Abstract
Background
Human pleuro-pulmonary endometriosis (PPE) is rare. Recently, we identified several cases of abdominal endometriosis in baboons that developed PPE.
Materials and Methods
Ten cases of PPE and 4 of intra-abdominal endometriosis (3 simultaneous) were identified at necropsy in baboons (Papio spp.) found dead due to natural causes. The endometriotic lesions were evaluated using immunohistochemistry.
Results
The stromal (CD10+) and epithelial cells in intra-abdominal cases were estrogen and progesterone receptor (ER/PR) positive and thyroid transcription factor 1 (TTF-1) negative similar to that seen in humans. In contrast, the PPE cases displayed TTF-1 positive epithelium lining the cystic spaces while the stroma was ER/PR positive similar to that in abdominal endometriosis. Both lymph nodes and spindle cell rests in lung interstitium contained ER/PR positive stromal cells.
Conclusion
The lung lesions were different from the abdominal lesions in having a TTF-1 positive lining epithelium. The deep pulmonary interstitial and lymph node endometrial stromal rests probably arrive via lymphatic route. The endometrial stroma is the driving force in PPE upon which the lung specific epithelium condenses and may require a novel approach to therapy.
Keywords: Thoracic endometriosis, immunohistochemistry, TTF-1, baboons, pathophysiology
Introduction
Endometriosis is one of the most common gynecologic disorders in humans, with up to 10% of reproductive age women [11] and 30-55% of infertile women being affected [14]. Pleuro-pulmonary endometriosis (PPE) is rare in humans. Pleuro-pulmonary or thoracic endometriosis is a rare but well-defined condition. Thyroid transcription factor 1 (TTF-1) has not been reported thus far in baboon or in human PPE. Our current knowledge of the pathogenesis of endometriosis in general remains unclear and the etiologic mechanism for the development of pleuro-pulmonary endometriosis is also unclear. Three major theories have been proposed: coelomic metaplasia [22, 23], lymphatic or hematogenous embolization from the uterus or pelvis [20], and Sampson’s theory of retrograde menstruation with subsequent transperitoneal-transdiaphragmatic migration of endometrial tissue [28]. The optimal management strategy for these cases is not agreed upon. PPE has not been described in baboons prior to our observations and provides an excellent model for experimental studies on endometriosis. Recently a case of pulmonary endometriosis was reported in the macaque monkey. [3] These cases may serve to help further our understanding of the etiology of PPE in humans [18].
Materials and Methods
This study was conducted at the Texas Biomedical Research Institute and University of Texas Health Science Center, San Antonio, Texas. All animal care and procedures were approved by the Texas Biomedical Research Institute Animal Care and Use Committee. The Texas Biomedical Research Institute is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International. Ten cases of PPE (three with additional uterine surface endometriosis) and one case of colonic surface endometriosis were identified at necropsy in baboons (Papio spp.). These necropsies were carried out on baboons that died of natural causes. One baboon died suddenly and was speculated to die from pneumothorax secondary to pleural cystic endometriosis. The lesions were fixed in 10% neutral buffered formalin, processed conventionally, embedded in paraffin, cut at 5 μM, stained with hematoxylin and eosin and examined microscopically. Immunohistochemical staining using the Ventana XT automated immunostainer was performed on sections using antibodies to estrogen receptor (ER; Ventana Medical Systems Inc., AZ, USA, prediluted), progesterone receptor (PR; Ventana Medical Systems Inc., AZ, USA, prediluted), thyroid transcription factor-1 (TTF-1; Dako, CA, USA, 1: 200 dilution), CD10 Leica Microsystems Inc., IL, USA, 1:50 dilution, and the proliferation marker Ki-67 (MIB-1, Ventana Medical Systems Inc., AZ, USA, prediluted). An elastic stain (elastic Von Giemsa) was also performed on select cases with pleuro-pulmonary involvement.
Results
Necropsy was performed on 11 female baboons found dead due to natural causes. Grossly, hemorrhagic and cystic lesions were noted on the lung surfaces and within the lung parenchyma bilaterally in 10 baboons (Fig-1A). An additional case was identified with similar lesions noted on the colonic serosa. Three of the above cases also had lesions noted on the uterine surface. Histologically, these lesions demonstrated similar histology of a biphasic pattern of spindled to plump stromal cells in association with an epithelial lining of columnar or flattened cuboidal cells consistent with endometriosis (Fig-1B). The lesions identified in the lungs varied from being nodular aggregates of stromal cells deep in the interstitium with peripherally located epithelial cells to subpleural cystic lesions of varying sizes with stromal and epithelial components. Hemosiderin laden macrophages and scattered mononuclear cells were noted. Some of the lesions extended from the pleural surface with invagination into the lung parenchyma, while others were entirely nodular and confined to the parenchyma. ER/PR positive stromal cells were identified in the subcapsular area of one hilar lymph node.
Fig-1A. Hemorrhagic cystic lesions in baboon lung.
