Abstract
OBJECTIVES
Absent pulmonary valve syndrome (APVS) is a rare cardiac malformation that is usually associated with aneurysmal dilatation of pulmonary arteries and respiratory distress. The surgical mortality of neonates and infants with APVS has decreased tremendously, from 60% in 1980s to 10–20% recently. This study retrospectively reviews surgical outcomes of our 10-year experience in patients with APVS.
METHODS
From 2002 to 2012, 42 patients with APVS underwent surgical correction. Thirty-seven patients had APVS as a variant of tetralogy of Fallot, 4 with double outlet right ventricle and 1 with ventricular septal defect. Respiratory distress was present in 12 infants. Four patients needed continuous positive airway pressure and 5 required intubation with mechanical ventilation before surgery.
RESULTS
There was no hospital death and 3 late deaths. The mean follow-up time was 62.71 ± 34.31 months. Significant differences were found in the duration of postoperative ventilation between patients with or without respiratory distress (P = 0.009) and patients with left or right aortic arch (P = 0.012). The Kaplan–Meier curve indicated that overall survival at 5 and 10 years was 92.4%. The survival rates between patients with or without respiratory distress were 72.7 and 100%, respectively (P = 0.003). Overall mortality was associated with longer cardiopulmonary bypass time (P = 0.004) and lower weight at operation (P = 0.042). There were no significant differences in survival and postoperative data such as the duration of ventilation or intensive care unit stay and New York Heart Association class among the three methods of right ventricular outflow tract (RVOT) reconstruction.
CONCLUSIONS
Surgical treatment of APVS has got favourable outcomes in terms of mortality and reoperation rate. Different methods of RVOT reconstruction do not affect the surgical outcome. Patients required long-term follow-up for postoperative respiratory complications secondary to persistent bronchomalacia.
Keywords: Congenital heart disease, Pulmonary valve, Surgery
INTRODUCTION
Absent pulmonary valve syndrome (APVS) is a rare congenital cardiac malformation, first described by Chevers [1] in 1846. Rarely isolated, it is mostly associated with tetralogy of Fallot (TOF), comprising ∼3–6% of patients in TOF [2, 3]. The anatomy of this syndrome has its own specific features of agenesis or absent pulmonary valve leaflets, pulmonary annulus stenosis, moderate-to-severe pulmonary insufficiency, aneurysmal dilatation of pulmonary arteries (PAs) and its first- and second-order branches, leading to the compression of the tracheobronchial tree especially in neonates and infants, who may require preoperative intubation and ventilation after birth.
In the past decades, surgical management for the repair of this cardiac anomaly as well as relief of the obstruction of the tracheobronchial tree has evolved. Surgical mortality for APVS in neonates and infants has decreased tremendously, from 60% in 1980s [4] to 10–20% [5–7] in recent years. Nevertheless, rates of reintervention for persistent respiratory symptoms and pulmonary insufficiency remain high years after surgery.
We retrospectively reviewed 42 patients with APVS in our hospital to determine the best treatment for APVS and to assess our surgical approach with regard to the management of dilated PAs and reconstruction of the right ventricular outflow tract (RVOT).
PATIENTS AND METHODS
Forty-two patients with APVS underwent surgical correction from July 2002 to July 2012. Patients were identified by echocardiography, computed tomography scan and cardiac catheterization. There were 21 males and 21 females. Median age and weight at repair were 9.5 (range: 1–84 months) and 8.0 (range, 3.5–18 kg), respectively. Twenty-two children were infants aged 31 days to 1 year. No neonates were included. Twelve infants had apparent symptoms of severe respiratory distress before surgery. Among them, 4 patients needed continuous positive airway pressure and 5 required intubation with mechanical ventilation. The 12 infants also had preoperative bronchoscopy for a fundamental measure of the degree of airway distress. Left bronchial compression was found in 6 patients and right bronchial compression in 4. The remaining 2 patients had both left and right bronchial compression. All patients had preoperative pulmonary insufficiency, 16 of whom displayed severe pulmonary regurgitation. No one underwent a palliative procedure.
