Abstract
Abnormalities in humoral immunity typically reflect a generalized or selective failure of effective B cell development. The developmental processes can be followed through analysis of cell surface markers such as IgM, IgD, CD10, CD19, CD20, CD21, and CD38. Early phases of B cell development are devoted to the creation of immunoglobulin and testing B cell antigen receptor signaling. Failure leads to the absence of B cells and immunoglobulin in the blood from birth. As the developing B cells begin to express a surface B cell receptor, they become subject to negative and positive selection pressures and increasingly depend on survival signals. Defective signalling can lead to selective or generalized hypogammaglobulinemia even in the presence of normal numbers of B cells. In the secondary lymphoid organs, some B cells enter the splenic marginal zone where pre-activated cells lie ready to rapidly respond to T-independent antigens, such as the polysaccharides that coat some microorganisms. Other cells enter the follicle and, with the aid of cognate follicular T cells, divide to help form a germinal center after their interaction with antigen. In the germinal center, B cells can undergo the processes of class switching and somatic hypermutation. Failure to properly receive T cell signals can lead to Hyper IgM syndrome. B cells that leave the germinal center can develop into memory B cells, short lived plasma cells, or long lived plasma cells. The latter ultimately migrate back to the bone marrow where they can continue to produce protective antigen-specific antibodies for decades.
Keywords: B cell Development, Human, B cell immune deficiencies, Review
Abnormal humoral immune function can manifest as immune deficiency, autoimmunity, or allergy. Many humoral immune disorders are caused by loss-of-function or altered-function mutations in genes involved in the regulation of B cell differentiation, tolerance, or function. Others reflect mutations in the immunoglobulin genes themselves. In some conditions, the genetic etiology has been well defined. In others, a genetic predisposition may have been recognized, but the underlying molecular or cellular defect remains unclear. The typical patient with a B cell deficiency will present with a history of recurrent upper respiratory or pulmonary infections and will exhibit reduced serum concentrations of one or more classes or subclasses of IgM, IgG, or IgA. However, specific deficits in the ability to mount a protective response against certain antigens or organisms may occur even in the presence of normal serum immunoglobulin levels may exhibit, and some virtually agammaglobulinemic patients can be remarkably asymptomatic. Perhaps paradoxically, some patients who have difficulty defending against exogenous antigens may mount pathogenic responses to self including red blood cells (autoimmune hemolytic anemia [AIHA]), platelets (idiopathic thrombocytopenic purpura [ITP]), nuclear antigens (systemic lupus erythematosus [SLE]), and tissues and organs of the endocrine system (e.g. autoimmune thyroid disease [ATD] including Grave’s disease and Hashimoto’s thyroiditis). Others may be overrepresented in allergy clinics.
The diseases of humoral immunity often arise as a result of specific defects in the production or regulation of B cells and their antibody products. Clinical evaluation typically begins with a complete blood count and an assessment of serum immunoglobulin levels. Patients with a complete absence of immunoglobulin tend to also lack B cells, indicating an early block in B cell development; whereas disorders of regulation typically result in abnormal levels of immunoglobulin in the presence of normal numbers of B cells (Table 1).
Table 1.
Selected Manifestations of Abnormal B cell Development
| Humoral Immunity | |||||||
|---|---|---|---|---|---|---|---|
| Serum Immunoglobulins | Antibody Responses |
Common Infections | Other Immunologic and Clinical Features | ||||
| M | G | A | E | ||||
| PREDOMINANTLY B CELL DEFICIENCY | |||||||
| Failure to create immunoglobulin | |||||||
| Recombinase activating gene (RAG 1/2) deficiency | ↓ | ↓ | ↓ | ↓ | − | bacteria, viruses, fungi | May present with Omenn Syndrome |
| Artemis deficiency (SCIDA) | ↓ | ↓ | ↓ | ↓ | − | " | Radiation sensitivity, may present with Omenn Syndrome |
| Cernunnos Deficiency | ↓ | ↓ | ↓ | ↓ | − | " | Radiation sensitivity |
| DNA Ligase IV (LIG4) deficiency | ↓ | ↓ | ↓ | ↓ | − | " | Radiation sensitivity, may present with Omenn syndrome |
| Failure to create the BCR | |||||||
| Autosomal agammaglobulinemia | |||||||
| Recessive: λ5, VpreB, Igα (CD79a), Igβ (CD79a) | ↓ | ↓ | ↓ | ↓ | − | bacteria, Giardia lamblia | Normal numbers of pro-B cells |
| Failure to permit the BCR to transduce its signal | |||||||
| Autosomal agammaglobulinemia | |||||||
| Recessive: BLNK deficiency | ↓ | ↓ | ↓ | ↓ | − | bacteria, Giardia lamblia | Normal numbers of pro-B cells |
| X-linked agammaglobulinemia (BTK deficiency) | ↓ | ↓ | ↓ | ↓ | − | " | Normal numbers of pro-B cells |
| PREDOMINANTLY ANTIBODY DEFICIENCY | |||||||
| Failure of follicular T cell:B cell interactions | |||||||
| Hyper IgM syndrome | |||||||
| X-linked CD40 ligand deficiency | N/↑ | ↓ | N/↓ | ↓ | +/− | bacteria, viruses, fungi | ITP, AIHA, neutropenia, liver and biliary disease |
| X-linked IKK-gamma (NEMO) deficiency | N/↑ | ↓ | ↓ | ↓ | +/− | " | Anhidrotic epidermal dysplasia, anti-polysaccharide deficiencies |
| CD40 deficiency | N/↑ | ↓ | N/↓ | ↓ | +/− | " | Neutropenia, gastrointestinal and liver/biliary tract disease |
| Failure to modify immunoglobulin genes | |||||||
| Hyper IgM syndrome | |||||||
| Activation-induced cytidine deaminase (AID) deficiency | N/↑ | ↓ | ↓ | ↓ | +/− | bacteria | Lymphadenopathy |
| Uracil-DNA Glycosylase (UNG) deficiency | N/↑ | ↓ | ↓ | ↓ | +/− | " | Lymphadenopathy |
| Alteration of BCR or B cell survival signals | |||||||
| CD20 deficiency | N/↑ | N/↓ | N | +/− | bacteria | Antipolysaccharide deficiency, decreased memory cells | |
| Selective IgA deficiency (MHC-associated, TACI) | N | N | ↓ | N | +/− | bacteria, Giardia lamblia | SLE (2–5%), CD (3%), RA (2%), ITP (0.5%), AR, allergic rhinitis |
| Common variable immune deficiency (ICOS, TACI, BAFFR, CD19) | N/↓ | ↓ | ↓ | ↓ | − | " | AIHA, ITP, ATD, SLE |
| Unknown | |||||||
| Selective inability to respond to bacterial polysaccharides | N | N | N | N | +/− | bacteria | |
| Selective IgA deficiency (MHC-associated) | N | N | ↓ | N | +/− | bacteria, Giardia lamblia | SLE (2–5%), CD (3%), RA (2%), ITP (0.5%), AR |
| IgG subclass deficiency | N | N/↓ | N/↓ | N | +/− | +/− bacteria | |
| Common variable immune deficiency (MHC-associated) | N/↓ | ↓ | ↓ | ↓ | − | bacteria, Giardia lamblia | AIHA, ITP, ATD, SLE |
AIHA - Autoimmune Hemolytic Anemia, ITP - Idiopathic Thrombocytopenic Purpura, SLE - systemic lupus erythematosus, ATD - Autoimmune Thyroid Disease CD - Celiac Disease, IDDM - Insulin Dependent Diabetes MKellitus, AR - Allergic Rhinitis, RA - rheumatoid arthritis
B cell development begins in the primary lymphoid organs
B lymphocytes arise from multipotent hematopoietic stem cells that first appear in the embryo within the para-aortic splanchnopleure1. At 7 weeks fetal gestation, these cells take up residence in the liver. By the middle of the second trimester they can be found in bone marrow, which just prior to birth becomes their exclusive home. In these organs, daughter cells give rise to lymphoid primed multipotent progenitors (LMPPs) that produce common lymphoid precursors (CLPs), which can generate T cells, B cells, NK cells, and dendritic cells2, 3. Final B cell differentiation requires the exposure of CLP daughter cells to specialized microenvironments such as those found in the fetal liver and the bone marrow3. B cells continue to be produced in the bone marrow throughout the life of the individual, although the rate of production decreases with age4.