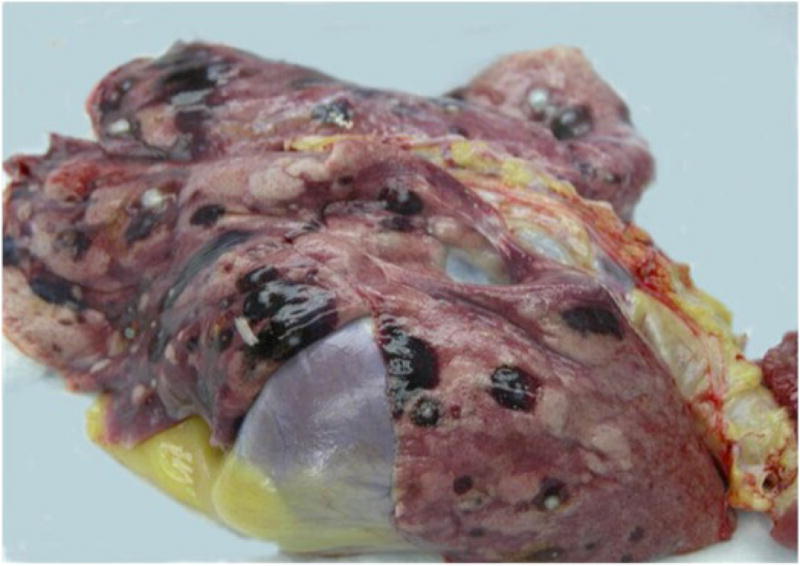
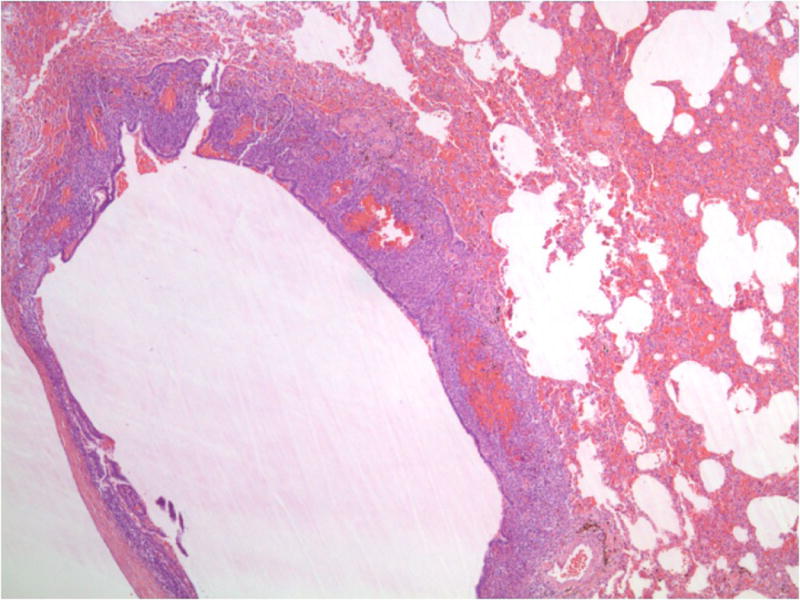
Fig-1B. Biphasic lesions of endometriosis in baboon lungs comprising of endometrial glands in a spindle cell stromal background (H&E, 40x).
Immunohistochemical stains of the endometriotic lesions of the uterine surface in the abdomen and the colonic surface showed consistent strong ER (Fig-2A) and PR (Fig-2B) positivity in both the stromal and epithelial components. CD10 was positive in the stroma of all cases and in 2 of 4 cases the epithelial component also marked with CD10 (Fig-2C). TTF-1 was negative in all of three cases.
Fig-2A. Colonic endometriotic foci with ER positivity in both glandular and stromal component and (20x).
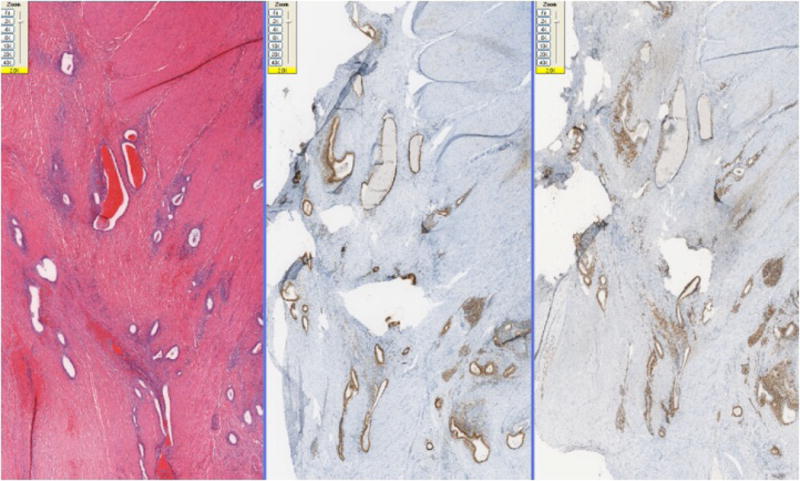
Fig-2B. Colonic endometriotic foci with PR positivity in both glandular and stromal component (100x).