Thirty-seven patients had APVS as a variant of TOF, 4 with double outlet right ventricle and 1 with ventricular septal defect (VSD). Other associated cardiovascular lesions included atrial septal defect (ASD) in 23 patients, right aortic arch in 8, left persistent superior vena cava in 3, major aorto-pulmonary collateral arteries (MAPCAs) in 3 and anomalous right coronary artery crossing the RVOT in 2.
Surgical technique
Complete correction was performed with the aid of cardiopulmonary bypass, moderate systemic hypothermia (25–28°C) and intermittent cold-blood cardioplegia. All patients received correction as a one-stage repair. A longitudinal incision was made initially in the main PA and extended into the infundibulum of the RVOT, which allowed an overall assessment of the anterior malalignment VSD, infundibular muscle bundles, hypoplastic pulmonary annulus and the dilated PAs. VSD was closed through a transatrial and transpulmonary approach using different patches, including autologous pericardium (n = 18), xenograft pericardium (n = 1) and Dacron patch (n = 23). Infundibular resection was performed in patients with TOF to relieve obstruction of the RVOT. ASD was closed by direct suture for most of the patients except 4 for elevated central venous pressure after bypass.
The surgical treatment of dilated PAs differed widely from patients in consideration of their varying degrees of dilation and airway distress. Seven patients did not receive any intervention on their main PA or PA branches. A reduction pulmonary arterioplasty was performed in the remaining 35 patients (Table 1). Plication of dilated PAs was only used in patients with mid-respiratory distress. Posterior wall excision, or a combination of plication and wall reduction were not performed.
Table 1:
Different types of PA arterioplasty and RVOT reconstruction
| PA arterioplasty and RVOT reconstruction | Number of patients |
|---|---|
| Number of patients | 42 |
| Type of PA arterioplasty | |
| Plication of RPA | 3 |
| Plication of LPA and RPA | 4 |
| Anterior resection of RPA | 8 |
| Anterior resection of LPA and RPA | 17 |
| Anterior resection of MPA, LPA and RPA | 3 |
| Type of RVOT reconstruction | |
| Transannular patch | 17 |
| Monocusp valve | 18 |
| Homograft valved conduit | 2 |
| Contegra bovine jugular vein valved conduit | 4 |
| Gore-Tex conduit | 1 |
RPA: right pulmonary artery; LPA: left pulmonary artery; MPA: main pulmonary artery.
RVOT reconstruction was then accomplished following the management of dilated PAs, including transannular patch, monocusp valve, homograft valved conduit, Contegra bovine jugular vein valved conduit and Gore-Tex conduit (Table 1). A monocusp valve was made of either autologous pericardium (n = 17) or polytetrafluoroethylene (n = 1).
The Lecompte manoeuvre was applied to 2 patients by dividing the ascending aorta and translocation of the mobilized PAs anterior to the aorta, away from the tracheobronchial tree. In 3 patients, MAPCAs were treated with transcatheter closure before surgery.
Two patients with anomalous right coronary arteries crossing the RVOT underwent different techniques of surgical correction. In 1 case, the main PA was transected above the annulus and was pulled down over the anomalous right coronary artery to a transverse incision in the RVOT. A direct anastomosis was made between the posterior wall of the divided main PA and the transverse incision. The anterior wall was enlarged with a monocusp valve patch. In the other patient, the type of RVOT reconstruction provided a palliative shunt between the main PA and RVOT with the aid of an extracardiac Gore-Tex conduit.
Statistical analysis
Data were analysed with the statistical computing package SPSS 20.0. The continuous data were expressed as median and mean ± standard deviation. Two-group comparisons were assessed with Student's t-tests for continuous variables that were normally distributed and Fisher's exact tests for categorical data. Three-group comparisons for the continuous data were tested by one-way analysis of variance. An overall survival rate was estimated with a Kaplan–Meier curve. Differences between survival curves were identified with the log-rank test. A Cox regression hazards model was used to evaluate several variables as potential risk factors for long-term survival, including age and weight at operation, preoperative airway distress, preoperative ventilation, left or right aortic arch, duration of cardiopulmonary bypass and aortic cross-clamping time, type of PA arterioplasty and RVOT reconstruction. P-values <0.05 were considered statistically significant.
