Abstract
Breast cancer is the most common cancer among women. To date, 22 common breast cancer susceptibility loci have been identified accounting for ~ 8% of the heritability of the disease. We followed up 72 promising associations from two independent Genome Wide Association Studies (GWAS) in ~70,000 cases and ~68,000 controls from 41 case-control studies and nine breast cancer GWAS. We identified three new breast cancer risk loci on 12p11 (rs10771399; P=2.7 × 10−35), 12q24 (rs1292011; P=4.3×10−19) and 21q21 (rs2823093; P=1.1×10−12). SNP rs10771399 was associated with similar relative risks for both estrogen receptor (ER)-negative and ER-positive breast cancer, whereas the other two loci were associated only with ER-positive disease. Two of the loci lie in regions that contain strong plausible candidate genes: PTHLH (12p11) plays a crucial role in mammary gland development and the establishment of bone metastasis in breast cancer, while NRIP1 (21q21) encodes an ER co-factor and has a role in the regulation of breast cancer cell growth.
Breast cancer is one of the most commonly occurring epithelial malignancies in women with an estimated one million new cases and over 400,000 deaths annually worldwide1. Familial aggregation and twin studies have demonstrated the substantial contribution of inherited susceptibility to breast cancer 2, 3. Over the last four years, we and others have conducted several genome-wide association studies (GWAS) and reported breast cancer susceptibility variants at 21 loci 4-14 with an additional locus (CASP8) identified through a candidate gene approach 15. These variants are associated with modest risks of the disease (per-allele odds ratios <1.3), and explain ~ 8% of the excess familial risk of breast cancer, while other rarer high and moderate risk loci contribute less than 20%, suggesting that other loci remain to be identified 16.
To identify further breast cancer susceptibility loci, we selected 72 SNPs that were genotyped and found to be significantly associated with breast cancer at P<0.0001 in either of two breast cancer GWAS in the UK (UK2 and BBCS) 17, 18. We attempted to genotype these SNPs in up to 41 case-control studies through the Breast Cancer Association Consortium (BCAC). After quality control (QC) exclusions (see Methods), we analysed data on 54,588 cases of invasive breast cancer, 2401 cases of Ductal Carcinoma in Situ (DCIS) and 58,098 controls. In addition, we utilised data from 7 additional breast cancer GWAS from which summary results had been obtained based on imputation to Hapmap 2 CEU. Results from the GWAS and BCAC replication were then combined to derive the overall evidence of association for each SNP based on 69,564 cases and 68,150 controls.
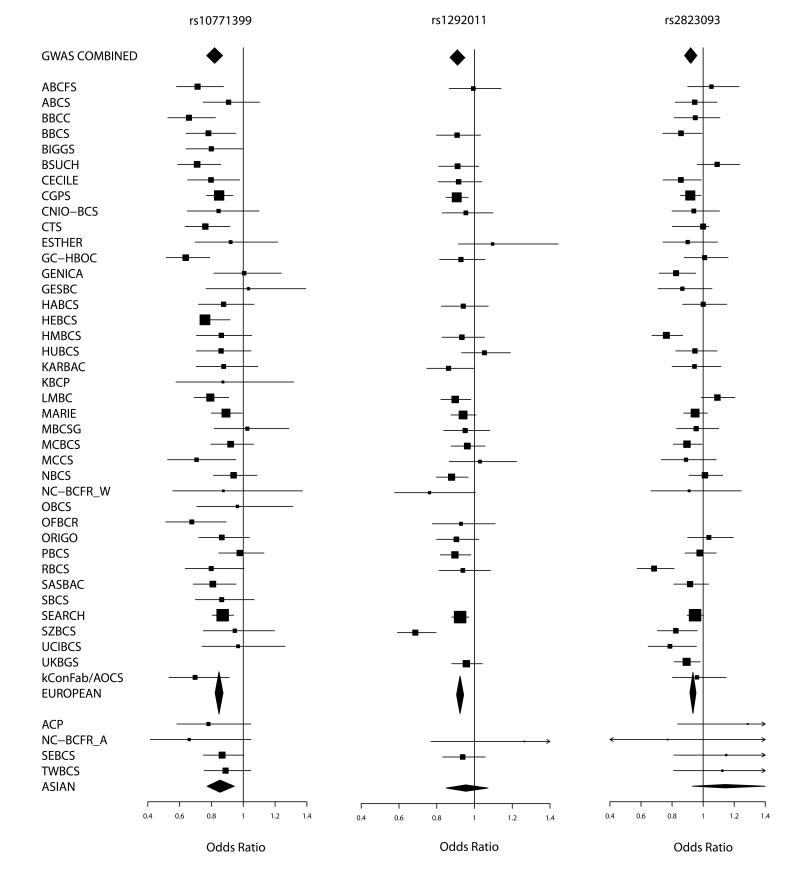
Three SNPs showed strong evidence for association in European women, consistent with the effect seen in the original GWAS (Table 1 and Figure 1). In each case, the genotype-specific odds ratios (ORs) were consistent with an allele dose (log-additive) model (Supplementary Table 1). SNP rs2823093 showed some evidence of heterogeneity in the per-allele ORs among studies in the replication stage (P=0.002), with particularly marked associations in two studies (HMBCS, RBCS; Figure 1). The association in the replication stage remained highly significant, however, even after excluding these two studies (P=7.1×10−7). The other two loci showed no evidence of heterogeneity among studies. Two additional SNPs on 17q21, rs2532348 and rs199523 (correlated at r2=0.80 in the UK2 GWAS), gave more limited evidence of replication (P=0.000078 and P=0.0063) and reached P=5.8×10−7 and P=2.6×10−6 respectively when combined with the GWAS data (Supplementary Table 2). These SNPs were only genotyped in the UK2 GWAS. They could not be imputed using HapMap, and were only successfully genotyped in 12 studies in the BCAC replication. Moreover, for SNP rs2532348 there was evidence of heterogeneity among studies in the per-allele ORs in BCAC (P=0.001). Further data will be required to determine whether this SNP is associated with breast cancer risk. Three other SNPs (rs10940235 on 5q11, rs4403040 on 4q21 and rs6027564 on 20q13) showed evidence of replication at P<0.01 but none reached genome-wide levels of statistical significance (Supplementary Table 2).
