Abstract
OBJECTIVES
The diagnosis of small lung nodules has increased in recent years; limited resection and minimally invasive surgery are highly desirable in patients with these lesions. While wedge resection may be curative for small lung nodules, the technique is sometimes difficult to perform when the tumour nodule is near the pulmonary hilum. In such situations, either anatomical segmentectomy or subsegmentectomy can obtain an adequate surgical margin; port-access thoracoscopic surgery is the preferred type of minimally invasive surgery. Three-dimensional (3D) computed tomography (CT) simulations are reportedly useful in planning and performing thoracoscopic surgery. We use 3D CT simulation to aid thoracoscopic segmentectomy for small lung nodules and subsegmentectomy for even smaller nodules and conduct here a retrospective evaluation of the clinical results of subsegmentectomy. We present our technique for 3D CT simulation-assisted port-access thoracoscopic subsegmentectomy in the superior segment of the left lower lobe.
METHODS
Between July 2008 and June 2012, 15 patients underwent port-access thoracoscopic subsegmentectomy. We evaluated the pathological diagnoses, the tumour sizes, the indications, the operative times and the volumes of blood loss.
RESULTS
Seven patients were diagnosed with lung cancer (LC) and eight had metastatic lung tumours (MLT). The median tumour size was 12 mm. The indication for using this surgical technique was to secure surgical margins in 13 patients (LC, 6; MLT, 7) and because of poor surgical risk in two patients (LC, 1; MLT, 1). The mean surgical time was 166 min and the median blood loss was 19 ml. There were no recurrences.
CONCLUSIONS
Port-access thoracoscopic lung subsegmentectomy using 3D CT simulation can be safely performed and is able to secure adequate surgical margins.
Keywords: Thoracoscopy, Segmentectomy, Subsegmentectomy, Computed tomography, Simulation
INTRODUCTION
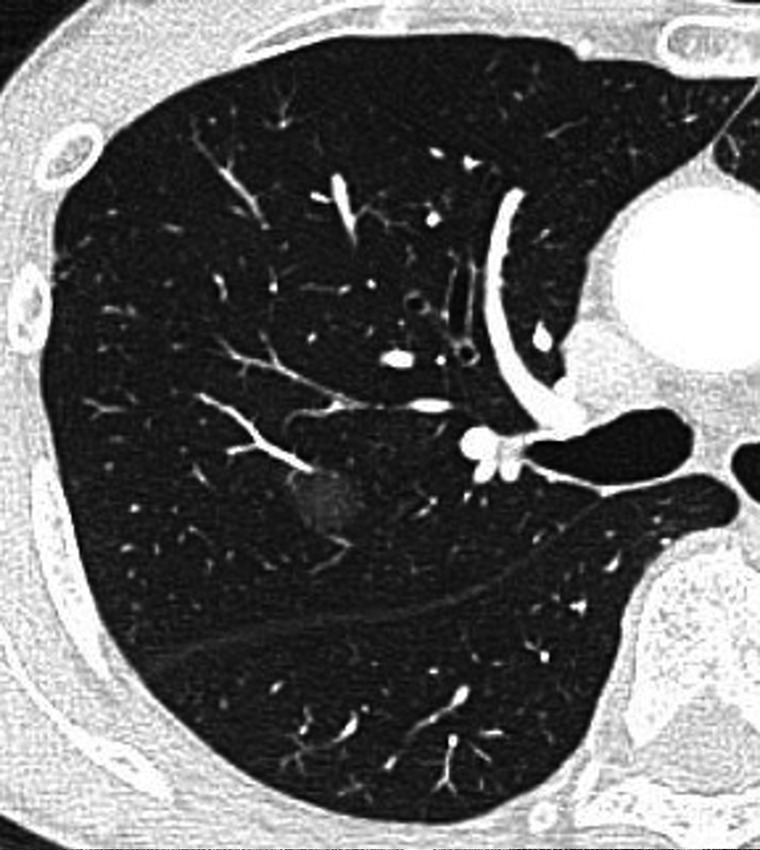
In recent years, the diagnosis of small lung nodules and lung cancers (LCs) with ground-glass opacity (GGO) has been increasing due to developments in computed tomography (CT). It is reported that the prognosis of small nodules with GGO is good, with a limited resection such as segmentectomy or wedge resection [1–3]. Limited resection techniques and minimally invasive surgery techniques are therefore in great demand. Although wedge resections may be sufficient for curing small LCs with GGO, they are sometimes difficult to perform, particularly when the nodule is located near the pulmonary hilum (Fig. 1). Anatomical segmentectomy is preferred in such cases, both to secure an adequate surgical margin and to preserve pulmonary function [4, 5]. However, it can sometimes be unnecessary to perform an entire segmentectomy for smaller lung nodules; a few reports have described the usefulness of subsegmentectomy for smaller lesions [6–8].
