Abstract
During testis development, fetal Leydig cells increase their population from a pool of progenitor cells rather than from proliferation of a differentiated cell population. However, the mechanism that regulates Leydig stem cell self-renewal and differentiation is unknown. Here, we show that blocking Notch signaling, by inhibiting γ-secretase activity or deleting the downstream target gene Hairy/Enhancer-of-split 1, results in an increase in Leydig cells in the testis. By contrast, constitutively active Notch signaling in gonadal somatic progenitor cells causes a dramatic Leydig cell loss, associated with an increase in undifferentiated mesenchymal cells. These results indicate that active Notch signaling restricts fetal Leydig cell differentiation by promoting a progenitor cell fate. Germ cell loss and abnormal testis cord formation were observed in both gain- and loss-of-function gonads, suggesting that regulation of the Leydig/interstitial cell population is important for male germ cell survival and testis cord formation.
Keywords: Notch, Stem cells, Leydig cell, Germ cell, Testis cord, Hes1, Mouse
INTRODUCTION
Mouse embryonic gonad development begins at ~10.0 days post coitum (dpc) with the thickening of the epithelial layer (the coelomic epithelium) overlying the mesonephric tubules (Brennan and Capel, 2004). Expression of the Y chromosome gene Sry in a subset of somatic progenitor cells initiates the differentiation of Sertoli cells and leads to rapid morphological changes in the gonad. Approximately 36–48 hours from the onset of Sry expression, a well-organized testis structure is formed: Sertoli cells aggregate around germ cells, segregating the tubular testis cords from the interstitial space. Peritubular myoid cells tightly surround the outside of testis cords. The interstitial region between testis cords contains steroid-secreting Leydig cells, other uncharacterized mesenchymal cells and the vasculature, including a characteristic coelomic vessel along the surface of the gonad. This well-organized structure provides a supportive environment for male germ cell development in fetal and adult life.
Fetal Leydig cells are first apparent in the testis at 12.5 dpc and their number declines shortly after birth (Habert et al., 2001). The embryonic origin of Leydig cell precursors is not yet clear. Some evidence suggests that precursors arise from the coelomic epithelium (Brennan et al., 2003; Karl and Capel, 1998), whereas other evidence suggests that Leydig progenitors migrate from the mesonephros into the gonad before 11.0 dpc and remain undifferentiated until 12.5 dpc (Jeays-Ward et al., 2003). The fetal Leydig cell population increases at least twofold before birth; however, no mitotic activity has been detected in differentiated Leydig cells (Byskov, 1986; Kerr et al., 1988; Migrenne et al., 2001; Orth, 1982). The increase in Leydig cell number has been attributed to differentiation of a population of progenitor/stem cells located in the interstitium (Orth, 1982). However, the mechanism that controls mesenchymal stem cell differentiation and self-renewal during Leydig cell development is unknown (Habert et al., 2001).
Notch, a transmembrane receptor that mediates local communication between cells, is involved in cell fate determination, particularly in stem cell maintenance and differentiation in many animal systems (Lai, 2004). For example, Notch signaling restricts neural differentiation by repressing the expression of proneural genes during Drosophila neural-epidermal fate decisions (Parks et al., 1997). A failure of Notch signaling causes all proneural cluster cells to express high levels of proneural proteins and become neurons. Constitutive Notch signaling has the opposite effect, and suppresses neural differentiation. During mammalian embryogenesis, Notch signaling has been found to regulate progenitor cell differentiation in both neuronal and pituitary development (Jensen et al., 2000; Raetzman et al., 2007), and to regulate adult stem cell maintenance and differentiation in the hematopoietic and intestinal stem cell systems (Duncan et al., 2005; Fre et al., 2005).
In mammals, four Notch receptors (Notch1–Notch4) interact with structurally similar Notch ligands, delta-like 1 (also called Delta1), delta-like 3, delta-like 4, jagged 1 and jagged 2. After binding with its ligand, Notch is activated by γ-secretase-dependent proteolysis within the transmembrane domain to release the Notch intracellular domain (NICD) (De Strooper et al., 1999; Huppert et al., 2000; Schroeter et al., 1998). Blocking γ-secretase function, for example, by use of the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl-L-alanyl]-S-phenylglycine-t-butyl Ester (DAPT), has been shown to affect kidney development by blocking Notch activity (Cheng et al., 2003). After cleavage, NICD translocates into the nucleus and associates with the constitutive DNA-binding protein CSL (after CBF1, suppressor of hairless and Lag1) to activate transcription of downstream targets. The hairy/enhancer of split genes, encoding the basic helix-loop-helix transcription factors Hes1 and Hes5, are the most well-defined targets of the NICD-CSL complex (Kageyama et al., 2007).
Based on the expression of multiple Notch receptors and downstream targets in the gonad, we investigated whether Notch signaling is involved in gonadal somatic cell differentiation by analyzing gonads in which Notch signaling was gained or lost during testis development. Our findings indicate that Notch signaling regulates Leydig progenitor cell maintenance and differentiation during fetal life.
MATERIALS AND METHODS
Animals
NotchlacZ mice were generated by replacing most of the ankyrin repeat region with lacZ as described previously (Hamada et al., 1999). Notch3lacZ mice, in which lacZ is recombined into the Notch3 EGF repeat region, were generously provided by Drs Marc Tessier-Lavigne and Bill Skarnes (Leighton et al., 2001). Hes1+/− mice (generously provided to L.R. by R. Kageyama, Kyoto University, Japan) were maintained on C57BL/6 or mixed genetic backgrounds (Ishibashi et al., 1995). RosaNotch mice (generously provided by Douglas A. Melton, Harvard University) (Murtaugh et al., 2003) and Sf1-cre mice (generously provided by Keith L. Parker, UTSMC) (Bingham et al., 2006) were maintained on a hybrid 129; C57BL/6 background, and are healthy and fertile as heterozygotes or homozygotes. RosaNotch; Sf1-cre embryos were generated by crossing a RosaNotch female with an Sf1-cre male mouse. Hs-cre transgenic XY mice (generously provided by Argiris Efstratiadis, Columbia) were crossed with RosaNotch, and Cre expression was induced as described (Dietrich et al., 2000). Expression experiments were performed on random bred CD1 mice (Jackson Laboratory). All experiments were conducted in accordance with the principles and procedures outlined in the NIH Guidelines for the Care and Use of Experimental Animals.