RESULTS
Early results
No hospital death occurred in this group. Right atrial thrombosis was found in 1 patient 4 days after surgery, but this patient was discharged against advice for financial problems. Postoperative data are summarized in Table 2. Several variables were evaluated among the groups, such as cardiopulmonary bypass and aortic cross-clamping time, duration of intensive care unit (ICU) stay and postoperative ventilation. The major differences were revealed in the duration of postoperative ventilation between patients with or without respiratory distress (P = 0.009) and patients with left or right aortic arch (P = 0.012). The cardiopulmonary bypass time of patients undergoing transannular patch was shorter than that of those with the monocusp valve (P = 0.031) and valved conduit (P = 0.001).
Table 2:
Early and late results of patients with APVS
| Postoperative data | Mean/median (range) or number |
|---|---|
| Number of patients | 42 |
| Cardiopulmonary bypass time (min) | 106.30 ± 38.40 (55–283) |
| Aortic cross-clamping time (min) | 64.16 ± 19.71 (27–135) |
| Intensive care unit stay (days) | 4 (2–64) |
| Duration of ventilation (h) | 31 (11–955) |
| Early death (n) | 0 |
| Late death (n) | 3 |
| Complications | |
| Respiratory complications | |
| Mild bronchomalasia (n) | 5 |
| Severe bronchomalasia (n) | 2 |
| Mild left bronchial distress (n) | 1 |
| Cardiac complications | |
| Low cardiac output (n) | 5 |
| Slight residual VSD (n) | 4 |
| Main PA stenosis (n) | 6 |
| Reoperation (n) | 2 |
Postoperative complications can be divided into two major groups, unsatisfactory respiratory relief and cardiac repair. Unsatisfactory respiratory relief was closely related to persistent bronchomalacia caused by long-term compression of the tracheobronchial tree (Table 2). Postoperative bronchoscopy was necessary in these patients to evaluate the severity of bronchomalacia. One patient undergoing Lecompte manoeuvre was found to have mild left bronchial distress, caused by the posterior translocated ascending aorta instead of the dilated PAs. All of them were ventilated for >120 h, with the longest duration of 955 h. Cardiac complications were less severe than respiratory complications, including low cardiac output in 5 patients and slight residual VSD in 4.
Late results
Three late deaths occurred during the follow-up. The cause of death was mainly attributed to severe pneumonia 2, 5 and 7 months, postoperatively. All 3 patients were infants with preoperative ventilation and prolonged postoperative ventilator support for unsatisfactory respiratory relief, including severe bronchomalacia (n = 2) and mild left bronchial distress (n = 1). The follow-up data were available for all but 1 patient, who gave up therapy automatically. The mean follow-up time was 62.71 ± 34.31 (range: 1–120 months). The Kaplan–Meier curve indicated that overall survival at 5 and 10 years was 92.4%. The survival rates between patients with or without respiratory distress were 72.7 and 100%, respectively (P = 0.003, log-rank test; Fig. 1). There were no significant differences in survival among the three different types of RVOT reconstruction (P = 0.052, log-rank test) (Fig. 2). On univariate analysis, overall mortality was associated with longer cardiobypass time (P = 0.004) and lower weight at operation (P = 0.042). However, we were unable to identify any risk factor for long-term survival on multivariate analysis.
Figure 1:
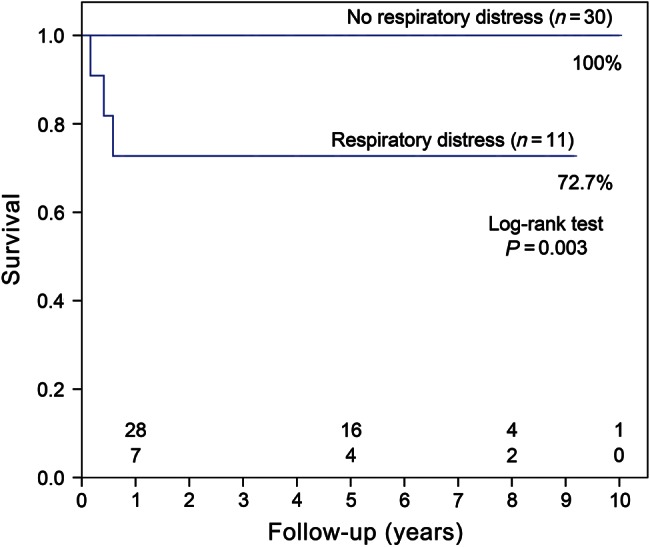
Kaplan–Meier survival between patients with or without respiratory distress.