Table 1.
| SNP | Chromosome Position1 |
Alleles2 | MAF | Stage | Per-allele OR (95%CI)3 |
P | Combined P |
|---|---|---|---|---|---|---|---|
| rs10771399 | 12p11 28046347 |
AG | 0.12 | UK2 | 0.79 (0.71-0.87) |
3.1×10−6 | |
| 0.11 | BBCS | 0.84 (0.74-0.96) |
.008 | ||||
| 0.10 | Other GWAS | 0.83 (0.75-0.91) |
5.7×10−5 | ||||
| 0.12 | BCAC replication |
0.85 (0.83-0.88) |
3.3×10−27 | 2.7×10−35 | |||
| rs1292011 | 12q24 114320905 |
AG | 0.41 | UK2 | 0.88 (0.83-0.94) |
5.8×10−5 | |
| 0.42 | BBCS | 0.95 (0.88-1.03) |
0.23 | ||||
| 0.40 | Other GWAS | 0.91 (0.86-0.96) |
.0008 | ||||
| 0.41 | BCAC replication |
0.92 (0.91-0.94) |
6.2×10−14 | 4.3×10−19 | |||
| rs2823093 | 21q21 15442703 |
GA | 0.26 | UK2 | 0.96 (0.89-1.03) |
0.21 | |
| 0.26 | BBCS | 0.88 (0.76-0.92) |
.00013 | ||||
| 0.26 | Other GWAS | 0.91 (0.85-0.97) |
.0032 | ||||
| 0.27 | BCAC replication |
0.94 (0.92-0.96) |
1.7×10−9 | 1.1×10−12 |
Build 36
Minor allele listed second
Per copy of the minor allele
Figure 1.
Forest plots for the 3 SNPs showing evidence of association with breast cancer. Squares represent the estimated per-allele odds ratio (OR) for individual studies. The area of square is inversely proportional to the precise of the estimate. Diamonds represent the summary OR estimates for the subgroups indicated. Horizontal lines represent 95% confidence limits.
For women of Asian ancestry, SNP rs10771399 (12p11) was also associated with breast cancer risk, with the estimated OR being similar to that in women of European ancestry (Supplementary Table 3). There was no significant evidence of association for either SNPs rs1292011 (12q24) or rs2823093 (21q21) in women of Asian ancestry. For rs2823093, the estimated OR was in the opposite direction than that in women of European ancestry, but the estimates did not differ significantly (Supplementary Table 3).
SNP rs10771399 showed strong evidence of association with both estrogen receptor (ER)-positive and ER-negative breast cancer, with the estimated per-allele ORs being similar (based on 24,775 ER-positive and 7,122 ER-negative cases; Supplementary Table 4a). In contrast, for SNPs rs1292011 and rs2823093, the association was confined to ER-positive breast cancer, with no evidence of association for ER-negative disease (Supplementary Table 4a). These latter results conform to the general pattern of a preponderance of common susceptibility loci for ER-positive disease identified through GWAS based on cases unselected for disease subtype 19, 20. In terms of per-allele OR, SNP rs10771399 has one of the strongest effects identified to date for ER-negative breast cancer (OR 0.85, 95%CI 0.80-0.90). For all three SNPs, the per-allele OR for DCIS was similar to that for invasive disease (based on up to 2,148 DCIS cases; Supplementary Table 4b). For SNP rs10771399, the estimated OR was higher for 10 studies in which cases were selected for a positive family history and/or bilaterality, as would be expected under a polygenic model 21 (P=0.027, Supplementary Table 5); however, exclusion of data from these studies made little difference to the estimated OR. There was no evidence for difference in the per-allele OR by age at diagnosis for any SNP (Supplementary Table 4c).