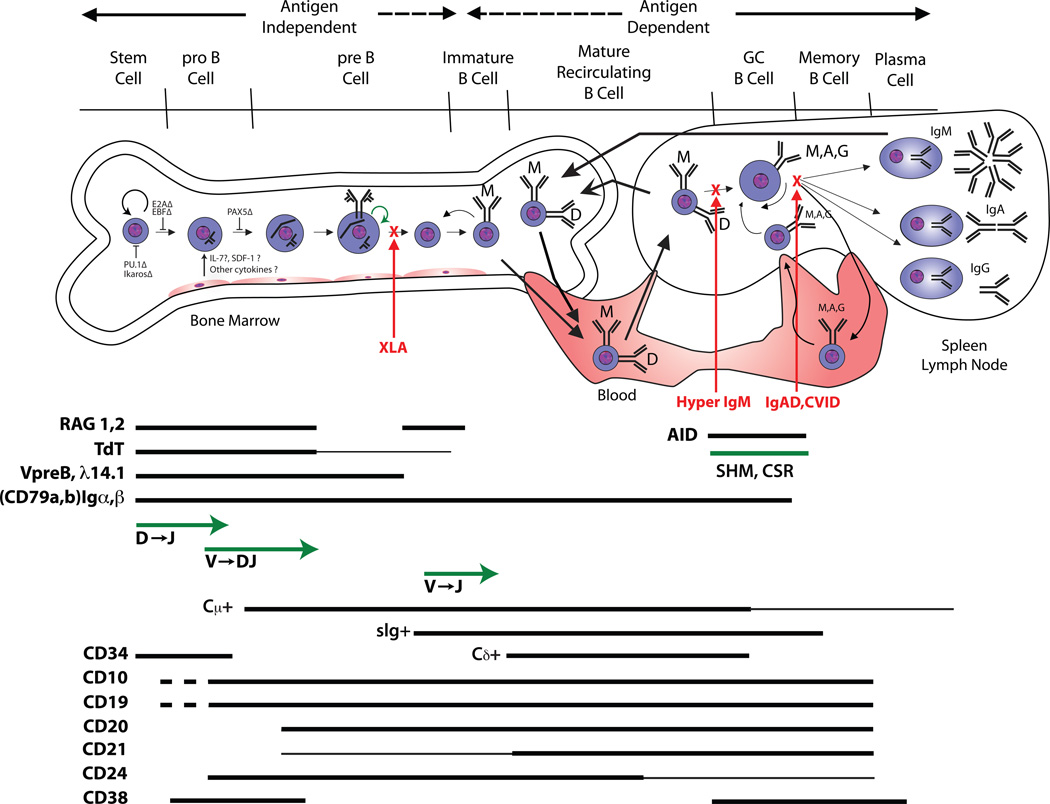
Although a sophisticated reader will recognize the oversimplification of what is a very complex process, B cell differentiation (Figure 1) is typically conceptually viewed as a linear pathway defined by the regulated expression of specific sets of transcription factors, immunoglobulin gene products, and cell surface molecules. Deficiencies in the function of the transcription factors that control early B cell development often block B cell production (Figure 1). B lymphocyte development requires the action of cytokines and transcription factors that positively and negatively orchestrate gene expression. In mice, stromal cell–derived interleukin-7 promotes V to DJ rearrangement and transmits survival signals5. In humans, however, IL-7 does not appear to be essential for B-lineage differentiation and there are active, ongoing efforts to define the bone marrow stromal cell–derived cytokines that transduce the signals necessary for B-cell development in human6, 7.
Figure 1.
B cell development illustrated as a linear progression through developmental checkpoints. Proper assembly of the B cell antigen receptor complex is required. The expression pattern of key surface molecules during this process is marked by bars. Also illustrated are the developmental checkpoints at which loss of function (Δ) of selected transcription factors can influence B cell development.
Immunoglobulin is the primary product of B cells, and early studies viewed B cell development through the prism of antibody generation. Immunoglobulins are the product of a complex process of V[D]J gene rearrangement events which typically proceed in a hierarchical pattern. Pro-B cells are marked by the initiation of RAG1/RAG2 mediated rearrangement of the DH-JH locus. RAG mediated rearrangement also underlies production of the T cell receptor, hence RAG dysfunction can prevent production of both T cells and B cells, yielding a severe combined immune deficiency. Incomplete blocks in V[D]J rearrangement permit the oligoclonal survival of B cells and T cells. This condition can manifest as Omenn Syndrome, which typically presents with pruritic skin lesions, fever, lymphadenopathy, anemia, eosinophilia, and chronic diarrhea8.
DH→JH joining is followed by VH→DJH rearrangement. Terminal deoxynucleotidyl transferase (TdT) catalyzes the addition of random N nucleotides between these rearranging gene segments, and is thus critical for the development of a diverse antigen receptor repertoire. Human patients with a loss of function of TdT have yet to be identified.
Immunoglobulins have a very short cytoplasmic domain. They depend on their association with Igα (CD79a) and Igβ (CD79b) to form a B cell receptor complex that can transduce the binding signal into the cell. Igα and Igβ, as well as VpreB, and λ14.1 [λ5], which together form the surrogate light chain (ψLC) are constitutively expressed by pro-B cells. Activation of these immunoglobulin-associated genes requires transcription factors. PU.1, an ETS family loop-helix-loop (winged helix) transcription factor, appears to help regulate expression of CD79a, RAG1, and TdT, as well as the three immunoglobulin loci: μ, κ and λ. Ikaros, a zinc finger transcription factor, also promotes expression of TdT, λ14.1 (λ5) and VpreB9, 10. The E2A locus encodes two basic helix-loop-helix transcription factors that represent two alternately spliced products, E12 and E4711. E47 promotes TdT and RAG-1 expression better than E12, whereas E12 promotes expression of EBF and PAX5 better than E47. Early B cell factor (EBF), another helix-loop-helix transcription factor, promotes early B cell development. EBF is expressed at all stages of differentiation except plasma cells and in mice has been shown to be critical in the progression of B cells past the early pro-B cell stage. PAX5, a paired-box transcription factor, is expressed exclusively in B lineage cells. In mice, B cell precursors require PAX5 in order to progress beyond the pro-B cell stage2. The presence of PAX5 also prevents early B lineage progenitors from transiting into other hematopoietic pathways. Aiolos, a zinc finger transcription factor, is expressed after commitment to the B cell lineage. Deficiencies of these transcription factors have not yet been defined in human, but in mice aiolos deficiency leads to autoantibody production and B cell lymphomas12, 13.
