Abstract
OBJECTIVES
This study aimed to report on original aortic valve reconstruction for patients on dialysis.
METHODS
Aortic valve reconstruction has been performed on 404 cases from April 2007 through September 2011. Among them, 54 cases on haemodialysis were retrospectively studied. Forty-seven patients had aortic stenosis, 5 had aortic regurgitation (AR), and 2 had infective endocarditis. Mean age was 70.2 ± 8.5 years. There were 35 males and 19 females. There were 27 primary aortic valve reconstructions, 11 with CABG, 6 with ascending aortic replacement, 5 with mitral valve repair and 4 with maze. First, in the procedure, harvested pericardium was treated with 0.6% glutaraldehyde solution. After resecting the cusps, we measured the distance between commissures with original sizing instrument. Then, the pericardium was trimmed with the original template. Three cusps were sutured to each annulus.
RESULTS
Peak pressure gradient averaged to 66.0 ± 28.2 mmHg preoperatively, and decreased to 23.4 ± 10.7, 13.8 ± 5.5 and 13.3 ± 2.3 mmHg, 1 week, 1 year, and 3 years after the operation, respectively. No calcification was detected with echocardiographic follow-up. Recurrence of AR was not recorded with the mean follow-up of 847 days except for 1 case reoperated on for infective endocarditis 2.5 years after the operation. Three hospital deaths were recorded due to non-cardiac causes. Other patients were in good condition. There was no thromboembolic event.
CONCLUSIONS
Medium-term results are excellent. Since warfarin for dialysis patients becomes problematic, a postoperative warfarin-free status is desirable. Aortic valve reconstruction can provide patients with a better quality of life without warfarin.
Keywords: Aortic valve reconstruction, Autologous pericardium, Dialysis
INTRODUCTION
In the past, chronic haemodialysis for end-stage renal disease (ESRD) was considered a contraindication to major cardiac surgery. In 1968, Lansing recorded the first successful valve replacement in a patient on haemodialysis [1]. The longer the life expectancy of dialysis patients becomes, the more patients may likely become candidates for major cardiac surgery [2]. Life expectancy after the initiation of haemodialysis for ESRD patients has reached 11 ± 9 years in Japan. Major reasons for death of patients on dialysis were heart failure, cerebral infarction, cerebral haemorrhage, gastrointestinal bleeding and infection. For the prevention of these factors, we need better heart valve surgery with good haemodynamics and a postoperative warfarin-free condition, and the avoidance of foreign-body implantation as much as possible. In terms of aortic valve surgery for patients on dialysis, prosthetic valve selection is still controversial. Although mechanical prostheses have been preferred before, because of the anxiety regarding calcification of bioprostheses, the bioprosthesis is becoming popular because of the warfarin-related complication of the mechanical prosthesis [3–6]. However, the haemodynamics resulting from the bioprosthesis is still clearly inferior compared with that from the native aortic valve. Moreover, the systemic calcification of dialysis patients is still a serious concern with using a bioprosthesis. In reality, we have not found the ideal surgical treatment of aortic valve disease in haemodialysis patients yet. To seek the ideal surgical treatment for aortic valve disease, our original aortic valve reconstruction using glutaraldehyde-treated autologous pericardium for dialysis patients was reviewed.
METHODS
Our new original aortic valve reconstruction and the clinical study of this procedure were approved by the institutional review board of Toho University Ohashi Medical Center. All patients underwent this operation after written informed consent had been obtained.
Aortic valve reconstruction using glutaraldehyde-treated autologous pericardium was performed on 404 patients from April 2007 through September 2011 [7]. Among them, 54 patients had chronic end-stage renal disease (ESRD) on haemodialysis preoperatively. These 54 patients were retrospectively studied to investigate the adequacy of this new operation for dialysis patients. Among all 54 patients, 48 showed aortic stenosis (AS) including 1 case due to prosthetic valve endocarditis with preoperative echocardiography. Four patients with AS also showed severe aortic regurgitation (AR). On the contrary, 6 patients showed pure AR including 1 due to native valve infective endocarditis. Three patients had congenital bicuspid aortic valves. Preoperative echocardiography revealed that the aortic surgical annular diameter averaged 20.1 ± 2.4 mm. Peak pressure gradient through the aortic valve averaged to 66.0 ± 26.2 mmHg. Mean age of the 54 patients was 70.2 ± 8.5 years (40–83 years old). There were 35 males and 19 females. Patients' characteristics are summarized in Table 1. There were 27 isolated aortic valve reconstructions including 1 case of redo aortic valve surgery for prosthetic valve endocarditis needing patch plasty of sinus of Valsalva perforation. Concomitant procedures included 11 coronary artery bypass graft (CABG) operations, 6 ascending aortic replacements, 6 mitral valve replacements, 5 mitral valve repairs, 4 maze procedures, 3 tricuspid valve annuloplasties, 1 repair of ruptured sinus of Valsalva, and 1 atrial septal defect closure. Many concomitant procedures have been done in combination.