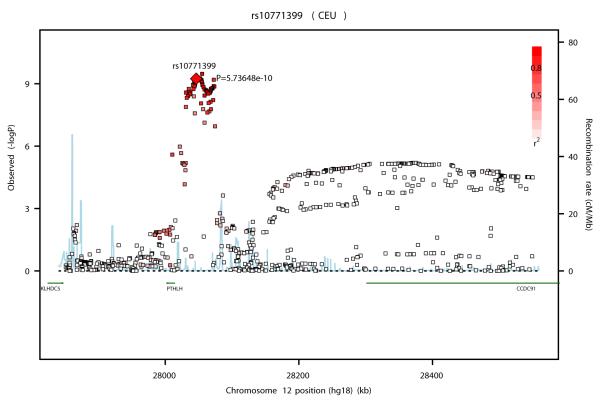
SNP rs10771399 lies in a ~300kb linkage disequilibrium (LD) block on 12p11 that contains one known gene, PTHLH (Parathyroid Hormone like Hormone isoform 1), also called PTHrP (Parathyroid hormone–related protein; Figure 2a). PTHrP is expressed in a wide variety of tissues and in many malignancies, including 60% of breast tumors and is required for normal mammary gland and bone development 22-25. During lactation it is released by the mammary gland to regulate the transfer of calcium from the skeleton to the milk 26, 27. Tumor secreted PTHrP mimics the action of parathyroid hormone (PTH) by binding to its receptor PTH1R 28 promoting humoral hypercalcemia as well as metastasis of breast cancer cells to the bone 23, 29-31. It has been suggested that PTHrP enhances tumorigenesis through its pro-proliferative and anti-apoptotic activity by promoting survival in cells subjected to apoptosis 32, 33. However, conflicting data regarding the correlation of PTHrP expression level and breast cancer survival have been found 24, 34-36. Moreover, a recent study reported that loss of PTHrP accelerates tumor incidence in DCIS and is associated with monocyte infiltration 37.
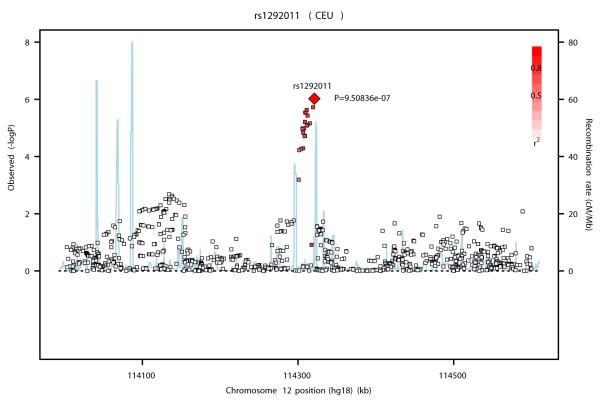
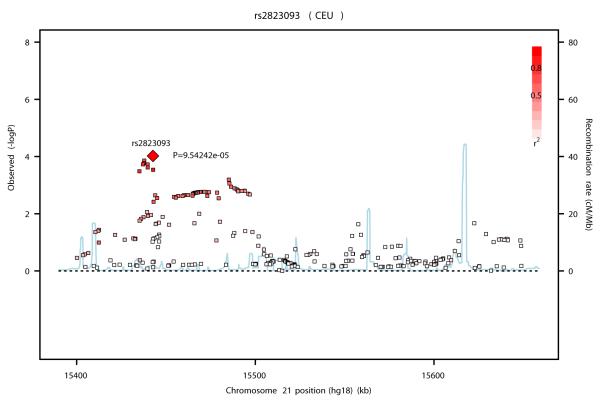
Figures 2a, b and c.
Association plots for the three new breast cancer susceptibility loci at (a) 12p11 (b) 12q24 and (c) 21q21 drawn using the SNAP software35 68. Genotyped and imputed SNPs are plotted based on their chromosomal position in build 36 on the X axis and their overall P values (as −log10 values) from the UK2 and BBCS GWAS on the Y axis. For each region, the most strongly associated SNP is represented by a diamond. The intensity of the red shading reflects the strength of correlation (r2) between the best SNP and the other SNPs in the region. Genes present in the region (if any) are indicated in green.
SNP rs1292011 on 12q24 lies in a ~ 100 kb LD block that contains no known genes (Figure 2b). SNPs in this region have been found to be associated with squamous esophageal carcinoma, renal cell carcinoma, liver adenoma, heart disease and type 1 diabetes as well as blood pressure and PSA levels 38-47. Two plausible cancer candidate genes, MAPKAPK5 (mitogen-activated protein kinase-activated protein kinase 5, also called MK5/PRAK) and TBX3 (T-box3), lie within 2 Mb of rs1292011. MAPKAPK5 is a member of the serine/threonine kinase family and is directly activated by Myc 48. TBX3 plays a role in mammary gland development49 and its haplo-insufficiency is associated with Ulnar-Mammary disorder50. TBX3 was found to be amplified and over-expressed in several cancers including breast cancer 51-54 and at high levels in plasma from breast and ovarian cancer patients 52. Recently, it has been shown that estrogen regulates the expansion of breast cancer stem cells through the FGF/FGFR/TBX3 pathway 52, 55 and that TBX3 is a direct downstream target of the Wnt/beta-catenin pathway 56. The expression of TBX3 was found to be significantly higher (P<0.0001) in ER-positive than in ER-negative breast cancer tumors in two independent datasets containing 781 tumors (with HGU-133A Affymetrix expression data) 57 and 244 tumors (with 44k Agilent expression data) 58. These data suggest that the association of rs1292011 with ER-positive breast cancer could be mediated through its effect on TBX3.