Production of a properly functioning B cell receptor is essential for development beyond the pre-B cell stage, which is classically defined by the cytoplasmic expression of a complete μ H chain protein. μ H chains in pre-B cell attempt to associate with VpreB and λ14.1 [λ5]. Successful pairing creates a pre-B cell receptor, which signals the termination of further H chain rearrangement (allelic exclusion)14. This is followed by four to six cycles of cell division2; a process associated with a progressive decrease in cell size. Late pre-B daughter cells reactivate RAG1 and RAG2 and begin to undergo VL→JL rearrangement. Successful production of a complete κ or λ light chain permits expression of conventional IgM on the cell surface membrane (mIgM), which identifies the immature B cell. It is at this stage that the antigen specificity of the antibody begins to play the critical role in further development.
An intact and functional B cell antigen receptor (BCR) complex, which consists of the Igα and Igβ co-receptors in association with either the pre-B receptor or mature membrane-bound immunoglobulin (mIg), must be present in order for the developing B cell to survive. Thus loss of function mutations of the μ heavy chain, of the components of the surrogate light chain, or of CD79 all yield a B cell deficient agammaglobulinemia15–18.
The early repertoire appears to be enriched for self-reactive antibodies19. Many of these self reactive cells undergo repeated rounds of light chain rearrangement that lessen, but do not necessarily abolish, the self-specificity of their BCR, a process termed receptor editing20. Other potentially pathogenic self-reactive B cells are inactivated by cell anergy or by apoptosis of the host cell, although the details of this process in human are not yet well described. Self reactivity can also be beneficial and some self-reactive antibodies, especially those enriched for germline sequence, contribute to what is termed the germline natural antibody repertoire21. These antibodies are felt to form the first line of defense against common pathogens and are thus considered to belong to the innate portion of the adaptive immune response22. Some of these antibodies are expressed in the marginal zone of the spleen. In patients with congenital asplenia, this population does not survive, and this line of defense never forms23.
Immature B cells that have successfully produced an acceptable IgM B cell receptor extend transcription of the H chain locus to include the Cδ exons downstream of Cμ. Alternative splicing permits co-production of IgM and IgD. These now newly mature IgM+IgD+ B cells enter the blood and migrate to the periphery where they form the majority of the B cell pool in the spleen and the other secondary lymphoid organs. The IgM and IgD on each of these cells share the same variable domains. The function of IgD remains controversial.
Tyrosine kinases can play key roles in B cell development
It is not enough to simply express a B cell receptor complex, that complex must be able to transduce a signal into the cell. Unsurprisingly, loss of function mutations in components of the signal transduction cascade also inhibit B cell development. X-linked agammaglobulinemia (XLA), the first genetic form of B cell immune deficiency to be recognized, serves as the classic example of the need for an intact signal cascade through the phospholipase Cγ (PLCγ) pathway. Mutations in Bruton’s tyrosine kinase (BTK) are the genetic basis of XLA and account for 85% of patients24. BTK, a member of TEC family of cytoplasmic tyrosine kinases, is part of the BCR and pre-BCR signal transduction pathway25. The B cell linker protein (BLNK) is a SRC homology 2 (SH2) domain-containing signal transduction adaptor. When phosphorylated by SYK, BLNK serves as a scaffold for the assembly of cell activation targets that include GRB2, VAV, NCK, and PLCγ26, 27. The SH2 domains of activated BTK and PLCγ2 bind to the scaffold protein BLNK, allowing BTK to phosphorylate PLCγ2. This leads to activation of other kinases, resulting in calcium influx and also activation of several transcription factors, which is essential for the B lineage development and function28–30. Both BTK and BLNK deficiency result in the arrest of B cell development at the pre-B cell stage31,32, yielding an agammaglobulinemic state.
Cell surface antigens associated with B cell development
Immunoglobulin is poorly or not expressed on the cell surface prior to the immature B cell stage; and after that stage immunoglobulin is expressed throughout B cell development until the plasma cell stage. Hence, early and late stages of development are typically identified through the analysis of the surface expression of other cell surface markers. Of these, CD10, CD19, CD20, CD21, CD24, CD34, and CD38 are of particular importance (Figure 1) and assessment of their expression is often used clinically to either identify specific functionality or as a target for clinical intervention.
CD34 is expressed on a small population (1–4%) of bone marrow cells that includes hematopoietic stem cells. It is a highly glycosylated Type I transmembrane glycoprotein that binds to CD62L (L-selectin) and CD62E (E-selectin) that likely aids in cell trafficking. CD34 expression is often used to assess, identify, or extract hematopoietic stem cells from cord blood or bone marrow.
CD10 is expressed just prior to initiation of DJ rearrangement. It is also known as neprilysin, neutroendopeptidase, or the common acute lymphocytic leukemia antigen (CALLA). CD10 is a Type II membrane glycoprotein metalloprotease that is thought to down-regulate cellular responses to peptide hormones and cytokines by virtue of its protease activity. Because of its early expression pattern, CD10 is often used as a marker for pre-B acute lymphocytic leukemias and for certain lymphomas33.
CD19 is a cell-surface glycoprotein of the immunoglobulin superfamily. It is exclusively expressed throughout B cell development from the pro-B cell stage up to, but not including, the plasma cell stage (Figure 1)34. This broad expression pattern makes it the best cell surface marker for enumerating B cells in clinical studies. Internally, CD19 associates with PI-3 kinase and the tyrosine kinase VAV.
CD19 exists in a complex with CD21 (complement receptor 2: CDR2), CD81 (TAPA-1) and Leu 13. CD21 (complement receptor 2: CR2) is a cell surface protein that contains a small cytoplasmic domain and an extracellular domain consisting of a series of short consensus repeats termed complement control protein (CCP) domains. These extracellular domains can bind three different products of complement C3 cleavage, iC3b, C3dg, and C3d. With the help of CD21, CD19 can bind the complement C3 cleavage product C3d. The simultaneous binding of sIgM and CD19 to a C3d-antigen complex enables CD19 and the BCR to interact and thereby provide a link between innate and adaptive immune responses. EBV can use CD21 as a receptor, allowing entry into mature B cells. Once integrated into the cell, EBV gene products transform the cells permitting creation of long-lived B cell lines. These lines are an excellent source of replenishable DNA or monoclonal human immunoglobulins.