In situ hybridization, β-gal staining and immunocytochemistry
Gonads were dissected and fixed overnight in 4% paraformaldehyde/phosphate-buffered saline (PFA-PBS). In situ hybridization was performed as previously described on whole-mount tissue (Nieto et al., 1992). RNA antisense probes were made for Notch1 (1.8 kb), Notch2 (900 bp; intracellular domain), Notch3 (~2.6 kb) and Notch4 (1.8 kb; intracellular domain) from plasmids kindly provided by Tom Gridley, and for Hes1, Hes3 and Hes5, from plasmids kindly provided by Tom Vogt. RNA probes were synthesized using the DIG RNA labeling kit (Roche) following manufacturer's instructions. β-Gal staining was performed as previously described (Yao et al., 2002). Stained gonads were embedded, cryosectioned and imaged with a Zeiss axiophot microscope. Immunocytochemistry was performed on cryosections and whole-mount tissue as previously described (Brennan et al., 2002). Antibodies used were rabbit anti-3-HSD and rabbit anti-SF1 (the kind gifts of Ken-ichirou Morohashi; both used at a dilution of 1:2000), rat anti-PECAM1 (Pharmingen, San Diego, CA, USA; 1:500), rabbit anti-laminin (the kind gift of Harold Erikson, 1:500), rabbit anti-SOX9 (the kind gift of Francis Poulat, 1:1000), rabbit anti-cleaved caspase 3 (Cell Signaling, 1:500), rabbit anti-SCP3 (Novus, 1:500), rabbit anti-GFP (Invitrogen, 1:1000), rabbit anti-LHX9 (the kind gift of T. Jessell, Columbia, 1:2500) and anti-β-galactosidase (1:2000, Cappel-ICN). Cy2-, Cy3- or Cy5-conjugated secondary antibodies (Jackson ImmunoResearch) were used at a dilution of 1:500. DNA was stained with Syto13 (Invitrogen, 1:5000). Samples were mounted in DABCO as described (Karl and Capel, 1998) and imaged on a Zeiss LSM510 confocal microscope. Each image shown in the figures is representative of at least three sections of three independent gonads.
Histology
Testes were dissected and fixed overnight in Bouin's fixative at 4°C. Samples were then dehydrated and embedded in paraffin. Sections were cut (8 μm), and stained using standard Hematoxylin and Eosin procedures, mounted and photographed using a Zeiss Axioplan 2 microscope. For immunostaining, peroxidase-conjugated secondary antibodies and HistoMark BLACK Peroxidase System kit (KPL) were used following the manufacturers' protocol. After peroxidase staining, sections were stained with Hematoxylin and Eosin.
Organ culture
Genital ridges (11.5 dpc) were cultured in agar blocks at 37°C with 5% CO2/95% air in 30 μl Dulbecco's Minimal Eagle Medium (DMEM) for 48 hours, supplemented with 10% fetal calf serum and 50 μg/ml ampicillin (Martineau et al., 1997). DAPT (Calbiochem, 100 μM final concentration) or DMSO was added to the medium.
Statistical analysis
For each embryo, positive cells were counted on 10 evenly spaced sections (10 μm×460.07 μm×460.07 μm) through the whole gonad, and the total number of cells was calculated. For each genotype we present the mean±s.e.m. of total cell numbers from at least three independent gonads. The two-tailed P values were determined by using Student's t-test. P<0.05 is considered statistically significant.
Reverse transcription and quantitative PCR (QPCR)
Gonads were separated from the mesonephros and total RNA was isolated using TRIzol (Invitrogen). Reverse transcription reactions were performed using the IScript cDNA Synthesis Kit (BioRad). The BioRad MyiQ iCycler and SYBR Green Supermix were used for performing the Q-PCR reaction. The iCycler software was used for QPCR data analysis. Primers were as follows: hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1), TCAGTCAACGGGGGACATAAA and GGGGCTGTACTGCTTAACCAG; Sox9, GCGGAGCTCAGCAAGACTCTG and ATCGGGGTGGTCTTTCTTGTG; Amh, GGCTTCGGGCTCATCTTAACC and TGAAACAGCGGGAATCAGAGC; Dhh, GCCTGATGACAGAGCGTTGC and GAGTGAATCCTGTGCGTGGTG; Ptgds, GGCTCCTGGACACTACACCT and CTGGGTTCTGCTGTAGAGGGT; Pdgfra, TCCATGCTAGACTCAGAAGTCA and TCCCGGTGGACACAATTTTTC; Pdgfa, GAGGAAGCCGAGATACCCC and GGCACATGGTTAATGGCATGG; Lhx9, AGATGGAGCGCAGATCCAAG and GCAGATAGTACCTGTCGGAGA; Arx, CAAGGATGGTGAGGACAGC and TCTGGAACCACACCTGGACT; Ptch1, AAAGAACTGCGGCAAGTTTTTG and CTTCTCCTATCTTCTGACGGGT; Cyp11a (Scc), TGGCCCCATTTACAGGGAGAA and GGCATCTGAACTCTTAAACAGGA; and Cyp17a1, CAGAGAAGTGCTCGTGAAGAAG and AGGAGCTACTACTATCCGCAAA. Independent cDNA samples from 4 pairs of mutant and control gonads were examined. Each PCR reaction was repeated 3 times. Gene expression level was normalized to Hprt1 and compared using the Student's t-test.
RESULTS
Notch signaling components are expressed during embryonic gonad development
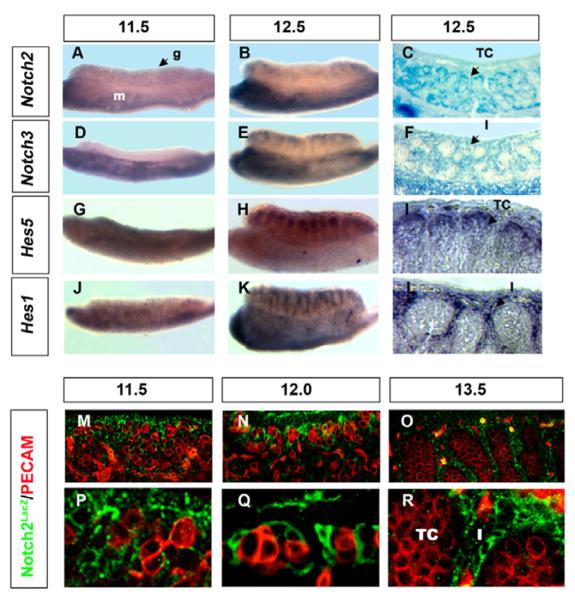
The expression patterns of Notch receptors and downstream targets were examined in XY gonads between 11.5 and 13.5 dpc, when progenitor cells arise and commit to the various testicular cell fates. Expression of Notch1 has been previously reported in the arterial coelomic vessel in the XY gonad and is coincident with Notch4 (Brennan et al., 2002) (data not shown). Notch2 expression was detected by mRNA in situ hybridization (Fig. 1A,B), and by using a lacZ reporter targeted to the Notch2 locus (Notch2LacZ) (Fig. 1C,M–R) (Hamada et al., 1999). Notch2 is expressed at higher levels in XY than in XX gonads throughout these stages (see Fig. S1 in the supplementary material). Using both methods, Notch2 was detected at low levels at 11.5 dpc (Fig. 1A,M,P). lacZ-positive cells (detected using an antibody against β-gal) were found in the coelomic epithelium and in deeper layers of the XY gonad (Fig. 1M,P). At 12.0 dpc, Notch2 expression was localized to cells that are clustered around groups of germ cells in the position of pre-Sertoli cells at the beginning of testis cord development (Fig. 1N,Q). Using a histochemical X-gal detection method, Notch2 was expressed in Sertoli cells inside testis cords at 12.5 dpc (Fig. 1C). At 13.5 dpc, Notch2 expression declined in Sertoli cells, and shifted to the interstitium (Fig. 1O,R; see Fig. S1G in the supplementary material).