Table 1:
Baseline characteristics of all 54 patients
| Mean ± standard deviation or number (%) | |
|---|---|
| Age (years) | 70.2 ± 8.5 |
| Female gender | 19 (35.2) |
| Diagnosis of aortic stenosis | 48 (88.9) |
| Diagnosis of aortic regurgitation | 10 (18.5) |
| Combined disease of stenosis and regurgitation | 4 (7.4) |
| Echocardiographic surgical annular diameter (mm) | 20.1 ± 2.4 |
| Peak pressure gradient through aortic valve (mmHg) | 66.0 ± 26.2 |
| Echocardiographic grade of aortic regurgitation | 1.31 ± 1.02 |
| Infective endocarditis | 2 (3.7) |
| Congenital bicuspid aortic valve | 3 (5.6) |
| Isolated aortic valve reconstruction | 27 (50.0) |
| Cardiopulmonary bypass time for isolated operation (min) | 156.5 ± 30.6 |
| Aortic cross-clamp time for isolated operation (min) | 113.1 ± 19.0 |
| Operations with concomitant procedures | 27 (50.0) |
Detailed concomitant procedures: CABG = 6, CABG + maze + Asc.Ao.replacement = 1, CABG + MVR = 1, CABG + Asc.Ao. replacement = 1, CABG + MV repair = 1, CABG + MV repair + TAP + ASD closure = 1, Asc.Ao. replacement = 4, MVR = 4, MV repair = 3, MVR + TAP = 1, TAP = 1, maze = 2, Maze + repair of ruptured sinus of Valsalva = 1.
Data are mean ± SD unless otherwise specified.
CABG: coronary artery bypass graft; maze: maze procedure; Asc.Ao.replacement: graft replacement of ascending aorta including hemi-arch aortic replacement; MVR: mitral valve replacement; MV repair: mitral valve repair; TAP: tricuspid valve annuloplasty; ASD closure: closure of atrial septal defect.
The surgical technique of our original aortic valve reconstruction was previously reported [8]. After the cleansing of redundant tissue, autologous pericardium with an area of at least 7 × 8 cm was harvested. Then, it was treated with 0.6% glutaraldehyde solution with a buffer for 10 min. The treated pericardium was rinsed three times in physiological saline solution.
All aortic valve reconstructive procedures were performed during cardioplegic arrest on cardiopulmonary bypass.
Diseased cusps were excised meticulously. In case of severe calcification, Cavitron Ultrasonic Surgical Aspirator (CUSA) (SonoSurg, Olympus, Tokyo, Japan) was very helpful in removing calcium without damaging the annular tissue. Particularly in patients on dialysis, there could be the serious obstacle of aortic wall calcification. CUSA played a great role in ascending aortic replacement in the case of a porcelain aorta. In this case, because of the danger of clamping the ascending aorta, we replaced the ascending aorta during hypothermic circulatory arrest after removal of calcium by CUSA.
The reconstructive procedure was started from the measurement of the distance between each commissure with an original sizing instrument. Correct measurement is important. The new cusp with the size corresponding to the measured value was trimmed with an original template from glutaraldehyde-treated autologous pericardium. Then, the annular margin of the pericardial cusp was sutured continuously with 4–0 monofilament stitches to each annulus. Commissural coaptation was secured with additional 4–0 monofilament stitches. The coaptation of three new cusps was always insured with direct vision under negative pressure made by a left ventricular vent before closure of the aortotomy.