CD19 is not essential for transduction to occur, thus it is not required for B cell production. However, it is required for appropriate modulation of that signal. In the absence of CD19, full activation and maturation of the B cell is inhibited, yielding panhypogammaglobulinemia in the presence of normal numbers of B cells in the blood35. This phenotype is characteristic common variable immune deficiency (CVID), the most common phenotype of antibody deficiency under the care of the clinical immunologist; although CD19 deficiency contributes to only a small subset of patients with CVID.
CD20 appears to function as a B cell Ca2+ channel subunit and regulates cell cycle progression. It is a member of the CD20/FcεRIβ superfamily of leukocyte surface antigens. CD20 can interact directly with MHC Class I and II molecules, as well as members of another family of four transmembrane domain proteins known as the TM4SF, e.g., CD43, CD81, and CD82. It also appears to interact indirectly with LYN, FYN, and LCK and can thus also modulate signal transduction in B cells36. Deficiency of CD20 can lead to hypogammaglobulinemia in the presence of normal B cell numbers, and an inability to mount anti-polysaccharide responses37.
The pattern of expression of CD20, present on mature B cells through plasmablasts but not on early stem cells or on plasma cells, makes it an outstanding target for B cell ablative therapy. Antibodies directed against CD20, such as Rituximab, effectively reduce B-cell numbers without affecting the ability to produce new B cells or inhibiting the production of protective antibodies by long-lived plasma cells. While originally developed to treat lymphoma, it is being used successfully for the treatment of rheumatoid arthritis and other autoimmune diseases38.
CD24 is a GPI-linked sialoprotein that serves as a ligand for P-selectin (CD62P). It is expressed on progenitor, immature, and mature B cells. Its expression decreases in activated B cells and is lost entirely in plasma cells. This cell surface marker can be used to characterize the state of B cell development for diagnostic purposes.
CD38 is first expressed on pre-B cells, but is down regulated in immature and mature B cells. It is a bifunctional enzyme that can synthesize cyclic ADP-ribose (cADPR) from nicotinamide adenine dinucleotide (NAD+) and also hydrolyze cADPR to ADP-ribose. It is presumed that the enzyme exists to ADP-ribosylate target molecules. CD38 is re-expressed in activated B cells and early plasma cells; but its expression is again lost in mature plasma cells. This pattern of expression makes CD38 an excellent marker to identify B cells from the blood or lymphoid organs that are activated or proceeding to the plasmablast stage. CD38 expression has also been used to distinguish between aggressive and more benign forms of chronic lymphocytic leukemia (CLL)39.
BAFF and APRIL can play key roles in the development of mature B cells
B cells leave the bone marrow while still undergoing initial maturation, demonstrating progressively higher levels of IgD expression with a commensurate lowering of IgM. They need the splenic environment in order to complete this maturation process. Immigrant splenic maturing B cells pass through a series of transitional stages, which balance responses between B cell activating factor of the tumor necrosis family (BAFF) and its receptor, BAFF-R; with activation of the BCR by self-antigen. Only a minority of newly arisen cells will successfully make this transition. Death signals triggered through interaction of the B cell receptor with self antigen may be counter balanced by stimulation of BAFF-R, which is expressed primarily on B cells40. BAFF-R activation enhances expression of survival factors such as Bcl-2 and at the same time down regulates pro apoptotic factors.
BAFF and a second TNF family member APRIL (a proliferation-inducing ligand) can also induce isotype switching in naive human B cells41. APRIL, as well as BAFF, can bind to B-cell maturation antigen (BCMA) and transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI), both of which are members of the TNF-R family. BCMA is exclusively expressed on B cells, whereas TACI is expressed on B cells and activated T cells.
The importance of BAFF-R and TACI for the long term survival of B cells is underscored by the effect of loss of function or altered function mutations, which promote the development of the phenotypes of selective IgA deficiency or panhypogammaglobulinemia with normal numbers of newly arisen B cells (CVID).
B cell development in the periphery
The life span of mature B cells expressing surface IgM and IgD appears entirely dependent on exposure to antigen. Mature unstimulated cells may live for only days or weeks. Antigens from peripheral sites are transported into the secondary lymphoid organs by dendritic cells, macrophages, and other highly specialized cells transport of entry. Circulating lymphocytes within these organs then survey the antigens. As originally postulated by Burnet´s "clonal selection" theory, B cells are rescued from apoptosis by their response to a cognate antigen. The reaction to antigen leads to activation, which may then be followed by diversification.
The Spleen
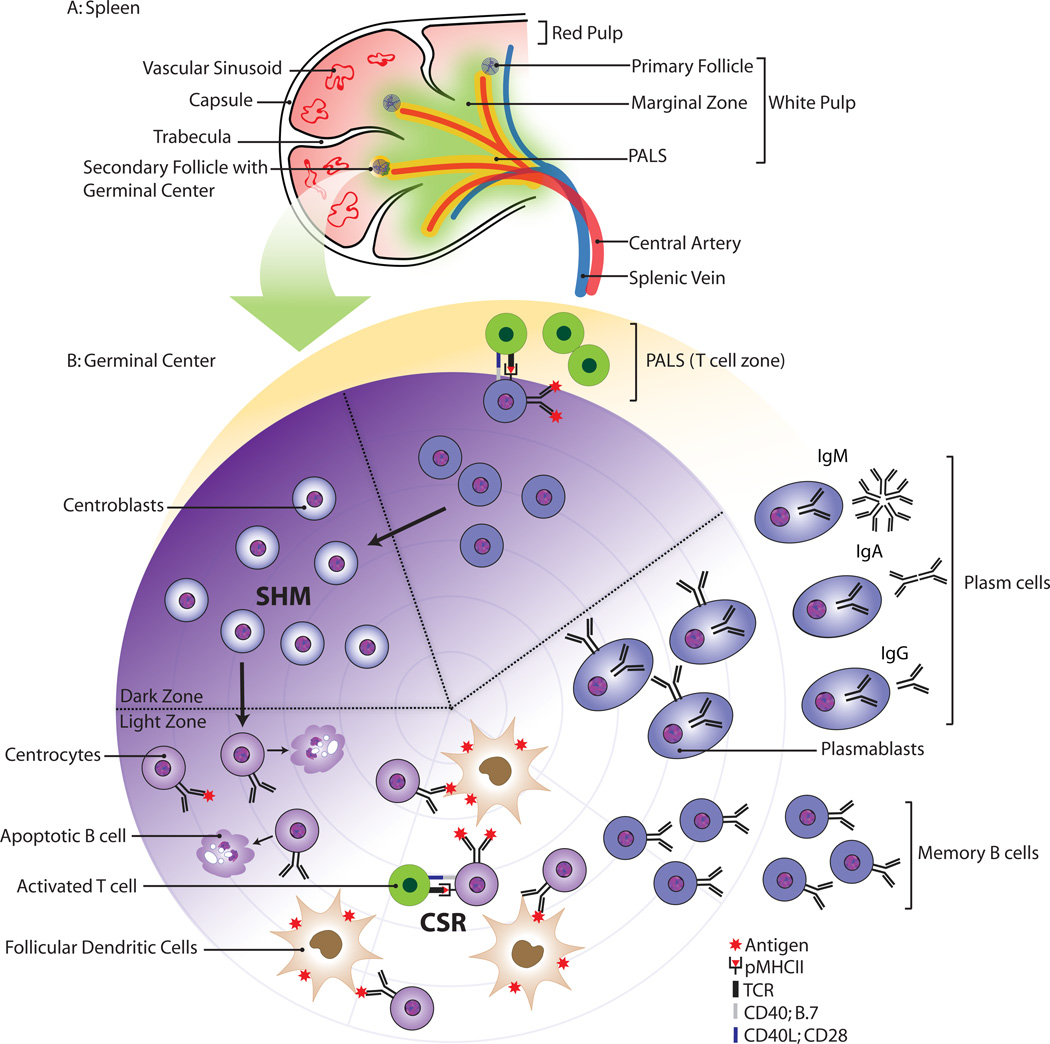
In the secondary lymphoid organs, T cells and B cells are segregated into clearly defined areas. Figure 2 illustrates the pattern observed in the white pulp of the spleen. It is in these areas where the antigen-dependent B cell activation occurs and where the cells subsequently undergo further differentiation. The marginal sinuses, the site of entry for lymphocytes, macrophages, and dendritic cells, separate the white pulp from the red pulp. These sinuses are lined with a mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expressing endothelium. In the marginal sinuses, one finds a specialized layer of metallophilic macrophages that are thought to control the entry of antigen into the white pulp.