Fig. 1. Dynamic expression of the Notch signaling pathway suggests a role in mouse gonad development.
The coelomic domain of the gonad is upwards, anterior is leftwards and posterior is rightwards. Whole mount in situ hybridization of Notch2 (A,B), Notch3 (D,E), Hes5 (G,H) and Hes1 (J,K) were performed on 11.5 and 12.5 dpc XY whole gonads, and in some cases, stained gonads were sectioned (I,L). Whole-mount β-gal staining was performed on 12.5 dpc XY Notch2LacZ (C) and Notch3LacZ (F) gonads prior to sectioning. (M–R) XY Notch2LacZ gonads were stained with antibodies against β-gal (green) to detect Notch2, and PECAM1 (red, germ cells and vasculature) at 11.5, 12.0 and 13.5 dpc. Low (M–O) and high (P–R) magnification images are shown at each stage. Notch2 was detected throughout the XY gonad at 11.5 dpc (M,P), and localized to cells that are clustered around groups of germ cells at the beginning of testis cord development (N,Q). The Sertoli cell expression pattern was apparent at 12.5 dpc (C). At 13.5 dpc, β-gal expression was undetectable in Sertoli cells and shifted to interstitial cells in Notch2LacZ mice (O,R). Notch3 was not detected in XY gonads at 11.5 dpc (D). At 12.5 dpc, Notch3 was detected in the interstitium of the XY gonad (E,F). Hes5 and Hes1 were expressed in 11.5 dpc XY gonads (G,J). At 12.5 dpc, Hes5 was detected inside the cords (H,I), whereas Hes1 was detected in the interstitium (K,L). A,B,D,E,G,H,J,K were taken at 12× magnification; C,F were taken at 20× magnification; I,L,M–O were taken at 25× magnification; P–R were taken at 63× magnification. g, gonad; m, mesonephros; TC, testis cord; I, interstitium.
The Notch3 expression pattern was determined by in situ hybridization and X-gal staining of Notch3LacZ gonads. Notch3 was not detected in XX or XY gonads at 11.5 dpc (Fig. 1D; see Fig. S1D in the supplementary material). At 12.5–13.5 dpc, Notch3 was expressed in the interstitium of the XY gonad and at relatively lower levels in XX gonads (Fig. 1E,F; see Fig. S1E,F in the supplementary material). β-Gal activity was never detected inside testis cords at any stages examined, suggesting that Notch3 is not expressed in Sertoli cells or their progenitors, but overlaps with Notch2 expression after 12.5 dpc (Fig. 1E,F; see Fig. S1G,H in the supplementary material).
The expression of Hes genes was examined by in situ hybridization to substantiate the activation of Notch signaling and to gain further insight into the roles of the different Notch receptors. At 12.5 dpc, Hes5 was detected in Sertoli cells inside testis cords, whereas Hes1 expression was restricted to the interstitium (Fig. 1G–L). These expression patterns correspond to the pattern of Notch2 (Hes5), and the overlapping domain of Notch2 and Notch3 (Hes1). Hes3 expression was not detected at 11.5–12.5 dpc in either XX or XY gonads (data not shown). These results reveal that Notch receptors and downstream target genes are expressed at the time when gonadal somatic cell populations appear and initiate differentiation, suggesting that the Notch pathway may be important for their fate decisions.
Blocking Notch signaling via a γ-secretase inhibitor promotes Leydig cell differentiation in XY gonads
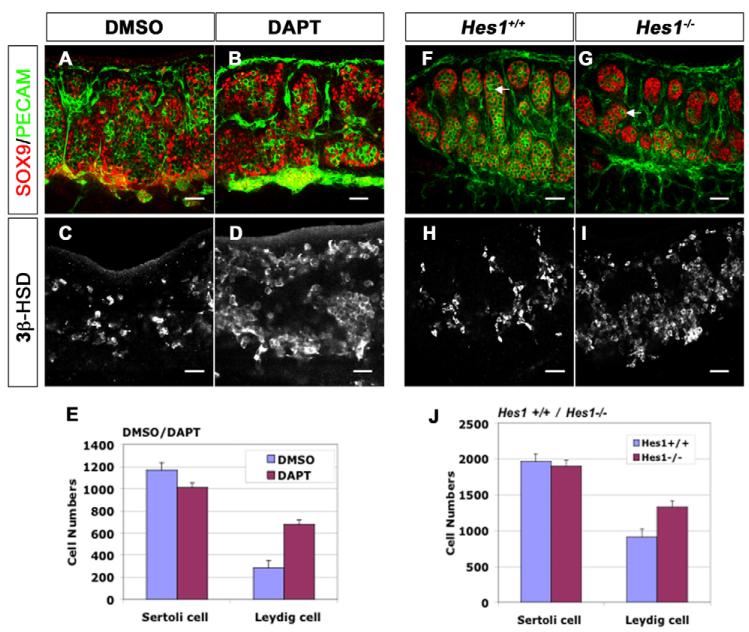
To investigate Notch function, we cultured XY gonads with the γ-secretase inhibitor DAPT to block the Notch signaling pathway. Mouse embryonic gonads (11.5 dpc) were cultured for 2 days in DMEM with DAPT or DMSO as a control. Cultured male gonads stained with antibodies against SOX9 (a marker of Sertoli cells) and PECAM1 (a marker of endothelial cells and germ cells) appeared normal, with a male-specific coelomic vessel and organized testis cords (Fig. 2A,B). However, when treated and untreated gonads were stained with an antibody against 3β-HSD (3beta-hydroxysteroid dehydrogenase) to detect differentiated Leydig cells, Leydig cell numbers were significantly increased (138%; n=8 gonads; P<0.001) in DAPT treated gonads compared with DMSO controls (Fig. 2C–E) (n=8). By contrast, Sertoli cell numbers were equal to those in the DMSO control but decreased with respect to the DAPT-treated gonads (Fig. 2A,B,E) (n=3 gonads; P=0.1792). This result suggests that Notch signaling specifically restricts Leydig cell differentiation.
Fig. 2. Loss of Notch signaling results in an increase in fetal Leydig cells.
The coelomic domain of the gonad is upwards, anterior is leftwards and posterior is rightwards. (A–E) 11.5 dpc mouse XY gonads were cultured in DMEM for 48 hours with DMSO (A,C) or the γ-secretase inhibitor DAPT (B,D). (A,B) Sertoli cells were stained with antibodies against SOX9 (red), and vasculature and germ cells, with antibodies against PECAM1 (green). (C,D) Leydig cells, detected with antibodies against 3β-HSD were significantly increased (138%; n=8 gonads; P<0.001) in DAPT-treated gonads (D,E), compared with the DMSO control (C,E). Numbers of Sertoli cells are not significantly affected (E; n=3 gonads; P=0.1792). (F–J) Deletion of the Notch downstream target gene Hes1 (Hes1−/−) resulted in normal numbers of SOX9-positive Sertoli cells (red, G,J; n=3 gonads; P=0.2138), but an increase of 3β-HSD-positive Leydig cells (I,J; 47%; n=3 gonads; P=0.039) and a loss of germ cells (PECAM1, green; white arrows in F,G). Data presented are mean±s.e.m. of cell numbers from at least three independent gonad samples. Scale bars: 50 μm.