Mean cardiopulmonary bypass time and aortic cross-clamp time with isolated aortic valve reconstruction were 156.5 ± 30.6 min and 113.1 ± 19.0 min, respectively. Operative data are also given in Table 1.
Echocardiographic evaluation was done 1 week, 1 month, 3 months and every 6 months after surgeries. Data are presented as mean ± standard deviation. Survival rate and freedom from reoperation rate were calculated by the Kaplan–Meier method.
RESULTS
No operation was converted to the conventional aortic valve replacement. There were 3 hospital deaths due to non-cardiac cause consisting of pneumonia, enterocolitis, and thrombotic thrombocytopenic purpura. Initial postoperative echocardiographic study of these 3 patients showed good cardiac function with well-working new aortic valves. The mean follow-up period was 28.2 ± 13.7 months. Survival rates were 84.6% at 30 months and 79.6% at 50 months (Fig. 1). The numbers of patients left in the risk group at 30, 40 and 50 months were 21, 9 and 2. Freedom from reoperation rate was 95.2% both at 30 and 50 months. Reoperation was recorded for 1 patient due to infective endocarditis 30 months after surgery. Recurrence of more-than-mild AR was not recorded aside from 1 reoperated patient (Fig. 2). Peak pressure gradient, which had averaged to 66.0 ± 28.2 mmHg preoperatively, decreased to 23.4 ± 10.7, 13.8 ± 5.5 and 13.3 ± 2.3 mmHg 1 week, 1 year and 3 years after the operation (Fig. 3), respectively. No calcification of pericardial cusp was detected with echocardiographic follow-up. All surviving patients were in good condition with New York Heart Association functional class 1. There was no thromboembolic event recorded during the follow-up period while patients received a small dose of daily aspirin. In terms of our original aortic valve reconstruction, we did not provide anticoagulation postoperatively. Anticoagulation with warfarin was employed for patients who had undergone the concomitant procedure including CABG, mitral valve replacement, mitral valve repair and tricuspid valve repair.
Figure 1:
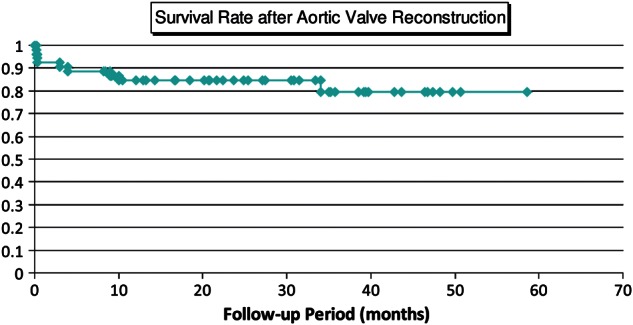
Survival curve after aortic valve reconstruction. Survival rate was 79.6% with 58.6 months follow-up.
Figure 2:
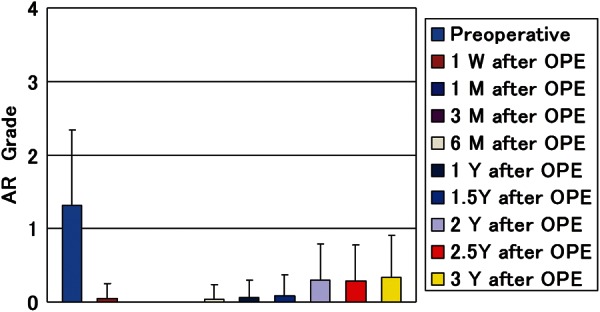
Postoperative follow-up of aortic regurgitation (AR) with echocardiographic evaluation (echocardiographic evaluation at 1 week, 1 month, 3 months, and every 6 months after surgeries).
Figure 3:
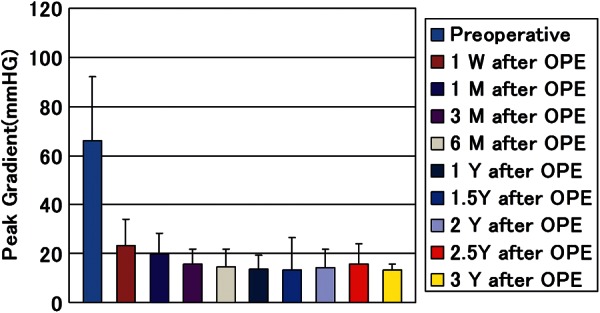
Postoperative follow-up of peak pressure gradient through newly created aortic valve with echocardiographic evaluation (echocardiographic evaluation at 1 week, 1 month, 3 months, and every 6 months after surgeries).