Figure 2.
Schematic morphology of the spleen (A) and the germinal center reaction (B). The white pulp consists of a central artery surrounded by T cells; the marginal zone and the follicles. Antigen-specific B cells and T cells interact at the border of the follicle. The B cells differentiate into centroblasts and undergo clonal expansion in the germinal center dark zone.
Follicular dendritic cells (FDC) are highly specialized cells that present antigen to B cells. In contrast to other types of dendritic cells, FDC do not process antigen. Instead, FDC have abundant complement receptors and Ig Fc receptors that allow accumulation of antigen in the form of immune complexes within the B follicle. FDC are crucial for B cell maintenance as well as for their activation and differentiation.
Mature B cells are not homogenous. Functionally and developmentally distinct subsets exist. In the spleen, follicular B cell have a key role in the adaptive immune response, whereas marginal zone B cells are now seen as major players at the interface between the initial innate immune response and the delayed adaptive response42. The ability of MZ B cells to rapidly respond to encapsulated bacteria by differentiating into antigen specific plasma cells helps keep such infections under control. MZ B cells take time to develop and the marginal zone is not fully populated until after the age of two. Asplenic patients have difficulty producing normal numbers of marginal zone B cells,23 and a poor response to blood borne infections can be observed43.
T Independent Responses
The nature of the activation process is critical. Unlike T cells, which require presentation of antigen by other cells, B cells can respond directly to an antigen as long as that antigen is able to cross-link the antibodies on the B cell surface. Such antigens, especially those that by nature cannot be recognized by T cells (e.g., DNA or polysaccharides) can induce a B cell response independent of T cell help. T independent responses can lead to class switching, depending on the cytokine milieu. For example, anti-polysaccharide responses are typically linked to production of IgG2. B cells that are activated by antigen alone do not take part in a germinal center reaction.
Clinical evaluation of the ability of the patient to produce IgM T independent responses can be made by assessing serum isohemagglutinin titers, since the sugars that define blood type A and B are also found on bacteria. Vaccination with 23 valent Pneumovax with a subsequent comparison of pre and post vaccination titers to pneumococcal polysaccharides permits assessment of the ability to produce T independent IgG antibodies.
T Dependent Responses
Binding to a cognate antigen activates antigen specific B cells and T cells. Activated B cells express both MHC class I and class II molecules on their cell surface. They can thus present both intracellular and extracellular antigens to CD4 T helper and CD8 T cytotoxic lymphocytes. Their role as antigen presenting cells is enhanced when they present peptides from the same antigen they have taken up with their antibodies. Cognate recognition of the same antigen by both a B cell and a T cell permits each of these cells to reciprocally activate the other. In particular, T cell activated B cells express the costimulatory molecules CD80 and CD86, which are in turn required for activation of T cells via CD28 as well as their inactivation by CD152 (CTLA-4). Since B cells do not express interleukin-12, they do not induce expression of IFN-γ in the activated T cells but rather favor the differentiation of activated T cells into IL-4, IL-5, IL-10, and IL-13 expressing Th2 cells. These cytokines can support the CD40 induced expansion of memory B cells (IL-4), OX40 or CD27 induced differentiation into short-lived and long-lived plasma cells (IL-10), and CD40 induced class switch recombination (CSR) to IgG4 or IgE (IL-4). Co-stimulation also permits T helper cells to induce somatic hypermutation (SHM), permitting affinity maturation44.
Germinal Centers
The germinal center (GC) is the micro-environment where affinity maturation of the humoral immune response and class switching takes place [reviewed in45,46]. Germinal centers develop only after T cell-dependent activation of B cells. Their full function is dependent on the interaction between CD40 expressed on B cells and CD40L (CD154) expressed on activated T cells. Patients with loss of function mutations in CD40L have high serum levels of IgM and suffer from recurrent infections (Hyper-IgM syndrome)47. And, following the common pattern, disruption of normal regulation through CD40:CD40L, including through its signal transduction cascade (e.g. NEMO deficiency), permits the production of pathogenic IgM autoantibodies that are frequently directed against hematopoetic cells including red blood cells, white blood cells, and platelets.
It takes about a week for the complex GC structure to develop in a primary immune response. After activation of antigen specific B cells and T cells, small clusters of proliferating B cells are observed at the border of the T cell zone and the primary B cell follicle48. The rapidly expanding antigen-activated B cell clone seems to push the naive B cells towards the edge of the primary follicle. Naive B cells form a mantle zone around the newly developing GC, and the primary follicle changes into a secondary follicle containing within it a network of follicular dendritic cells and follicular T cells. About the second week after immunization, the GC matures into a classical structure that contains a dark zone and a light zone. In the fully developed GC, dividing cells are termed centroblasts, whereas differentiating cells within the FDC network are termed centrocytes (Figure 2b).
In the dark zone, proliferating B cells induce somatic hypermutation of their immunoglobulin variable domains, consisting of single nucleotide changes or microdeletions49. By chance one or more of these mutations may give rise to a receptor with altered affinity for the stimulating antigen, creating a clone of variants expressing antigen receptors of various affinities. B cells expressing higher affinity receptors are presumed to compete more effectively for the antigen, which becomes rate limiting. B cells can move from the dark zone to the light zone and back again50. In the light zone, B cells can undergo class switching.
Only the few B cells with a high affinity receptor for the antigen ultimately survive. The rest of the B cell variants die by apoptosis, presumably due to the lack of sufficient stimulation through the B cell antigen receptor complex. In the light zone, surrounded by a network of FDC, the B cells can differentiate into plasma cells or memory cells (Figure 2b).