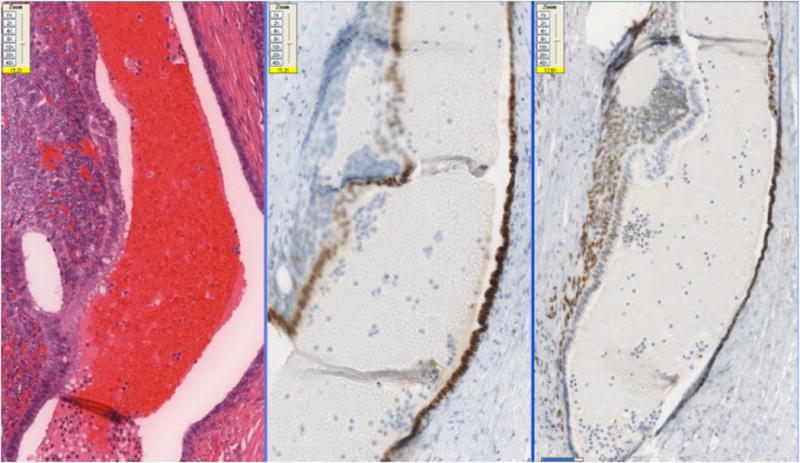
Fig-2C. Endometriotic foci with CD10 positivity in both glandular and stromal component (100x).
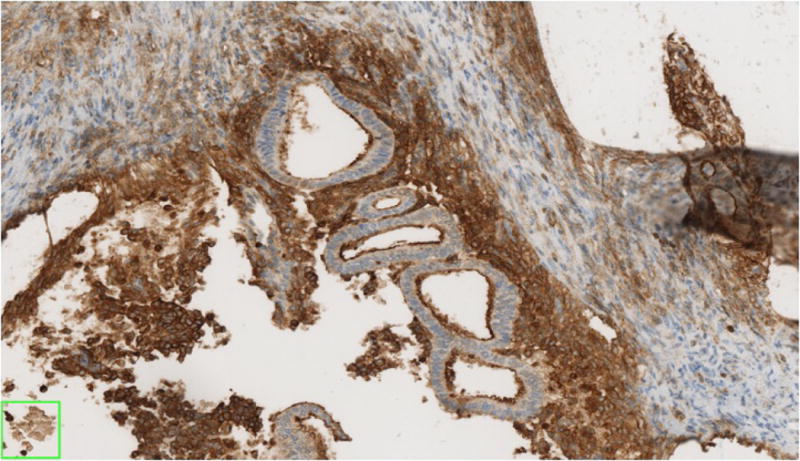
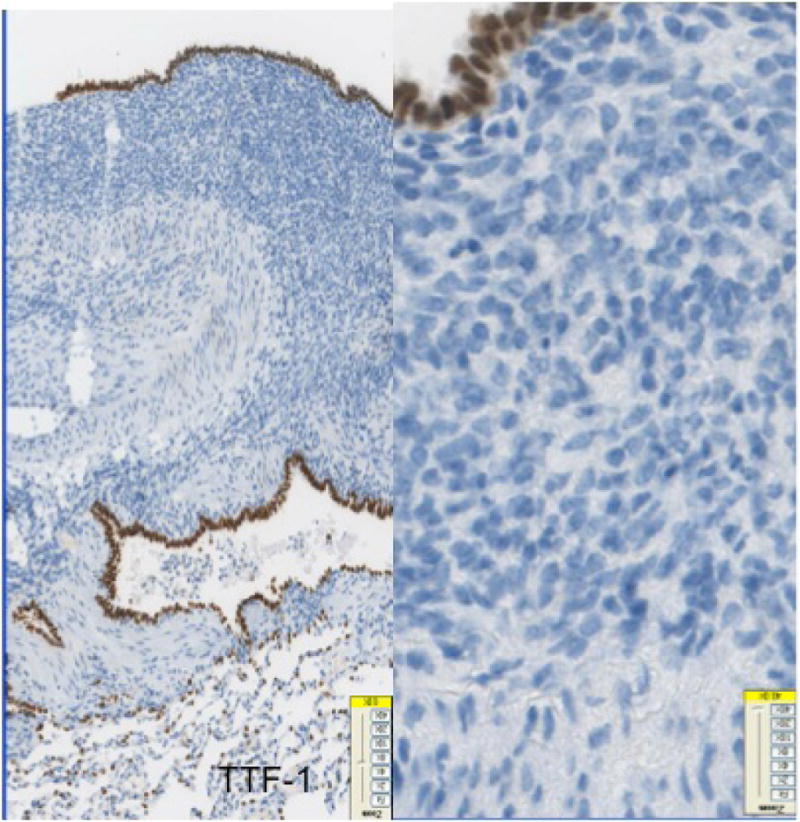
The 10 cases of pleuro-pulmonary endometriosis showed an identical immunohistochemical profile. Vimentin and keratin stains highlighted the biphasic spindle cell and epithelial components. All cases were ER, PR (Fig-3A, 3B, 3C) and CD10 positive in the stroma. These stains were consistently negative in the epithelial component. The stromal component in the lymph node in one case was also positive for ER/PR (Fig-3D). TTF-1 (Fig-3E) was positive in 10/10 cases in the epithelial component both in the deep parenchymal lesions as well as the surface pleural-based lesions unlike in abdominal endometriosis. Elastic stain performed on select cases showed abnormal staining with loss of elastic tissue within the lesions in the lungs. Immunohistochemistry for the proliferation marker Ki-67 of the endometriotic lesions in the lungs did not show any increased staining in the stromal or epithelial component. The lesions were negative for CD34, S-100 and CD68 in both stromal and epithelial components consistently.
