, WRAT word reading, Calculations, Abstractions and similarities, BNT |
CVLT, California Verbal Learning Test; WAIS, Wechsler Adult Intelligence Scale; VOSP, Visual Object and Space Perception Battery; GDS, Geriatric Depression Scale; WRAT, Wide Range Achievement Test; BNT, Boston Naming Test.
All enrollees in the ADRC obtain a final cognitive diagnosis by consensus conference using published diagnostic criteria. Conferences are multidisciplinary and attended by nurses, neuropsychologists, and behavioral neurologists. For the purpose of HIV evaluations, in addition to the standard team present, all conferences were attended by a clinician with more than 10 years of experience diagnosing HIV-related cognitive disorders (V.V.). We applied HAND diagnoses using clinical acumen and the 2007 (Frascati) criteria as a guide.5 Subjects with mild to moderately impaired performance (1 to 2 SD below the mean, adjusted for age and education) were diagnosed as ANI (without report of functional symptoms) or MND (report of functional symptoms). Those diagnosed as HAD demonstrated severe impairment (typically worse than −2 SD) in two cognitive domains with report of functional deficits. We relied on a combination of patient self-report and proxy reporting of function to establish symptoms. Patients were stratified based on cognitive diagnoses from the most recent main study visit (the median time elapsed between functional and NP assessment was 8.5 months and the average time elapsed was similar across all HIV+ groups).
Assessments of functional performance and self-awareness
Participants in this study attended one 3-h visit during which they completed tests of functional performance (FP) from the Neuropsychological Assessment Battery (NAB).27 We administered a modified, shortened version of the NAB, which did not require alterations to the instructions of the NAB but was designed to evaluate participants' self-awareness of their capacity to perform daily activities, as previously described.8 Briefly, the FP battery consists of five subtests that assess memory, language and calculation, judgment, spatial ability, and attention/executive functions using tasks that approximate activities from daily life. Before each subtest subjects were asked to predict their performance based on a brief description of the task and how they thought they would perform on similar tasks in everyday life. They were asked to compare themselves to healthy peers, and to predict their performance using percentiles. A graph of a bell-shaped curve was provided as a visual aid (Fig. 1), and in order to ensure that they interpreted the curve correctly, they were reminded that the majority of their healthy peers would perform at an “average” level, or 50th percentile. After completing each subtest, they were again asked to rate their performance relative to their peers, using the same curve.
FIG. 1.
Bell curve visual aid. This picture was presented to patients when asking them to give predictions or self-assessments of performance. Percentiles and “average,” “worst,” and “best” are indicated below the curve as guides for self-assessment.
Raw FP testing scores were converted to percentiles and t-scores, corrected for age, education, and gender based on a normative dataset published in the NAB manual, and corrected scores were compared between diagnostic groups. We calculated a posttest discrepancy score by subtracting the actual percentile performance from the posttest self-assessment percentile; increasingly negative or positive scores indicate greater discrepancy between self-assessment and actual performance. A negative discrepancy score indicates that subjects overestimated their performance, and a positive discrepancy score indicates underestimation. All statistical analyses were carried out in SAS (v9.2, Cary, NC). We used nonparametric modeling to compare scores on all FP tests (Kruskal–Wallis and Wilcoxon ranked sums with exact statements to compute Monte Carlo estimates; post hoc pairwise comparisons were performed using Dunn's tests), and did not control for covariates, given the small sample size and the relatively matched important parameters across groups. T-scores of performance were used in all statistical analyses, and percentiles were used to present the findings graphically.
Results
The groups were similar on most demographic parameters, although controls were more often women (Table 2). The HIV+ individuals who participated in this pilot study were of similar age, education, CD4+ count, and cART status as the larger UCSF HIV Over 60 Cohort. All HIV+ cases were on cART with plasma HIV RNA <400 copies/ml. The majority of HIV+ subjects were white men who have sex with men (MSM) and one endorsed risk associated with past intravenous drug use. Within the HIV+ groups, the median GDS score was 6, with 71% of participants having a GDS score <10.
Table 2.