SNP rs2823093 lies in a ~ 130 Kb LD block containing no known genes. The nearest gene, ~900 Kb downstream, is NRIP1 (Nuclear Receptor interacting protein 1) (Figure 2 c) or also called RIP140 (Receptor-interacting protein 140). RIP140 acts as a strong transcriptional repressor for nuclear receptors 59, 60. It interacts with estrogen receptor α (ERα), represses the ER signalling and inhibits its mitogenic effects 61. This repression is mediated through interaction with FHL1, a protein involved in suppressing cancer cell growth and migration 62. Several lines of evidence suggest that RIP140 plays an important role in the regulation of breast cancer cell growth. Knockdown of RIP140 was found to induce growth promotion in an ER-positive breast cancer cell line 61. This protein was also highly induced following the treatment of human breast cancer cells with retinoids, known for their breast cancer growth suppression and their anti-estrogenic effects 63-66. A Spanish case-control study, which genotyped SNPs in 91 breast cancer candidate genes in ~700 cases and ~700 controls, identified a relatively rare SNP at this locus (rs926184 - MAF~2%), located 175 Kb upstream of rs2823093, which showed a modest association with breast cancer 67. These two SNPs are, however, not correlated (r2=0 in HapMap CEU). The expression of NRIP1 has been shown to be significantly higher in ER-positive than ER-negative tumors (p<0.0001) 57, 58 suggesting that the association of rs28323093 with ER-positive breast cancer could be mediated through its effect on NRIP1 expression 57, 58.
The three novel susceptibility variants identified in this study are relatively common (MAF 0.11-0.41) and together explain ~0.7% of the familial risk of breast cancer, and bring the total contribution of common low-penetrance breast cancer susceptibility loci to ~9%. The relative risks associated with these variants are modest, with the per-allele ORs for the risk allele ranging from 1.07 to 1.22 fold, but the causal variants underlying some of these loci might confer more substantial risks. The present work highlights the importance of combining GWAS and large-scale replication studies with tumor subtyping in the identification and characterisation of breast cancer susceptibility loci.
The genes in these regions (if proven to be the causal genes) underscore that diverse mechanisms are likely to be relevant to breast cancer pathogenesis. Re-sequencing of these loci, combined with fine-scale mapping and functional analyses will provide more insights into the genetic architecture of breast cancer and the pathogenesis of the disease.
Methods
GWAS analysis
Primary genotype data were obtained for nine breast cancer GWAS in populations of European ancestry (Supplementary Table 6). Standard QC was performed on all scans, as follows. We excluded all individuals with low call rate (<95%), extreme high or low heterozygosity (P<10−5), and all individuals evaluated to be of non-European ancestry (>15% non-European component, by multidimensional scaling using the three Hapmap2 populations as a reference). We excluded SNPs with: call rate <95%; call rate <99% and MAF<5%, all SNPs with MAF<1%, and SNPs whose genotype frequencies departed from Hardy-Weinberg equilibrium at P<10−6 in controls or P<10−12 in cases. For highly significant SNPs the genotype intensity cluster plots were examined manually to judge reliability, either centrally or by contacting the original investigators.
Data were imputed for all scans for ~2.6M SNPs using HapMap version 2 CEU as a reference, using the program Mach v1.0. Estimated per-allele ORs and standard errors were generated from the imputed genotypes using Probabel69. For two studies (UK2 and HEBCS), estimates were adjusted by the first three principal components, since this was found to materially reduce the inflation. Residual inflation was then adjusted for by multiplying the variance by a genomic control adjustment factor, based on the ratio of the median chi-squared test statistic to its expected value. BBCS and UK2 used the same control data (WTCCC2) but different genotyping platforms. These studies were imputed separately. For the combined analysis, the control set was divided randomly between the two studies, in proportion to the size of case series, to provide disjoint strata. For a limited subset of SNPs that could not be imputed (including rs2532348 and rs199523 on 17q21), genotype data from the original scan(s) were used in the analysis.
Replication stage
SNPs for replication were genotyped in 46 studies, of which 4 were case-only studies that did not contribute to the current analysis (Supplementary Table 7). Data from BBCS were excluded as the same cases were included in the GWAS. Seven studies (HABCS, HMBCS, HUBCS, KARBAC, RBCS, SEARCH and SEBCS) were analysed by Fluidigm for 72 SNPs (Supplementary Table 2). We selected 63 SNPs selected from UK2: one replaced by a better surrogate, and one failed, so only data were available for 61 SNPs. Ten SNPs were selected from BBCS and one SNP was selected from both scans (The original SNP, rs1975930, also referred to as rs56003999, did not work by Fluidigm and in some iPlex analyses and was replaced by a surrogate rs10771399, r2=0.95, which was typed in all studies). Samples from 27 studies were genotyped by iPlex for 29 SNPs that showed the strongest associations. Seven additional studies (ABCFS, CGPS, MCCS, NC-BCFR, OFBCR, PBCS, UKBGS) were genotyped by Taqman for up to 4 SNPs that showed association after the Fluidigm and iPlex genotyping, including all three 3 SNPs discussed in detail here. We restricted the analysis to individuals of European or East Asian ancestry, since the sample size for other ethnicities was too small to give meaningful results.