Fig-3A. Positive staining for ER in the stromal component and absence of staining in glandular component in foci of pleuro-pulmonary endometriosis (20x).
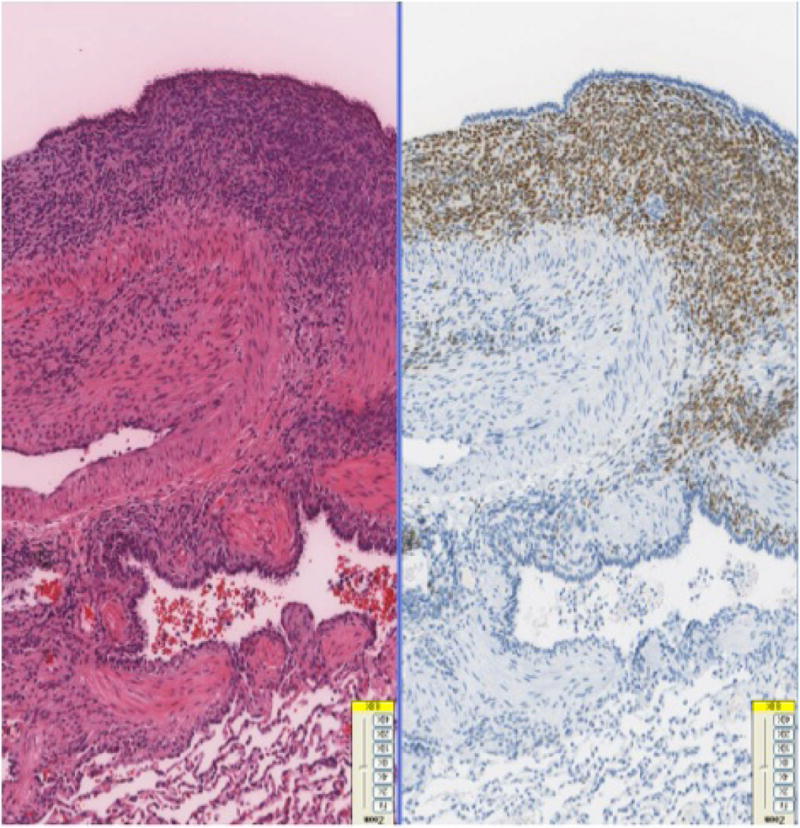
Fig-3B. Positive staining for PR in the stromal component and absence of staining in glandular component in foci of pleuro-pulmonary endometriosis (40x).
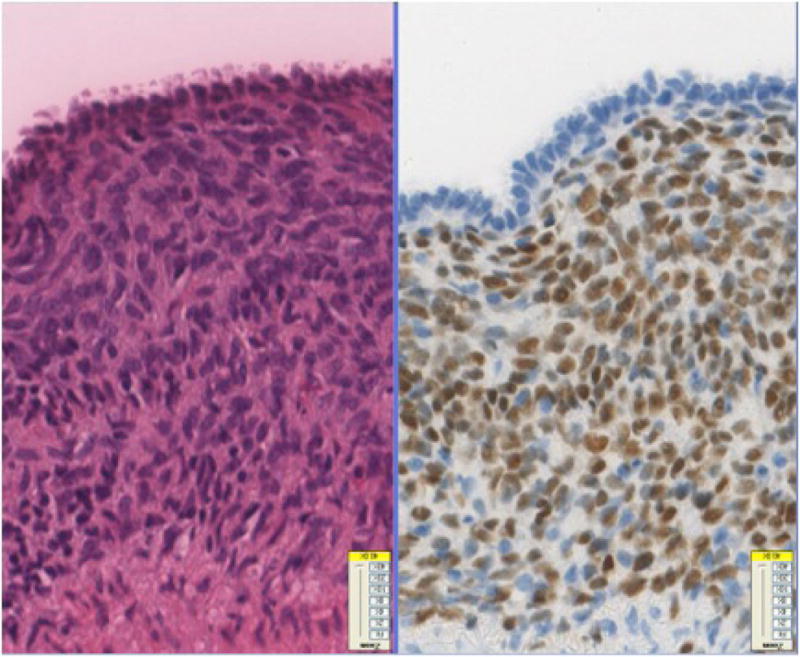
Fig-3C. ER/PR positive stroma in the endometriotic foci in lung (40x).