Demographic Constitution of the Groups
| Control | NL | ANI | MND | p-value | |
|---|---|---|---|---|---|
| Sample size, n | 10 | 8 | 8 | 8 | |
| Age, mean years (SD) | 64 (9.4) | 65 (4.1) | 66 (6.4) | 67 (3.2) | 0.566 |
| Gender (% male) | 40% | 100% | 100% | 100% | <0.001 |
| Education, mean years (SD) | 17 (2.8) | 17 (2.5) | 16 (2.6) | 16 (1.6) | 0.866 |
| Ethnicity, white | 8/8a | 8/8 | 8/8 | 8/8 | |
| Risk for HIV, MSM only | — | 7/8 | 7/8 (87.5) | 7/8 | |
| CD4 count, mean (SD) | 600 (279) | 525 (210) | 550 (264) | 0.938 |
Ethnicity for two subjects not reported.
NL, HIV+ normal cognition; ANI, asymptomatic neurocognitive impairment; MND, mild neurocognitive disorder; MSM, men who have sex with men.
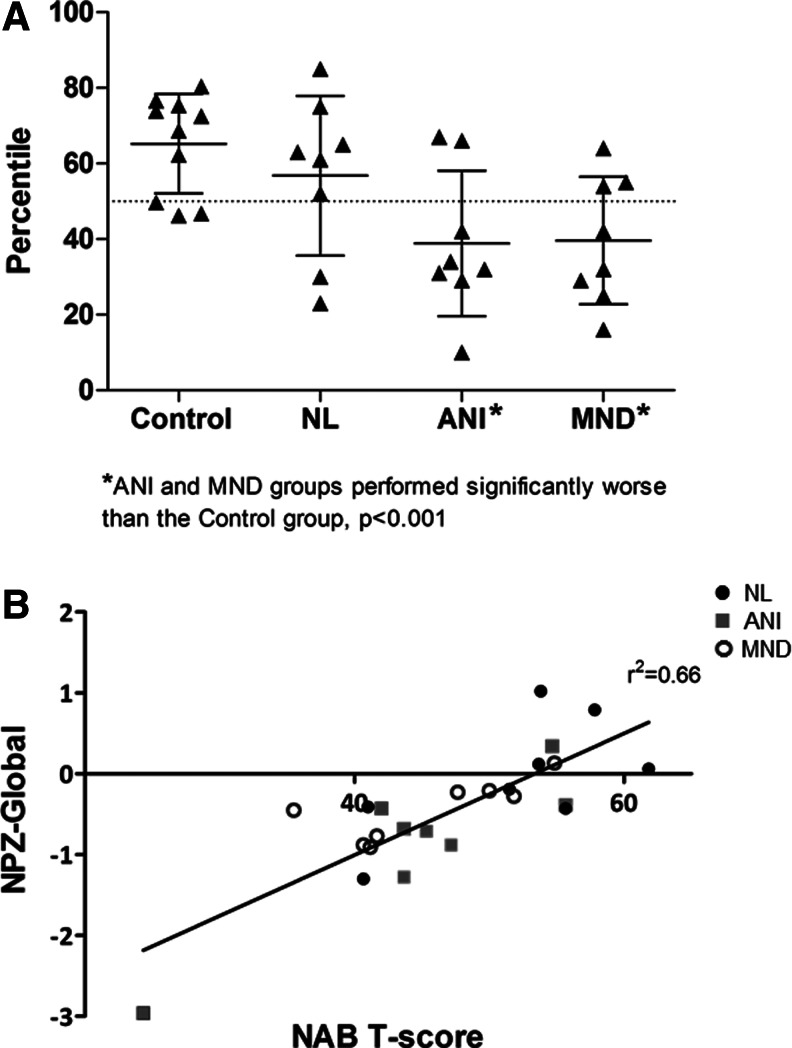
There was considerable variability in FP scores across the HIV+ groups. Most HIV-NL subjects performed above the 50th percentile (6/8), whereas most ANI and MND subjects performed below the 50th percentile (6/8 and 5/8, respectively, Fig. 2a). Few cases performed below the 25th percentile for any of the groups (1/8 HIV-NL, 1/8 ANI, and 1/8 MND). There appeared to be less heterogeneity in performance among controls, with 8/10 performing at or above the 50th percentile. Furthermore, the majority of impaired subjects (6/8 ANI and 5/8 MND) performed >2 SD below controls, and analysis revealed that performance on the NAB differed across groups (p=0.005, Wilcoxon). Compared to HIV-negative controls, pairwise comparisons identified worse performance for the ANI (p<0.001) and MND groups (p<0.001), and using nonparametric testing, t-scores differed across groups for the judgment and driving subtests (p<0.05). However, there were no differences between the MND and ANI groups nor were there differences between the HIV-negative controls and HIV-NL. Some subtests (e.g., judgment) appeared to have a ceiling effect whereas others (e.g., driving) had a broader range of distribution across groups (Table 3). There was a strong correlation between FP t-scores and summary NP test scores across all HIV+ groups (r2=0.66; Fig. 2b).