DISCUSSION
Cardiac valve operation for dialysis patients is still challenging. Patients with ESRD requiring heart surgeries have higher risk for operative mortality and morbidity compared with non-ESRD patients [9]. The previous report showed that the mortality rate associated with cardiac valve operation in renal failure patients was ∼10–15% [4–6]. In terms of aortic valve replacement in dialysis patients, one report from Japan showed a hospital mortality of 6.8% and an overall actuarial survival rate at 3 years of 74.6% [10].
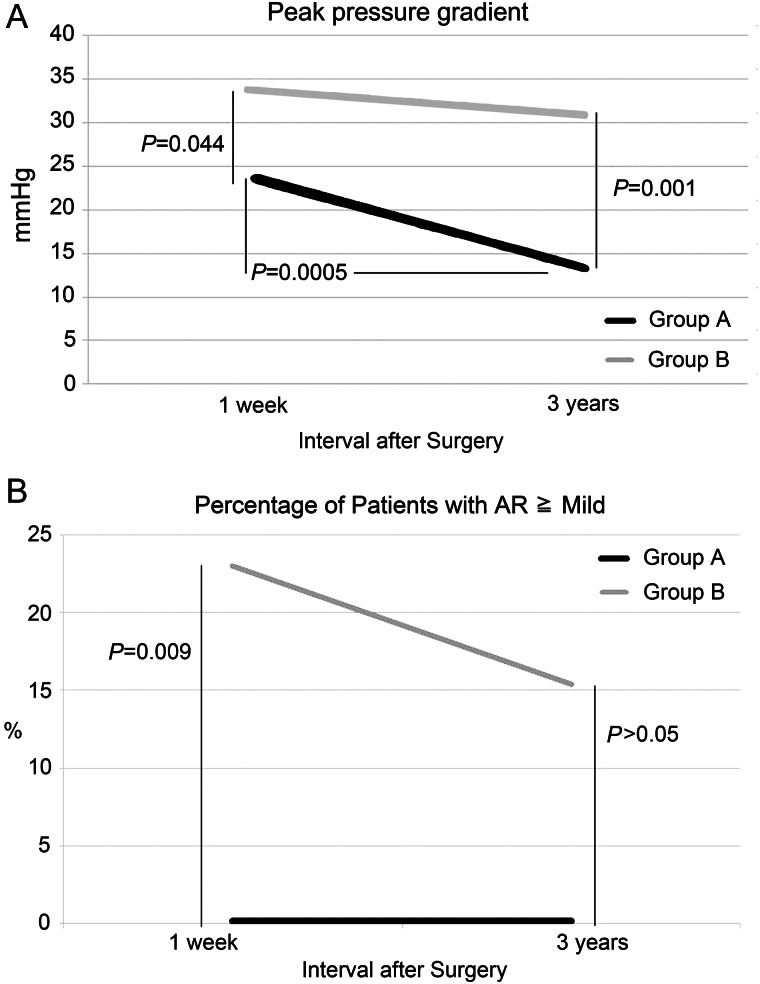
Our current study showed that the in-hospital mortality rate after original aortic valve reconstruction in dialysis patients was 5.6%. Also, all 3 deaths were due to non-cardiac causes. Moreover, postoperative haemodynamics was excellent, with up to 58 months follow-up. Survival rate was 79.6%, and freedom from reoperation rate was 95.2% with 58.6 months follow-up. This result has also been compared with our own data on aortic prosthetic valve replacement cases. From January 2004 through March 2010, 78 conventional aortic valve replacements with prosthetic valves have been performed at our institution. Among them, 13 patients were on dialysis preoperatively. The data on 54 patients in the current study were compared with those of 13 patients on dialysis with prosthetic valve replacement. Age and gender were well matched between the two groups. Survival rate and freedom from reoperation rate did not show significant difference between the two groups. Postoperative haemodynamics was significantly better in the aortic valve reconstruction group than in the conventional prosthetic replacement group (Fig. 4). The short-term and medium-term results of aortic valve reconstruction with glutaraldehyde-treated autologous pericardium were excellent, and this procedure seemed to be feasible as a surgical treatment for aortic valve disease in dialysis patients.