Increase of Leydig cell numbers in Hes1−/− XY gonads
To investigate whether the effects of Notch signaling on Leydig cell development are mediated through the transcriptional repressor Hes1, we analyzed Hes1−/− mouse gonads using antibodies against SOX9, PECAM1 and 3β-HSD. In both Hes1+/− and wild-type littermates, germ cells and Sertoli cells were well organized into testis cords, with Leydig cells and vessels located between the cords. In Hes1−/− male gonads, SOX9-positive and 3-HSD-positive cells were present in the testis at the appropriate stages, 11.5 dpc (data not shown) and 13.5 dpc (Fig. 2F–J), indicating that Hes1 is not required for initiation of Sertoli or Leydig cell differentiation. However, Leydig cell numbers significantly increased in Hes1−/− gonads relative to wild-type controls (Fig. 2H–J) (47%; n=3 gonads; P=0.039). By contrast, Sertoli cell numbers were relatively normal (Fig. 2F,G,J) (n=3 gonads; P=0.2138).
Both the in vitro and in vivo disruption of Notch signaling caused an increase in Leydig cell number, indicating that the Notch-Hes1 pathway normally functions to restrict Leydig cell differentiation from progenitor cells during testis development. The discrepancy between these two experiments may result from the fact that the inhibitor blocks all Notch signaling, whereas loss of Hes1 disrupts only one downstream pathway.
Notch signaling inhibits Leydig cell development
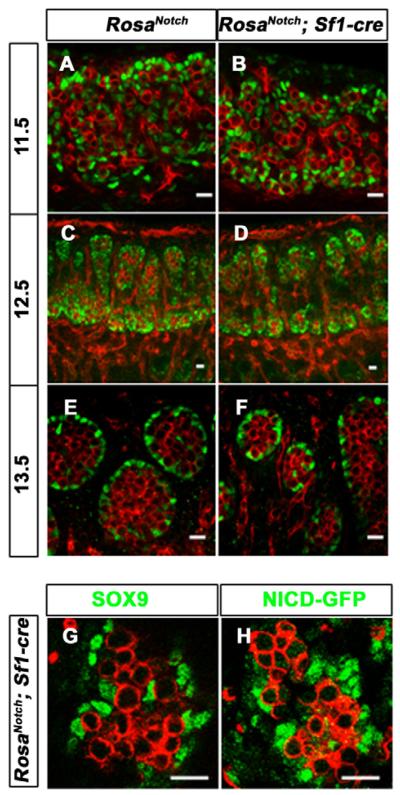
To test our hypothesis further, we developed a gain-of-function assay using RosaNotch; Sf1-cre mice. In RosaNotch mice (Murtaugh et al., 2003), the NICD transgene and an EGFP reporter are targeted to the Rosa locus behind a floxed transcriptional STOP sequence. Expression of NICD and EGFP is constitutively activated when Cre recombinase deletes the floxed STOP fragment. Previous studies have shown that Cre recombinase activity in Sf1-cre mice reflects the endogenous SF1 expression pattern in gonadal somatic cells beginning at 11.5 dpc (Bingham et al., 2006; Kim et al., 2007). To verify the timing and cell-specificity of Cre expression in RosaNotch; Sf1-cre gonads, we used antibodies against Cre recombinase, and antibodies against GFP to detect NICD expression. Although NICD-GFP was not detected above background at 10.5 dpc, nuclear accumulation occurred by 11.5 dpc in many somatic cells, and expression was detected in differentiated Sertoli and Leydig cells at all later stages examined (see Fig. S2 in the supplementary material and data not shown).
Despite the constitutive activation of Notch signaling in RosaNotch; Sf1-cre XY Sertoli progenitors at 11.5 dpc and after, SOX9 expression was similar to wild type at all stages examined (Fig. 3A–F). In consecutive sections of the mutant gonad, cells ectopically expressing NICD (GFP positive) were also SOX9 positive (Fig. 3G,H). This indicates that ectopic expression of Notch at 11.5 dpc and later does not restrict Sertoli cell differentiation, or alter the differentiated Sertoli cell fate.
Fig. 3. Constitutively active Notch signaling in Sertoli cells does not change Sertoli cell fate.
The coelomic domain of the gonad is upwards, anterior is leftwards and posterior is rightwards.
(A–F) Immunofluorescent staining of PECAM1 (red) and SOX9 (green) on wild-type (A,C,E) and RosaNotch; Sf1-cre (B,D,F) mouse gonads at 11.5 (A,B), 12.5 (C,D) and 13.5 dpc (E,F). SOX9 appeared normal at all stages, although some mutant cords were smaller than wild type at 13.5 dpc (F). (G,H) Immunofluorescent staining of SOX9 (green, G) and GFP (green, a marker for NICD expression, H) on two adjacent sections of a 13.5 dpc mutant gonad showed that SOX9-positive Sertoli cells were also Notch active cells. Scale bars: 20 μm.
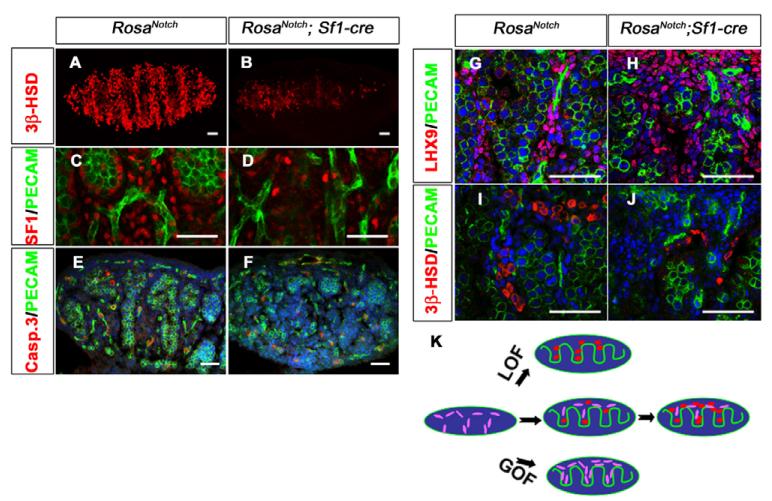
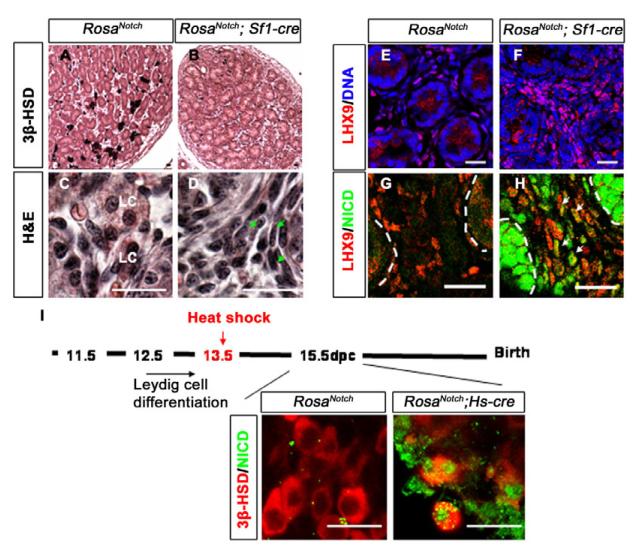
By contrast, based on antibody staining, the number of 3β-HSD and SF-1-positive cells significantly decreased in RosaNotch; Sf1-cre XY gonads at 13.5 dpc compared with wild type or mice with only one of the two transgenes (Fig. 4A–D; see Fig. S2F,G in the supplementary material). To determine whether the decrease in Leydig cell number is due to apoptosis, we stained the gonad with an antibody against caspase 3. We observed no significant increase in apoptosis in the gonads of RosaNotch; Sf1-cre mice (Fig. 4E,F) (n=9 sections from three independent gonads of each genotype; P=0.7813).