Figure 2:
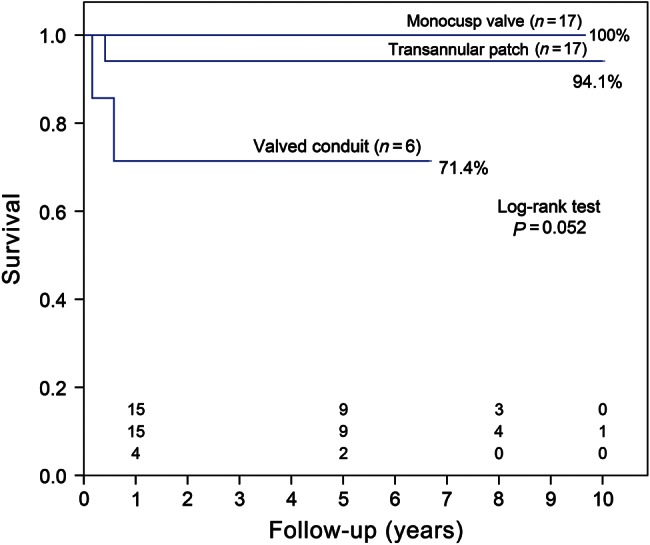
Kaplan–Meier survival among the three methods of RVOT reconstruction.
Echocardiography and electrocardiogram examinations were suggested for all patients during the follow-up period. Moderate pulmonary regurgitation was present in the surviving 38 patients, regardless of whether a pulmonary valve was inserted. PA stenosis was another cardiac complication that mattered for patients with APV and TOF. Six patients in our group suffered from main PA stenosis. The mean gradient across the stenosis site was 44.78 ± 12.21 (range: 31.9–67 mmHg).
Reoperation was performed in 2 patients for distal main PA stenosis. These 2 patients were back to hospital 1 month and 6 months later for surgical enlargement of narrowing main PA, respectively. The patch augmentation was also extended to the origin of the right PA in 1 patient. No signs or symptoms indicated reoperation in any patient with pulmonary insufficiency.
Two patients were readmitted to the hospital 2 months after initial operation for pneumonia and tachypnoea caused by persistent bronchomalacia. Both of them were reintubated and underwent bronchoscopy to exclude other causes of airway compression. After positive pressure ventilation and effective anti-infection therapy, they fully recovered from pneumonia and were discharged from the hospital.
All survivors remained in good condition, in New York Heart Association (NYHA) Class I or II. No differences were found in postoperative NYHA classification among the three types of RVOT reconstruction.
DISCUSSION
The pathological anatomy of aneurysmal dilatation of PAs in APVS is an issue of the utmost importance to surgeons. The dilatated PAs, especially the right PAs, compress the tracheobronchial tree in varying degrees. In a study by Momma et al. [8], the diameter of the right PA inversely correlated with the diameter of the right bronchi. In other words, patients with increased right PA dilatation may experience more severe symptoms.
It is interesting that some patients were able to survive in the neonatal period and infancy with mild or even no respiratory symptoms, while others suffered from severe airway complications immediately after birth. It is so far unclear what makes the differences between the two groups. Degrees of dilatated PAs may be one of the answers. But we are wondering if the aortic arch plays an important role in the mechanism as well. At our institute, none of the 8 patients with right aortic arch presented any symptom of respiratory distress. Two of them were even <6 months. Similarly, Nölke et al. [9] reported a case of a 5-month infant with no clinical signs. This drew our attention to the fact that patients with a right aortic arch may experience less severe airway compression than those with a left aortic arch. The duration of ICU stay and postoperative ventilation were significantly shorter in patients with a right aortic arch.