Figure 1:
A lung nodule near the pulmonary hilum, containing ground-glass opacity, which was resected by S2b and S3a combined subsegmentectomy.
The minimally invasive port-access thoracoscopic approach is preferred over thoracotomy because of its several advantages: decreased postoperative pain, shortened chest-tube duration, shortened length of hospital stay, faster return to preoperative activity levels and preserved pulmonary function [9, 10]. We therefore perform thoracoscopic subsegmentectomy for smaller indeterminate lung nodules that do not lend themselves to adequate surgical margins with wedge resection.
Three-dimensional (3D) CT simulation provides useful information for thoracoscopic surgery [11]. We previously reported the use of thoracoscopic lung segmentectomy with 3D CT for small nodules [12]; there are few reports, however, of thoracoscopic lung subsegmentectomy. This report describes and evaluates the technique of port-access thoracoscopic lung subsegmentectomy using 3D CT for small lung nodules.
MATERIALS AND METHODS
Our institutional ethics committee approved this retrospective study, and the chairperson waived the need for obtaining study consent from individual patients as consent was obtained for the original surgical procedure. This study involved 15 consecutive patients, six men and nine women, who underwent port-access thoracoscopic subsegmentectomy between July 2008 and June 2012. The median age of the patients was 62 years at the time of surgery (range: 54–82 years).
The numbering and symbols used to denote pulmonary segments or subsegments followed the method described in Yamashita's Roentgenologic Anatomy of the Lung [13]. Subsegmentectomy was defined as a pulmonary segmentectomy at the subsegmental (third order) arterial and bronchial branches.
Indications for port-access thoracoscopic subsegmentectomy
Our patient selection criteria for port-access thoracoscopic subsegmentectomy were as follows: (i) lung tumours of indeterminate nature, but considered suspicious for malignancy; (ii) intentional resection planned of a cT1aN0M0 primary LC tumour, < 2 cm in diameter with a GGO ratio of greater than 80% by high-resolution CT, in patients with good pulmonary function who were able to tolerate lobectomy; (iii) compromised resection in patients who were considered to be poor candidates for lobectomy due to limited cardiopulmonary reserve or other organ failure; (iv) metastases and (v) benign tumours. Wedge resection was considered to be inappropriate in all cases due to the tumour size or its location deep in the parenchyma.
Three-dimensional computed tomography simulation
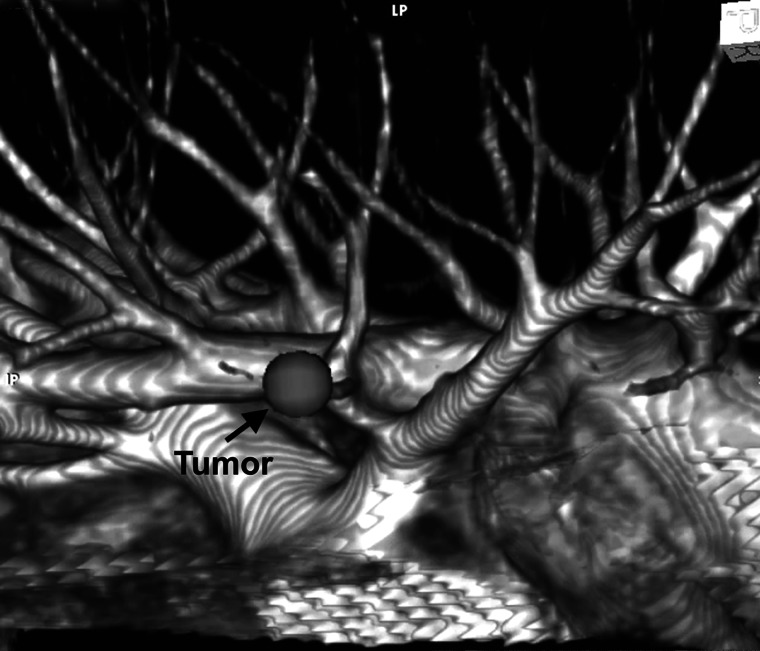
We recorded multidetector CT (MDCT) images after injecting an iodinated contrast medium and saved the Digital Imaging and Communications in Medicine data on a computer server. We used workstations or a client viewer (AquariusNET: TeraRecon, Inc., San Mateo, CA, USA) for image analyses and determined pulmonary arteriovenous structure by using a 3D volume-rendering method; the surgeon processed 3D images within 5 min of their capture. The window level and the window width were adjusted to identify differences in the density of the contrast agent, in order to distinguish between arteries and veins. In the absence of such differences, the arteries and veins were distinguished on the basis of measurements such as axis direction and vessel diameter. The simulation images were used to identify segmental arterial branches, intersegmental veins that were to be preserved and venous branches in the affected segment that were to be divided; these data were used in planning and performing the surgical procedure. The operations were performed while comparing and contrasting the simulation images with real-time conditions in the surgical field, by rotating and resizing the 3D CT images.