Somatic hypermutation (SHM) and class switch recombination (CSR)
Immunoglobulin SHM and CSR are essential mechanisms for the generation of a high affinity, adaptive humoral immune response. They allow the generation of effector plasma cells secreting high affinity IgG, IgA, and IgE antibodies.
Hypermutation occurs only during a narrow window in B cell development. The mechanism is induced during B cell proliferation within the GC dark zone51. With a high rate of about 10−3 to 10−4 / base pair / generation, single nucleotide exchanges are introduced in a stepwise manner into the rearranged V-region and its 3´ and 5´ flanking sequences. Mutations are randomly introduced, although there is a preference for transitions (cytidine → thymidine or adenosine → guanine) over transversions. Analysis of the pattern of somatic mutations has revealed that the sequence of the complementarity determining regions (CDRs), the loops that form the antigen binding site, have been selected to form mutation hot spots.
B cells can switch from expression of their VHDJH-exon with Cμ to expression of the same VHDJH-exon with any of the downstream CH genes (e.g., Cα1,2, Cγ1,2,3,4, or Cε) in a process known as class switching. Class switching, like somatic hypermutation, is a hallmark of B cell activation. It can be induced by T cell-independent signals (e.g., LPS) or by signals derived from T cells (e.g., CD40L). CD40L-deficient humans (X-linked hyper IgM syndrome) are severely impaired in the expression of Ig classes other than IgM47.
The choice of CH gene targeted for switch recombination in a particular B cell appears to be dependent on external cytokine signals, e.g., from T cells. IFN-γ targets CSR to IgG2, IL-4 to IgG4 and IgE, and transforming growth factor-β (TGF-β) to IgA.
Both molecular mechanisms, CSR and SHM are dependent on an activation-induced cytidine deaminase (AID)52,53, 54 and uracil-DNA glycosylase (UNG). AID converts Cytidine nucleotides into Uracil. UNG removes the uracil, permitting its replacement with anyone of the four nucleotides, facilitating somatic hypermutation. AID activity at sites of runs of cytidine, such as the switch regions upstream of individual heavy chain genes, followed by UNG elimination of the resultant runs of uracil allow the DNA double helix to fray, permitting the double stranded breaks necessary to allow class switching. Patients with homozygous AID or UNG loss-of-function mutations present with a Hyper-IgM syndrome55; but one that is not associated with the production of autoantibodies.
Both mechanisms, SHM and CSR, have to be tightly controlled, since the introduction of mutations and double strand breaks into the DNA can not only pose a risk to the longevity of the B cell, but also lead to activating translocations and mutations of oncogenes56. For example, for Burkitt lymphoma cells and for plasma cell derived myeloma cells the translocation and ectopic expression of the c-myc gene is an apparent consequence of abnormal SHM and CSR.
B cell memory
Plasma cells
One of the key features of the immune system is immunological memory for antigens encountered in the past. Immunological memory of B lymphocytes is dependent on T helper lymphocytes. While primary B cell responses start with secreted low affinity IgM antibodies after a lag-phase of 1 – 2 days and only gradually develop high affinity antibodies of other classes, secondary responses start faster and with high affinity antibodies of IgM and other classes. This protective humoral memory is provided by long-lived plasma cells57, which are generated in the secondary lymphoid organs and then migrate to the bone marrow or to a site affected by inflammation. In the bone marrow, they can survive for long periods, providing long term protection against previous activating antigens.
Memory B cells
Memory B cells are derived from naive B cells activated by antigen and T cell help in extrafollicular or germinal center reactions (Figure 2b). The differentiation to memory B cells is critically dependent on CD40 of the B cell and CD40L expressed by T cells and follicular dendritic cells. Inactivation of the CD40 or CD40L genes by targeted mutation in the murine germline or by accidental mutation in humans leads to a hyper-IgM syndrome and a general lack of B cell memory58.
In human, CD27 expression is often used as a marker of the memory B cell phenotype. Three types of CD27+ memory B cells have been identified in the circulation: IgM+IgD−CD27+ IgM producing memory B cells, IgM−IgD−CD27+ class switched memory B cells, and IgM+IgD+CD27+ marginal zone-like memory B cells59. This latter population has been shown to produce anti-polysaccharide antibodies. Recently it has been shown that some memory B cells may not express CD2760, but instead express Fc receptor-like 4 (FCRL4)61, 62 complicating the analysis of this critical B cell subset.
Local production of immunoglobulin in the mucosal tissues
The gastrointestinal and respiratory mucosal epithelia, which are constantly exposed to external environment, are sites of complex interactions between the body microorganisms ranging from commensal bacteria to parasites. Proper defense appears to require the local production of immunoglobulin, especially IgA and IgE. Although serum IgA concentration is a fraction of the level of IgG, local mucosal production of IgA make it most abundant immunoglobulin isotype in the body, comprising at least 70% of the total immunoglobulin produced.63 Indeed,approximately 80% of IgA secreting cells reside in the gut mucosa. The mechanisms that regulate the preferential migration of IgA producing cells to the gut and other mucosal surfaces, such as mammary glands of lactating woman, salivary glands and alveolar tract, are an active area of investigation. Special adhesion molecules and local environment appear to be contributing elements64.
In the absence of parasites, local IgE production in the epithelia and mucosa often permits allergic reactions. Local restriction to the mucosal tissues, at sites of exposure to antigen, reduces the likelihood of systemic anaphylaxis65.
Development of ectopic lymphoid tissue in autoimmune disease
In immune diseases such as myasthenia gravis, Sjögren´s Syndrome, Hashimoto´s thyroiditis, or rheumatoid arthritis, ectopic lymphoid tissue may develop in the affected tissue or organ66, 67. The presence of proinflammatory cytokines may support the development of additional lymphoid tissue. In some patients well-organized, large lymphocyte structures may develop that are similar in appearance to the lymphoid follicles seen in the secondary lymphoid organs. At the center of these cell clusters one finds a network of FDC. Antigen presented by the FDC appears to activate the B cells, which induces proliferation. A layer of T cells, which may support B cell differentiation into plasma cells, surrounds the central B cells. There are indications that normal processes of antibody repertoire selection may not work properly within such ectopic lymphoid tissue, contributing to the production of potentially hazardous antibodies. This and other questions remain active topics of investigation.
Concluding remarks
The study of B cell development has led to a greater understanding of the mechanisms that underlie the pathogenesis of B cell immune deficiencies as well as to the development of reagents, such as antibodies directed against critical cell surface molecules, that can be used for the diagnosis or the treatment of immune disorders. Conversely, the study of immune deficiencies has yielded greater understanding of the mechanisms that underlie normal development of the humoral immune response and the recognition that B cell immune deficiencies are largely the consequence of abnormal B cell development. Although the molecular nature of the majority of known B cell immune deficiencies have now been defined, the molecular nature of the most common B cell immune deficiencies, e.g. selective IgA deficiency, symptomatic IgG subclass deficiencies, selective deficits in the ability to respond to bacterial polysaccharides, and common variable immune deficiency, remain unknown. Based on past history, it is likely that the dynamic of twin investigations into normal development and development in disease will ultimately yield the answers to these remaining clinical conundrums and permit the development of new diagnostic tools and therapeutic agents.