The debate of surgical management of APVS mainly concentrates on two points: reduction PA arterioplasty and reconstruction of RVOT. Both are dedicated to minimizing postoperative mortality and complications. There is no doubt that reduction PA arterioplasty should be applied to all patients with aneurysmal dilatation of PAs. But this procedure is frequently individualized to each patient during surgery after an overall assessment of the degree of PA dilatation. At our institute, plication and anterior wall excision were the only two methods used for patients. To some extent, plication can decrease wall tension as well as reduce the diameter of dilatated PAs. But the limited capability of PA reduction makes plication only suitable for patients with mild respiratory symptoms.
Some surgeons have also recommended total resection of the dilatated PAs [10]. Nevertheless, we think it is unnecessary to perform such procedures. Although simply removing the entire dilatated PA theoretically eliminates most of the abnormal PA walls, which can largely avoid the possibility of further PA dilatation and airway compression, no patients in our group undergoing plication or resection presented postoperative PA re-dilatation. Secondly, the anastomosis between the posterior walls of the divided right and left PAs may provide a high-tension suture. Thirdly, the use of valved conduit to reconstruct the RVOT significantly increases the reoperation rate.
No matter what methods were used, all patients underwent the procedure of right PA reduction. As mentioned above, the right PA dilatation is closely related to airway distress, hence it is important to resect the dilatated right PAs to an appropriate size. Dissolution of the right PAs from branch to hilum is beneficial to further arterioplasty, which can also avoid postoperative distortion and stenosis. Moreover, it is recommended not to excise much tissue at the angle of the main and right PAs in the case of a too-acute angle at this point [11].
Reconstruction of RVOT is another complicated part of the surgery. In constrast to that in isolated TOF, the primary concern of RVOT reconstruction in APVS is whether a pulmonary valve should be inserted or not. However, there is no consensus on the optimal method of RVOT management. In general, postoperative pulmonary valve insufficiency has some side effects on the outcome. On one hand, an incompetent pulmonary valve will largely increase the volume load of the right ventricle, thus leading to long-term right ventricular dysfunction and continuous exercise intolerance, or even sudden death caused by acute arrhythmias [12, 13]. On the other hand, due to the increased pulmonary blood flow, pulmonary valve regurgitation will enhance the risk of persistent PA dilation and airway distress [14].
We reviewed the methods and opinions on RVOT reconstruction in APVS of several groups (Table 3). Some surgeons are in favour of the surgical strategy that a pulmonary valve should be inserted, while others find it unnecessary. No matter what methods were used, the outcome was favourable in both groups in terms of survival and reoperation rate. The reason why some surgeons did not support insertion of a pulmonary valve is because their follow-up data of patients with transannular patch were also remarkable and uneventful [15, 16]. Meanwhile, the benefit of an inserted pulmonary valve in maintaining stable haemodynamics was too limited to be evaluated. Though monocusp valve has a potential advantage in the management of RVOT, the outcome may be far from our previous expectation. But the fact remains that no group can be absolutely certain that all patients without implantation of a pulmonary valve will be free of more than moderate pulmonary regurgitation.
Table 3:
Reports of methods of RVOT reconstruction in patients with APVS
| Study | Total patients | RVOT reconstruction |
Support valve inserted | |||
|---|---|---|---|---|---|---|
| Valved conduit | Monocusp/bioprosthetic valve | Transannular patch | Other | |||
| Hew et al. [10] | 54 | 15 | 0 | 27 | 12 | No |
| Brown et al. [7] | 20 | 8 | 6 | 5 | 1 | Yes |
| Chen et al. [11] | 20 | 4 | 0 | 13 | 3 | No |
| Norgaard et al. [17] | 36 | 5 | 14 | 17 | 0 | No difference |
| Alsoufi et al. [18] | 61 | 31 | 26 | 4 | 0 | Yes |
At our institute, there were no significant differences in survival and postoperative data such as duration of ventilation or ICU stay and NYHA class among the three methods of RVOT reconstruction. Until now, no patients have experienced more than moderate pulmonary valve insufficiency in each group and shown any sign of right ventricular dysfunction. It is supposed that the types of RVOT reconstruction do not affect surgical outcome in the early- and mid-term follow-up. But we lack pre- and postoperative data of changes in the right ventricle volume and the comparison among the different methods.