Fig. 4. Constitutively active Notch signaling results in a loss of Leydig cells.
The coelomic domain of the gonad is upwards, anterior is leftwards and posterior is rightwards. (A–D) Compared with wild type (A,C), RosaNotch; Sf1-cre mouse gonads (B,D) showed a loss of Leydig cells at 13.5 dpc based on immunofluorescent staining for 3β-HSD (red, A,B) or SF1 (red, C,D). PECAM1 (green) labels vasculature and germ cells in C and D. (E,F) In view of the low levels of activated caspase 3 (red), this loss was not due to cell death (PECAM1, green; Syto13 stains DNA, blue; n=3 gonads; P=0.7813). (G–J) LHX9 (red), which is expressed in interstitial somatic cells (G), shows an increase in RosaNotch; Sf1-cre gonads (H; n=3 gonads; P<0.01), whereas Leydig cell numbers are decreased based on 3β-HSD staining (I,J). Images shown are representative staining of at least three sections from three independent gonads. (K) A summary diagram of Notch/Hes1 results: loss of Notch function (LOF) leads to Leydig cell differentiation (red, oval cells), whereas gain of Notch function (GOF) maintains interstitial mesenchymal progenitor cells (purple, spindle shaped), and restricts their differentiation into Leydig cells. Scale bars: 50 μm.
An alternative explanation for the reduction in Leydig cell numbers is that Leydig cells fail to differentiate when Notch is activated in progenitors. To investigate this possibility, we stained with an antibody against LIM homeobox gene 9 (Lhx9). LHX9 is essential for the initial formation of the gonad and is expressed in somatic progenitors in the epithelium and subjacent mesenchymal cells at the earliest stages of gonad formation (see Fig. S3A in the supplementary material) (Birk et al., 2000). At 13.5 dpc and later stages, some LHX9-positive cells are normally detected in the coelomic domain and in the interstitial space between cords (see Fig. S3B in the supplementary material), suggesting that some cells maintain undifferentiated characteristics during gonad development. The number of LHX9-positive progenitor cells was increased in RosaNotch; Sf1-cre male gonads at 13.5 dpc (Fig. 4G,H) (n=9 sections from three independent gonads; P<0.01), whereas the number of differentiated Leydig cells was reduced based on expression of 3β-HSD (Fig. 4I,J) (n=9 sections from three independent gonads; P<0.01), suggesting that upregulation of Notch signaling promotes the maintenance of progenitor cells, and restricts their differentiation into Leydig cells (Fig. 4K).
Undifferentiated progenitors in RosaNotch; Sf1-cre gonads are maintained throughout embryonic development
One possible explanation for the increase in numbers of LHX9-positive cells at 13.5 dpc is that Notch signaling causes a developmental delay in the gonad, which is resolved at later stages. To test this possibility, we allowed RosaNotch; Sf1-cre embryos to develop until after birth. At postnatal day (P) 1, investigation of the Leydig cell population using antibodies against 3β-HSD indicated that Leydig cell differentiation was not restored in RosaNotch; Sf1-cre testes, although testis development had proceeded (Fig. 5A,B). Histological staining with Hematoxylin-Eosin revealed an increase of spindle-shaped interstitial mesenchymal cells in the RosaNotch; Sf1-cre testis when compared with wild type, where typical Leydig cells can be easily recognized by their cell shape and staining pattern (Fig. 5C,D). A spindle-shaped cell of similar description has been identified as the adult Leydig stem cell (Ariyaratne and Chamindrani Mendis-Handagama, 2000; Davidoff et al., 2004; Kerr et al., 1988). The increase of spindle shaped mesenchymal cells was also correlated with an increase of LHX9-positive cells (Fig. 5E,F). Many of the LHX9-positive cells were also constitutively expressing NICD at P1 in RosaNotch; Sf1-cre testes (Fig. 5G,H), suggesting that constitutive Notch signaling in some interstitial progenitor cells maintains their undifferentiated state and prevents their differentiation into Leydig cells throughout fetal development. The gain-of-function mutant (RosaNotch; Sf1-cre) has a relatively small epididymis and descent of the testes is partial at P1. As expected, we did not detect a defect in male reproductive tract development in Hes1 mutant embryos (where excessive Leydig cells differentiate).
Fig. 5. Constitutive Notch signaling restricts Leydig cell differentiation throughout fetal gonad development, but cannot change the fate of differentiated Leydig cells.
The coelomic domain of the gonad is upwards, anterior is leftwards and posterior is rightwards. (A–D) H&E staining of mouse testis sections at P1, with (A,B) or without (C,D) immunostaining for 3β-HSD (black) in RosaNotch; Sf1-cre gonads (B,D) compared with gonads from mice carrying only one of the two transgenes (A,C). Spindle shaped cells increased in RosaNotch; Sf1-cre gonads (green arrows in D), whereas Eosin-staining Leydig cells (LC) declined. (E–H) Immunofluorescent staining for LHX9 (red, nuclear staining; cytoplasmic staining is nonspecific background) and DNA (Syto13, blue in E,F) or NICD (green in G,H) shows increased LHX9-positive cells in RosaNotch; Sf1-cre gonads, some of which were expressing Notch (white arrows in H, yellow staining cells). Broken lines outline testis cords. (I) RosaNotch; Hs-cre mice were heat-shocked at 13.5 dpc, after Leydig cell differentiation is initiated. At 15.5 dpc, many 3β-HSD-positive cells were detected (red), some also expressing NICD (green). Scale bars: 20 μm.
Notch signaling does not affect committed Leydig cell fate
To test whether constitutive Notch signaling can cause the dedifferentiation of mature Leydig cells, we crossed RosaNotch female mice to mice in which Cre is driven by the Hsp70 promoter (Hs-cre) to induce NICD expression after Leydig cell differentiation (Fig. 5I). RosaNotch; Hs-cre embryos were heat-shocked at 13.5 dpc, a stage when a large number of Leydig cells are already differentiated. After heat shock, Notch signaling was ectopically activated in many cells in the RosaNotch; Hs-cre embryos by 15.5 dpc. However, many 3β-HSD positive cells were present, and many of these were expressing constitutive Notch based on co-expression of GFP. This result suggests that Notch signaling can restrict progenitor cell differentiation, but cannot reverse the differentiation of Leydig cells.