FIG. 2.
Performance on the Neuropsychological Assessment Battery (NAB) across diagnostic groups (A) and correlated with neuropsychological summary score (B). (A) The majority of individuals in the asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND) groups performed >2 standard deviations below HIV-negative controls. (B) NAB t-scores correlated well with performance on neuropsychological testing, as summarized in an average z-score across all domains (NPZ-Global).
Table 3.
Mean (SD) Scores on Subtests of the Neuropsychological Assessment Battery Stratified by Diagnostic Group
| Task | Control | NL | ANI | MND |
|---|---|---|---|---|
| Immediate recall | 53.7 (±20.5) | 61.4 (±40.5) | 46.1 (±27.7) | 37.0 (±33.3) |
| Delayed recall | 55.4 (±24.8) | 65.4 (±26.4) | 36.1 (±35.0) | 27.1 (±36.1) |
| Bill payment | 62.5 (±7.4) | 40.9 (±25.9) | 32.3 (±31.9) | 34.9 (±29.7) |
| Judgment | 89.1 (±24.3) | 77.3 (±20.0) | 54.6 (±21.0)a | 76.1 (±18.9) |
| Navigation | 59.1 (±36.4) | 52.0 (±38.1) | 31.6 (±29.7) | 31.8 (±32.9) |
| Driving | 71.6 (±27.6) | 42.8 (±33.3) | 30.9 (±25.6)a | 31.0 (±26.2)a |
| NAB total | 65.2 (±13.1) | 56.6 (±21.2) | 38.6 (±19.3)a | 39.6 (±16.8)a |
Differs from control group at p<0.05.
Statistically significant group differences noted for judgment and driving subtests, and total Neuropsychological Assessment Battery (NAB) scores.
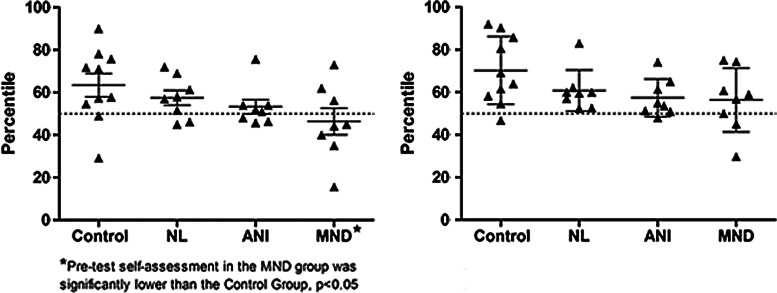
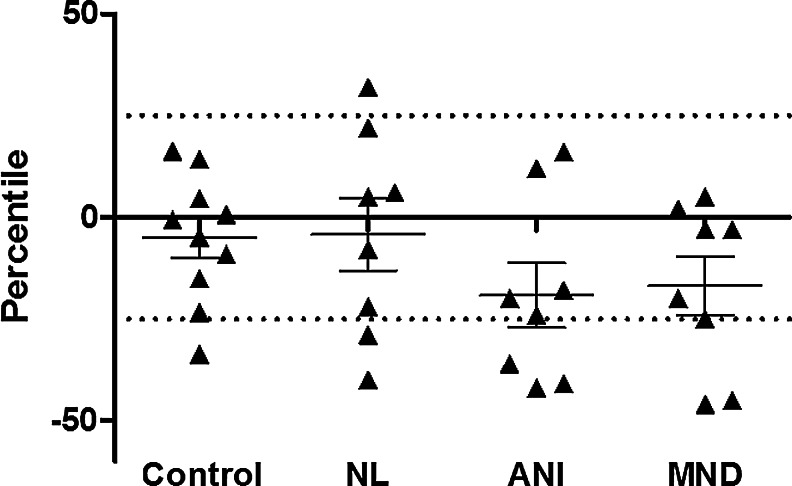
Many subjects anticipated their performance to be at or above the 50th percentile in the pretest assessment (6/10 controls, 6/8 HIV-NL, 5/8 ANI, but only 3/8 MND cases); however, all but four subjects consistently felt they performed at or above the 50th percentile in the posttest scenario (Fig. 3). When comparing posttest estimated performance to actual performance, we identified a wide range of discrepancy, with more consistent negative discrepancies (i.e., overestimations of performance) among impaired HIV+ subjects (ANI and MND, Fig. 4). There were no statistically significant differences in posttest discrepancy scores between any groups at a p<0.05 level; however, the model η2 was 0.18, thus with this small effect size our analyses may have been underpowered to detect group differences.
FIG. 3.
Self-assessment of NAB performance by diagnostic group before (left) and after (right) completing the tests. Statistical analysis of self-assessment by group utilizing a generalized linear model (GLM) and Dunnett's post hoc testing revealed a significant difference in pretest self-assessment between the MND and control groups. No significant differences between patient and control groups were found at posttest self-assessment.
FIG. 4.
Discrepancy between performance and self-assessment of performance measured posttesting. Individuals with ANI and MND had the greatest discrepancies driven by poorer performance.
Discussion