All studies complied with BCAC genotyping QC standards by including at least 2% of samples in duplicate and a common set of 93 CEPH DNAs used by the HapMap Consortium (HAPMAPPT01, Coriell Institute for Medical Research, Cambden, NJ). Genotype data were excluded for: any sample that consistently failed genotyping for >20% of the SNPs typed; all samples on any one plate that had a SNP call rate <90%; all genotype data for any SNP where overall call rate was <95%; and all genotype data for any SNP where duplicate concordance was <98% (based on 2% of samples genotyped in duplicate). In addition, for any SNP for which the P-value for departure from Hardy-Weinberg equilibrium for controls was <0.005, clustering of the intensity plots was reviewed manually and the data excluded if clustering was judged to be poor. After QC exclusions we analysed data on 54,588 cases of invasive breast cancer, 2,401 cases of DCIS and 58,098 controls.
Per-allele and genotype-specific odds ratios for the replication stage were estimated using logistic regression, adjusted for study. Women of European and Asian ancestry were analysed separately. NC-BCFR contributed cases and controls to both European and Asian analyses; for the remaining studies the subjects were either predominantly European or predominantly Asian, and subjects from other minority ethnicities were excluded.
Statistical significance levels from the GWAS and BCAC replication phases were obtained by combining the logOR estimates and standard errors as in a fixed effect meta-analysis. Heterogeneity in the OR association with each SNP by ER status was evaluated using a case-only analysis, by logistic regression. Heterogeneity by age was evaluated by fitting a linear age × genotype interaction term.
Supplementary Material
Acknowledgements
We would thank the following individuals for their contribution to this project (study in brackets): Sten Cornelissen, Richard van Hien, Linde Braaf , Laura Van’t Veer, Bas Bueno-de-Mesquita and Sander Canisius (ABCS); Niall McInerney, Gabrielle Colleran, Andrew Rowan and Nicola Miller (BIGGS); Anne Langheinz (BSUCH) ; José Ignacio Arias Pérez, Pilar Zamora, Primitiva Menendez, Tais Moreno and Guillermo Pita (CNIO-BCS); Muriel Adank, Margreet Ausems and Senno Verhoef (DFBBCS); Ute Hamann, Yon-Dschun Ko, Christian Baisch, Hans-Peter Fischer, Beate Pesch, Sylvia Rabstein and Volker Harth (GENICA); Kirsimari Aaltonen, Päivi Heikkilä, Tuomas Heikkinen, Dario Greco, RN Hanna Jäntti and Irja Erkkilä (HEBCS); Helena Kemiläinen, Eija Myöhänen and Aija Parkkinen (KBCP); Tracy Slanger, Elke Mutschelknauss, S. Behrens, R. Birr, M.Celik, U. Eilber, B. Kaspereit, N. Knese and K. Smit (MARIE); Paolo Radice, Bernard Peissel, Monica Barile, Marco A. Pierotti (MBCSG); Teresa Selander, Mona Gill, Lucine Collins and Nayana Weerasooriya (OFBCR); Mervi Grip, Kari Mononen and Meeri Otsukka (OBCS); E. Krol-Warmerdam, and J. Blom (ORIGO); Dr. Prat Boonyawongviroj and Dr. Pornthep Siriwanarungsan (ACP). For full acknowledgements including funding see Supplementary Note.
Footnotes
URLs HapMap http://hapmap.ncbi.nlm.nih.gov/
Conflicts of interest The authors have no conflict competing financial interests to disclose.