Sertoli cell markers were not significantly different in RosaNotch; Sf1-cre gonads, but markers of differentiated Leydig cells were significantly downregulated
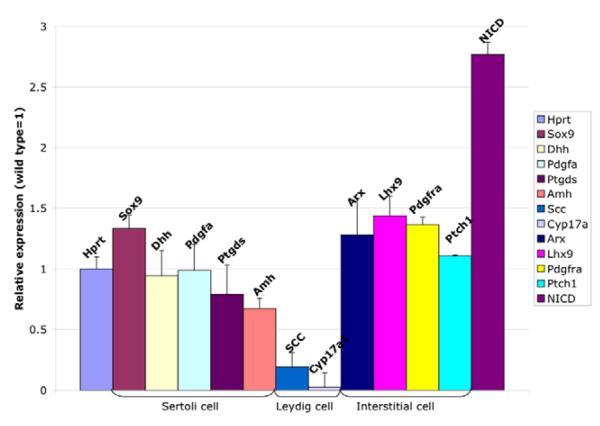
To investigate the interaction between the Notch pathway and other molecules and pathways involved in gonad development, gene expression profiles were compared between 13.5 dpc RosaNotch; Sf1-cre and control testes by Q-RT-PCR (Fig. 6). Sertoli cell-specific genes, such as Sox9 (P=0.0534), Dhh (P=0.7942), Pdgfa (P=0.964) and Ptgds (P=0.4774) were expressed at similar levels to wild-type controls. Only Amh was downregulated (P=0.0323). This result is consistent with the observation that RosaNotch; Sf1-cre gonads exhibit normal Sertoli cell histology and SOX9 immunostaining (see Figs 3 and 5). Two genes specific to differentiated Leydig cells, Scc and Cyp17a1, were significantly downregulated in the mutant testes (P=0.0057 and 0.0046 respectively), as expected from the observed Leydig cell loss. By contrast, the interstitial cell gene, Lhx9, which is not expressed in differentiated Leydig cells, was significantly increased in RosaNotch; Sf1-cre gonads (P=0.0212), consistent with the observed increase in undifferentiated progenitor cells in these gonads (see Figs 4 and 5). Expression of Arx, Pdgfra and Ptch1 in interstitial cells has been reported previously, and shown to be important for Leydig cell differentiation (Brennan et al., 2003; Kitamura et al., 2002; Yao et al., 2002); however, their cell-type specificity is not known. Expression levels of these genes were not significantly different in RosaNotch; Sf1-cre gonads (P=0.0881, 0.2915 and 0.5526, respectively), suggesting that they are likely to be expressed in progenitor cells and are not repressed by Notch signaling.
Fig. 6. Sertoli cell markers are not significantly different in RosaNotch; Sf1-cre gonads, but markers of differentiated Leydig cells are significantly downregulated.
mRNA was isolated from a single pair of mouse gonads, and expression was compared by Q-RTPCR between RosaNotch; Sf1-cre and wild-type gonads, normalized to Hprt. Sertoli cell-specific genes were similar (Sox9, Dhh, Pdgfa and Ptgds) or significantly downregulated (Amh) relative to normal expression levels (P=0.0534, 0.7942, 0.964, 0.4774 and 0.0323, respectively). Two differentiated Leydig cell genes, Scc and Cyp17a1, were significantly decreased in the mutant testis (P=0.0057, 0.00460). Interstitial markers, Arx, Pdgfra and Ptch1, were similar to wild type, while Lhx9 was significantly increased in RosaNotch; Sf1-cre gonads (P=0.0881, 0.2915, 0.5526 and 0.0212, respectively). NICD was significantly upregulated as expected. Data presented are mean±s.e.m. of Q-RT-PCR results from a total of four independent pairs of mutant and control gonad samples (each sample repeated at least three times for each gene).
Testis cord formation and germ cell survival are affected by both gain and loss of Notch signaling
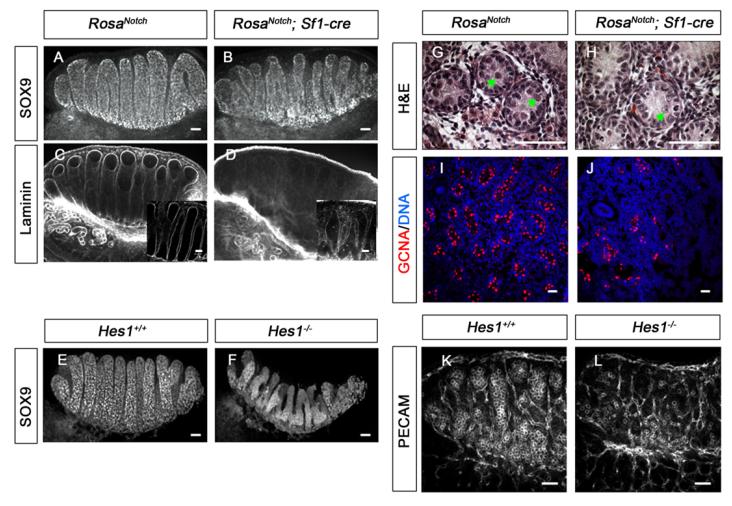
In the XY gonad, peritubular myoid cells surround Sertoli and germ cells to form testis cords bounded by a basal lamina. Although testis cords formed in both Hes1−/− and RosaNotch; Sf1-cre gonads, both SOX9 and laminin staining revealed that they were smaller and irregularly shaped compared with wild type, despite the fact that Sertoli cell differentiation appeared to occur normally (Fig. 7A-F). Additionally, a significant germ cell loss was found in XY RosaNotch; Sf1-cre gonads at later stages, based on both histological staining and immunofluorescent staining with germ cell nuclear antigen 1 (GCNA1) (Fig. 7G-J) (P<0.001; n=3). Activated caspase 3 was present in the interior of testis cords, suggesting germ cells undergo apoptosis at P1 (see Fig. S4 in the supplementary material). In Hes1−/− mice with increased Leydig cell numbers, there was also a significant male-specific germ cell loss on the C57BL/6 background at 13.5 dpc (Fig. 7K,L, compare also Fig. 2F,G) (n=3; P<0.001), despite the fact that Hes1 is not expressed in Sertoli or germ cells. No obvious germ cell loss was observed in XX mutant gonads, suggesting that the effect of Hes1 on germ cell numbers occurs after sexually dimorphic development of germ cells begins in the gonads. These findings imply that Leydig cells or their progenitors have functions that strongly affect both testis cord formation and germ cell maintenance in the XY gonad.
Fig. 7. Testis cord formation and germ cell survival are affected by gain or loss of Notch signaling in gonadal somatic cells.