Figure 4:
Comparison of postoperative haemodynamics between aortic valve reconstruction and prosthetic valve replacement. Group A: 54 patients operated by means of original aortic valve reconstruction, Group B: 13 patients operated by means of conventional aortic valve replacements including both bioprosthesis and mechanical prosthesis. (A) Comparison of postoperative peak pressure gradient of aortic valve. (B) Comparison of postoperative aortic regurgitation (AR).
The remaining issue could be the durability of this reconstruction. Some surgeons might question the durability of glutaraldehyde-treated autologous pericardium itself. However, human autologous pericardium has been used in many aspects of cardiac surgery. Human pericardium has been used for patch repair or right ventricular outflow tract reconstruction in congenital heart disease. It has also been used for leaflet extension in mitral valve repair. Chauvaud et al. [11] reported valve extension with glutaraldehyde-preserved autologous pericardium in mitral valve repair. In their series, they found no calcification of autologous pericardium in 64 cases with 6 months to 9 years follow-up. In terms of aortic valve reconstruction or repair, the usage of fresh autologous pericardium has been reported in 1963 [12]. The rather negative findings of early reports, basically due to thickening and retraction, resulted in discarding fresh autologous pericardium from the valve armamentarium. On the contrary, we found that tensile strength of glutaraldehyde-treated human pericardium was four times higher than native aortic valve leaflets [13]. Glutaraldehyde-treated autologous pericardium has been used for the aortic valve by Al Halees et al. [14] in their report of up to 16 years follow-up of aortic valve reconstruction with pericardium. They found that aortic valve reconstruction was feasible with good haemodynamics, low mortality and thromboembolic rate. Moreover, its behaviour at 10 years was comparable to that of stentless aortic valve bioprosthesis. Chan et al. [15] reported truly stentless autologous pericardial aortic valve replacement. Their freedom from structural valve deterioration was 100% with a mean follow-up period of 6.5 years. In our current series, haemodynamics was well kept with less-than-mild regurgitation with up to 58 months follow-up. Moreover, no structural valve deterioration was noticed.
In the haemodialysis patients, renal failure itself is one of the known comorbidities associated with rapid progression of AS. Mao et al. [16] reported a case suggesting that circulating circumstance in ESRD and dialysis could accelerate the progression of prosthetic aortic valve stenosis. Moreover patients on dialysis tended to have ectopic calcification due to hyperphosphatemia from insufficient excretion of phosphorus. Calcification has always been considered as one of the important factors accelerating AS in dialysis patients. On the other hand, warfarin-induced calcification was clearly mentioned in the 2011 Clinical Guidelines for the Evaluation and the Treatment of Cardiovascular Complications in Haemodialysis Patients by the Japanese Society for Dialysis Therapy. Warfarin induces calcification by inhibiting gamma-carboxylation of the matrix Gla protein (MGP) and thereby inactivating the putative calcification-inhibitory activity of the protein [17]. Some authors reported that warfarin might be associated with severity of aortic valve calcification in dialysis patients [18, 19]. After our original aortic valve reconstruction, patients did not need warfarin at all from the very beginning of the postoperative course. In the current series, patients did not basically need warfarin postoperatively, and no calcification was detected with the follow-up echocardiography.
To consider an ideal aortic valve surgery for dialysis patients, we need better heart valve surgery with good haemodynamics to prevent heart failure and a postoperative warfarin-free condition to prevent bleeding or thromboembolic complications, and to avoid foreign body implantation to keep the risk of infection as low as possible. Our original aortic valve reconstruction using glutaraldehyde-treated autologous pericardium had good postoperative haemodynamics, a postoperative warfarin-free status, and no foreign body situation. This warfarin-free status could also prevent warfarin-induced calcification. Hence, this procedure has the possibility of being the treatment of choice for aortic valve disease in dialysis patients.
LIMITATION
This study was an uncontrolled retrospective study with a limited number of patients and limited period of follow-up. To investigate the durability of this operation, longer follow-up studies should be conducted continuously.