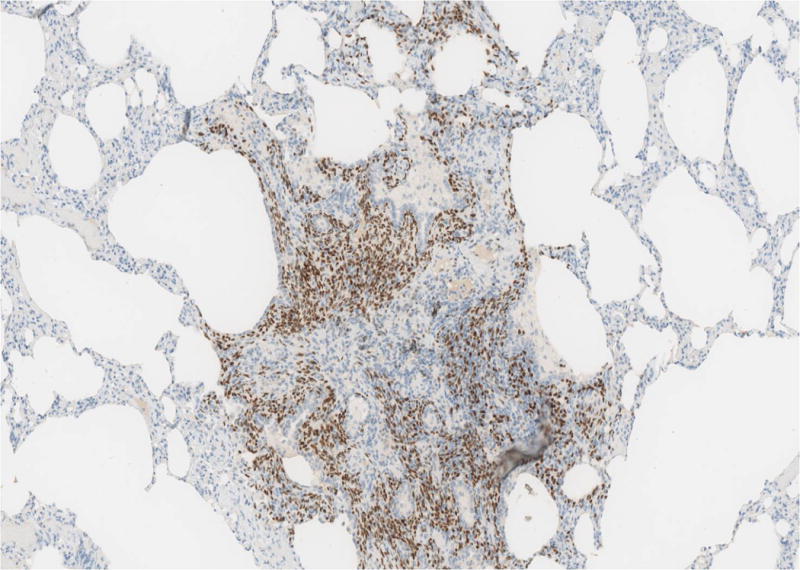
Fig-3D. ER/PR positive endometrial stroma in lymphnode (20x).
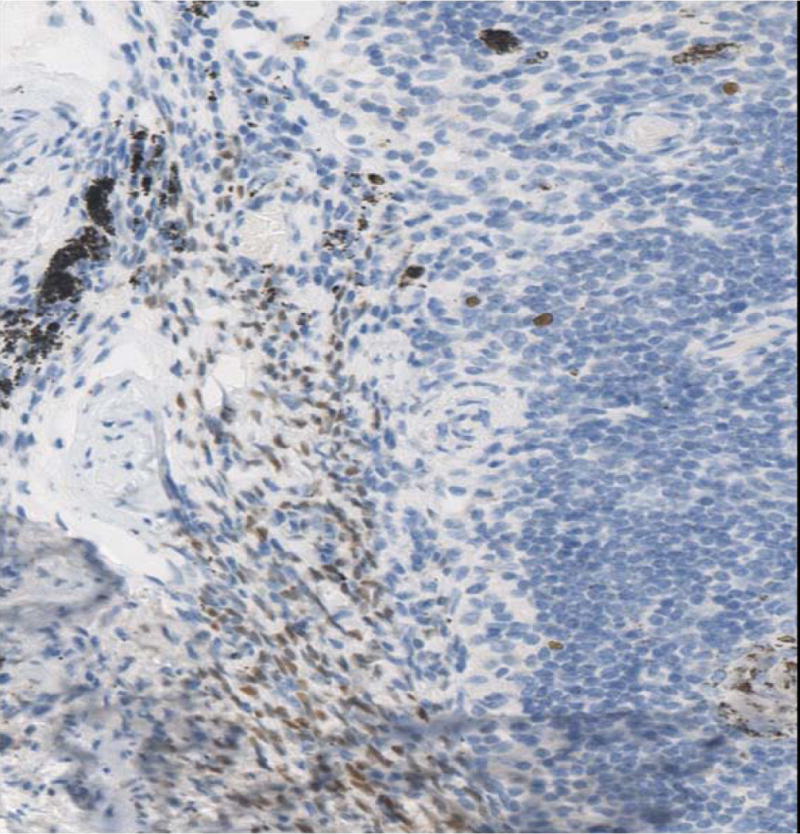
Fig-3E. Glandular component staining positively with TTF-1 in endometriotic foci of pleuro-pulmonary endometriosis (40x).
Discussion
Similarities between baboons and humans
Baboons share 95-98% genetic homology with humans, but unlike humans, gene amplifications and duplications are more common than mutations in baboons [19]. Several of the spontaneously occurring diseases in baboons are similar to humans and offer an excellent research tool to provide an insight in to pathogenesis of these diseases. Necropsies on spontaneously dying baboons have demonstrated eosinophilic bronchitis-like lesion [5] and pneumoconiosis that bear marked similarity to the analogous human disease. Endometriosis and adenomyosis are also diseases that are common to both baboons and humans [6].
Endometriosis in baboons
The pelvic anatomy of baboons is very similar to humans and they have monthly menstrual cycles and spontaneous menses with a prevalence of endometriosis that increases with duration of captivity [9, 10]. After more than 2 years in captivity, the prevalence of biopsy-proven endometriosis is as high as 27% [8]. In established multigenerational breeding colonies, the prevalence has been reported as high as 60% in infertile animals. Also in large, established colonies, many cases of severe disease are encountered at the time of necropsy. Because of this fact they are an established model for endometriosis research [7, 30]. PPE has not been hitherto recognized in baboons.