The coelomic domain of the gonad is upwards, anterior is leftwards and posterior is rightwards. (A–F) Immunofluorescent staining for SOX9 (A,B,E,F) and Laminin (C,D, box inserts show 40× images of a confocal section) were used to evaluate mouse testis cord structure. At 13.5 dpc abnormal cord formation was detected in both, RosaNotch; Sf1-cre gonads (B,D) and Hes1−/− gonads (F). (G,H) At postnatal day (P) 1, fewer germ cells were detected inside testis cords based on Hematoxylin and Eosin staining (green arrows), although Sertoli cells appeared normal. (I,J) A significant decrease in the number of GCNA-positive cells (red; n=3, P<0.001) reflects germ cell loss in RosaNotch; Sf1-cre gonad at P1 (Syto13 stains DNA, blue). (K,L) C57BL/6 Hes1−/− gonads showed a significant germ cell loss at 13.5 dpc based on PECAM1 fluorescent immunostaining (P<0.001, n=3). Scale bars: 50 μm.
DISCUSSION
During organogenesis, expansion of each cell population relies on proliferation of differentiated cells or differentiation from an expanding progenitor cell population. In the embryonic male gonad, Sertoli cells are the most proliferatively active of the differentiated cells during gonad development, consistent with the observation that after the initial steps of Sertoli cell fate commitment and testis cord formation, expansion relies on cell divisions of committed cells rather than recruitment from a progenitor pool (Baker and O'Shaughnessy, 2001; Orth, 1982). By contrast, cell division is rarely detected among differentiated Leydig cells, implying that the increase in differentiated Leydig cell numbers during gonad growth relies on recruitment from an undifferentiated progenitor pool of cells in the interstitial compartment that continuously proliferate and differentiate (Orth, 1982). Our results strongly support this model and suggest that Notch signaling regulates fetal Leydig progenitor cell maintenance.
Notch signaling can regulate asymmetric divisions and/or maintain the undifferentiated status of a stem cell population (Lai, 2004). Based on the early expression patterns of Notch2 and Hes5 in Sertoli progenitors, we hypothesized that Notch was involved in the fate decision that gives rise to Sertoli cells, possibly by regulating an asymmetric division of a progenitor of both the Sertoli and Leydig cell lineages. However, in these experiments, constitutively active Notch driven by Sf1-cre (RosaNotch; Sf1-cre) restricts the differentiation of Leydig cells from the progenitor pool, but does not significantly affect establishment of the Sertoli cell population, suggesting that Notch signaling does not control a potential Leydig versus Sertoli cell fate decision at any of the stages we studied. We found that Leydig cell differentiation cannot be disrupted by constitutive expression of Notch after 13.5 dpc, suggesting that only undifferentiated progenitor cells are responsive to active Notch signaling. It is possible that Sertoli cell commitment is established prior to the accumulation of NICD using Sf1-cre as an activator. Although it has been shown that Cre recombinase activity starts as early as 10.5 dpc in Sf1-cre mice (Bingham et al., 2006), we did not detect NICD-GFP expression in RosaNotch; Sf1-cre at 10.5 dpc (see Fig. S2A in the supplementary material). Mouse strain background or the recombination and transcriptional efficiency of NICD-GFP could delay the accumulation of nuclear NICD-GFP in our experiments.
Instead of an increase in the number of Sertoli cells, there was an increase of spindle-shaped LHX9-positive mesenchymal cells in the interstitium of RosaNotch; Sf1-cre testes. LHX9 is expressed in the epithelium and the interstitial mesenchyme in the primordial gonad at very early stages (9.5–11.5 dpc), and is maintained in some interstitial cells throughout male gonad development (Mazaud et al., 2002). Because the Lhx9-null mutant gonads fail to develop, and regress completely by 13.0 dpc (Birk et al., 2000; Mazaud et al., 2002), a later role for Lhx9 in maintaining interstitial progenitor cells has not been tested. Lhx2, the closest homolog of Lhx9, is expressed in hair follicle stem cells, and regulates the maintenance of this population (Rhee et al., 2006). Lhx9 may have a similar function in the maintenance of the gonadal progenitor cell pool.
In adult testes, interstitial mesenchymal cells have been identified as progenitors for Leydig cells in immunohistological studies and in the ethane dimethane sulfonate (EDS)-treated adult rat testis during regeneration of Leydig cells (Davidoff et al., 2004). Based on our results, we hypothesize that mesenchymal Leydig stem/progenitor cells arise in the gonad at early stages. Some of these cells differentiate into fetal Leydig cells during embryonic gonad development, and others retain their undifferentiated characteristics and serve as precursor/stem cells for the expansion of Leydig cells during postnatal testis development (Habert et al., 2001). It has been recognized that fetal Leydig cells do not give rise to adult Leydig cells. However, it is not clear whether the same stem/progenitor cell population that gives rise to fetal Leydig cells also gives rise to adult Leydig cells. It is possible that the stem cell population established at fetal stages enters a quiescent phase after birth, and becomes active again prior to puberty to establish and maintain the adult Leydig cell population. An appropriate cell lineage tracing experiment will be needed to prove this hypothesis. Even if the fetal and adult Leydig stem/progenitor population is the same, the mechanisms of self-renewal and differentiation may be different at fetal and adult stages. A conditional block or activation of Notch signaling in adult Leydig stem cells will uncover whether Notch also regulates adult stem cell maintenance and Leydig differentiation.
Some steroidogenic markers of Leydig cell progenitors appear in the gonad as early as 12.5 dpc, suggesting that the first wave of Leydig cell fate commitment might occur as early as 11.5 dpc (Val et al., 2006). This may be the reason why a gain-of-Notch-function did not completely abolish the differentiated Leydig cell population: perhaps some progenitors are committed to Leydig cell differentiation prior to the stage when Sf1-cre is active and NICD accumulates. Further experiments are needed to clarify this possibility.
Notch2 and Notch3 are expressed in an overlapping pattern in the interstitial compartment. Mice mutant for Notch3 appear normal (Krebs et al., 2003). As Notch2−/− mice are embryonic lethal, we examined Notch2 function using a Notch2 hypomorphic mutant Notch2del/del (McCright et al., 2001) and conditional allele Notch2flox/− (Saito et al., 2003) crossed with the Sf1-cre or Hs-cre line to conditionally delete Notch2 in SF1-positive somatic cells or throughout the gonad. However, no difference was observed between wild-type and mutant gonads, which may reflect a redundancy between Notch receptors. We have not identified the ligand responsible for Notch signaling to Leydig precursors. Jagged 1 (Jag1) is expressed in the interstitium (Brennan et al., 2002), and is a good candidate. As Jag1-null mutants are embryonic lethal, a conditional allele will be necessary for a functional approach (High et al., 2008). Jagged 2 is expressed in the XY specific coelomic vessel and its branches as reported previously (Brennan et al., 2002). Other ligands, delta-like 1, delta-like 3 and delta-like 4, are also expressed in XY gonads at 11.5 and 12.5 dpc (Nef et al., 2005) (data not shown). Further expression and functional analysis will be necessary to identify the ligands that activate the Notch pathway in Leydig progenitor cells.