Surgical technique
Patients were placed in the lateral decubitus position, and the lung was isolated using a double-lumen endotracheal tube. The surgeon stood near the patient's anterior chest wall, facing a monitor. The assistant stood against the patient's back, facing another monitor positioned to provide an inverted image. The first trocar was inserted along the midaxillary line, through the fourth intercostal space in the case of upper-lobe lesions and through the sixth intercostal space in the case of lower-lobe lesions. An endoscopic rod-lens telescope (5 mm, 30°) was positioned at this port. One 15 or 20 mm flexible trocar and two trocars of 5 mm were inserted subsequently. The arterial branches were dissected and the hilar lymph nodes were subsequently resected; frozen section was performed in patients with LC or suspected malignancy. Only hilar lymph nodes were dissected, and mediastinal lymph nodes dissections were omitted because hilar lymph node metastases were not recognized by the frozen section. Suture ligation was the preferred mode of vessel division. Prior to August 2010, the bronchus was divided before bilateral lung inflation. From August 2010 onwards, the bronchus was threaded and ligated using a monofilament slip knot which was customized based on the previously reported modified Roeder knot [14] (http://www.youtube.com/watch?v=XH2jt7kL3mo). This enabled the surgeon to close the bronchus and to identify the inflation–deflation line between subsegments. The bilateral lungs were inflated, and the bronchus then was divided with a stapler or ligated according to the bronchial diameter.
The individual operative dissections were simulated by the 3D CT volume-rendering method. The parenchyma was dissected along the intersubsegmental pulmonary veins by using either electrocautery or a sealing device, and the venous branches running into the affected subsegment were divided. Staplers were used to divide the parenchyma in the peripheral lung. The resection line was designed on the subsegment line in order to obtain a sufficient surgical margin around the tumour. When it was not possible to obtain adequate surgical margins with simple subsegmentectomy, a few adjacent subsegments were removed in a combined style. The resected lung was removed via the larger trocar incision without enlarging the incision; a chest tube was then inserted at this site.
RESULTS
Patient characteristics
Table 1 presents the preoperative CT findings of subsegmentectomy patients. All pulmonary nodules were indeterminate in nature but considered suspicious for malignancy. Eight patients exhibited nodules of solid density and seven exhibited nodules with GGO. Of the GGO nodules, four of seven had a GGO ratio of less than 100% but greater than 80%, and three were purely GGO. Nodule size ranged from 5 to 16 mm (median: 12 mm) in diameter.
Table 1:
Preoperative CT lung nodule findings
| Preoperative CT findings | Patients (n = 15) |
|---|---|
| Solid | 8 |
| Non-solid | 7 |
| GGO ratio 80–100% | 4 |
| 100% | 3 |
| Tumour size | Median: 12 mm (5–16 mm) |
GGO: ground glass opacity; CT: computed tomography.
Pathological evaluation
Seven patients were diagnosed with primary LC, and the remaining eight were diagnosed with metastatic lung tumours (MLTs) on pathology. All primary LC nodules represented adenocarcinoma [15]: adenocarcinoma in situ (AIS) in six patients and minimally invasive adenocarcinoma (MIA) in one patient (Table 2). There were no lymph node metastases, and all cancers were stage IA. The tumour size ranged from 3 to 18 mm (median: 11 mm) in diameter. All nodules containing GGO on CT were LC, and all solid nodules on CT were MLTs.