CONCLUSIONS
Medium-term results were excellent. Aortic valve reconstruction with glutaraldehyde-treated autologous pericardium could give better quality of life for dialysis patients without warfarin. This operation seemed to be feasible as the surgical treatment for aortic valve disease for dialysis patients. Longer-term follow-up results will be disclosed in the future.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr H. Schäfers (Hamburg/Saar, Germany): One could discuss controversially whether the topic of simply replacing all cusps with autologous pericardium is really aortic valve reconstruction or whether it is not more similar to stentless autologous valve replacement. One needs to keep in mind that the technique is still relatively young, at least in your group, so conclusions need to be drawn carefully. If I remember correctly, you started your activities roughly five years ago, so at this point in time, five-year data are probably of limited value, and we should rather focus on things like two-year freedom from relative complications.
I have two questions specific to your paper. Number one, you mentioned in the overall experience that you had a relevant proportion, roughly 25% to 30% of patients, with aortic regurgitation. I assume that there was a large proportion of patients with deformed but otherwise pliable cusps. How do you deal with that scenario? Do you cut out normal cusp tissue that is simply deformed and replace it with pericardium? And if so, what is the reason?
Second, you pointed out very well the paper by Al Halees and colleagues that showed that the difference between autologous and bovine pericardium over 15 years was actually non-existent, even though different conclusions were drawn from these curves. What is your opinion, does it have to be autologous or would not a simple heterologous pericardial stentless valve replacement do the same?
Dr Kawase: First of all, if there are pliable cusps in the aortic valve, what should we do? In our centre, we are doing this type of reconstruction by the technique of replacement of the three cusps. The bottom line, the basis of the thinking, is different from the usual aortic valve plasty. There are two major points related to our technique. We are measuring the distance between commissures, not the diameter of the annulus. And the second point is that we are independently replacing the three cusps; it's not one piece of material for the aortic valve. I'm not sure whether it should be called reconstruction or replacement, but I think the rationale is different for those two techniques. At some point in the future we can give corroboration, but since it is a different concept, I think it's natural to do our procedure for all the patients, even where there is one cusp that can be preserved.
Dr J. Scott Rankin (Nashville, TN, USA): I'd like to comment on the Al Halees study. Even though the freedom from reoperation was the same with those two tissues, the autologous pericardial patients primarily were reoperated on for annular dilatation, and the autologous pericardium, over the long term, remained pliable, supple and did not calcify. But annular stabilization was a problem and the patients dilated 15 years out and had to be reoperated on for aortic incompetence. In contrast, the bovine pericardial cusps all calcified and the patients experienced terrible inflammatory calcification of the root which was difficult to reoperate on.
So that leads to this paper, and what I believe is a great idea, because if there is one difficult group in valve surgery, it's the dialysis patients. Tissue valves calcify, the patients don't tolerate anticoagulation with mechanical valves, and your approach potentially is a solution, again given more long-term data and follow-up. But the idea is good.
Dr M. Nosal (Bratislava, Slovakia): From published data and even from our own experience, we found that the pericardial extensions are quite prone to infective endocarditis; from the published data, the incidence goes up to 5% of patients. I saw you had only one patient with infective endocarditis coming back, and I wanted to ask you if you use a specific antibiotic prophylaxis protocol in this difficult group of patients?
Dr Kawase: We don't use a specific technique for the prevention of future infection. And I'm not sure of the reason why, even for the 404-case series, we have only two patients with infective endocarditis afterwards. We should look at that in the future.