Endometriosis in Humans
Endometriosis is a potentially painful condition in women in which endometrium is found inside the peritoneal cavity and may involve the pelvic and abdominal organs. The less common sites of endometriosis include the ovaries, rectovaginal septum, colon, appendix, lymph nodes, brain and lungs [26]. Endometriosis affects about 10% of the general female population and accounts for 50% of pelvic pain/infertility cases in women [14, 26]. Thoracic endometriosis syndrome (TES) [20] in humans is a rare condition with four distinctly recognized clinical enitites: catamenial pneumothorax (CP), catamenial hemothorax, catamenial hemoptysis and lung nodules. Flieder and colleagues described 9 cases of PPE [13]. In another study, Alifano et al described CP as an underlying cause of spontaneous pneumothorax in 8 of 32 women hospitalized over an 18-month period for surgical management [2]. This may reflect that thoracic endometriosis is perhaps an under-recognized cause of secondary spontaneous pneumothorax in women [16, 27]. TTF-1 has not been studied in human PPE.
The 10 cases of baboon PPE in this series showed an interesting and hitherto unreported immunohistochemical profile. The stroma in all cases was immunoreactive with ER, PR and CD10. These stains were consistently negative in the epithelial component. In contrast, TTF-1 was positive in 10/10 cases in the epithelial component both in the deep cystic parenchymal lesions as well as the surface pleural-based lesions unlike in abdominal endometriosis. The immunohistochemical profile of these lesions cannot be explained completely by the existing models of pathogenesis of endometriosis.
Pathogenesis of Endometriosis
The spontaneous disease model of endometriosis in baboons
The results of this study provide some new insights into the pathogenesis of endometriosis in baboons that provide an excellent experimental model for extrapolating the results to human endometriosis. This is the first study describing TTF-1 positivity in the lining cells of the endometriotic lesions in the lung parenchyma as well as lesions on the pleural surface. TTF-1 is a 38-kDa, homeodomain protein that regulates gene expression in the thyroid, lungs and diencephalon during embryogenesis. It is normally expressed in alveolar pneumocytes, Clara cells, ciliated respiratory epithelial cells, and bronchial basal cells of the lung [24].
In the present study, PPE in baboons differs from abdominal endometriosis in that the epithelium is derived locally and is TTF-1 positive in contrast to abdominal endometriosis in which the epithelium is ER/PR positive and similar to that in the uterine endometrium. This finding is different from the human PPE cases described by Flieder et al who reported strong ER positivity in the glandular cells [13]. They did not perform TTF-1.
The immunohistochemical profile of the PPE lesions suggests a different pathogenesis from abdominal endometriosis. Minute foci of ER positive spindle stromal cells are seen in the lung interstitium implying that the stroma is the primary driving force in the pathogenesis of these lesions. The stroma probably induces the laying down of epithelium locally via local irritation or via mediators. The lack of ER/PR staining in the glandular component contradicts the theory of regurgitation and implantation. The presence of deep parenchymal lesions and the occurrence of bilateral lesions cannot be fully explained by Myers’ theory of celomic metaplasia. The presence of stromal cells in the lymph node supports the embolization theory for extra abdominal endometriosis. It appears that it is only the stromal component that embolizes. The embolization of the stromal component in the lung parenchyma then induces the epithelial component from the local tissue defined stem cell population. The cysts form next due to expansion of the lung during inspiration without fibrosis unlike that seen in abdominal endometriosis where fibrosis is common. Since the lining is not ER/PR positive it might explain why hormonal treatment has failed and recurrences are common with behavior simulating a low grade malignancy with metastasis / or benign metastasis.
Different theories have been postulated over the years to explain the occurrence of endometrial glands and stroma outside the confines of the uterus. The foremost amongst these are Sampsons’ theory of regurgitation and implantation; Myers’ theory of celomic metaplasia; and embolization from the uterus and abdominal endometriosis. However, it appears that endometriosis is a multifactorial disease entity and none of the existing theories can explain all cases of endometriosis.
The Sampsons’ theory is most favored currently. This theory states that “peritoneal circulation” causes a preferential flow of fluids from the pelvis to the right subdiaphragmatic area through the right paracolic gutter [21]. Congenital or acquired defects in the diaphragm that frequently exist in many individuals predominantly on the right side could facilitate the transdiaphragmatic movement of endometrial tissue. Once the viable endometrial tissue has entered the pleural space, it may colonize either the diaphragm or the rest of the pleural space [2, 21, 4]. Retrograde menstruation has been found by laparoscopy to be a common phenomenon occurring in 76%-90% of women with patent fallopian tubes. In comparison, only a small number of menstruating women suffer from endometriosis.
The theory of coelomic metaplasia proposed by Myers is based on the similar histogenesis for endometrium and pleural and peritoneal mesothelium. Pathogenic stimuli (inflammation or hormonal) could be responsible for inducing precursor cells in the pleura or the peritoneum to differentiate into endometrial cells. This theory cannot explain the occurrence of intrapulmonary endometriosis or the right-sided predominance.