Table 2:
Postoperative lung nodule pathology
| Lung cancer | 7 |
| Adenocarcinoma | 7 |
| Adenocarcinoma in situ | 6 |
| Minimally invasive adenocarcinoma | 1 |
| Metastatic lung tumour | 8 |
| Renal cancer | 4 |
| Colon cancer | 2 |
| Malignant melanoma | 1 |
| Laryngeal cancer | 1 |
| Total | 15 |
Resected subsegments
The resected subsegments are shown in Table 3. Of the 15 patients, 12 underwent single subsegmentectomy and three had combined subsegmentectomy.
Table 3:
Resected lung subsegments
| Right | Left | ||
|---|---|---|---|
| 2a | 1 | 3a | 1 |
| 3a | 1 | ||
| 3b | 1 | ||
| 2b + 3a | 1 | ||
| 1b + 3a | 1 | ||
| 4a | 1 | 4a | 1 |
| 6c | 1 | 6b | 1 |
| 7a | 1 | 6c | 1 |
| 8a | 2 | 8a + 9a | 1 |
| Total | 10 | Total | 5 |
Indications for subsegmentectomy
Table 4 presents the indications for subsegmentectomy. In 13 patients, six with LC and seven with MLT, subsegmentectomy was chosen over wedge resection in order to obtain adequate surgical margins; these patients had anatomical features that prompted this choice, such as a tumour location near the pulmonary hilum. Two patients, one with LC and one with MLT, were considered poor surgical risks for segmentectomy or lobectomy because of post-pulmonary resection status in one and coronary heart disease in the other.
Table 4:
Indications for subsegmentectomy
| Variables | Patients (n = 15) |
|---|---|
| Securing surgical margins (insufficient margin for wedge resection) | 13 (LC, 6; MLT, 7) |
| Poor surgical risk | 2 (LC, 1; MLT, 1) |
LC: lung cancer; MLT: metastatic lung tumour.
Surgical parameters
Surgical parameters are given in Table 5. The median operative time was 166 min (range: 71–302 min) and the median blood loss was 19 ml (range: 0–882 ml). One patient, with 882 ml of bleeding from the pulmonary artery, required blood transfusion. The chest tubes were maintained in position for 1–7 days (median: 1 day) after surgery. No hilar lymph node involvement was observed. There were few complications during the peri- or postoperative period. No tumour recurrence was noted during the follow-up period, which ranged from 3 to 49 months (median: 22 months).
Table 5:
Surgical parameters
| Variables | Median (range) |
|---|---|
| Operative time (min) | 166 (71–302) |
| Bleeding (ml) | 19 (0–882) |
| Chest tube duration (days) | 1 (1–7) |
Representative case report
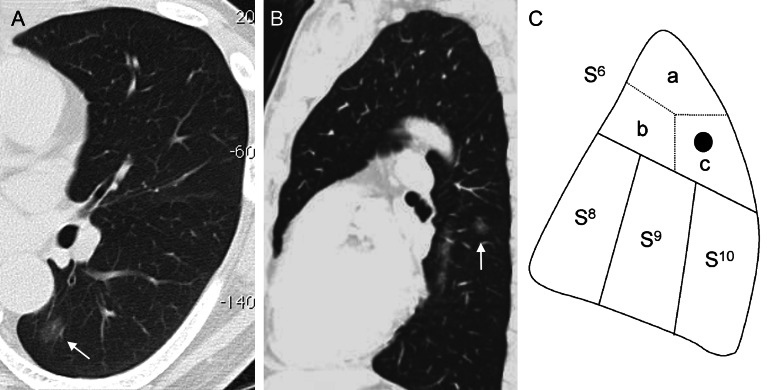
A 54-year old man was admitted to our hospital with a 12-mm non-solid tumour in the superior segment of the left lower pulmonary lobe that was suspicious for bronchioloalveolar carcinoma (Fig. 2A and B). Port-access thoracoscopic subsegmentectomy was planned because of the location of the nodule (Fig. 2C). Vessels and bronchi were identified pre- or intraoperatively using MDCT with the 3D volume-rendering method (Fig. 3). The intersegmental vein was dissected, and the peripheral lung was divided using electrocautery along the vein. The intrasegmental pulmonary artery and bronchus were identified from the posterior approach; the artery was ligated and divided using a bipolar sealing device (BSD), and the bronchus was dissected, ligated using a monofilament thread and cut after the left lung was inflated; the inflation allowed for visualization of the anatomical intersegmental plane for dissection. The intrasegmental veins were identified and ligated; the intersegmental vein was preserved, based on the 3D image. The parenchyma was dissected using electrocautery and the BSD along the inflation–deflation line. The peripheral parenchyma containing the nodule was dissected using a stapling device, allowing a sufficient surgical margin. Frozen section revealed adenocarcinoma, AIS. The operative time was 197 min and blood loss was 127 ml.