What do we know?
The anatomic sites of early B cell development and the basic mechanisms that permit rearrangement of immunoglobulin genes and production of soluble antibody
The molecular basis of most Mendelian immune deficiency disorders
The molecular basis of basic BCR signal transduction pathways
What is still unknown?
The signals that control early B cell development in human
The signals that control the migration and activation of IgA and IgE producing cells in the mucosa, and the development of marginal zone B cells
The mechanisms that underlie the development of most cases of common variable immune deficiency
The mechanisms that underlie most selective anti-polysaccharide deficiencies
The mechanisms that regulate the composition of the natural antibody repertoire
The mechanisms that determine which B cells will be permitted to form long lived plasma cells and which will form memory B cells
The non-genetic (environmental?) mechanisms that predispose to the development of common variable immune deficiency and hypogammaglobulinemia
The mechanisms that trigger autoimmunity in immune deficient hosts
Acknowledgments
Funding: This work is supported, in part, by NIH AI48115, AI078449, and AI079741.
Abbreviations
- AID
activation-induced cytidine deaminase
- AIHA
autoimmune hemolytic anemia
- APRIL
a proliferation-inducing ligand
- AR
allergic rhinitis
- AT
autoimmune thyroid disease
- BAFF
B cell activating factor of the tumor necrosis family
- BAFF-R
B cell activating factor of the tumor necrosis family receptor
- BCMA
B cell maturation antigen
- BCR
B cell receptor
- BLNK
B cell linker protein
- BTK
Bruton tyrosine kinase
- CALLA
common acute lymphocytic leukemia antigen
- CCP
complement control protein
- CD
Celiac disease
- CD40L
CD40 ligand
- CDR
complementarity determining region
- CLP
common lymphoid progenitor
- CR2
complement receptor 2
- CSR
class switch recombination
- CVID
common variable immune deficiency
- EBV
Epstein-Barr Virus
- FCRL4
Fc receptor-like 4
- FDC
follicular dendritic cell
- GC
germinal center
- H chain
immunoglobulin heavy chain
- IDDM
insulin dependent diabetes melllitus
- IgA
Immunoglobulin A
- IgD
immunoglobulin D
- IgE
immunoglobulin E
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- IL-10
interleukin 10
- IL-4
interleukin 4
- IL-7
interleukin 7
- ITP
idiopathic thrombocytopenic purpura
- LMPP
lymphoid primed multipotent progenitor
- MAdCAM-1
mucosal addressin cell adhesion molecule 1
- MHC
major histocompatibility complex
- MZ
marginal zone
- NK
natural killer
- PLCγ
phospholipase Cγ
- RA
rheumatoid arthritis
- RAG
rearrangement associated gene
- SH2
SRC homology 2 domain
- SHM
somatic hypermutation
- SLE
systemic lupus erythematosus
- TACI
transmembrane activator and calcium-modulator and cyclophilin ligand interactor
- TdT
terminal deoxynucleotidyl transferase
- TGF-β
transforming growth factor-β
- TNF
tumor recrosis family
- TNF-R
tumor necrosis family receptor
- UNG
uracil-DNA glycosylase
- XLA
X-linked agammaglobulinemia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andre M Vale, Division of Clinical Immunology and Rheumatology, Department of Medicine, University of Alabama at Birmingham, SHEL 176, 1530 3rd Avenue South, Birmingham, AL 35294-2182, avale@uab.edu, TEL: (205) 934-6826, FAX: (205) 975-6352.
Harry W Schroeder, Jr, Division of Clinical Immunology and Rheumatology, Departments of Medicine, Microbiology, and Genetics, University of Alabama at Birmingham, SHEL 176, 1530 3rd Avenue South, Birmingham, AL 35294-2182, hwsj@uab.edu, TEL: (205) 934-6826, FAX: (205) 975-6352.
References
- 1.Tavian M, Robin C, Coulombel L, Peault B. The human embryo, but not its yolk sac generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15:487–495. doi: 10.1016/s1074-7613(01)00193-5. [DOI] [PubMed] [Google Scholar]
- 2.Matthias P, Rolink AG. Transcriptional networks in developing and mature B cells. Nat Rev Immunol. 2005;5:497–508. doi: 10.1038/nri1633. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6:107–116. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 4.Nunez C, Nishimoto N, Gartland GL, Billips LG, Burrows PD, Kubagawa H, et al. B cells are generated throughout life in humans. J Immunol. 1996;156:866–872. [PubMed] [Google Scholar]
- 5.Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Semin Immunol. 2006;18:20–30. doi: 10.1016/j.smim.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 6.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBien TW. Fates of human B-cell precursors. Blood. 2000;96:9–23. [PubMed] [Google Scholar]
- 8.Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, et al. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124:1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinh LA, Ferrini R, Cobb BS, Weinmann AS, Hahm K, Ernst P, et al. Down-regulation of TDT transcription in CD4(+)CD8(+) thymocytes by Ikaros proteins in direct competition with an Ets activator. Genes Dev. 2001;15:1817–1832. doi: 10.1101/gad.905601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabbattini P, Lundgren M, Georgiou A, Chow C, Warnes G, Dillon N. Binding of Ikaros to the lambda5 promoter silences transcription through a mechanism that does not require heterochromatin formation. EMBO J. 2001;20:2812–2822. doi: 10.1093/emboj/20.11.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothenberg EV, Telfer JC, Anderson MK. Transcriptional regulation of lymphocyte lineage commitment. Bioessays. 1999;21:726–742. doi: 10.1002/(SICI)1521-1878(199909)21:9<726::AID-BIES4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Wang JH, Avitahl N, Cariappa A, Friedrich C, Ikeda T, Renold A, et al. Aiolos regulates B cell activation and maturation to effector state. Immunity. 1998;9:543–553. doi: 10.1016/s1074-7613(00)80637-8. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Matthias G, Mihatsch MJ, Georgopoulos K, Matthias P. Lack of the transcriptional coactivator OBF-1 prevents the development of systemic lupus erythematosus-like phenotypes in Aiolos mutant mice. J Immunol. 2003;170:1699–1706. doi: 10.4049/jimmunol.170.4.1699. [DOI] [PubMed] [Google Scholar]
- 14.Loffert D, Ehlich A, Muller W, Rajewsky K. Surrogate light chain expression is required to establish immunoglobulin heavy chain allelic exclusion during early B cell development. Immunity. 1996;4:133–144. doi: 10.1016/s1074-7613(00)80678-0. [DOI] [PubMed] [Google Scholar]
- 15.Yel L, Minegishi Y, Coustan-Smith E, Buckley RH, Trubel H, Pachman LM, et al. Mutations in the mu heavy-chain gene in patients with agammaglobulinemia. N Engl J Med. 1996;335:1486–1493. doi: 10.1056/NEJM199611143352003. [DOI] [PubMed] [Google Scholar]