Authors contribution M.G. and D.F.E. wrote the manuscript. K.M., M.G. and D.F.E. performed the statistical analysis. O.F., N.J., N.O., I.dosS.S., M.L. and J.P. led the BBCS GWAS. D.F.E., P.D.P.P., A.M.D., C.T. and N.R. led the UK2 GWAS. Q.Waisfisz and H.M.-H. led the DFBBCS GWAS, with support from A.G.U. and F.R.. P.Hall, K.C., A.I. and J.Liu led the SASBAC GWAS. H.N., K.A. and C.Blomqvist led the HEBCS GWAS. AMeindl, R.K.S. and B.M.-M. led the GC-HBOC GWAS. J.C.-C., R.H., S.N. and D.F.-J. led the MARIE GWAS. J.L.H., M.Southey, H.T., E.M., D.S. and M.Bui led the ABCFS/kConFab GWAS. D.J.H. and S.J.C. led the CGEMS GWAS. E.D. and J.D. provided bioinformatics support. Q.Wang, M.K.H. and K.D. provided data management support for BCAC. C.Baynes, D.C., M.M. and S.A. managed centralised genotyping for BCAC samples. M.K.S. provided gene expression analysis. J.L.H., M.Southey, C.A. and D.J.P. co-ordinated the ABCFR study. M.K.S., A.B., S.V. and F.B.L.H. co-ordinated ABCS. P.A.F. co-ordinated the BBCC study. E.S., I.T. and M.K. co-ordinated BIGGS. F.Marme, ASchneeweiss, C.Sohn and B.Burwinkel co-ordinated the BSUCH study. P.G., T.T., E.C.-D. and F.Menegaux co-ordinated the CECILE study. S.E.B., B.G.M. and S.F.N. co-ordinated CGPS. R.L.M., R.A., A.G.-N. and J.Benítez co-ordinated CNIO-BCS. H.A.-C., A.Z., L.Bernstein and C.C.D. co-ordinated CTS. H.Brenner, H.M., V.A. and C.Stegmaier co-ordinated the ESTHER study. C.J., H.Brauch and B.P. co-ordinated the GENICA study. J.C.-C., S.W.-G. and U.E. co-ordinated the GESBC study. T.D., P.S., M.Bremer and P.Hillemanns co-ordinated HABCS. N.V.B., N.N.A., Y.I.R., J.H.K. and T.D. co-ordinated HMBCS. M.Bermisheva, D.P., N.V.B., T.D., and E.K. co-ordinated HUBCS. A.Lindblom and S.Margolin co-ordinated the KARBAC study. A.Mannermaa, V.Kataja, V.-M.K. and J.M.H. co-ordinated the KBCP study. D.L., B.T.Y., G.F. and K.L. co-ordinated the LMBC study. S.Manoukian, B.Bonanni, S.F. and P.P. co-ordinated the MBCSG study. F.J.C., X.W., K.S. and A.Lee co-ordinated the MCBCS study. M.Southey, G.G.G., L.Baglietto, G.S. and C.M. co-ordinated MCCS. G.G.A., V.Kristensen and A.-L.B.-D. co-ordinated NBCS. E.M.J. and A.Miron co-ordinated the NC-BCFR study. R.W., K.P., A.J.-V. and S.K. co-ordinated OBCS. I.L.A., G.G. and A.M.M. co-ordinated the OFBCR study. P.D. C.J.vanA., R.A.E.M.T. and C.Seynaeve co-ordinated the ORIGO study. J.D.F., M.G.-C., L.Brinton and J.Lissowska co-ordinated PBCS. M.J.H., A.H., R.A.O. and A.M.W.v.d.O. co-ordinated RBCS. A.Cox and M.W.R.R. co-ordinated SBCS. B.A.P. initiated SEARCH with P.D.P.P. and D.F.E.. M.Shah co-ordinated SEARCH. A.J., J.Lubinski, K.J. and K.Durda co-ordinated SZBCS. M.J., M.Schoemaker, A.A. and A.Swerdlow co-ordinated UKBGS. G.C.-T. led the contribution of kConFab cases and AOCS controls to BCAC, J.Beesley and X.C. performed iPLEX genotyping for several of the BCAC sites. K.R.M., A.Lophatananon, S.R. and A.Chaiwerawattana co-ordinated the ACP study. D.K., K.-Y.Y. and D.-Y.N. co-ordinated SEBCS. C.-Y.S. J.-C.Y., P.-E.W. and C.-N.H. co-ordinated TWBCS. A.P., R.S. and L.V. co-ordinated DBCSS. D.M.E., W.J.T., S.M.G. and N.J.G. co-ordinated the POSH study.
Reference List
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein P, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 3.Peto J, Mack TM. High constant incidence in twins and other relatives of women with breast cancer. Nat Genet. 2000;26:411–414. doi: 10.1038/82533. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S, et al. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet. 2009;41:585–590. doi: 10.1038/ng.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoniou AC, et al. A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor-negative breast cancer in the general population. Nat Genet. 2010;42:885–892. doi: 10.1038/ng.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Easton DF, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher O, et al. Novel breast cancer susceptibility locus at 9q31.2: results of a genome-wide association study. J Natl Cancer Inst. 2011;103:425–435. doi: 10.1093/jnci/djq563. [DOI] [PubMed] [Google Scholar]
- 8.Haiman CA, et al. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet. 2011 doi: 10.1038/ng.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter DJ, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stacey SN, et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2007;39:865–869. doi: 10.1038/ng2064. [DOI] [PubMed] [Google Scholar]
- 11.Stacey SN, et al. Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2008;40:703–706. doi: 10.1038/ng.131. [DOI] [PubMed] [Google Scholar]
- 12.Thomas G, et al. A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1) Nat Genet. 2009;41:579–584. doi: 10.1038/ng.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbull C, et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet. 2010;42:504–507. doi: 10.1038/ng.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng W, et al. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet. 2009;41:324–328. doi: 10.1038/ng.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox A, et al. A common coding variant in CASP8 is associated with breast cancer risk. Nat Genet. 2007;39:352–358. doi: 10.1038/ng1981. [DOI] [PubMed] [Google Scholar]