The theory of transplantation of endometrium through lymphatic or vascular embolization could explain not only the intrapulmonary endometriosis but also the other extrapelvic localizations. Trauma or manipulation of uterine tissue would be a factor predisposing to microembolization. In the review by Joseph and Sahn [20] pelvic endometriosis was documented in 84% of the patients with extraabdominal endometriosis.
The mechanism of survival of ectopic endometrial stromal tissue is not well understood, but it is hypothesized that abnormal matrix metalloproteinase (MMP) and their tissue inhibitor expression, altered immune milieu, aberrant local aromatase activity, genetic and environmental factors may be involved [17]. Endometriotic tissues show statistically significantly stronger staining for MMP-9 and reduced beta-catenin expression when compared with proliferative endometrium. Endometrial endometrioid carcinoma also shows decreased E-cadherin expression in comparison with proliferative endometrium [29, 12]. Furthermore, the vasculature that promotes growth and viability of the endometriotic lesions has been shown to express increased levels of angiogenic factors such as CYR61 and vascular endothelial growth factor –A. [17]
Differential Diagnosis
The differential diagnosis of biphasic hemorrhagic cystic lesions on the pleural surfaces and in the lung parenchyma in humans would include recreational drug related lesions, lymphangioleiomyomatosis, eosinophilic granuloma, pleuro-pulmonary blastoma and other biphasic lesions like synovial sarcoma, spindle cell lesions, fibrohistiocytic tumors, and angiosarcoma, besides idiopathic causes.
Treatment options
The etiological mechanisms of PPE are not well understood and therefore there are no well-defined guidelines for optimal management. The treatment options are both medical and surgical [25]. The different modalities of surgical treatment are video-assisted thoracoscopy, diaphragmatic resection, partial pleurectomy and bullectomy, and tubal ligation and hysterectomy with bilateral salpingo-oopherectomy. Different medications that have been to show variable results are oral contraceptives, the progestational drug danazol and gonadotrophin-releasing hormone agonists. The aim of medical management is to induce a pseudopregnancy or pseudomenopause, thereby inhibiting the proliferation of endometrial tissue [25, 1, 15]. However, failure rates as high as 50% are seen in patients with CP [20]. A combined approach is now regarded as the best option for treating patients and is gaining increasing popularity [2]. The recurrence rate of CP is much higher after treatment as compared to primary pneumothorax irrespective of the treatment modality. The recurrence could be due to mutifocal microscopic stromal rests deep in the lung tissue that are inaccessible to surgeons as well as the TTF-1 cells lining the cysts not responding to hormonal manipulation.
In summary, the epithelial component of baboon PPE is negative for ER/PR and appears to be locally derived from tissue defined TTF-1 positive “stem” cells under the influence of ER/PR positive endometrial stromal cells which may get to the lung possibly via a lymphatic route. The catastrophic hemorrhage that derives from breakdown of the glandular component in turn may not be amenable to hormonal manipulation currently being applied to abdominal endometriosis.
Conclusions
Abdominal endometriosis differs from PPE. The TTF-1 positive cells lining the cystic areas in PPE suggest that the pathogenesis of PPE is other than what has been previously postulated. The presence of deep parenchymal interstitial aberrant nodular stromal rests suggests that the stroma is the driving force in PPE upon which the lung specific epithelium condenses. Treatment of PPE may require a novel approach to therapy.
References
- 1.Abu Hashim H. Gonadotrophin-releasing hormone analogues and endometriosis: current strategies and new insights. Gynecol Endocrinol. 2012;28(4):314–21. doi: 10.3109/09513590.2011.650751. [DOI] [PubMed] [Google Scholar]
- 2.Alifano M, Roth Th, Camilleri Broet S, Schussler O, Magde- leinat P, Regnard JF. Catamenial pneumothorax. A prospective study. Chest. 2003;124:1004–8. doi: 10.1378/chest.124.3.1004. [DOI] [PubMed] [Google Scholar]
- 3.Assaf BT, Miller AD. Pleural Endometriosis in an Aged Rhesus Macaque (Macaca mulatta):A Histopathologic and Immunohistochemical Study. Veterinary Pathology. 2012;49(4):636–64. doi: 10.1177/0300985811406890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagan P, Le Pimpec Barthes F, Assouad J, Souilamas R, Riquet M. Catamenial pneumothorax: retrospective study of surgical treatment. Ann Thorac Surg. 2003;75:378–81. doi: 10.1016/s0003-4975(02)04320-5. [DOI] [PubMed] [Google Scholar]