Figure 2:
A pulmonary nodule with ground-glass opacity in the superior segment of the left lower pulmonary lobe (CT), which was resected by S6c subsegmentectomy.
Figure 3:
The 3D volume-rendering method used for simulation.
DISCUSSION
In recent years, the discovery of small lung tumours has been increasing as the resolution of CT continues to develop. It is reported that the prognosis is good for patients who undergo resection of small LC nodules [16, 17], and minimal surgery such as wedge resection or segmentectomy is considered sufficient for resection. Although wedge resection is a very simple procedure, it is sometimes insufficient to obtain adequate surgical margins, e.g. with pulmonary nodules in the hilum that are not near the peripheral parenchyma. Wedge resection is also sometimes difficult in patients with indeterminate GGO nodules on CT imaging, as the nodules are sometimes not palpable due to their air content. In such cases, anatomic segmentectomy is reportedly superior to wedge resection for securing surgical margins and diagnosing indeterminate nodules. Recently, Schuchert et al. [18] reported performing anatomical segmentectomy for suspected malignancy in 86.2% of indeterminate pulmonary nodules; anatomical segmentectomy is beneficial in both the diagnosis and the treatment of these nodules.
Port-access thoracoscopy offers the advantages of reduced postoperative pain and pulmonary function preservation and depends on reducing the operative risk in patients who have poor respiratory function or heart disease preoperatively [9, 10]. Minimally invasive surgery techniques are therefore in great demand.
Recent advances in image processing and 3D CT angiography allow the demonstration of the precise structures of pulmonary arteries and veins, thereby enabling surgeons to perform thoracoscopic lung lobectomy and segmentectomy; we described thoracoscopic lung segmentectomy using 3D CT simulation in an earlier article [12]. Moreover, subsegmentectomy reduces the extent of lung volume reduction [6] and is therefore considered more minimally invasive for smaller nodules than segmentectomy. The current study reports the usefulness of port-access thoracoscopic lung subsegmentectomy for small lung nodules.
There have been few reports describing thoracoscopic subsegmentectomy. In our series, we suggested that the procedure should be used for small nodules, suspicious for LC, with a GGO ratio of greater than 80%; over the last decade, several authors have reported that a large GGO ratio is a good prognostic factor in the limited resection of small LC nodules [1, 19–21]. Nakata et al. [20] and Kodama et al. [21] have proposed that patients with tumours that have GGO ratios of greater than 50% should be considered possible candidates for limited resection, although patients with a GGO ratio of 50% exhibited vessel infiltration and experienced local recurrence after wedge resection [20]. We therefore set a larger GGO ratio for subsegmentectomy in our series.
In a postoperative evaluation, we determined that subsegmentectomy is appropriate for LC patients in whom wedge resection would provide insufficient surgical margins or for patients who are considered a poor surgical risk for segmentectomy or lobectomy. The pathological diagnosis in cases suspicious for LC was almost uniformly adenocarcinoma with AIS that has a good prognosis with limited resection. Only one patient had MIA, with its inherent possibility of local recurrence [15, 22]. The indication for subsegmentectomy in this patient was compromised pulmonary function, constituting a poor surgical risk. In this patient with a prior contralateral lobectomy for LC, two tumours were resected with simultaneous S1 segmentectomy and S8a subsegmentectomy because an adequate surgical margin could not be obtained with wedge resection, even though lobectomy are preferred for this pathological type of tumour. Therefore, our indication for subsegmentectomy was an appropriate case for wedge resection in the pathological type but an inappropriate case for wedge resection to secure a surgical margin. We set surgical margins to be larger than the nodule itself or 2 cm [23]. The prognosis of limited resection in patients with small GGO nodules is known to be good [1–3], and we determined that port-access thoracoscopic subsegmentectomy is useful for securing adequate surgical margins in small nodules with a GGO ratio of greater than 80%, although this study is only a preliminary report.