This study was designed to determine the feasibility of objectively evaluating functional abilities and self-awareness in HIV+ patients, with the intention of measuring deficits in self-awareness in individuals with cognitive impairment. All HIV+ subjects with ANI in this study evidenced neuropsychological deficits sufficient to meet MND or HAD criteria, but lacked evidence of functional impairment by self-report or by proxy interview. These pilot data were limited by sample size, HIV+ groups being all male, and a gap in time between cognitive testing and functional assessments; nevertheless, the data suggest a relationship between cognitive impairment, functional performance, and insight that would benefit from further exploration. Our sample consisted of individuals who were accessing medical care and had undetectable viral load, which allows us to understand barriers in the context of optimal care but may not more broadly inform the general HIV+ population.
The majority of subjects performed within a normal range on FP tasks and self-assessment across groups was quite similar, although we identify great variability in performance among ANI individuals, with most individuals performing below the 50th percentile compared to peers. When averaged across tasks, performance within the ANI group was comparable to that in the MND group, and both ANI and MND groups performed significantly worse than HIV-negative controls. Despite the lapse in time between NP testing and functional assessment, FP scores across all HIV+ groups correlated well with NP test performance, indicating a strong relationship between cognitive abilities and function as captured by objective measures.
We further demonstrate that self-awareness of performance is often discrepant from actual performance, with the majority of both ANI and MND participants tending to overestimate their abilities. Although only a pilot study, these data suggest that functional deficits in ANI patients, and in particular older HIV+ patients, may go unreported, and may be captured by objective measures that could increase the accuracy of diagnosis. In a recently published study, researchers reported that combining self-report with performance-based measures assessing function increased the detection of symptomatic HAND, and 9% of their sample demonstrated evidence of functional impairment via performance-based but not self-report measures.28
There may be aspects of the functional testing that influenced insight into performance. We did not ask participants to rate performance directly, but instead to rate this in comparison to peers. Social isolation is common in the older HIV population, and may limit an individual's understanding of peer performance.29–32 Thus, overestimations and underestimations may reflect a lack of insight into, or awareness of, the abilities of others, rather than a lack of personal insight. There is also the possibility that a subject might be unfamiliar with the task approximated by the FP test, for instance the subject may not drive or be responsible for paying bills, and this may make it difficult to provide an accurate pretest self-assessment. For this reason, we focused our analysis of self-assessment discrepancy on posttest assessment.