- 5.Christal JL, Hubbard GB, Dick EJ., Jr Eosinophilic bronchitis-like lesion as the cause of death in a Macaca mulatta: a first case report. J Med Primatol. 2008;37(2):63–66. doi: 10.1111/j.1600-0684.2007.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Hooghe TM, Bambra CS, De Jonge I, Lauweryns JM, Koninckx PR. The prevalence of spontaneous endometriosis in the baboon Papio anubis, (Papio cynocephalus) increases with the duration of captivity. Acta Obstet Gynecol Scand. 1996;75:98–101. doi: 10.3109/00016349609033298. [DOI] [PubMed] [Google Scholar]
- 7.D’Hooghe TM, Bambra CS, Raeymaekers SCM, De Jonge I, Lauweryns JM, Koninckx PR. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis) Am J Obstet Gynecol. 1995;173:125–134. doi: 10.1016/0002-9378(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 8.D’Hooghe TM, Bambra CS, Raeymaekers BM, Koninckx PR. Serial laparoscopies over 30 months show that endometriosis in captive baboons (Papio anubis, Papio cynocephalus) is a progressive disease. Fertil Steril. 1996;65:645–649. [PubMed] [Google Scholar]
- 9.D’Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update. 2002;8:84–88. doi: 10.1093/humupd/8.1.84. [DOI] [PubMed] [Google Scholar]
- 10.D’Hooghe TM, Riday AM, Bambra CS, Suleman MA, Raeymaekers MS, Koninckx PR. The cycle pregnancy rate is normal in baboons with stage I endometriosis but decreased in primates with stage II and III-IV disease. Fertil Steril. 1996;66:809–813. [PubMed] [Google Scholar]
- 11.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–358. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 12.Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun SA. A modified model for endometriosis. Ann N Y Acad Sci. 2002;955:308–317. doi: 10.1111/j.1749-6632.2002.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 13.Flieder DB, Moran CA, Travis WD, Koss MN, Mark EJ. Pleuro-pulmonary endometriosis and pulmonary ectopic deciduosis: a clinico-pathologic and immunohistochemical study of 10 cases with emphasis on diagnostic pitfalls. Hum Pathol. 1998;29:1495–503. doi: 10.1016/s0046-8177(98)90021-1. [DOI] [PubMed] [Google Scholar]
- 14.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 15.Benagiano Gluseppe, Brosens Ivo. Endometriosis, a modern syndrome. Indian Journal of Medical Research. 2011;133(6):581. [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta D, Hansell A, Nichols T, Duong T, Ayres J, Strachan D. Epidemiology of pneumothorax in England. Thorax. 2000;55:666–71. doi: 10.1136/thorax.55.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastings JM, et al. A baboon model for endometriosis: implications for fertility. Repro Biol and Endo. 2006;4(Suppl 1):57. doi: 10.1186/1477-7827-4-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehoux Jean-Paul, Defrere Sylvie, Squifflet Jean, et al. Is the baboon model appropriate for endometriosis studies? Fertility and Sterility. 2011;96(3) doi: 10.1016/j.fertnstert.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Jolly CJ. A proper study for mankind: Analogies from the Papionin monkeys and their implications for human evolution. Am J Phys Anthropol. 2001;(Suppl 33):177–204. doi: 10.1002/ajpa.10021. [DOI] [PubMed] [Google Scholar]
- 20.Joseph J, Sahn SA. Thoracic endometriosis syndrome: new observations from an analysis of 110 cases. Am J Med. 1996;100:164–70. doi: 10.1016/s0002-9343(97)89454-5. [DOI] [PubMed] [Google Scholar]
- 21.Kirschner PA. Porous diaphragm syndromes. Chest Surg Clin North Am. 1998;8:449–72. [PubMed] [Google Scholar]
- 22.Lauchlan SC. The secondary muellerian system. Obstet Gynecol Surv. 1972;27:133–46. doi: 10.1097/00006254-197203000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Meyer R. The current question of adenomyositis and adenomyomas in general and particularly seroepithelial adenomyositis and sarcomatoid adenomyometritis. Zentralbl Gynakol. 1919;43:745–50. [Google Scholar]
- 24.Nakamura N, Miyagi E, Murata S, et al. Expression of thyroid transcription factor-1 in normal and neoplastic lung tissues. Mod Pathol. 2002;15:1058–1067. doi: 10.1097/01.MP.0000028572.44247.CF. [DOI] [PubMed] [Google Scholar]
- 25.Olive DL, Pritts EA. Treatment of endometriosis. N Engl J Med. 2001;345:266–75. doi: 10.1056/NEJM200107263450407. [DOI] [PubMed] [Google Scholar]
- 26.Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759–69. doi: 10.1056/NEJM199306173282407. [DOI] [PubMed] [Google Scholar]
- 27.Sadikot RT, Green T, Meadows K, et al. Recurrence of primary spontaneous pneumothorax. Thorax. 1997;52:805–9. doi: 10.1136/thx.52.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissues into the peritoneal cavity. Am J Obstet Gynecol. 1927;24:422–69. [Google Scholar]
- 29.Shaco-Levy R, et al. Matrix metalloproteinases 2 and 9, E-cadherin, and beta-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):226–32. doi: 10.1016/j.ejogrb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Stevens VC. Some reproductive studies in the baboon. Hum Reprod Update. 1997;3:533–40. doi: 10.1093/humupd/3.6.533. [DOI] [PubMed] [Google Scholar]