Fetal Leydig cells promote Wolffian duct development and regulate other aspects of XY embryo masculinization (Geissler et al., 1994; Jost et al., 1973). Our data strongly suggest that Leydig cells also have an important impact on germ cell survival and testis cord structure. In gonads with either an increase or a decrease in Leydig cell number, we observed germ cell loss and cord structure disruptions. This is consistent with loss of function mutants of Dhh (Pierucci-Alves et al., 2001; Yao et al., 2002) and Pdgfra (Brennan et al., 2003) in which Leydig cells were lost, and cord structure was also affected. Germ cell loss was also observed in Dhh mutant gonads (Clark et al., 2000; Yao et al., 2002). In Dhh mutants, it was possible that testis cord and germ cell defects were caused by defects in Sertoli cells, which express Dhh and are known to be important for aggregation of germ cells into testis cords (Clark et al., 2000). As both Sertoli and Leydig progenitor cells activate Notch signaling in the RosaNotch; Sf1-cre gonad, it is possible that defects from either or both of them affect cord structure and germ cell survival in the gain-of-function mutants. However, this cannot be the case in Hes1 loss-of-function mutants as no Hes1 expression was detected in Sertoli or germ cells. These results support the idea that Leydig cells, and perhaps the number, types and distribution of other interstitial cells, have an impact on shaping cord structure and on the survival of male germ cells. It is possible that Notch signaling directly regulates the interaction between Sertoli and interstitial cells (including peritubular myoid cells or other interstitial progenitors), or that abnormal Leydig cell development indirectly affects cord formation.
Overall, our results strongly support the presence of Leydig progenitor cells in the mouse embryonic testis, and uncover the molecular mechanism that regulates maintenance of these cells. Leydig cells play a crucial role during spermatogenesis in adult life. Our data strongly imply that Leydig cells or their progenitors are also important for germ cell survival and cord formation during fetal life. This study may shed light on the function of Notch signaling in the regulation of progenitor/stem cell populations in other systems, and could lead to therapeutic approaches to regulate Leydig cell number in adolescent and adult life.
Supplementary Material
Acknowledgments
We thank Douglas A. Melton for RosaNotch mice, Ryoichiro Kageyama for Hes1+/− mice, Tom Gridley for Notchdel mice, Marc Tessier-Lavigne for Notch3lacZ mice, Keith L. Parker for Sf1-cre mice, Argiris Efstratiadis for Hs-cre mice and Hisamaru Hirai For Notch2loxp/− mice. We thank Ken-ichirou Morohashi for anti-3β-HSD and anti-SF1 antibodies, Francis Poulat for anti-SOX9 antibody, Thomas Jessell for anti-LHX9 antibody and Harold Erikson for anti-Laminin antibody. We thank Tom Gridley and Tom Vogt for mRNA in situ probe plasmids. We thank Barbara Ahrens and Jordan Batchvarov for breeding mice. We thank all Capel laboratory members for helpful suggestions. This work was funded by grants from the NIH to B.C. (HD39963 and HL63054), and to L.R. (DK076647A), and by a Stem Cell Research Fellowship from Duke University Medical Center to H.T.
Footnotes
Supplementary material Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/135/22/3745/DC1
References
- Ariyaratne HB, Chamindrani Mendis-Handagama S. Changes in the testis interstitium of Sprague Dawley rats from birth to sexual maturity. Biol. Reprod. 2000;62:680–690. doi: 10.1095/biolreprod62.3.680. [DOI] [PubMed] [Google Scholar]
- Baker PJ, O'Shaughnessy PJ. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122:227–234. doi: 10.1530/rep.0.1220227. [DOI] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, et al. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature. 2000;403:909–913. doi: 10.1038/35002622. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat. Rev. Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Brennan J, Karl J, Capel B. Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev. Biol. 2002;244:418–428. doi: 10.1006/dbio.2002.0578. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol. Rev. 1986;66:71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development. 2003;130:5031–5042. doi: 10.1242/dev.00697. [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol. Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Muller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J. Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Dietrich P, Dragatsis I, Xuan S, Zeitlin S, Efstratiadis A. Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm. Genome. 2000;11:196–205. doi: 10.1007/s003350010037. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Geissler WM, Davis DL, Wu L, Bradshaw KD, Patel S, Mendonca BB, Elliston KO, Wilson JD, Russell DW, Andersson S. Male pseudohermaphroditism caused by mutations of testicular 17 beta-hydroxysteroid dehydrogenase 3. Nat. Genet. 1994;7:34–39. doi: 10.1038/ng0594-34. [DOI] [PubMed] [Google Scholar]
- Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol. Cell Endocrinol. 2001;179:47–74. doi: 10.1016/s0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Kadokawa Y, Okabe M, Ikawa M, Coleman JR, Tsujimoto Y. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development. 1999;126:3415–3424. doi: 10.1242/dev.126.15.3415. [DOI] [PubMed] [Google Scholar]
- High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc. Natl. Acad. Sci. USA. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature. 2000;405:966–970. doi: 10.1038/35016111. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Jost A, Vigier B, Prepin J, Perchellet JP. Studies on sex differentiation in mammals. Recent Prog. Horm. Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev. Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Risbridger GP, Knell CM. Stimulation of interstitial cell growth after selective destruction of foetal Leydig cells in the testis of postnatal rats. Cell Tissue Res. 1988;252:89–98. doi: 10.1007/BF00213829. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Capel B. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc. Natl. Acad. Sci. USA. 2007;104:16558–16563. doi: 10.1073/pnas.0702581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat. Genet. 2002;32:359–369. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis. 2003;37:139–143. doi: 10.1002/gene.10241. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr. Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Mazaud S, Oreal E, Guigon CJ, Carre-Eusebe D, Magre S. Lhx9 expression during gonadal morphogenesis as related to the state of cell differentiation. Gene Expr. Patterns. 2002;2:373–377. doi: 10.1016/s1567-133x(02)00050-9. [DOI] [PubMed] [Google Scholar]
- McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- Migrenne S, Pairault C, Racine C, Livera G, Geloso A, Habert R. Luteinizing hormone-dependent activity and luteinizing hormone-independent differentiation of rat fetal Leydig cells. Mol. Cell Endocrinol. 2001;172:193–202. doi: 10.1016/s0303-7207(00)00339-7. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev. Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Gilardi-Hebenstreit P, Charnay P, Wilkinson DG. A receptor protein tyrosine kinase implicated in the segmental patterning of the hindbrain and mesoderm. Development. 1992;116:1137–1150. doi: 10.1242/dev.116.4.1137. [DOI] [PubMed] [Google Scholar]
- Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat. Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- Parks AL, Huppert SS, Muskavitch MA. The dynamics of neurogenic signalling underlying bristle development in Drosophila melanogaster. Mech Dev. 1997;63:61–74. doi: 10.1016/s0925-4773(97)00675-8. [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the Desert hedgehog-null mouse testis. Biol. Reprod. 2001;65:1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev. Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, Yamaguchi T, Yamamoto G, Seo S, Kumano K, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev. Biol. 2006;299:250–256. doi: 10.1016/j.ydbio.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.