Given that we evaluated only individuals over 60 years of age, there may also be a sense that functional deficits are simply normal changes associated with aging, and thus subjects rate their performance as average. Likewise, since functional deficits likely developed gradually in many individuals, accommodation to reduced levels of function over time could contribute to poor self-awareness. Furthermore, apathy is a common symptom of HIV disease, and poor performance or inaccurate self-assessment may reflect lack of effort rather than deficits in function or insight due to cognitive impairment.33 Finally, it should be considered that our control subjects were healthy volunteers and more likely to be women; this could impact comparisons between HIV+ and HIV-negative control groups.
In other patient populations with cognitive impairment, such as AD, physicians, and researchers, often rely on third-party informants, typically a spouse or relative, to inform the patients' symptoms and functional abilities.34–36 However, in the HIV+ population, this may not be a reliable approach. Evidence in the HIV literature suggests that older HIV+ patients often lack close social networks and that stigma (for HIV and cognitive disorders) and social isolation in HIV+ elders may hinder a proxy's ability to inform function.29,31 At the UCSF MAC we contact proxy informants to obtain collateral information as a standard part of our protocol. Within the UCSF HIV Over 60 Cohort, we were able to contact only 85% of informants for 87 participants. Among these informants, only 40% lived with the patient. During the same time period at our center, we saw 28 patients with AD, 47 patients with mild cognitive impairment (MCI), and 239 healthy controls of age between 60 and 70 years. Among these, we were able to reach 100% of the participants' informants, of whom 64% lived with the participant. Thus, although proxy reporting has proven to be useful in the context of other neurodegenerative diseases, it may not be feasible to rely on third-party reporting to bolster diagnostic accuracy in HIV, since physical distance is likely to impact the quality of information.
Our data provide proof of principle that self-awareness and self-report of functional abilities may be inaccurate in HIV+ patients. This in turn implies that the current estimates of symptomatic impairment (MND and HAD) may be underestimated. Our findings are consistent with a recent independent report demonstrating dissociation between reporting of functional compromise and objective measures among younger HIV+ subjects.37 These observations are further bolstered by knowledge that neuropathological abnormalities are identified in some ANI cases.38 The consequences of underestimating symptomatic disease may be sizable. From a research perspective, the field has largely failed to identify accurate biomarkers of disease in the current era and treatment trials have a high rate of failure.39 Clinically, assumptions that the vast majority of impaired subjects are asymptomatic may reduce efforts toward assessment and treatment of HAND given the competing priorities of busy clinics. Clarification of diagnosis will benefit clinical-neuropathological correlations and help define the long-term consequences associated with the currently employed diagnostic criteria.
This study raises further awareness that ANI should not be considered a continuum of impairment severity between normal cognition and MND. This finding concurs with that of a recent publication noting similar-to-worse performance on objective tests of everyday functioning among ANI subjects compared to MND and several reports identifying fluctuation in cognition in HIV rather than progressive decline.5,40,41 Since the major difference between ANI and other impaired groups is the reporting of functional deficits, our data lend support to the possibility that such a high transition rate may be, in part, related to an inaccurate understanding of functional capabilities at the time of diagnosis. Our data highlight a weakness in the diagnostic criteria, such that there may be no true clinical or neuropathological difference between ANI and MND, but the ANI category instead may capture a subset of cognitively impaired individuals who have deficits in self-awareness.
In sum, in this small sample of HIV+ patients over 60 years of age we identified a pattern of functional compromise associated with cognitive impairment, with little difference in frequency of functional limitations between those with and without symptoms (ANI vs. MND). Correlation between objectively measured functional and cognitive abilities suggests that tools to measure functional performance may improve the accuracy of diagnosis. We further note a lack self-awareness of functional performance and atypical social networks that may not allow reliance on third parties to inform functional deficits in HIV+ patients. If confirmed, these data have important implications for the applicability of current diagnostic criteria as they relate to clinical parameters and outcomes.