It may seem that port-access thoracoscopic subsegmentectomy is technically complex and difficult to perform. The separation of the segments and subsegments is very important in anatomical segmentectomy or subsegmentectomy and the inflation–deflation line is essential in determining the dissecting plane; the bronchus must be divided and then selectively supplied with air in order to demonstrate the inflation–deflation line. This selective air supply is difficult to apply to the subsegmental bronchus, as it is very small; the operative time was consequently quite long at the beginning of our series. Before August 2010, we had been using the bronchus-pushing method to create the inflation–deflation line. In August 2010, we blindly ligated a bronchus, using a monofilament suture with a knot-pusher, after the subsegmental bronchus was supplied with air. This led to injury of the pulmonary artery with a high blood loss. To avoid this complication in the future, we introduced a method of threading the bronchus with a monofilament suture before bilateral lung inflation; the monofilament suture is pulled to close the bronchus after inflation. With the introduction of this method, subsegmentectomy became easier to perform. We now use a modified Roeder knot to close the bronchus and to create the inflation–deflation line before ligating and cutting the bronchus. The ligation for the bronchus is available because Japanese thoracic surgical literature reports the safety and usefulness of the procedure for lobectomy for LC [24].
There were a few complications experienced in our patient series. One patient required a chest tube for 7 days, for a prolonged air leak after simultaneous S1 segmentectomy and S8a subsegmentectomy. Two patients with severe parenchymal adhesions had a high blood loss during adhesiolysis of pleural adhesion.
No patients in our series experienced tumour recurrence, although the observation period was short. It may stand to reason that recurrences were not recognized because subsegmentectomy was performed in patients in whom a wedge resection was considered adequate to cure their LC [2].
Our procedure is applicable to various anatomical subsegments. We have performed ∼140 port-access thoracoscopic segmentectomies or subsegmentectomies from September 2004; the current series represents 15 subsegmentectomies performed during this period. There are no technical differences between the procedures of thoracoscopic segmentectomy and subsegmentectomy. However, in thoracoscopic subsegmentectomy, the preservation of lung parenchyma is more expected than it is in segmentectomy (Fig. 2c), and it is expected to be better than our previously reported feasibility and safety in thoracoscopic segmentectomy [12, 25]. We therefore consider the subsegmentectomy technique to be both feasible and safe. The follow-up time was too short in this series to properly evaluate postoperative pulmonary function. It is necessary to evaluate this point in the future.
In conclusion, port-access thoracoscopic lung subsegmentectomy can be safely performed, utilizing 3D CT simulation, in order to secure adequate surgical margins for small nodules.
Conflict of interest: none declared.
References
- 1.Nakayama H, Yamada K, Saito H, Oshita F, Ito H, Kameda Y, et al. Sublober resection for patients with peripheral small adenocarcinomas of the lung: surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg. 2007;84:1675–9. doi: 10.1016/j.athoracsur.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida J, Nagai K, Yokose T, Nishimura M, Kakinuma R, Ohmatsu H, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg. 2005;129:991–6. doi: 10.1016/j.jtcvs.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 3.Asamura H. Minimally invasive approach to early, peripheral adenocarcinoma with ground-glass opacity appearance. Ann Thorac Surg. 2008;85:S701–4. doi: 10.1016/j.athoracsur.2007.10.104. [DOI] [PubMed] [Google Scholar]