- 16.Minegishi Y, Coustan-Smith E, Wang YH, Cooper MD, Campana D, Conley ME. Mutations in the human lambda5/14.1 gene result in B cell deficiency and agammaglobulinemia. J Exp Med. 1998;187:71–77. doi: 10.1084/jem.187.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Kanegane H, Sanal O, Tezcan I, Ersoy F, Futatani T, et al. Novel Igalpha (CD79a) gene mutation in a Turkish patient with B cell-deficient agammaglobulinemia. Am J Med Genet. 2002;108:333–336. doi: 10.1002/ajmg.10296. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari S, Lougaris V, Caraffi S, Zuntini R, Yang J, Soresina A, et al. Mutations of the Igbeta gene cause agammaglobulinemia in man. J Exp Med. 2007;204:2047–2051. doi: 10.1084/jem.20070264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 20.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 22.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 23.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 24.Conley ME, Mathias D, Treadaway J, Minegishi Y, Rohrer J. Mutations in btk in patients with presumed X-linked agammaglobulinemia. Am J Hum Genet. 1998;62:1034–1043. doi: 10.1086/301828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki Y, Isselbacher KJ, Pillai S. Bruton tyrosine kinase is tyrosine phosphorylated and activated in pre-B lymphocytes and receptor-ligated B cells. Proc Natl Acad Sci U S A. 1994;91:10606–10609. doi: 10.1073/pnas.91.22.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 27.Ishiai M, Sugawara H, Kurosaki M, Kurosaki T. Cutting edge: association of phospholipase C-gamma 2 Src homology 2 domains with BLNK is critical for B cell antigen receptor signaling. J Immunol. 1999;163:1746–1749. [PubMed] [Google Scholar]
- 28.Rawlings DJ. Bruton's tyrosine kinase controls a sustained calcium signal essential for B lineage development and function. Clin Immunol. 1999;91:243–253. doi: 10.1006/clim.1999.4732. [DOI] [PubMed] [Google Scholar]
- 29.Webb CF, Yamashita Y, Ayers N, Evetts S, Paulin Y, Conley ME, et al. The transcription factor Bright associates with Bruton's tyrosine kinase, the defective protein in immunodeficiency disease. J Immunol. 2000;165:6956–6965. doi: 10.4049/jimmunol.165.12.6956. [DOI] [PubMed] [Google Scholar]
- 30.Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 31.Conley ME, Cooper MD. Genetic basis of abnormal B cell development. Curr Opin Immunol. 1998;10:399–406. doi: 10.1016/s0952-7915(98)80112-x. [DOI] [PubMed] [Google Scholar]
- 32.Minegishi Y, Rohrer J, Coustan-Smith E, Lederman HM, Pappu R, Campana D, et al. An essential role for BLNK in human B cell development. Science. 1999;286:1954–1957. doi: 10.1126/science.286.5446.1954. [DOI] [PubMed] [Google Scholar]
- 33.LeBien TW, McCormack RT. The common acute lymphoblastic leukemia antigen (CD10)--emancipation from a functional enigma. Blood. 1989;73:625–635. [PubMed] [Google Scholar]
- 34.Haas KM, Tedder TF. Role of the CD19 and CD21/35 receptor complex in innate immunity, host defense and autoimmunity. Adv Exp Med Biol. 2005;560:125–139. doi: 10.1007/0-387-24180-9_16. [DOI] [PubMed] [Google Scholar]
- 35.van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354:1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 36.Popoff IJ, Savage JA, Blake J, Johnson P, Deans JP. The association between CD20 and Src-family Tyrosine kinases requires an additional factor. Mol Immunol. 1998;35:207–214. doi: 10.1016/s0161-5890(98)00042-x. [DOI] [PubMed] [Google Scholar]
- 37.Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, et al. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 120:214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 39.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 40.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 41.Castigli E, Geha RS. Molecular basis of common variable immunodeficiency. J Allergy Clin Immunol. 2006;117:740–746. doi: 10.1016/j.jaci.2006.01.038. quiz 7. [DOI] [PubMed] [Google Scholar]
- 42.Lopes-Carvalho T, Foote J, Kearney JF. Marginal zone B cells in lymphocyte activation and regulation. Curr Opin Immunol. 2005;17:244–250. doi: 10.1016/j.coi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 44.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 45.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 46.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 47.Facchetti F, Appiani C, Salvi L, Levy J, Notarangelo LD. Immunohistologic analysis of ineffective CD40-CD40 ligand interaction in lymphoid tissues from patients with X-linked immunodeficiency with hyper-IgM. Abortive germinal center cell reaction and severe depletion of follicular dendritic cells. J Immunol. 1995;154:6624–6633. [PubMed] [Google Scholar]
- 48.Camacho SA, Kosco-Vilbois MH, Berek C. The dynamic structure of the germinal center. Immunol Today. 1998;19:511–514. doi: 10.1016/s0167-5699(98)01327-9. [DOI] [PubMed] [Google Scholar]
- 49.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 50.Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 51.Wagner SD, Neuberger MS. Somatic hypermutation of immunoglobulin genes. Annu Rev Immunol. 1996;14:441–457. doi: 10.1146/annurev.immunol.14.1.441. [DOI] [PubMed] [Google Scholar]
- 52.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 53.Neuberger MS, Harris RS, Di Noia J, Petersen-Mahrt SK. Immunity through DNA deamination. Trends Biochem Sci. 2003;28:305–312. doi: 10.1016/S0968-0004(03)00111-7. [DOI] [PubMed] [Google Scholar]
- 54.Honjo T, Muramatsu M, Fagarasan S. AID: how does it aid antibody diversity? Immunity. 2004;20:659–668. doi: 10.1016/j.immuni.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 56.Kuppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20:5580–5594. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- 57.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 58.Durandy A, Revy P, Imai K, Fischer A. Hyper-immunoglobulin M syndromes caused by intrinsic B-lymphocyte defects. Immunol Rev. 2005;203:67–79. doi: 10.1111/j.0105-2896.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 59.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fecteau JF, Cote G, Neron S. A new memory CD27−IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol. 2006;177:3728–3736. doi: 10.4049/jimmunol.177.6.3728. [DOI] [PubMed] [Google Scholar]
- 61.Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202:783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ehrhardt GR, Hijikata A, Kitamura H, Ohara O, Wang JY, Cooper MD. Discriminating gene expression profiles of memory B cell subpopulations. J Exp Med. 2008;205:1807–1817. doi: 10.1084/jem.20072682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 64.Fagarasan S, Honjo T. Regulation of IgA synthesis at mucosal surfaces. Curr Opin Immunol. 2004;16:277–283. doi: 10.1016/j.coi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 66.Weyand CM, Klimiuk PA, Goronzy JJ. Heterogeneity of rheumatoid arthritis: from phenotypes to genotypes. Springer Semin Immunopathol. 1998;20:5–22. doi: 10.1007/BF00831996. [DOI] [PubMed] [Google Scholar]
- 67.Kim HJ, Berek C. B cells in rheumatoid arthritis. Arthritis Res. 2000;2:126–131. doi: 10.1186/ar77. [DOI] [PMC free article] [PubMed] [Google Scholar]