Acknowledgments
We thank our study participants. This work was funded by NIH grants K23-AG032872 (V.V.) and P50-AG023501 (B.M.). Additional support was from AG00688 (H.R.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008;16(2):94–98. [PubMed] [Google Scholar]
- 2.Heaton RK. Franklin DR. Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiesi A. Vella S. Dally LG, et al. Epidemiology of AIDS dementia complex in Europe. AIDS in Europe Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11(1):39–44. doi: 10.1097/00042560-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Valcour VG. Shikuma CM. Watters MR. Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: Prevalence and potential mechanisms. AIDS. 2004;18(Suppl 1):S79–86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antinori A. Arendt G. Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaton RK. Clifford DB. Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmon E. Perani D. Collette F, et al. A comparison of unawareness in frontotemporal dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatr. 2008;79(2):176–179. doi: 10.1136/jnnp.2007.122853. [DOI] [PubMed] [Google Scholar]
- 8.Williamson C. Alcantar O. Rothlind J. Cahn-Weiner D. Miller BL. Rosen HJ. Standardised measurement of self-awareness deficits in FTD and AD. J Neurol Neurosurg Psychiatr. 2010;81(2):140–145. doi: 10.1136/jnnp.2008.166041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham DP. Kunik ME. Doody R. Snow AL. Self-reported awareness of performance in dementia. Brain Res Cogn Brain Res. 2005;25(1):144–152. doi: 10.1016/j.cogbrainres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Melrose RJ. Tinaz S. Castelo JM. Courtney MG. Stern CE. Compromised fronto-striatal functioning in HIV: An fMRI investigation of semantic event sequencing. Behav Brain Res. 2008;188(2):337–347. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Cysique LA. Maruff P. Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: A combined study of two cohorts. J Neurovirol. 2004;10(6):350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 12.Hinkin CH. van Gorp WG. Satz P, et al. Actual versus self-reported cognitive dysfunction in HIV-1 infection: Memory-metamemory dissociations. J Clin Exp Neuropsychol. 1996;18(3):431–443. doi: 10.1080/01688639608408999. [DOI] [PubMed] [Google Scholar]
- 13.Rourke SB. Halman MH. Bassel C. Neuropsychiatric correlates of memory-metamemory dissociations in HIV-infection. J Clin Exp Neuropsychol. 1999;21(6):757–768. doi: 10.1076/jcen.21.6.757.852. [DOI] [PubMed] [Google Scholar]
- 14.Gorman AA. Foley JM. Ettenhofer ML. Hinkin CH. van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev. 2009;19(2):186–203. doi: 10.1007/s11065-009-9095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivithanaporn P. Heo G. Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival. A population-based study. Neurology. 2010;75:1150–1158. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK. Marcotte TD. Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 17.Vigil O. Posada C. Woods SP, et al. Impairments in fine-motor coordination and speed of information processing predict declines in everyday functioning in hepatitis C infection. J Clin Exp Neuropsychol. 2008;30(7):805–815. doi: 10.1080/13803390701802354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mindt MR. Cherner M. Marcotte TD, et al. The functional impact of HIV-associated neuropsychological impairment in Spanish-speaking adults: A pilot study. J Clin Exp Neuropsychol. 2003;25(1):122–132. doi: 10.1076/jcen.25.1.122.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thames AD. Kim MS. Becker BW, et al. Medication and finance management among HIV-infected adults: The impact of age and cognition. J Clin Exp Neuropsychol. 2011;33(2):200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinkin CH. Castellon SA. Durvasula RS, et al. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59(12):1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcotte TD. Heaton RK. Wolfson T, et al. The impact of HIV-related neuropsychological dysfunction on driving behavior. The HNRC Group. J Int Neuropsychol Soc. 1999;5(7):579–592. doi: 10.1017/s1355617799577011. [DOI] [PubMed] [Google Scholar]