- 4.Schuchert MJ, Pettiford BL, Keeley S, D'Amato TA, Kilic A, Close J, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–32. doi: 10.1016/j.athoracsur.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Keenan RJ, Landreneau RJ, Maley RH, Jr, Singh D, Macherey R, Bartley S, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78:228–33. doi: 10.1016/j.athoracsur.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Nakamoto K, Omori K, Nezu K Lung Cancer Project Group of West-Seto Inland Sea, Japan. Superselective segmentectomy for deep and small pulmonary nodules under the guidance of three-dimensional reconstructed computed tomographic angiography. Ann Thorac Surg. 2010;89:877–84. doi: 10.1016/j.athoracsur.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimoto K, Nomori H, Mori T, Ohba Y, Shiraishi K, Ikeda K. Combined subsegmentectomy: post operative pulmonary function compared to multiple segmental resection. J Cardiothorac Surgery. 2011;6:17. doi: 10.1186/1749-8090-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okui M, Kohno M, Izumi Y, Asakura K, Nomori H. Combined subsegmentectomy for S2b (horizontal subsegment of the posterior segment) and S3a (lateral subsegment of the anterior segment) in the right upper pulmonary lobe. Gen Thorac Cardiovasc Surg. 2011;59:632–5. doi: 10.1007/s11748-010-0738-0. [DOI] [PubMed] [Google Scholar]
- 9.Kaseda S, Aoki T, Hangai N, Shimizu K. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg. 2000;70:1644–6. doi: 10.1016/s0003-4975(00)01909-3. [DOI] [PubMed] [Google Scholar]
- 10.Atkins BZ, Harpole DH, Jr, Manngum JH, Toloza EM, D'Amico TA, Burfeind WR., Jr Pulmonary segementectomy by thoracotomy or thoracoscopy: Reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg. 2007;84:1107–13. doi: 10.1016/j.athoracsur.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Akiba T, Marushima H, Harada J, Kobayashi S, Morikawa T. Importance of preoperative imaging with 64-row three-dimensional multidetector computed tomography for safer video-assisted thoracic surgery in lung cancer. Surg Today. 2009;39:844–7. doi: 10.1007/s00595-009-3965-1. [DOI] [PubMed] [Google Scholar]
- 12.Oizumi H, Kanauchi N, Kato H, Endoh M, Suzuki J, Fukaya K, et al. Anatomical thoracoscopic pulmonary segmentectomy under three-dimensional multi-detector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg. 2011;141:678–82. doi: 10.1016/j.jtcvs.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita H. Roentgenologic Anatomy of the Lung. Tokyo: Igaku-Shoin; 1978. pp. 1–389. [Google Scholar]
- 14.Zhilin OV. Modified roeder loop for laparoscopic surgery. Surg Laparosc Endosc. 1996;6:76–7. [PubMed] [Google Scholar]
- 15.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Port JL, Kent MS, Korst RJ, Libby D, Pasmantier M, Altorki NK. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest. 2003;124:1828–33. doi: 10.1378/chest.124.5.1828. [DOI] [PubMed] [Google Scholar]
- 17.Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–75. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 18.Schuchert MJ, Abbas G, Awais O, Pennathur A, Nason KS, Wilson DO, et al. Anatomic segmentectomy for the solitary pulmonary nodule and early-stage lung cancer. Ann Thorac Surg. 2012;93:1780–7. doi: 10.1016/j.athoracsur.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 19.Takashima S, Maruyama Y, Hasegawa M, Yamanda T, Honda T, Kadoya M, et al. Prognostic significance of high-resolution CT findings in small peripheral adenocarcinoma of the lung: a retrospective study on 64 patients. Lung Cancer. 2002;36:289–95. doi: 10.1016/s0169-5002(01)00489-5. [DOI] [PubMed] [Google Scholar]
- 20.Nakata M, Sawada S, Yamashita M, Saeki H, Kurita A, Takashima S, et al. Objective radiologic analysis of ground-glass opacity aimed at curative limited resection for small peripheral non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;129:1226–31. doi: 10.1016/j.jtcvs.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Kodama K, Higashiyama M, Takami K, Oda K, Okami J, Maeda J, et al. Treatment strategy for patients with small peripheral lung lesion(s): intermediate-term results of prospective study. Eur J Cardiothorac Surg. 2008;34:1068–74. doi: 10.1016/j.ejcts.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi M, Morikawa A, Kawasaki M, Matsuno Y, Yamada T, Hirohashi S, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844–52. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Sawabata N, Ohta M, Matsumura A, Nakagawa K, Hirano H, Maeda H, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg. 2004;77:415–20. doi: 10.1016/S0003-4975(03)01511-X. [DOI] [PubMed] [Google Scholar]
- 24.Sato K, Li M, Ono N, Yokomise H. A study of a simple ligation method for bronchus. Jpn J Chest Surg. 2002;16:776–8. Japanese Ed. [Google Scholar]
- 25.Oizumi H, Kanauchi N, Kato H, Endoh M, Takeda S, Suzuki J, et al. Total thoracoscopic pulmonary segmentectomy. Eur J Cardiothorac Surg. 2009;36:374–7. doi: 10.1016/j.ejcts.2009.03.038. [DOI] [PubMed] [Google Scholar]