- 16.Varghese JS, Easton DF. Genome-wide association studies in common cancers--what have we learnt? Curr Opin. Genet Dev. 2010;20:201–209. doi: 10.1016/j.gde.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher O, et al. Novel breast cancer susceptibility locus at 9q31.2: results of a genome-wide association study. J Natl Cancer Inst. 2011;103:425–435. doi: 10.1093/jnci/djq563. [DOI] [PubMed] [Google Scholar]
- 18.Turnbull C, et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet. 2010;42:504–507. doi: 10.1038/ng.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broeks A, et al. Low penetrance breast cancer susceptibility loci are associated with specific breast tumor subtypes: findings from the Breast Cancer Association Consortium. Hum Mol Genet. 2011;20:3289–3303. doi: 10.1093/hmg/ddr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Closas M, et al. Heterogeneity of breast cancer associations with five susceptibility loci by clinical and pathological characteristics. PLoS Genet. 2008;4:e1000054. doi: 10.1371/journal.pgen.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoniou AC, Easton DF. Risk prediction models for familial breast cancer. Future Oncol. 2006;2:257–274. doi: 10.2217/14796694.2.2.257. [DOI] [PubMed] [Google Scholar]
- 22.Dunbar ME, Wysolmerski JJ, Broadus AE. Parathyroid hormone-related protein: from hypercalcemia of malignancy to developmental regulatory molecule. Am J Med Sci. 1996;312:287–294. doi: 10.1097/00000441-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Philbrick WM, et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996;76:127–173. doi: 10.1152/physrev.1996.76.1.127. [DOI] [PubMed] [Google Scholar]
- 24.Southby J, et al. Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Cancer Res. 1990;50:7710–7716. [PubMed] [Google Scholar]
- 25.Wysolmerski JJ, Stewart AF. The physiology of parathyroid hormone-related protein: an emerging role as a developmental factor. Annu. Rev Physiol. 1998;60:431–460. doi: 10.1146/annurev.physiol.60.1.431. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18:832–872. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 27.Wysolmerski JJ. Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann N Y. Acad Sci. 2010;1192:161–169. doi: 10.1111/j.1749-6632.2009.05249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juppner H, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 29.DeMauro S, Wysolmerski J. Hypercalcemia in breast cancer: an echo of bone mobilization during lactation? J Mammary. Gland. Biol Neoplasia. 2005;10:157–167. doi: 10.1007/s10911-005-5398-9. [DOI] [PubMed] [Google Scholar]
- 30.Dunbar ME, Wysolmerski JJ. Parathyroid hormone-related protein: a developmental regulatory molecule necessary for mammary gland development. J Mammary. Gland. Biol Neoplasia. 1999;4:21–34. doi: 10.1023/a:1018700502518. [DOI] [PubMed] [Google Scholar]
- 31.Wysolmerski JJ, Broadus AE. Hypercalcemia of malignancy: the central role of parathyroid hormone-related protein. Annu. Rev Med. 1994;45:189–200. doi: 10.1146/annurev.med.45.1.189. [DOI] [PubMed] [Google Scholar]
- 32.Henderson JE, et al. Nucleolar localization of parathyroid hormone-related peptide enhances survival of chondrocytes under conditions that promote apoptotic cell death. Mol Cell Biol. 1995;15:4064–4075. doi: 10.1128/mcb.15.8.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okoumassoun LE, Russo C, Denizeau F, Averill-Bates D, Henderson JE. Parathyroid hormone-related protein (PTHrP) inhibits mitochondrial-dependent apoptosis through CK2. J Cell Physiol. 2007;212:591–599. doi: 10.1002/jcp.21055. [DOI] [PubMed] [Google Scholar]
- 34.Henderson MA, et al. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 2006;66:2250–2256. doi: 10.1158/0008-5472.CAN-05-2814. [DOI] [PubMed] [Google Scholar]
- 35.Linforth R, et al. Coexpression of parathyroid hormone related protein and its receptor in early breast cancer predicts poor patient survival. Clin Cancer Res. 2002;8:3172–3177. [PubMed] [Google Scholar]
- 36.Yoshida A, et al. Significance of the parathyroid hormone-related protein expression in breast carcinoma. Breast Cancer. 2000;7:215–220. doi: 10.1007/BF02967463. [DOI] [PubMed] [Google Scholar]
- 37.Fleming NI, et al. Parathyroid hormone-related protein protects against mammary tumor emergence and is associated with monocyte infiltration in ductal carcinoma in situ. Cancer Res. 2009;69:7473–7479. doi: 10.1158/0008-5472.CAN-09-0194. [DOI] [PubMed] [Google Scholar]
- 38.Bluteau O, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312–315. doi: 10.1038/ng1001. [DOI] [PubMed] [Google Scholar]
- 39.Cho YS, et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 40.Cui R, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137:1768–1775. doi: 10.1053/j.gastro.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 41.Erdmann J, et al. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet. 2009;41:280–282. doi: 10.1038/ng.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gudbjartsson DF, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 43.Kato N, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purdue MP, et al. Genome-wide association study of renal cell carcinoma identifies two susceptibility loci on 2p21 and 11q13.3. Nat Genet. 2011;43:60–65. doi: 10.1038/ng.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soranzo N, et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat Genet. 2009;41:1182–1190. doi: 10.1038/ng.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Todd JA, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu C, et al. Genome-wide association study identifies three new susceptibility loci for esophageal squamous-cell carcinoma in Chinese populations. Nat Genet. 2011;43:679–684. doi: 10.1038/ng.849. [DOI] [PubMed] [Google Scholar]