Though surgical techniques have improved during the last decades, persistent bronchomalacia after surgery remains a great challenge for surgeons. Respiratory complications are mainly manifested by prolonged duration of ventilation and severe pulmonary infection. Generally, prolonged ventilation increases the risk of pulmonary infection while, continuous infection, in turn, extends the duration of ventilation. Even mild pulmonary infection would cause tachypnoea and stridor, leading to a poor recovery. Thus, intensive care should be given to patients for quite a long period after surgery. Proper methods such as prone position, positive pressure ventilation and effective anti-infection therapy are certainly advantageous to postoperative care.
LIMITATIONS
There are several limitations in this report. This retrospective review is not a randomized clinical trial so the results are undoubtedly influenced by selection bias such as the heterogeneousity of the patient and surgical methods. This single-centre series includes a relatively small group of patients and number of events to yield any meaningful comparison and results. It is a pity that we can only extend the follow-up time to a maximum of 10 years. Further investigation should be made to draw sound conclusions. We also lack detailed data of pre- and postoperative RV function in this report. Fortunately, a well-organized follow-up programme has been established recently, involving the measurement of RV function with the use of magnetic resonance image.
CONCLUSIONS
In conclusion, surgical treatment of APVS demonstrates favourable outcomes in terms of mortality and reoperation rate. It is our current strategy to use plication and anterior wall excision as methods of PA arterioplasty, in which right PA reduction should be paid massive attention. No major differences were found in survival rate and postoperative data among patients with the pulmonary valve and those without. Postoperative respiratory complications are difficult to handle and there remain unanswered questions, that only a long-term follow-up can answer.
Conflict of interest: none declared.
REFERENCES
- 1.Chevers N. Recherches sur les maladies de l'artère pulmonaire. Arch Gen Med. 1847;15:488–508. [Google Scholar]
- 2.Lev M, Eckner F. The pathologic anatomy of tetralogy of Fallot and its variations. Chest. 1964;45:251–61. doi: 10.1378/chest.45.3.251. doi:10.1378/chest.45.3.251. [DOI] [PubMed] [Google Scholar]
- 3.Rao B, Anderson R, Edwards J. Anatomic variations in the tetralogy of Fallot. Am Heart J. 1971;81:361–71. doi: 10.1016/0002-8703(71)90106-2. doi:10.1016/0002-8703(71)90106-2. [DOI] [PubMed] [Google Scholar]
- 4.Dunnigan A, Oldham NH, Benson DW. Absent pulmonary valve syndrome in infancy: surgery reconsidered. Am J Cardiol. 1981;48:117–22. doi: 10.1016/0002-9149(81)90580-4. doi:10.1016/0002-9149(81)90580-4. [DOI] [PubMed] [Google Scholar]
- 5.Godart F, Houyel L, Lacour-Gayet F, Serraf A, Sousa-Uva M, Bruniaux J, et al. Absent pulmonary valve syndrome: surgical treatment and considerations. Ann Thorac Surg. 1996;62:136–42. doi: 10.1016/0003-4975(96)00276-7. doi:10.1016/0003-4975(96)00276-7. [DOI] [PubMed] [Google Scholar]