- 22.Chernoff RA. Martin DJ. Schrock DA. Huy MP. Neuropsychological functioning as a predictor of employment activity in a longitudinal study of HIV-infected adults contemplating workforce reentry. J Int Neuropsychol Soc. 2010;16(1):38–48. doi: 10.1017/S1355617709990828. [DOI] [PubMed] [Google Scholar]
- 23.Bodenheimer T. Primary care—will it survive? N Engl J Med. 2006;355(9):861–864. doi: 10.1056/NEJMp068155. [DOI] [PubMed] [Google Scholar]
- 24.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 177–178. [DOI] [PubMed] [Google Scholar]
- 26.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24(4):709–711. [PubMed] [Google Scholar]
- 27.Stern R. White T. Psychological Assessment Resources, Inc.; 2003. Neuropsychological Assessment Battery (NAB) [Google Scholar]
- 28.Blackstone K. Moore DJ. Heaton RK, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: Self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18(01):79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grov C. Golub SA. Parsons JT. Brennan M. Karpiak SE. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care. 2010;22(5):630–639. doi: 10.1080/09540120903280901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao D. Pryor JB. Gaddist BW. Mayer R. Stigma, secrecy, and discrimination: Ethnic/racial differences in the concerns of people living with HIV/AIDS. AIDS Behav. 2008;12(2):265–271. doi: 10.1007/s10461-007-9268-x. [DOI] [PubMed] [Google Scholar]
- 31.Schrimshaw EW. Siegel K. Perceived barriers to social support from family and friends among older adults with HIV/AIDS. J Health Psychol. 2003;8(6):738–752. doi: 10.1177/13591053030086007. [DOI] [PubMed] [Google Scholar]
- 32.Vance DE. Childs G. Moneyham L. McKie-Bell P. Successful aging with HIV: A brief overview for nursing. J Gerontol Nurs. 2009;35(9):19–25. doi: 10.3928/00989134-20090731-04. quiz 26–17. [DOI] [PubMed] [Google Scholar]
- 33.Castellon SA. Hinkin CH. Wood S. Yarema KT. Apathy, depression, and cognitive performance in HIV-1 infection. J Neuropsychiatr Clin Neurosci. 1998;10(3):320–329. doi: 10.1176/jnp.10.3.320. [DOI] [PubMed] [Google Scholar]
- 34.Karlawish JH. Clark CM. Diagnostic evaluation of elderly patients with mild memory problems. Ann Intern Med. 2003;138(5):411–419. doi: 10.7326/0003-4819-138-5-200303040-00011. [DOI] [PubMed] [Google Scholar]
- 35.O'Keeffe FM. Murray B. Coen RF, et al. Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain. 2007;130(Pt 3):753–764. doi: 10.1093/brain/awl367. [DOI] [PubMed] [Google Scholar]
- 36.Grill JD. Raman R. Ernstrom K. Aisen P. Karlawish J. Effect of study partner on the conduct of Alzheimer disease clinical trials. Neurology. 2013;80(3):282–288. doi: 10.1212/WNL.0b013e31827debfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thames AD. Becker BW. Marcotte TD, et al. Depression, cognition, and self-appraisal of functional abilities in HIV: An examination of subjective appraisal versus objective performance. Clin Neuropsychol. 2011;25(2):224–243. doi: 10.1080/13854046.2010.539577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherner M. Cysique L. Heaton RK, et al. Neuropathologic confirmation of definitional criteria for human immunodeficiency virus-associated neurocognitive disorders. J Neurovirol. 2007;13(1):23–28. doi: 10.1080/13550280601089175. [DOI] [PubMed] [Google Scholar]
- 39.Uthman OA. Abdulmalik JO. Adjunctive therapies for AIDS dementia complex. Cochrane Database Syst Rev. 2008;((3)):CD006496. doi: 10.1002/14651858.CD006496.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Gandhi NS. Skolasky RL. Peters KB, et al. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. J Neurovirol. 2011;17(2):159–165. doi: 10.1007/s13365-011-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McArthur JC. Haughey N. Gartner S, et al. Human immunodeficiency virus-associated dementia: An evolving disease. J Neurovirol. 2003;9(2):205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]