- 48.Kress TR, et al. The MK5/PRAK kinase and Myc form a negative feedback loop that is disrupted during colorectal tumorigenesis. Mol Cell. 2011;41:445–457. doi: 10.1016/j.molcel.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 49.Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- 50.Bamshad M, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 51.Fan W, Huang X, Chen C, Gray J, Huang T. TBX3 and its isoform TBX3+2a are functionally distinctive in inhibition of senescence and are overexpressed in a subset of breast cancer cell lines. Cancer Res. 2004;64:5132–5139. doi: 10.1158/0008-5472.CAN-04-0615. [DOI] [PubMed] [Google Scholar]
- 52.Lomnytska M, Dubrovska A, Hellman U, Volodko N, Souchelnytskyi S. Increased expression of cSHMT, Tbx3 and utrophin in plasma of ovarian and breast cancer patients. Int J Cancer. 2006;118:412–421. doi: 10.1002/ijc.21332. [DOI] [PubMed] [Google Scholar]
- 53.Lyng H, et al. Gene expressions and copy numbers associated with metastatic phenotypes of uterine cervical cancer. BMC Genomics. 2006;7:268. doi: 10.1186/1471-2164-7-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowley M, Grothey E, Couch FJ. The role of Tbx2 and Tbx3 in mammary development and tumorigenesis. J Mammary. Gland. Biol Neoplasia. 2004;9:109–118. doi: 10.1023/B:JOMG.0000037156.64331.3f. [DOI] [PubMed] [Google Scholar]
- 55.Fillmore CM, et al. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc. Natl Acad Sci U. S. A. 2010;107:21737–21742. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renard CA, et al. Tbx3 is a downstream target of the Wnt/beta-catenin pathway and a critical mediator of beta-catenin survival functions in liver cancer. Cancer Res. 2007;67:901–910. doi: 10.1158/0008-5472.CAN-06-2344. [DOI] [PubMed] [Google Scholar]
- 57.Reyal F, et al. A comprehensive analysis of prognostic signatures reveals the high predictive capacity of the proliferation, immune response and RNA splicing modules in breast cancer. Breast Cancer Res. 2008;10:R93. doi: 10.1186/bcr2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 59.L’Horset F, Dauvois S, Heery DM, Cavailles V, Parker MG. RIP-140 interacts with multiple nuclear receptors by means of two distinct sites. Mol Cell Biol. 1996;16:6029–6036. doi: 10.1128/mcb.16.11.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tazawa H, et al. Regulation of subnuclear localization is associated with a mechanism for nuclear receptor corepression by RIP140. Mol Cell Biol. 2003;23:4187–4198. doi: 10.1128/MCB.23.12.4187-4198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White KA, Yore MM, Deng D, Spinella MJ. Limiting effects of RIP140 in estrogen signaling: potential mediation of anti-estrogenic effects of retinoic acid. J Biol Chem. 2005;280:7829–7835. doi: 10.1074/jbc.M412707200. [DOI] [PubMed] [Google Scholar]
- 62.Lin J, et al. Four and a half LIM domains 1 (FHL1) and receptor interacting protein of 140kDa (RIP140) interact and cooperate in estrogen signaling. Int J Biochem. Cell Biol. 2009;41:1613–1618. doi: 10.1016/j.biocel.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Kerley JS, Olsen SL, Freemantle SJ, Spinella MJ. Transcriptional activation of the nuclear receptor corepressor RIP140 by retinoic acid: a potential negative-feedback regulatory mechanism. Biochem. Biophys. Res Commun. 2001;285:969–975. doi: 10.1006/bbrc.2001.5274. [DOI] [PubMed] [Google Scholar]
- 64.White KA, et al. Negative feedback at the level of nuclear receptor coregulation. Self-limitation of retinoid signaling by RIP140. J Biol Chem. 2003;278:43889–43892. doi: 10.1074/jbc.C300374200. [DOI] [PubMed] [Google Scholar]
- 65.Demirpence E, et al. Antiestrogenic effects of all-trans-retinoic acid and 1,25-dihydroxyvitamin D3 in breast cancer cells occur at the estrogen response element level but through different molecular mechanisms. Cancer Res. 1994;54:1458–1464. [PubMed] [Google Scholar]
- 66.Fontana JA, Nervi C, Shao ZM, Jetten AM. Retinoid antagonism of estrogen-responsive transforming growth factor alpha and pS2 gene expression in breast carcinoma cells. Cancer Res. 1992;52:3938–3945. [PubMed] [Google Scholar]
- 67.Vega A, et al. Evaluating new candidate SNPs as low penetrance risk factors in sporadic breast cancer: a two-stage Spanish case-control study. Gynecol. Oncol. 2009;112:210–214. doi: 10.1016/j.ygyno.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Johnson AD, et al. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aulchenko YS, Struchalin MV, van Duijn CM. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics. 2010;11:134. doi: 10.1186/1471-2105-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.