- 6.Dodge-Khatami A, Backer CL, Holinger LD, Baden HP, Mavroudis C. Complete repair of tetralogy of Fallot with absent pulmonary valve including the role of airway stenting. J Card Surg. 1999;14:82–91. doi: 10.1111/j.1540-8191.1999.tb00955.x. doi:10.1111/j.1540-8191.1999.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown JW, Ruzmetov M, Vijay P, Rodefeld MD, Turrentine MW. Surgical treatment of absent pulmonary valve syndrome associated with bronchial obstruction. Ann Thorac Surg. 2006;82:2221–6. doi: 10.1016/j.athoracsur.2006.07.022. doi:10.1016/j.athoracsur.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Momma K, Ando M, Takao A. Fetal cardiac morphology of tetralogy of Fallot with absent pulmonary valve in the rat. Circulation. 1990;82:1343–51. doi: 10.1161/01.cir.82.4.1343. doi:10.1161/01.CIR.82.4.1343. [DOI] [PubMed] [Google Scholar]
- 9.Nölke L, Azakie A, Anagnostopoulos PV, Alphonso N, Karl TR. The Lecompte maneuver for relief of airway compression in absent pulmonary valve syndrome. Ann Thorac Surg. 2006;81:1802–7. doi: 10.1016/j.athoracsur.2005.12.001. doi:10.1016/j.athoracsur.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Hew CC, Daebritz SH, Zurakowski D, del Nido PI, Mayer JE, Jr, Jonas RA. Valved homograft replacement of aneurysmal pulmonary arteries for severely symptomatic absent pulmonary valve syndrome. Ann Thorac Surg. 2002;73:1778–85. doi: 10.1016/s0003-4975(02)03511-7. doi:10.1016/S0003-4975(02)03511-7. [DOI] [PubMed] [Google Scholar]
- 11.Chen JM, Glickstein JS, Margossian R, Mercando ML, Hellenbrand WE, Mosca RS, et al. Superior outcomes for repair in infants and neonates with tetralogy of Fallot with absent pulmonary valve syndrome. J Thorac Cardiovasc Surg. 2006;132:1099–104. doi: 10.1016/j.jtcvs.2006.05.049. doi:10.1016/j.jtcvs.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 12.Cheung MH, Konstantinov IE, Redington AN. Late complications of repair of tetralogy of Fallot and indications for pulmonary valve replacement. Semin Thorac Cardiovasc Surg. 2005;17:155–9. doi: 10.1053/j.semtcvs.2005.02.006. doi:10.1053/j.semtcvs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–81. doi: 10.1016/S0140-6736(00)02714-8. doi:10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 14.Donofrio M, Jacobs M, Rychik J. Tetralogy of Fallot with absent pulmonary valve: echocardiographic morphometric features of right-sided structures and their relationship to presentation and outcome. J Am Soc Echocardiogr. 1997;10:556–61. doi: 10.1016/s0894-7317(97)70010-5. doi:10.1016/S0894-7317(97)70010-5. [DOI] [PubMed] [Google Scholar]
- 15.Meijboom F, Szatmari A, Deckers JW, Utens EM, Roelandt JR, Bos E, et al. Cardiac status and health-related quality of life in the long term after surgical repair of tetralogy of Fallot in infancy and childhood. J Thorac Cardiovasc Surg. 1995;110:883–91. doi: 10.1016/s0022-5223(05)80154-0. doi:10.1016/S0022-5223(05)80154-0. [DOI] [PubMed] [Google Scholar]
- 16.Bacha EA, Scheule AM, Zurakowski D, Erickson LC, Hung J, Lang P, et al. Long-term results after early primary repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2001;122:154–61. doi: 10.1067/mtc.2001.115156. doi:10.1067/mtc.2001.115156. [DOI] [PubMed] [Google Scholar]
- 17.Norgaard MA, Alphonso N, Newcomb AE, Brizard CP, Cochrane AD. Absent pulmonary valve syndrome. Surgical and clinical outcome with long-term follow-up. Eur J Cardiothorac Surg. 2006;29:682–7. doi: 10.1016/j.ejcts.2006.01.050. doi:10.1016/j.ejcts.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 18.Alsoufi B, Williams WG, Hua Z, Cai S, Karamlou T, Chan CC, et al. Surgical outcomes in the treatment of patients with tetralogy of Fallot and absent pulmonary valve. Eur J Cardiothorac Surg. 2007;31:354–9. doi: 10.1016/j.ejcts.2006.12.001. doi:10.1016/j.ejcts.2006.12.001. [DOI] [PubMed] [Google Scholar]