REFERENCES
- 1.Lansing AM, Leb DE, Berman LB. Cardiovascular surgery in end-stage renal failure. JAMA. 1968;204:682–6. [PubMed] [Google Scholar]
- 2.McGovern E, Rooney R, Neligan MC. Open heart surgery in patients receiving chronic haemodialysis. Thorax. 1984;39:388–9. doi: 10.1136/thx.39.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boning A, Boedeker RH, Rosendahl UP, Niemann B, Haberer S, Roth P, et al. Long-term results of mechanical and biological heart valves in dialysis and non-dialysis patients. Thorac Cardiovasc Surg. 2011;59:454–9. doi: 10.1055/s-0030-1271028. [DOI] [PubMed] [Google Scholar]
- 4.Kaplon RJ, Cosgrove DM, Gillinov AM, Lytle BW, Blackstone EH, Smedira NG. Cardiac valve replacement in patients on dialysis: influence of prosthesis on survival. Ann Thorac Surg. 2000;70:438–41. doi: 10.1016/s0003-4975(00)01544-7. [DOI] [PubMed] [Google Scholar]
- 5.Brinkman WT, Williams WH, Guyton RA, Jones EL, Craver JM. Valve replacement in patients on chronic renal dialysis: implications for valve prosthesis selection. Ann Thorac Surg. 2002;74:37–42. doi: 10.1016/s0003-4975(02)03692-5. [DOI] [PubMed] [Google Scholar]
- 6.Chan V, Jamieson WR, Fleisher AG, Denmark D, Chan F, Germann E. Valve replacement surgery in end-stage renal failure: mechanical prostheses versus bioprostheses. Ann Thorac Surg. 2006;81:857–62. doi: 10.1016/j.athoracsur.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Ozaki S, Kawase I, Yamashita H, Uchida S, Nozawa Y, Matsuyama T, et al. A total of 404 cases of aortic valve reconstruction with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2012.11.012. pii: S0022-5223(12)01389-X. doi: 10.1016/j.jtcvs.2012.11.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Ozaki S, Kawase I, Yamashita H, Uchida S, Nozawa Y, Matsuyama T, et al. Aortic valve reconstruction using self-developed aortic valve plasty system in aortic valve disease. Interact CardioVasc Thorac Surg. 2011;12:550–3. doi: 10.1510/icvts.2010.253682. [DOI] [PubMed] [Google Scholar]
- 9.Cooper WA, O'Brien SM, Thourani VH, Guyton RA, Bridges CR, Szczech LA, et al. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: results from the society of thoracic surgeons national adult cardiac database. Circulation. 2006;113:1063–70. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K, Tajima K, Takami Y, Okada N, Terazawa S, Usui A, et al. Early and late outcomes of aortic valve replacement in dialysis patients. Ann Thorac Surg. 2010;89:65–70. doi: 10.1016/j.athoracsur.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 11.ChauVaud S, Jebara V, Chachques JC, el Asmar B, Mihaileanu S, Perier P, et al. Valve extension with glutaraldehyde-preserved autologous pericardium. Results in mitral valve repair. J Thorac Cardiovasc Surg. 1991;102:171–7. [PubMed] [Google Scholar]
- 12.Ross DN. Surgical reconstruction of the aortic valve. Lancet. 1963;1:571–4. doi: 10.1016/s0140-6736(63)92687-4. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita H, Ozaki S, Iwasaki K, Kawase I, Nozawa Y, Umezu M. Tensile strength of human pericardium treated with glutaraldehyde. Ann Thorac Cardiovasc Surg. 2012;18:434–7. doi: 10.5761/atcs.oa.11.01804. [DOI] [PubMed] [Google Scholar]
- 14.Al Halees Z, Al Shahid M, Al Sanei A, Sallehuddin A, Duran C. Up to 16 years follow-up of aortic valve reconstruction with pericardium: a stentless readily available cheap valve? Eur J Cardiothorac Surg. 2005;28:200–5. doi: 10.1016/j.ejcts.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Chan KMJ, Rahmen-Haley S, Mittal TK, Gavino JA, Dreyfus GD. Truly stentless autologous pericardial aortic valve replacement: an alternative to standard aortic valve replacement. J Thorac Cardiovasc Surg. 2011;141:276–83. doi: 10.1016/j.jtcvs.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Mao M, El Ters M, Mankad S, Keddis M, Park S, Qian Q. Prosthetic aortic valve stenosis in end-stage renal failure. Int J Nephrol. doi: 10.4061/2011/386368. doi:10.4061/2011/386368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–7. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 18.Holden RM, Sanfilippo AS, Hopman WM, Zimmerman D, Garland JS, Morton AR. Warfarin and aortic valve calcification in haemodialysis patients. J Nephrol. 2007;20:417–22. [PubMed] [Google Scholar]
- 19.Palaniswamy C, Sekhri A, Aronow WS, Kalra A, Peterson SJ. Association of warfarin use with valvular and vascular calcification: a review. Clin Cardiol. 2011;34:74–81. doi: 10.1002/clc.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]